Submitted:
23 May 2023
Posted:
25 May 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Results
2.1. Molecular sequence data and phylogenetic analysis
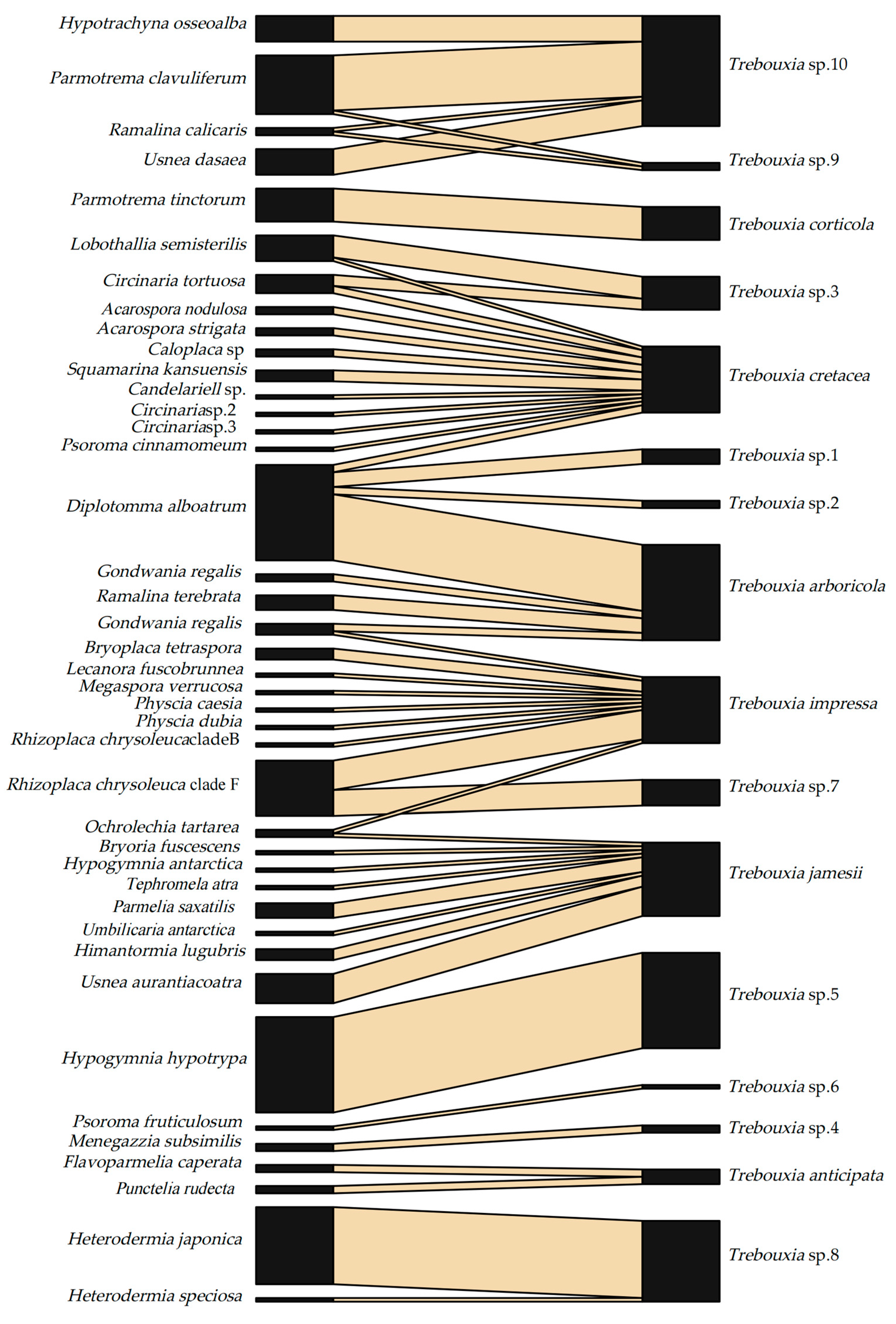
2.2. Mutualistic association pattern between LFF and LFA
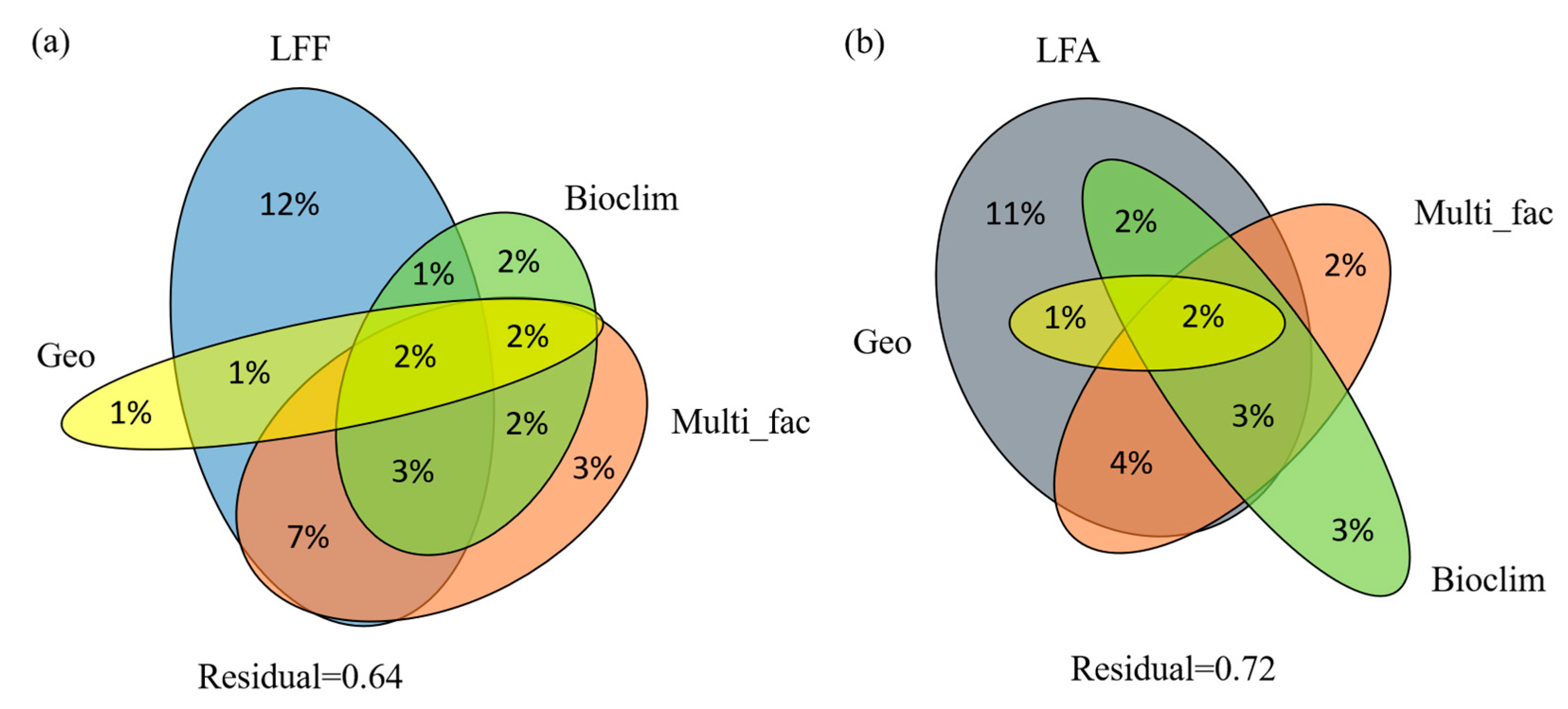
2.3. Correlation among LFF, LFA, and ecological factors
2.4. Cophylogenetic analyses
3. Discussion
4. Materials and Methods
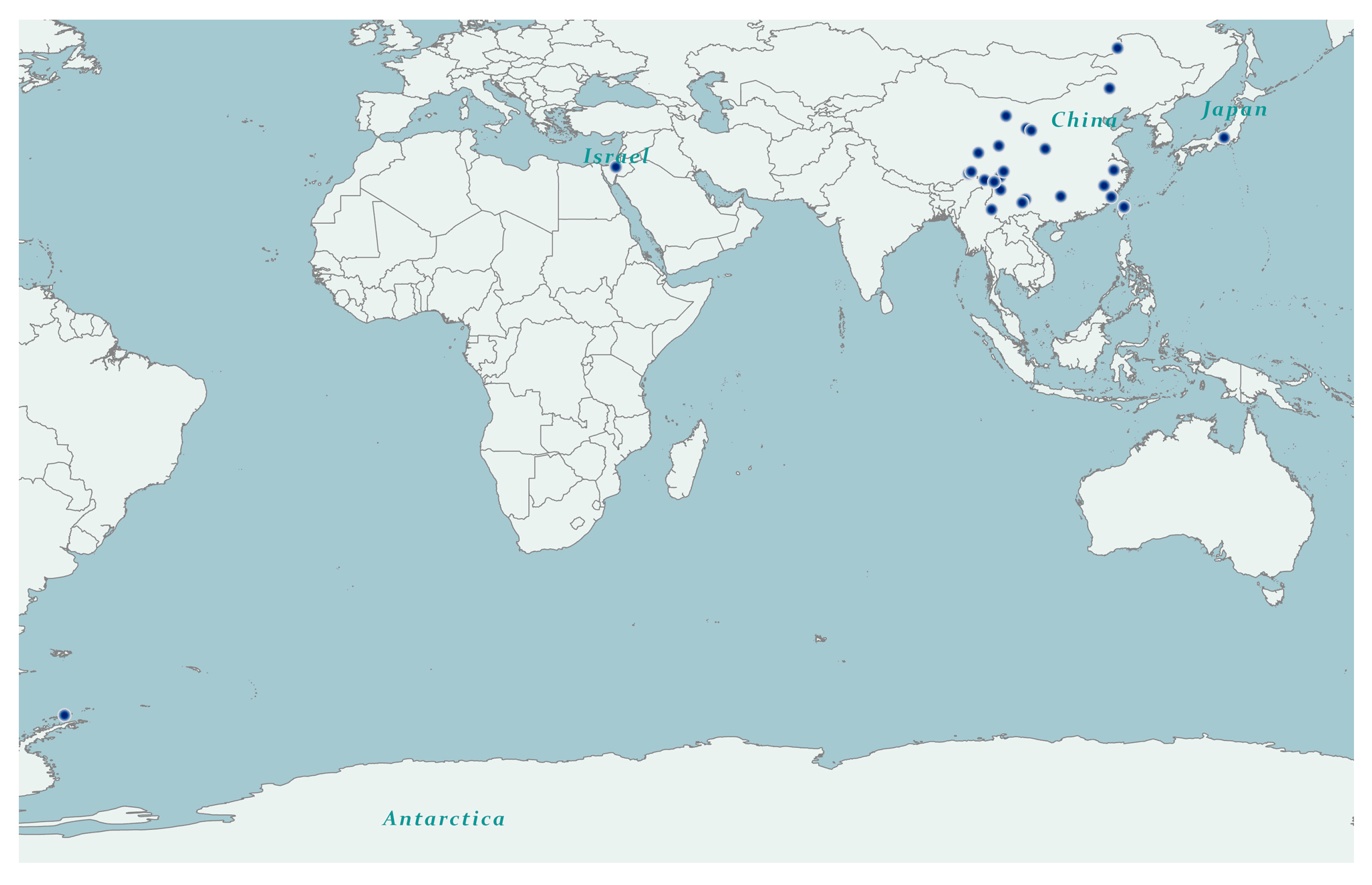
4.1. Taxon sampling
4.2. DNA extraction, amplification, and sequencing
4.3. DNA alignment and phylogenetic analysis
4.4. Species delimitation
4.5. Interaction network analyses
4.6. Variation partitioning analysis
4.7. Cophylogenetic analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota–Approaching one thousand genera. The Bryologist 2017, 119, 361–416. [Google Scholar] [CrossRef]
- Gargas, A.; DePriest, P.T.; Grube, M.; Tehler, A. Multiple origins of lichen symbioses in fungi suggested by SSU rDNA phylogeny. Science 1995, 268, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Lutzoni, F.; Pagel, M.; Reeb, V. Major fungal lineages are derived from lichen symbiotic ancestors. Nature 2001, 411, 937–940. [Google Scholar] [CrossRef]
- Lücking, R.; Nelsen, M.P. Ediacarans, protolichens, and lichen-derived Penicillium: A critical reassessment of the evolution of lichenization in fungi. Transformative Paleobotany 2018, 551–590. [Google Scholar] [CrossRef]
- Muggia, L.; Nelsen, M.P.; Kirika, P.M.; Barreno, E.; Beck, A.; Lindgren, H.; Lumbsch, H.T.; Leavitt, S.D. Formally described species woefully underrepresent phylogenetic diversity in the common lichen photobiont genus Trebouxia (Trebouxiophyceae, Chlorophyta): an impetus for developing an integrated taxonomy. Molecular Phylogenetics and Evolution 2020, 149, 106821. [Google Scholar] [CrossRef] [PubMed]
