1. Introduction
Advanced cancers sometimes result in malignant colorectal obstruction [
1], which may be caused by the colorectal cancer itself, tumor invasion of other organ cancers, or peritoneal dissemination. Although surgical resection of the obstructed colon and anastomosis with an intact colon or surgical bypass is ideal, surgery is often difficult, especially when the cancer is at an advanced stage. In such cases, colostomy or endoscopic colorectal stenting is an alternative treatment option. However, colostomies greatly compromise quality of life [
2], and endoscopic colorectal stenting for palliation (PAL) is therefore favored.
Endoscopic colorectal stenting for PAL still faces various challenges. First, there are many extracolonic stenoses and limited data exist regarding the short-term efficacy and safety of stenting [
3,
4,
5,
6,
7,
8]. In addition, data on the long-term outcomes are limited. There is also insufficient evidence regarding the safety of stenting in combination with drug therapy [
9,
10,
11]. Data on outcomes associated with different stents are also limited [
12,
13,
14], and numerous data are integrated with bridge-to-surgery (BTS) cases with limited data available for PAL only [
15,
16].
The Japan colonic stent safe procedure research group (JCSSPRG) was launched in 2012 to promote the safe use of colonic stents. We conducted several multicenter prospective studies to investigate the safety and efficacy of colonic stenting using various self-expandable metal stents (SEMSs). The WallFlex enteral colonic stent was the first SEMS approved by public insurance for endoscopic colonic stenting in Japan, and we conducted a multicenter study to evaluate its safety and efficacy. The study included both BTS and PAL cohorts. We previously reported the short-term outcomes of the entire cohort and the short- and long-term outcomes of the BTS cohort [
7,
13,
14]. We have accumulated data from the early days of stent introduction in Japan, and here, we report the short- and long-term outcomes of the PAL cohort.
2. Materials and Methods
2.1. Study Design
This single-arm, prospective, multicenter study by the JCSSPRG evaluated the efficacy and safety of colorectal stenting using an uncovered WallFlex enteral colonic stent. Fourteen academic centers and 32 community hospitals participated in the study. Institutional review board approval was obtained for patient enrollment at each institution prior to the start of the study. Each patient provided consent to undergo the procedure and registered for the study. This study was registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN00007953).
Patients with malignant large bowel obstruction were included in this study. Large bowel obstruction was diagnosed using abdominal radiography, colonoscopy, or computed tomography. Patients with a history of colonic stenting were excluded. Other exclusion criteria included enteral ischemia, suspected or impending perforation, intra-abdominal abscess, severe inflammatory changes around the obstruction, and contraindication to endoscopic treatment.
Although this study included both BTS and PAL cohorts, we selected only the PAL cohort and evaluated short- and long-term efficacy and safety of colonic stenting using the WallFlex enteral colonic stent.
2.2. Endoscopic stent placement
Stent placement was performed via colonoscopy under fluoroscopic guidance. Before starting this prospective study, we had developed the technical guidelines for safe colonic stenting (“the JCSSPRG Mini-Guidelines” [
17]) and tried to share tips for stent placement and points to avoid complication with the group members. Briefly, the colonoscope was gently inserted into the obstruction site, the catheter and guidewire were manipulated to pass through the stenosis, contrast medium was injected to identify the stenosis and measure the length of the obstruction site fluoroscopically, and finally the stent was deployed across the stenosis under endoscopic and fluoroscopic views using the through-the-scope method. Intraluminal marking with a clip was recommended to identify stricture location. Stricture dilation was avoided to prevent perforation. SEMS placement was performed or supervised by an endoscopy expert from the JCSSPRG. Only uncovered WallFlex enteral colonic stents (Boston Scientific Corporation, Natick, MA, USA), 22 and 25 mm in diameter and 6, 9, or 12 cm in length folded in 10 Fr. delivery system, were evaluated in this study.
2.3. Definition of outcomes
The colorectal obstructing scoring system (CROSS) was constructed by JCSSPRG based on a scoring system for malignant gastric outlet obstruction [
18,
19]. CROSS evaluates oral intake as follows: CROSS 0, requiring continuous decompression; CROSS 1, no oral intake; CROSS 2, liquid or enteral nutrient intake; CROSS 3, soft solids, low-residue, and full diet without symptoms of stricture.
Technical success was defined as successful deployment of the stent across the entire length of the stricture. Clinical success was defined as the resolution of symptoms and radiological relief of the obstruction within 24 hours, as confirmed by radiographic observation [
12].
Complications after SEMS placement included perforation, stent migration, recurrent colorectal obstruction (RCRO), bleeding, infection or fever, abdominal pain, tenesmus, fecal incontinence, and other minor complications. Bleeding that did not require treatment was defined as minor bleeding, whereas bleeding requiring treatment was defined as major bleeding. Early-onset was defined as occurring within 7 days and late-onset as occurring after 7 days. The incidence rate was calculated using the denominator of 200 patients enrolled in the PAL cohort.
