Submitted:
23 May 2023
Posted:
25 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Data Mining Methods
3. Results and discussion
3.1. Analysis of fluorinated greenhouse gases emissions in Taiwan
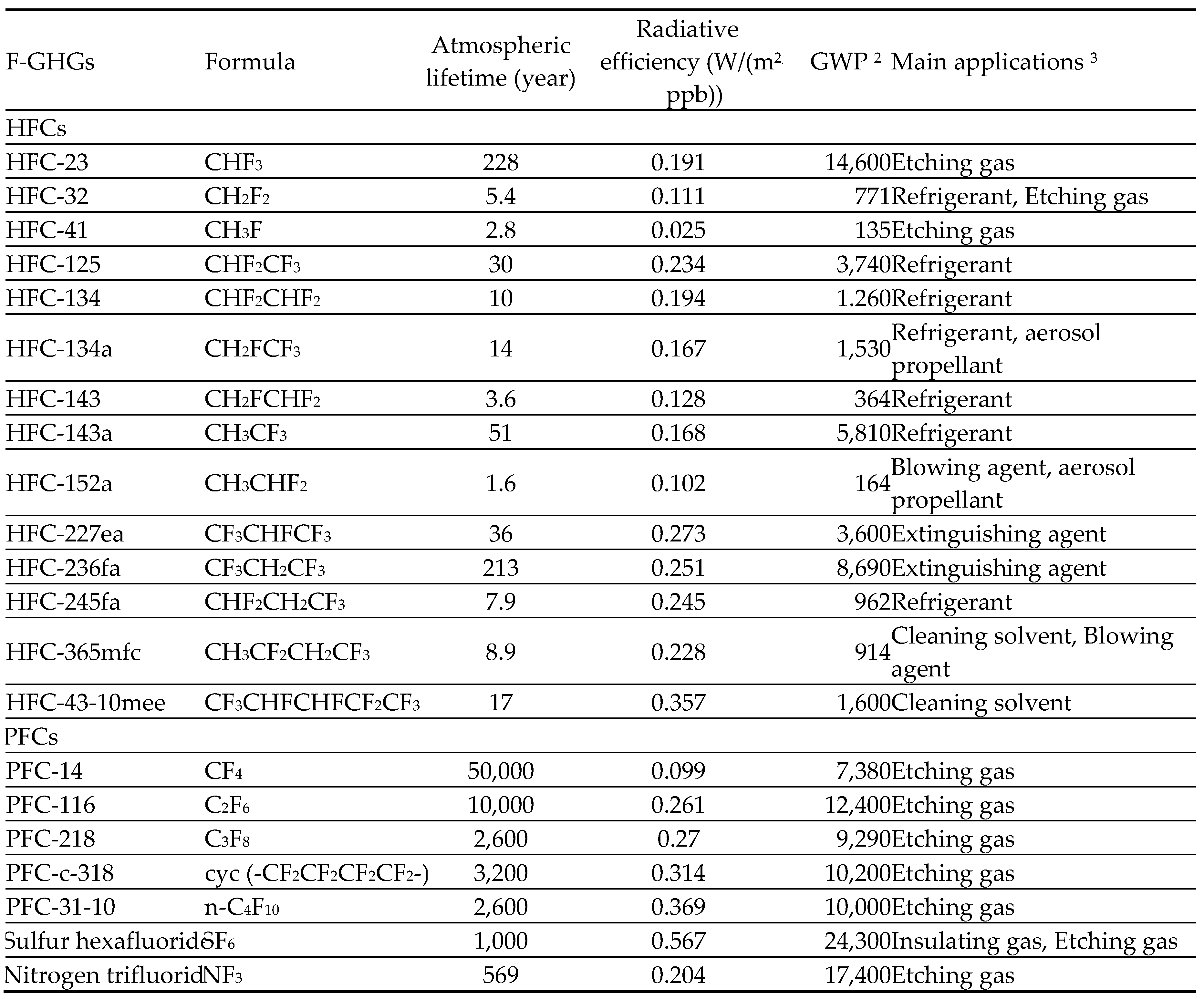
3.1.1. Hydrofluorocarbons (HFCs)
3.1.2. Perfluorocarbons (PFCs)
3.1.3. Sulfur hexafluoride (SF6)
3.1.4. Nitrogen trifluoride (NF3)
3.2. Regulatory strategies for controlling the emissions of fluorinated greenhouse gases
3.2.1. Air Pollution Control Act
- -
- The stationary sources that emit air pollutants shall comply with the emission standards of pipeline (or vent) by installing closed vent/collection system and air pollution control system.