- Lumbsch, H.T.; Rikkinen, J. Evolution of lichens. In The fungal community: its organization and role in the ecosystem., Dighton, J., White, J.F., Eds.; CRC Press: Boca Raton, Florida, 2017; pp. 53–62. [Google Scholar]
- Taylor, T.; Hass, H.; Kerp, H. The oldest fossil ascomycetes. Nature 1999, 399, 648–648. [Google Scholar] [CrossRef]
- Kroken, S.; Taylor, J.W. Phylogenetic species, reproductive mode, and specificity of the green alga Trebouxia forming lichens with the fungal genus Letharia. Bryologist 2000, 645–660. [Google Scholar] [CrossRef]
- Hill, D.J. Asymmetric co-evolution in the lichen symbiosis caused by a limited capacity for adaptation in the photobiont. The Botanical Review 2009, 75, 326–338. [Google Scholar] [CrossRef]
- Yahr, R.; Vilgalys, R.; Depriest, P.T. Strong fungal specificity and selectivity for algal symbionts in Florida scrub Cladonia lichens. Molecular Ecology 2004, 13, 3367–3378. [Google Scholar] [CrossRef]
- Thüs, H.; Muggia, L.; Pérez-Ortega, S.; Favero-Longo, S.E.; Joneson, S.; O’Brien, H.; Nelsen, M.P.; Duque-Thüs, R.; Grube, M.; Friedl, T.; et al. Revisiting photobiont diversity in the lichen family Verrucariaceae (Ascomycota). European Journal of Phycology 2011, 46, 399–415. [Google Scholar] [CrossRef]
- Leavitt, S.D.; Kraichak, E.; Nelsen, M.P.; Altermann, S.; Divakar, P.K.; Alors, D.; Esslinger, T.L.; Crespo, A.; Lumbsch, T. Fungal specificity and selectivity for algae play a major role in determining lichen partnerships across diverse ecogeographic regions in the lichen-forming family Parmeliaceae (Ascomycota). Molecular Ecology 2015, 24, 3779–3797. [Google Scholar] [CrossRef]