2.4. Data Collection and Statistical Analysis
All clinical data were prospectively reported using an electronic registration system. At enrollment, patient characteristics, including age, sex, Eastern cooperative oncology group (ECOG) performance status (PS), etiology, CROSS, and symptoms of obstruction were recorded. The obstruction number, type of obstruction, stricture length, and location of the obstruction were collected as tumor characteristics. The number of stent placements, length and diameter of the stent, balloon dilation before stent placement, procedure time, technical and clinical success, and causes of clinical failure were evaluated as the outcomes. Complications were investigated to assess safety, and the influence of concomitant chemotherapy evaluated.
Categorical variables are expressed as absolute numbers and percentages. Continuous variables are presented as medians and ranges. Overall survival and time-to-RCRO (TRCRO) were calculated using the Kaplan–Meier method. Risk factors for perforation were evaluated using the chi-square test and a logistic regression analysis model which included the following variables: age, sex, etiology, type of obstruction, length of stent, and chemotherapy after SEMS placement. All statistical analyses were performed using JMP Pro software (version 16.0; SAS Institute, Chicago, IL, USA).
3. Results
3.1. Patient characteristics
A total of 517 patients were enrolled in this study from March 2012 to October 2013. After excluding ineligible and BTS cases, 200 patients were included in the PAL cohort. The PAL cohort patient characteristics are shown in
Table 1. The median age was 74.5 years, and 55.5% of the patients were male. Sixty percent of the patients had ECOG PS 0 or 1, while the remaining 40% had ECOG PS 2 or higher. The most common etiology was colorectal cancer (72.5%), followed by gastric (15.5%) and pancreatic cancer (5.5%). Most patients (97.5%) had symptoms of obstruction. Only 39.0% had a CROSS of 0.
The details of the obstruction sites are summarized in
Table 2. Four cases exhibited two obstruction sites. Therefore, 204 obstruction sites were managed using colorectal stenting. Extracolonic obstruction accounted for 31.5% of the obstructions. There were 54 (27.0%) patients with stenosis with peritoneal dissemination. Median obstruction length was 4.0 cm. Most obstructions were located on the left side of the colon (69.6%), with sigmoid colon stenting being the most common.
3.2. Short-term outcomes of stent placement
Digestive tract decompression prior to stent placement was performed in 58 (29%) patients via nasogastric (n = 14), nasointestinal (n = 16), and transanal (n = 28) tube insertion. Cleansing enemas and oral bowel cleansing were performed as preparation for stent placement in 70 (35.0%) and 10 (5.0%) patients, respectively. Obstruction sites were marked with intraluminal clipping and extracorporeal marking in 107 (53.5%) and 19 (9.5%) patients, respectively.
The short-term outcomes are shown in
Table 3. Most patients were treated with a single stent (95.9%). The main stent type was 6 cm in length and 22 mm in diameter. Balloon dilation before stent placement was performed in only one patient (0.5%). The median procedure time was 30 min (range; 6–170).
The technical and clinical success rates were 98.5% and 94.5%, respectively. Technical failures resulted from failure of guidewire cannulation (n = 2) and perforation by a catheter (n = 1). Among the patients with technical success, clinical failure occurred in only eight cases due to insufficient stent expansion (n = 3), stent kinking (n = 1), stent migration (n = 1), perforation by stent injury (n = 1), perforation due to delayed decompression (n = 1), and proximal small bowel obstruction (n = 1).
Early-onset (≤7 days) complications occurred in 28 (14.0%) patients and are shown in
Table 4. There were four (2.0%) cases of perforation of which one case was perforated during the procedure and did not achieve technical success, two cases were perforated within 24 hours and did not achieve clinical success, and the remaining case was perforated by stent-edge injury 4 days after stent placement. Of these, three patients recovered after surgery. One patient had a poor prognosis and was treated conservatively but died. Perforations were caused by the catheter during procedure (n=1), stent-edge injury (n = 2) and delayed decompression (n = 1). There were two cases of perforation involving the stent and its surroundings. Stent migration occurred in one case and the stent was discharged from the anus. Other minor complications, such as bleeding (n = 4), infection or fever (n = 8), and abdominal pain (n = 9), improved upon conservative treatment.