- -
- The EPA in consultation with relevant agencies (i.e., Ministry of Economic Affairs) shall determine the emission standards based on specially designated industry categories, facilities, pollutant items or areas.
3.2.2. Climate Change Response Act
- -
- Process modification: Equipment renew by phase-out, and fluorinated gases (F-gases) reduction by environment-friendly substitutes and recovery/recycling/destruction system installed in the industrial sector.
- -
- Circular economy: Recovery/recycling/storage system installed in the waste (waste electronic appliances like air conditioner and refrigerator, and waste vehicles) management sector.
3.2.3. Waste management Act
3.3. Survey of current abatement technologies for controlling the emissions of fluorinated GHGs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greenhouse Gases (World Meteorological Organization). Available online: https://public.wmo.int/en/our-mandate/focus-areas/environment/greenhouse-gases (accessed on 21 May 2023).
- The Montreal Protocol on Substances that Deplete the Ozone Layer (United Nations Environment Programme). Available online: https://ozone.unep.org/treaties/montreal-protocol (accessed on 21 May 2023).
- Climate Change 2021: The Physical Science Basis (Intergovernmental Panel on Climate Change). Available online: https://www.ipcc.ch/report/sixth-assessment-report-working-group-i/ (accessed on 21 May 2023).
- Global Warming of 1.5 °C (Intergovernmental Panel on Climate Change). Available online: https://www.ipcc.ch/sr15/ (accessed on 21 May 2023).
- Sovacool, B.K.; Griffiths, S.; Kim, J.; Bazilian, M. Climate change and industrial F-gases: A critical and systematic review of developments, sociotechnical systems and policy options for reducing synthetic greenhouse gas emissions. Renew. Sustain. Energy Rev. 2021, 141, 110759. [Google Scholar] [CrossRef]
- Lindley, A.A.; McCulloch, A. Regulating to reduce emissions of fluorinated greenhouse gases. J. Fluor. Chem. 2005, 126, 1457–1462. [Google Scholar] [CrossRef]
- Müllerová, M.; Krtková, E.; Rošková, Z. F-gases: Trends, applications and newly applied gases in the Czech Republic. Atmosphere 2020, 11, 455. [Google Scholar] [CrossRef]
- Sheldon, D.J.; Crimmin, M.R. Repurposing of F-gases: Challenges and opportunities in fluorine chemistry. Chem. Soc. Rev. 2022, 51, 4977–4995. [Google Scholar] [CrossRef] [PubMed]
- Heath, E. Amendment to the Montreal Protocol on Substances that Deplete the Ozone Layer (Kigali Amendment). Int. Leg. Mater. 2017, 56, 193–205. [Google Scholar] [CrossRef]
- Höglund-Isaksson, L.; Purohit, P.; Amann, M.; Bertok, I.; Rafaj, P.; Schöpp, W.; Borken-Kleefeld, J. Cost estimates of the Kigali Amendment to phase-down hydrofluorocarbons. Environ. Sci. Policy 2017, 75, 138–147. [Google Scholar] [CrossRef]
- Castro, P.J.; Aráujo, J.M.M.; Martinho, G.; Pereiro, A.B. Waste management strategies to mitigate the effects of fluorinated greenhouse gases on climate change. Appl. Sci. 2021, 11, 4367. [Google Scholar] [CrossRef]
- Environmental Protection Administration (EPA). Taiwan Greenhouse Gases Inventory; EPA: Taipei, Taiwan, 2022. [Google Scholar]
- Trade Statistics (Customs Administration, Ministry of Finance, Taiwan). Available online: https://portal.sw.nat.gov.tw/APGA/GA30 (accessed on 12 May 2023).