- Chagnon, P.L.; Magain, N.; Miadlikowska, J.; Lutzoni, F. Strong specificity and network modularity at a very fine phylogenetic scale in the lichen genus Peltigera. Oecologia 2018, 187, 767–782. [Google Scholar] [CrossRef]
- Rikkinen, J. Cyanolichens: An Evolutionary Overview. In Cyanobacteria in Symbiosis, Rai, A.N., Bergman, B., Rasmussen, U., Eds.; Springer Netherlands: Dordrecht, 2002; pp. 31–72. [Google Scholar]
- Pardo-De la Hoz, C.J.; Magain, N.; Lutzoni, F.; Goward, T.; Restrepo, S.; Miadlikowska, J. Contrasting symbiotic patterns in two closely related lineages of trimembered lichens of the genus Peltigera. Frontiers in Microbiology 2018, 9, 2770–2770. [Google Scholar] [CrossRef] [PubMed]
- Vančurová, L.; Muggia, L.; Peksa, O.; Řídká, T.; Škaloud, P. The complexity of symbiotic interactions influences the ecological amplitude of the host: a case study in Stereocaulon (lichenized Ascomycota). Molecular Ecology 2018, 27, 3016–3033. [Google Scholar] [CrossRef]
- Lindgren, H.; Moncada, B.; Lücking, R.; Magain, N.; Simon, A.; Goffinet, B.; Sérusiaux, E.; Nelsen, M.P.; Mercado-Díaz, J.A.; Widhelm, T.J.; et al. Cophylogenetic patterns in algal symbionts correlate with repeated symbiont switches during diversification and geographic expansion of lichen-forming fungi in the genus Sticta (Ascomycota, Peltigeraceae). Molecular Phylogenetics and Evolution 2020, 150, 106860. [Google Scholar] [CrossRef] [PubMed]
- Zoller, S.; Lutzoni, F. Slow algae, fast fungi: exceptionally high nucleotide substitution rate differences between lichenized fungi Omphalina and their symbiotic green algae Coccomyxa. Molecular Phylogenetics and Evolution 2003, 29, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Piercey-Normore, M.D. Vegetatively reproducing fungi in three genera of the Parmeliaceae share divergent algal partners. The Bryologist 2009, 112, 773–785. [Google Scholar] [CrossRef]