3.3. Long-term outcomes of stent placement
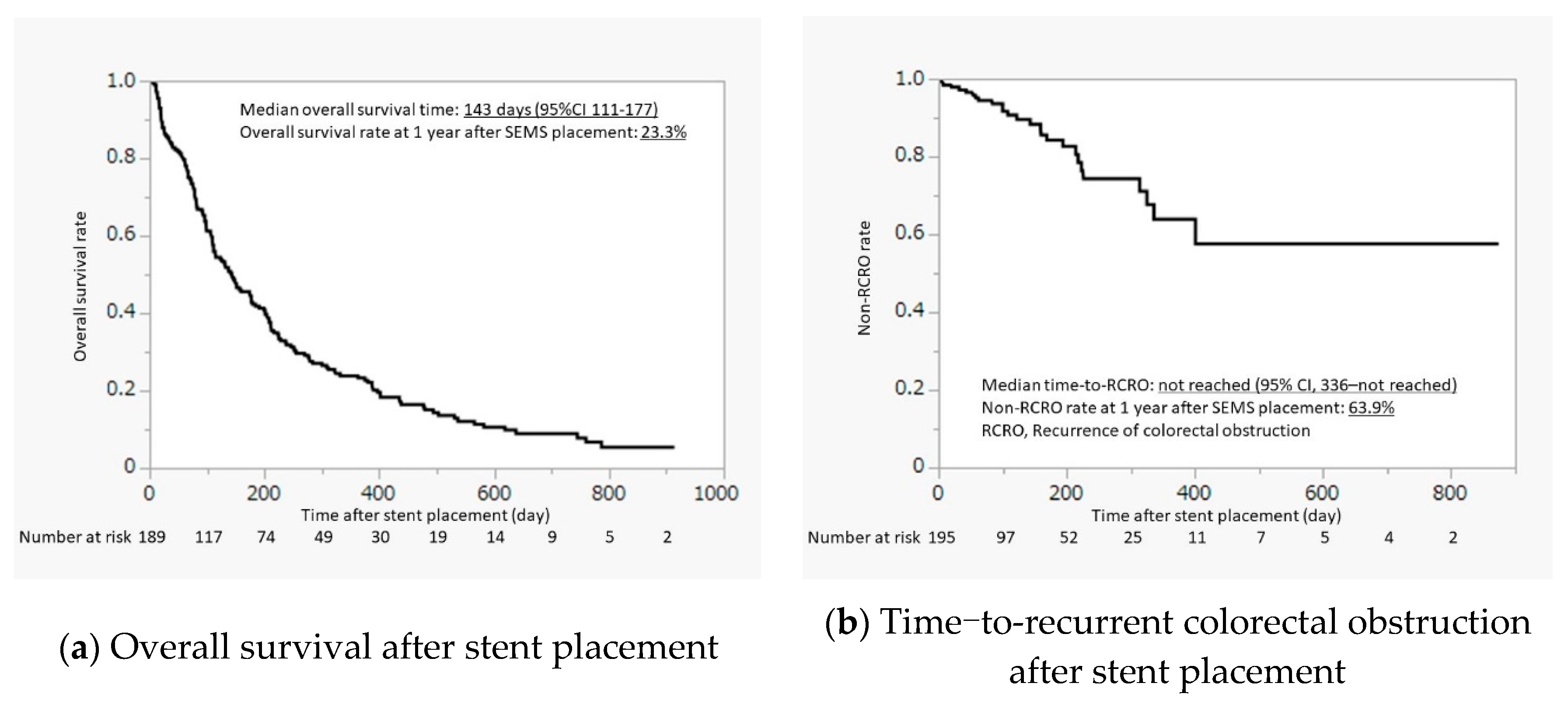
Overall survival and TRCRO after stent placement are shown in
Figure 1. The median overall survival was 143 days (95% confidence interval [CI], 111–177 days) and the overall survival rate at 1 year was 23.3%. The median duration of TRCRO was not reached (95% CI, 336–not reached), and non-RCRO rate at 1 year was 63.9%. Most patients (71.2%) remained free of RCRO until death or the last follow-up.
Late-onset (7 days<) complications occurred in 66 (33.0%) patients and are shown in
Table 4. There were 10 (5.0%) cases of perforation. Only two patients underwent surgery, and the other patients were considered to have a poor prognosis and were treated conservatively. Although most perforations were thought to be related to the stent and its surroundings (n = 5), there were cases with unclear causes (n = 4) or perforation of the tumor at another site (n = 1). Stent obstruction occurred in 24 (12.0%) patients of which four were treated surgically, nine underwent additional stenting, six underwent stent cleaning, one underwent ablation with argon plasma coagulation (APC), and four received conservative treatment. Proximal gastrointestinal obstruction was observed in 12 (6%) patients.
Fifty-six (28.0%) patients underwent chemotherapy after stent placement. There were 38 (26.2%) cases of 145 colorectal cancer cases, 15 (48.4%) cases of 31 gastric cancer cases, and one case each of cervical, ovarian, and pancreatic cancer (
Table 5). For colorectal cancer, oxaliplatin-based therapy was administered to 20 patients and irinotecan-based therapy was administered to 12 patients. Nine patients opted for the relatively less invasive oxaliplatin and irinotecan-free chemotherapy, as follows S-1, capecitabine, UFT + leucovorin, trifluridine/tipiracil, and cetuximab. Bevacizumab, an anti-vascular endothelial growth factor (VEGF) monoclonal antibody, was administered in five (13.2%) cases. Panitumumab or cetuximab, anti-epidermal growth factor receptor (EGFR) antibodies, was used in thirteen (34.2%) cases. Stent migration was observed in 5 (13.2%) of the 38 chemotherapy cases, two of which received molecular targeted drugs (panitumumab and bevacizumab). Perforation was observed in two (3.6%) patients, both were patients with colon cancer. One of whom received 5-fluorouracil plus folinic acid plus irinotecan (FOLFIRI) with bevacizumab until just before perforation, which occurred 145 days after stent placement. The patient was managed surgically. Another patient received 5-fluorouracil plus folinic acid plus oxaliplatin (FOLFOX), and perforation occurred 85 days after stent placement. The patient died nine days after the perforation.