- Singh, G.; Kukwa, M.; Dal Grande, F.; Łubek, A.; Otte, J.; Schmitt, I. A glimpse into genetic diversity and symbiont interaction patterns in lichen communities from areas with different disturbance histories in Białowieża forest, Poland. Microorganisms 2019, 7, 335. [Google Scholar] [CrossRef]
- Beck, A.; Kasalicky, T.; Rambold, G. Myco-photobiontal selection in a Mediterranean cryptogam community with Fulgensia fulgida. New Phytologist 2002, 153, 317–326. [Google Scholar] [CrossRef]
- Peksa, O.; Škaloud, P. Do photobionts influence the ecology of lichens? A case study of environmental preferences in symbiotic green alga Asterochloris (Trebouxiophyceae). Molecular Ecology 2011, 20, 3936–3948. [Google Scholar] [CrossRef]
- Dal Grande, F.; Rolshausen, G.; Divakar, P.K.; Crespo, A.; Otte, J.; Schleuning, M.; Schmitt, I. Environment and host identity structure communities of green algal symbionts in lichens. New Phytologist 2018, 217, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, D.P.; Aizen, M.A. Asymmetric specialization: a pervasive feature of plant-pollinator interactions. Ecology 2004, 85, 1251–1257. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Poulin, R.; Krasnov, B.R.; Shenbrot, G.I. Species abundance and the distribution of specialization in host-parasite interaction networks. Journal of Animal Ecology 2005, 946–955. [Google Scholar] [CrossRef]
- Vázquez, D.P.; Melián, C.J.; Williams, N.M.; Blüthgen, N.; Krasnov, B.R.; Poulin, R. Species abundance and asymmetric interaction strength in ecological networks. Oikos 2007, 116, 1120–1127. [Google Scholar] [CrossRef]
- Bowler, P.; Rundel, P. Reproductive strategies in lichens. Botanical Journal of the Linnean Society 1975, 70, 325–340. [Google Scholar] [CrossRef]
- Degelius, G. Biological studies of the epiphytic vegetation on twigs on Fraxinus excelsior. Acta Horti Gotoburgensis Medd Goteborgs Botany Tradgard 1964, 27, 11–55. [Google Scholar]
- Fernández-Mendoz, F.; Domaschke, S.; García, M.A.; Jordan, P.; Martín, M.P.; Printzen, C. Population structure of mycobionts and photobionts of the widespread lichen Cetraria aculeata. Molecular Ecology 2011, 20, 1208–1232. [Google Scholar] [CrossRef]
- Ertz, D.; Guzow-Krzemińska, B.; Thor, G.; Łubek, A.; Kukwa, M. Photobiont switching causes changes in the reproduction strategy and phenotypic dimorphism in the Arthoniomycetes. Scientific Reports 2018, 8, 4952. [Google Scholar] [CrossRef]
- Singh, G.; Dal Grande, F.; Divakar, P.K.; Otte, J.; Crespo, A.; Schmitt, I. Fungal–algal association patterns in lichen symbiosis linked to macroclimate. New Phytologist 2017, 214, 317–329. [Google Scholar] [CrossRef]
- Wirtz, N.; Lumbsch, H.T.; Green, T.G.A.; Türk, R.; Pintado, A.; Sancho, L.; Schroeter, B. Lichen fungi have low cyanobiont selectivity in maritime Antarctica. New Phytologist 2003, 160, 177–183. [Google Scholar] [CrossRef]
- Wiens, J.J.; Donoghue, M.J. Historical biogeography, ecology and species richness. Trends in Ecology & Evolution 2004, 19, 639–644. [Google Scholar] [CrossRef]
- Blasco-Costa, I.; Hayward, A.; Poulin, R.; Balbuena, J.A. Next-generation cophylogeny: unravelling eco-evolutionary processes. Trends in Ecology & Evolution 2021, 36, 907–918. [Google Scholar] [CrossRef]
- Buckley, H.L.; Rafat, A.; Ridden, J.D.; Cruickshank, R.H.; Ridgway, H.J.; Paterson, A.M. Phylogenetic congruence of lichenised fungi and algae is affected by spatial scale and taxonomic diversity. PeerJ 2014, 2, e573. [Google Scholar] [CrossRef] [PubMed]