3.4. Risk factors for perforation
Univariate analysis was performed on risk factors for perforation (
Table 6). Only the use of a long SEMS was a risk factor for perforation. Compared with 6 cm SEMS, the hazard ratio of 12 cm SEMS was 7.37 (95% CI, 1.53–35.58; p = 0.03), indicating that 12 cm SEMS was associated with perforation. The use of chemotherapy, including anti-VEGF antibody drugs, was also not a risk factor.
4. Discussion
This study investigated the short- and long-term efficacy and safety of SEMS placement of a WallFlex enteral colonic stent for palliation. This study is data on PAL at the time of introduction of colorectal stenting to Japan, and data on long-term prognosis of a large number of patients are also analyzed. The technical and clinical success rates were very high (98.5% and 94.5%, respectively) in the PAL cohort, and more than 70% of patients remained free from RCRO until death or the last follow-up. Stenting for PAL was beneficial even in patients with poor general condition or prognosis, with 28.0% of patients receiving chemotherapy, a one-year survival rate of 23.3%, and a 63.9% non-RCRO rate at one year. The cumulative perforation rate was 7%, with perforation during the procedure in one case, within 7 days after SEMS placement in three cases, and longer than 7 days after SEMS placement in ten cases. Only the use of a long SEMS was a risk factor for perforation.
The WallFlex enteral colonic stent was woven using only a cross-nitinol wire, which provides WallFlex with a high axial force, and an axial force zero border with a very small angle, which provides sustained pressure load on the intestinal wall [
20]. We previously reported the short-term efficacy and safety of colonic stenting with WallFlex in BTS and the long-term outcomes after BTS [
13,
14]. In this study, we found that short-term placement of WallFlex for colorectal cancer is safe and has minimal oncological impact.
Previous studies for PAL were summarized in
Table 7. As the definition of clinical success varied between papers, the total clinical success rate was calculated using the number of patients with malignant large bowel obstruction as the denominator. Unfortunately, many previous reports do not distinguish between short- and long-term complications. One report showed a comparable efficacy and safety for the WallFlex and Niti-S D-type, which was woven as hooks and cross stents [
21]. However, other studies using WallFlex reported a high perforation rate that was attributed to the structure or large diameter (including stents with a 25 / 30 mm in diameter) of the stent [
22,
23]. The cumulative perforation rate in this study was 7%, which was comparable to other studies. As shown in
Table 7, previous studies reported higher perforation rates for WallFlex than for Niti-S D-type stents[
12,
21,
22,
24,
25]. The short-term results of this study are comparable with those reported previously. Although the perforation rate with WallFlex in PAL cohort may be comparable, it is unclear whether there are differences in perforation rates between stents, as there are few reports of long-term placement of each stent.
Previous reports of endoscopic colorectal stenting for PAL tend to have lower clinical success rates (54.1–96.0%) than those for BTS because of numerous extracolonic obstructions [
8,
26,
29,
30]. Furthermore, peritoneal carcinomatosis is technically difficult, which lowers the technical success rate of PAL compared with that of BTS [
34]. A study limited to colorectal obstruction from gastric cancer reported technical success of 73.9% and clinical success of 54.1%, which is very low compared with that of primary colorectal cancer [
29]. In the present study, the technical and clinical success rates of extracolonic obstruction were 98.2% and 91.1%, which compare favorably with previous reports [
25,
27,
29,
30]. And the number of extracolonic cases in this study was 63 (31.5%), which tended to be a smaller proportion than in previous reports. This may also be a reason for the good clinical success.
The WallFlex colonic stent has a strong radial force, which may have helped maintain the stent lumen, contributing to its good outcomes in the present study [
20]. Evaluation of complication rates revealed that the previous reports for PAL had a higher perforation rate (7.9–8.9%) than those for BTS because of the long-term placement of the stent [
7,
15,
26]. In PAL cases, unlike BTS, perforation due to disease progression does not always result in surgery, and it is sometimes difficult to identify the site of perforation and whether the perforation was caused by the stent. In this study, there were 7 cases of perforation involving the stent and its surroundings. Age ≥70 years and sigmoid colonic location were found to be independently associated with occurrence of early perforation, and stent location in the flexure and absence of peritoneal carcinomatosis were significantly associated with delayed perforation [
24]. The three cases of early perforation in this study were among patients aged over 70 years, and three cases exhibited sigmoid colon or rectosigmoid obstruction, which corresponds to previous reports. Of the late perforations seen in the present study, five patients had peritoneal dissemination, and only one patient had a stent placed in flexion. Stenting in flexure or the sigmoid colon is a risk factor for perforation due to increased risk of stent-edge injury. A univariate analysis of perforation showed that a 12 cm long stent was a risk factor because it may be more prone to edge injury. However, the colon has many fixed and unfixed areas and is subject to peristalsis and intestinal floating, which makes it difficult to predict the associated risk of stent placement. In extrinsic cases, the intestine may become fixed, and care must therefore be taken when placing the stent, especially at bends.
The relationship between SEMS placement and overall survival could not be evaluated as this cohort included patients with different cancer origins. However, the median overall survival and TRCRO were both good, and 121 of 170 patients (71.2%) were free of RCRO until death or the last follow-up. SEMS placement could therefore improve quality of life, eliminating the need for additional surgery, and allow recovery from large bowel obstruction. Even in cases of RCRO, some cases can be treated with additional stents, APC ablation and stent cleaning.
Fifty-six (28.0%) patients underwent chemotherapy after stent placement. Despite the limited number of adverse events in the present study, chemotherapy was not a risk factor for perforation. Some reports indicate that stenting during and after chemotherapy is safe and effective, whereas others contraindicate stenting [
35,
36]. Furthermore, bevacizumab has been associated with higher complication rates, nearly tripling the risk of perforation [
28,
33]. In the present study, we observed one case of perforation on FOLFIRI + bevacizumab. In this case, the normal mucosa at the edge of the stent perforated. The perforation may have occurred because of delayed wound healing caused by angiogenesis inhibitors. According to previous reports, the perforation rate increased to 15.4% with bevacizumab, and the median time to perforation was 21 days [
28]. Perforations involving bevacizumab may be occurring late-onset. Considering the functional mechanism of anti-VEGF antibody drugs and the hazard ratio in this study, it is possible that anti-VEGF antibody drugs may be involved in perforation. The European Society of Gastrointestinal Endoscopy guidelines recommend that antiangiogenic therapy can be considered in patients following colonic stenting but that colonic stenting should be avoided during antiangiogenic therapy [
37]. As acute colorectal obstruction and perforation are potentially fatal, either surgery or stenting is required, even during systemic chemotherapy including bevacizumab therapy. Further studies are therefore needed to elucidate the relationship between SEMS placement and chemotherapy, in particular, stenting during treatment with anti-VEGF antibody drugs.
In recent years, hook and cross-type stents, which have lower axial and radial forces, have been widely used for colorectal stenting. These SEMSs were fold when longitudinal force is applied to the edge of the stent according to the hook structure. In contrast, cross-type stents are not folded and may increase the risk of stent tip or edge injury. Care must therefore be taken in stent selection because the flared portion of a stent often has a cross structure.
This study had several limitations. Although this study prospectively evaluated large number of patients compared with previous reports, it was a single-arm observational study. Future studies should include a prospective, randomized controlled trial comparing WallFlex enteral colonic stents with other stent types, such as hook and cross stents. Furthermore, clinical success and TRCRO were regarded as positive outcomes. However, these outcomes may be insufficient to judge the impact of the stenting on these patients and patient questionnaire should be used to assess quality of life. Finally, although administration of chemotherapy and anti-VEGF antibody drugs after stenting were investigated, the influence of these therapies prior to stenting were not evaluated, and the relationship between chemotherapy and perforation requires further elucidation.
5. Conclusions
This large, multicenter, prospective study demonstrated the efficacy and safety of palliative stenting with high-axial-force SEMSs for malignant colorectal obstruction. Most patients did not require additional surgery and remained free of RCRO until death or the last follow-up. However, care should be taken to avoid perforation at the stent edge with long-term stent placement when using a long SEMS.
Author Contributions
Conception and design of the study, analysis and interpretation of the data, and drafting of the article, R.I., T.S. (Takashi Sasaki), T.K., H.I., and Y.S.; conception and design of the study, data collection, and critical revision, T.M., T.Y., S.S., M.T., T.S.(Toshiyuki Shiratori), S.I., T.H., M.F., and I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Japan Gastroenterological Endoscopy Society, Japanese Foundation for Research and Promotion of Endoscopy Grant, and Colonic Stent Safe Procedure Research Group membership dues.
Institutional Review Board Statement
This study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of each institution. The protocol code was Ohashi 12-16 and the date of approval was March 26, 2013, at Toho University Ohashi Medical Center.
Informed Consent Statement
Informed consent was obtained from all patients involved in the study.
Data Availability Statement
Data sharing is not applicable.
Acknowledgments
The authors acknowledge Miyuki Tsuchida (Department of Gastroenterology,The University of Tokyo) for her role as a clinical study coordinator and data manager and Masafumi Yanagisawa for his assistance in website management. I would like to express my sincere gratitude to Dr. Shuntaro Yoshida for his support and cooperation in every aspect of my research based on his expertise.