- Piercey-Normore, M.D. The lichen-forming ascomycete Evernia mesomorpha associates with multiple genotypes of Trebouxia jamesii. New Phytologist 2006, 169, 331–344. [Google Scholar] [CrossRef] [PubMed]
- De Vienne, D.M.; Refrégier, G.; López-Villavicencio, M.; Tellier, A.; Hood, M.E.; Giraud, T. Cospeciation vs host-shift speciation: methods for testing, evidence from natural associations and relation to coevolution. New Phytologist 2013, 198, 347–385. [Google Scholar] [CrossRef]
- Beiggi, S.; Piercey-Normore, M.D. Evolution of ITS ribosomal RNA secondary structures in fungal and algal symbionts of selected species of Cladonia sect. Cladonia (Cladoniaceae, Ascomycotina). Journal of Molecular Evolution 2007, 64, 528–542. [Google Scholar] [CrossRef]
- Millanes, A.M.; Truong, C.; Westberg, M.; Diederich, P.; Wedin, M. Host switching promotes diversity in host-specialized mycoparasitic fungi: uncoupled evolution in the Biatoropsis usnea system. Evolution; international journal of organic evolution 2014, 68, 1576–1593. [Google Scholar] [CrossRef]
- Rogers, S.O.; Bendich, A.J. Extraction of DNA from plant tissues. In Plant Molecular Biology Manual; Springer, 1989; pp. 73–83. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 2000, 17, 540–552. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees; IEEE, 2010. [Google Scholar]
- Mason-Gamer, R.J.; Kellogg, E.A. Testing for phylogenetic conflict among molecular data sets in the tribe Triticeae (Gramineae). Systematic Biology 1996, 45, 524–545. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree. Tree figure drawing tool [WWW document] URL http://tree.bio.ed.ac.uk/software/figtree/, 2009.