Conflicts of Interest
T.S. (Takashi Sasaki) received personal fees from Boston Scientific Japan, Co., Ltd., Century Medical, Inc., JAPAN LIFELINE, Co., Ltd., SB-Kawasumi Laboratories, Inc. H.I. received personal fees from Boston Scientific Japan, Co., Ltd., Century Medical, Inc., TAEWOONG, Co., Ltd., JAPAN LIFELINE, Co.; received research grants from Boston Scientific Japan, Co., Ltd.; received scholarship donation from Boston Scientific Japan, Co., Ltd. T.M. received personal fee from Boston Scientific Japan. S.S. received personal fees from SB-Kawasumi Laboratories, Inc., Boston Scientific Japan, Co., Ltd., Century Medical, Inc. and JAPAN LIFELINE, Co., Ltd. I.M. has received research grants from Boston Scientific Corporation. Y.S. received personal fees from Century Medical, Inc., JAPAN LIFELINE, Co., Ltd., received research grants from Boston Scientific Japan, Co., Ltd. The other authors declare no conflicts of interest.
References
- Markogiannakis, H.; Messaris, E.; Dardamanis, D.; Pararas, N.; Tzertzemelis, D.; Giannopoulos, P.; Larentzakis, A.; Lagoudianakis, E.; Manouras, A.; Bramis, I. Acute mechanical bowel obstruction: clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007, 13, 432–437. [Google Scholar] [CrossRef]
- Zewude, W.C.; Derese, T.; Suga, Y.; Teklewold, B. Quality of Life in Patients Living with Stoma. Ethiop J Health Sci. 2021, 31, 993–1000. [Google Scholar]
- Sabbagh, C.; Browet, F.; Diouf, M.; Cosse, C.; Brehant, O.; Bartoli, E.; Mauvais, F.; Chauffert, B.; Dupas, J.L.; Nguyen-Khac, E.; et al. Is stenting as "a bridge to surgery" an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg. 2013, 258, 107–115. [Google Scholar] [CrossRef]
- van den Berg, M.W.; Sloothaak, D.A.; Dijkgraaf, M.G.; van der Zaag, E.S.; Bemelman, W.A.; Tanis, P.J.; Bosker, R.J.; Fockens, P.; ter Borg, F.; van Hooft, J.E. Bridge-to-surgery stent placement versus emergency surgery for acute malignant colonic obstruction. Br J Surg. 2014, 101, 867–873. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Ishida, H.; Yoshida, S.; Isayama, H.; Kuwai, T.; Maetani, I.; Shimada, M.; Yamada, T.; Saito, S.; Tomita, M.; et al. A Japanese prospective multicenter study of self-expandable metal stent placement for malignant colorectal obstruction: short-term safety and efficacy within 7 days of stent procedure in 513 cases. Gastrointest Endosc. 2015, 82, 697–707.e691. [Google Scholar] [CrossRef]
- Tomita, M.; Saito, S.; Makimoto, S.; Yoshida, S.; Isayama, H.; Yamada, T.; Matsuzawa, T.; Enomoto, T.; Kyo, R.; Kuwai, T.; et al. Self-expandable metallic stenting as a bridge to surgery for malignant colorectal obstruction: pooled analysis of 426 patients from two prospective multicenter series. Surg Endosc. 2019, 33, 499–509. [Google Scholar] [CrossRef]
- Ohki, T.; Yoshida, S.; Yamamoto, M.; Isayama, H.; Yamada, T.; Matsuzawa, T.; Saito, S.; Kuwai, T.; Tomita, M.; Shiratori, T.; et al. Determining the difference in the efficacy and safety of self-expandable metallic stents as a bridge to surgery for obstructive colon cancer among patients in the CROSS 0 group and those in the CROSS 1 or 2 group: a pooled analysis of data from two Japanese prospective multicenter trials. Surg Today. 2020, 50, 984–994. [Google Scholar] [CrossRef]
- Sasaki, T.; Yoshida, S.; Isayama, H.; Narita, A.; Yamada, T.; Enomoto, T.; Sumida, Y.; Kyo, R.; Kuwai, T.; Tomita, M.; et al. Short-Term Outcomes of Colorectal Stenting Using a Low Axial Force Self-Expandable Metal Stent for Malignant Colorectal Obstruction: A Japanese Multicenter Prospective Study. J Clin Med. 2021, 10. [Google Scholar] [CrossRef]
- Latenstein, A.E.J.; Hendriks, M.P.; van Halsema, E.E.; van Hooft, J.E.; van Berkel, A.M. Long-Term Colon Stent Patency for Obstructing Colorectal Cancer Combined with Bevacizumab. Case Rep Gastroenterol. 2017, 11, 711–717. [Google Scholar] [CrossRef]