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, automatic barcode gap discovery for primary species delimitation. Molecular Ecology 2012, 21, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef]
- Monaghan, M.T.; Wild, R.; Elliot, M.; Fujisawa, T.; Balke, M.; Inward, D.J.; Lees, D.C.; Ranaivosolo, R.; Eggleton, P.; Barraclough, T.G.; et al. Accelerated species inventory on Madagascar using coalescent-based models of species delineation. Systematic Biology 2009, 58, 298–311. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; Maio, N.D.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 2018, 67, 901–904. [Google Scholar] [CrossRef]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- Dormann, C.; Gruber, B.; Fründ, J. Introducing the bipartite package: analysing ecological networks. R News 8: 8–11; 2008. [Google Scholar]
- Delmas, E.; Besson, M.; Brice, M.H.; Burkle, L.A.; Dalla Riva, G.V.; Fortin, M.J.; Gravel, D.; Guimarães Jr, P.R.; Hembry, D.H.; Newman, E.A. Analysing ecological networks of species interactions. Biological Reviews 2019, 94, 16–36. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package. R package v.2.3-5 [WWW document] URL https://cran.r-project.org/web/packages/vegan/index.html. 2016.
- Fourment, M.; Gibbs, M.J. PATRISTIC: a program for calculating patristic distances and graphically comparing the components of genetic change. BMC Evolutionary Biology 2006, 6, 1–1. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Guarino, L.; Mathur, P. DIVA-Gis version 7.5.Manual. [WWW document] URL http://www.diva-gis.org. [Accessed 7 August 2021.]. 2012.
- Balbuena, J.A.; Míguez-Lozano, R.; Blasco-Costa, I. PACo: a novel procrustes application to cophylogenetic analysis. PLoS One 2013, 8, e61048. [Google Scholar] [CrossRef] [PubMed]
- Conow, C.; Fielder, D.; Ovadia, Y.; Libeskind-Hadas, R. Jane: a new tool for the cophylogeny reconstruction problem. Algorithms for Molecular Biology 2010, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Claude, J.; Strimmer, K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
| Temperate zone | Arctic zone | Tropic/subtropic zone | |
|---|---|---|---|
| Connectance | 0.25 | 0.22 | 0.15 |
| Links per species | 0.89 | 0.87 | 0.76 |
| Linkage density | 4.13 | 3.70 | 2.13 |
| Nestedness | 30.75 | 37.39 | 22.92 |
| Number of compartments | 3 | 3 | 6 |
| Cost regime | C-D-D&S-L-FDa | C | D | D&S | L | FD | Total cost |
|---|---|---|---|---|---|---|---|
| A | 0,1,2,1,1 | 1 | 8 | 6 | 91 | 35 | 146 |
| B | 0,1,2,1,-1 | 1 | 8 | 6 | 90 | 35 | 75 |
| C | 0,2,2,1,1 | 1 | 8 | 6 | 89 | 35 | 152 |
| D | 0,0,2,1,1 | 1 | 9 | 5 | 91 | 35 | 136 |
| E | 0,1,3,1,1 | 1 | 9 | 5 | 91 | 35 | 150 |
| F | 0,1,1,1,1 | 0 | 8 | 7 | 88 | 35 | 138 |
| G | 0,1,2,2,1 | 0 | 8 | 7 | 90 | 35 | 237 |
| H | 0,1,2,0,1* | 2 | 13 | 0 | 113 | 35 | 48 |
| I | 0,1,2,1,2 | 1 | 8 | 6 | 89 | 35 | 179 |
| J | 0,1,2,1,0 | 1 | 8 | 6 | 90 | 35 | 110 |
| K | 1,1,1,1,1 | 0 | 8 | 7 | 88 | 35 | 138 |
| L | 1,1,2,1,1 | 0 | 8 | 7 | 88 | 35 | 145 |
| M | 1,0,0,1,1 | 0 | 7 | 8 | 90 | 35 | 125 |
| N | -1,1,2,1,1 | 1 | 8 | 6 | 89 | 35 | 143 |
| O | 2,1,1,1,1 | 0 | 8 | 7 | 88 | 35 | 138 |
| P | 2,1,1,1,0 | 0 | 8 | 7 | 88 | 35 | 103 |
| Q | 2,1,1,0,0* | 0 | 8 | 7 | 108 | 35 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