- Bae, S.U. Is stent insertion for obstructing colon cancer a good prognostic factor in long-term oncologic outcomes in symptomatic obstructive colon cancer? J Minim Invasive Surg. 2021, 24, 123–125. [Google Scholar] [CrossRef]
- Matsuda, A.; Yamada, T.; Matsumoto, S.; Shinji, S.; Ohta, R.; Sonoda, H.; Takahashi, G.; Iwai, T.; Takeda, K.; Sekiguchi, K.; et al. Systemic Chemotherapy is a Promising Treatment Option for Patients with Colonic Stents: A Review. J anus, rectum and colon. 2021, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Watabe, H.; Isayama, H.; Kogure, H.; Nakai, Y.; Yamamoto, N.; Sasaki, T.; Kawakubo, K.; Hamada, T.; Ito, Y.; et al. Feasibility of a new self-expandable metallic stent for patients with malignant colorectal obstruction. Dig Endosc. 2013, 25, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yoshida, S.; Isayama, H.; Matsuzawa, T.; Kuwai, T.; Maetani, I.; Shimada, M.; Yamada, T.; Tomita, M.; Koizumi, K.; et al. A prospective multicenter study on self-expandable metallic stents as a bridge to surgery for malignant colorectal obstruction in Japan: efficacy and safety in 312 patients. Surg Endosc. 2016, 30, 3976–3986. [Google Scholar] [CrossRef]
- Kuwai, T.; Tamaru, Y.; Kusunoki, R.; Yoshida, S.; Matsuzawa, T.; Isayama, H.; Maetani, I.; Shimada, M.; Yamada, T.; Saito, S.; et al. Long-term outcomes of standardized colonic stenting using WallFlex as a bridge to surgery: Multicenter prospective cohort study. Dig Endosc. 2022, 34, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Han, K.S.; Hong, C.W.; Sohn, D.K.; Park, J.W.; Park, S.C.; Kim, S.Y.; Baek, J.Y.; Choi, H.S.; Chang, H.J.; et al. Clinical outcomes of palliative self-expanding metallic stents in patients with malignant colorectal obstruction. J Dig Dis. 2012, 13, 258–266. [Google Scholar] [CrossRef]
- Pais-Cunha, I.; Castro, R.; Libânio, D.; Pita, I.; Bastos, R.P.; Silva, R.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Endoscopic stenting for palliation of intra-abdominal gastrointestinal malignant obstruction: predictive factors for clinical success. Eur J Gastroenterol Hepatol. 2018, 30, 1033–1040. [Google Scholar] [CrossRef]
- Sasaki, T.; Isayama, H.; Kuwai, T.; Saito, S.; Endo, S.; Matsuda, A.; Sumida, Y.; Koizumi, K.; Yamada, T.; Ishibashi, R.; et al. Consensus guidelines for safe endoscopic colorectal stent placement. Available online: https://colon-stent.com/en?319_page.html (accessed on 20 March 2023).
- Adler, D.G.; Baron, T.H. Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol. 2002, 97, 72–78. [Google Scholar] [CrossRef]
- Saida, Y. Current status of colonic stent for obstructive colorectal cancer in Japan; a review of the literature. J anus, rectum and colon. 2019, 3, 99–105. [Google Scholar] [CrossRef]
- Sasaki, T.; Ishibashi, R.; Yoshida, S.; Fujisawa, T.; Shinagawa, H.; Gon, C.; Nakai, Y.; Sasahira, N.; Saida, Y.; Isayama, H. Comparing the mechanical properties of a self-expandable metallic stent for colorectal obstruction: Proposed measurement method of axial force using a new measurement machine. Dig Endosc. 2020, 1, 170–178. [Google Scholar] [CrossRef]
- Cheung, D.Y.; Kim, J.Y.; Hong, S.P.; Jung, M.K.; Ye, B.D.; Kim, S.G.; Kim, J.H.; Lee, K.M.; Kim, K.H.; Baik, G.H.; et al. Outcome and safety of self-expandable metallic stents for malignant colon obstruction: a Korean multicenter randomized prospective study. Surg Endosc. 2012, 26, 3106–3113. [Google Scholar] [CrossRef]
- van Hooft, J.E.; Fockens, P.; Marinelli, A.W.; Timmer, R.; van Berkel, A.M.; Bossuyt, P.M.; Bemelman, W.A. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008, 40, 184–191. [Google Scholar] [CrossRef] [PubMed]
- van Halsema, E.E.; van Hooft, J.E.; Small, A.J.; Baron, T.H.; García-Cano, J.; Cheon, J.H.; Lee, M.S.; Kwon, S.H.; Mucci-Hennekinne, S.; Fockens, P.; et al. Perforation in colorectal stenting: a meta-analysis and a search for risk factors. Gastrointest Endosc. 2014, 79, 970–982.e977, quiz 983.e972, 983.e975.. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Song, H.Y.; Park, J.H.; Ye, B.D.; Yoon, Y.S.; Kim, J.C. Metallic stent placement in the palliative treatment of malignant colonic obstructions: primary colonic versus extracolonic malignancies. J Vasc Interv Radiol. 2011, 22, 1727–1732. [Google Scholar] [CrossRef]
- Meisner, S.; González-Huix, F.; Vandervoort, J.G.; Repici, A.; Xinopoulos, D.; Grund, K.E.; Goldberg, P.; Registry Group, T.W. Self-Expanding Metal Stenting for Palliation of Patients with Malignant Colonic Obstruction: Effectiveness and Efficacy on 255 Patients with 12-Month's Follow-up. Gastroenterol Res Pract. 2012, 2012, 296347. [Google Scholar] [CrossRef]
- Sousa, M.; Pinho, R.; Proença, L.; Silva, J.; Ponte, A.; Rodrigues, J.; Carvalho, J. Predictors of Complications and Mortality in Patients with Self-Expanding Metallic Stents for the Palliation of Malignant Colonic Obstruction. GE Port J Gastroenterol. 2017, 24, 122–128. [Google Scholar] [CrossRef]
- Faraz, S.; Salem, S.B.; Schattner, M.; Mendelsohn, R.; Markowitz, A.; Ludwig, E.; Zheng, J.; Gerdes, H.; Shah, P.M. Predictors of clinical outcome of colonic stents in patients with malignant large-bowel obstruction because of extracolonic malignancy. Gastrointest Endosc. 2018, 87, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Small, A.J.; Coelho-Prabhu, N.; Baron, T.H. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010, 71, 560–572. [Google Scholar] [CrossRef]
- Kim, B.K.; Hong, S.P.; Heo, H.M.; Kim, J.Y.; Hur, H.; Lee, K.Y.; Cheon, J.H.; Kim, T.I.; Kim, W.H. Endoscopic stenting is not as effective for palliation of colorectal obstruction in patients with advanced gastric cancer as emergency surgery. Gastrointest Endosc. 2012, 75, 294–301. [Google Scholar] [CrossRef]
- Moon, S.J.; Kim, S.W.; Lee, B.I.; Lim, C.H.; Kim, J.S.; Soo, J.; Park, J.M.; Lee, I.S.; Choi, M.G.; Choi, K.Y. Palliative stent for malignant colonic obstruction by extracolonic malignancy: a comparison with colorectal cancer. Digestive Diseases and Sciences. 2014, 59, 1891–1897. [Google Scholar] [CrossRef]
- van den Berg, M.W.; Ledeboer, M.; Dijkgraaf, M.G.; Fockens, P.; ter Borg, F.; van Hooft, J.E. Long-term results of palliative stent placement for acute malignant colonic obstruction. Surg Endosc. 2015, 29, 1580–1585. [Google Scholar] [CrossRef]
- Kwon, S.J.; Yoon, J.; Oh, E.H.; Kim, J.; Ham, N.S.; Hwang, S.W.; Park, S.H.; Ye, B.D.; Byeon, J.S.; Myung, S.J.; et al. Factors Associated with Clinical Outcomes of Palliative Stenting for Malignant Colonic Obstruction. Gut and Liver. 2021, 15, 579–587. [Google Scholar] [CrossRef]
- Naruse, N.; Miyahara, K.; Sakata, Y.; Takamori, A.; Ito, Y.; Hidaka, H.; Sameshima, R.; Tsuruoka, N.; Shimoda, R.; Yamanouchi, K.; et al. Utility and safety of the self-expandable metallic colonic stent in Japanese patients who received systemic chemotherapy or palliative treatment for obstructive primary advanced colorectal cancer: A retrospective single-center medical chart evaluation. JGH Open. 2022, 6, 324–329. [Google Scholar] [CrossRef]
- Kuwai, T.; Yamaguchi, T.; Imagawa, H.; Yoshida, S.; Isayama, H.; Matsuzawa, T.; Yamada, T.; Saito, S.; Shimada, M.; Hirata, N.; et al. Factors related to difficult self-expandable metallic stent placement for malignant colonic obstruction: A post-hoc analysis of a multicenter study across Japan. Dig Endosc. 2019, 31, 51–58. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef]
- Fujisawa, G.; Niikura, R.; Kawahara, T.; Honda, T.; Hasatani, K.; Yoshida, N.; Nishida, T.; Sumiyoshi, T.; Kiyotoki, S.; Ikeya, T.; et al. Effectiveness and safety of chemotherapy for patients with malignant gastrointestinal obstruction: A Japanese population-based cohort study. World J Clin Cases. 2022, 10, 5253–5265. [Google Scholar] [CrossRef]
- van Hooft, J.E.; Veld, J.V.; Arnold, D.; Beets-Tan, R.G.H.; Everett, S.; Gotz, M.; van Halsema, E.E.; Hill, J.; Manes, G.; Meisner, S.; et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020, 52, 389–407. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).