- Tsai, W.T.; Lin, Y.Q. Trend Analysis of air quality index (AQI) and greenhouse gas (GHG) emissions in Taiwan and their regulatory countermeasures. Environments 2021, 8, 29. [Google Scholar] [CrossRef]
- Miller, B.R.; Rigby, M.; Kuijpers, L.J.M.; Krummel, P.B.; Steele, L.P.; Leist, M.; Fraser, P.J.; McCulloch, A.; Harth, C.; Salameh, P.; et al. HFC-23 (CHF3) emission trend response to HCFC-22 (CHClF2) production and recent HFC-23 emission abatement measures. Atmos. Chem. Phys. 2010, 10, 7875–7890. [Google Scholar] [CrossRef]
- Taiwan’s Pathway to Net-Zero Emissions in 2050 (National Development Council, Taiwan). Available online: https://www.ndc.gov.tw/en/Content_List.aspx?n=B927D0EDB57A7A3A&upn=A2B386E427ED5689 (accessed on 20 May 2023).
- Tsai, W.T.; Chen, H.P.; Hsieh, W.Y. (2002) A review of uses, environmental hazards and recovery/recycle technologies of perfluorocarbons (PFCs) emissions from the semiconductor manufacturing processes. J. Loss. Prev. Process Ind. 2002, 15, 65–75. [Google Scholar] [CrossRef]
- Tsai, W.T. An overview of environmental hazards and exposure risk of hydrofluorocarbons (HFCs). Chemosphere 2005, 61, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T. The decomposition products of sulfur hexafluoride (SF6): Reviews of environmental and health risk analysis. J. Fluor. Chem. 2007, 128, 1345–1352. [Google Scholar] [CrossRef]
- Tsai, W.T. Environmental and health risk analysis of nitrogen trifluoride (NF3), a toxic and potent greenhouse gas. J. Hazard. Mater. 2008, 159, 257–263. [Google Scholar] [CrossRef]
- Tsai, W.T. Environmental hazards and health risk of common liquid perfluoro-n-alkanes, potent greenhouse gases. Environ. Int. 2009, 35, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.T.; Hu, A.H. Voluntary GHG reduction of industrial sectors in Taiwan. Chemosphere 2012, 88, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Bartos, S.C.; Lee, W.M.; Li, S.N.; Lu, J. SF6 usage and emission trends in the TFT-LCD industry. Int. J. Greenhouse Gas Control 2013, 17, 106–110. [Google Scholar] [CrossRef]
- 2006 IPCC Guidelines for National Greenhouse Gas Inventories (Intergovernmental Panel on Climate Change). Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/ (accessed on 2 May 2023).
- Laws and Regulations Database (Ministry of Justice, Taiwan). Available online: https://law.moj.gov.tw/Eng/index.aspx (accessed on 3 May 2023).
- Taiwan Semiconductor Industry Association. Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/ (accessed on 2 May 2023).
- Taiwan Panel & Solution Association. Available online: http://www.tpasa.org.tw/ (accessed on 2 May 2023).
- Taiwan Semiconductor Manufacturing Company (TSMC) 2021 Sustainability Report. Available online: https://esg.tsmc.com/download/file/2021_sustainabilityReport/english/e-all.pdf (accessed on 12 May 2023).
- Weiss, R.F.; Mühle, J.; Salameh, P.K.; Harth, C.M. Nitrogen trifluoride in the global atmosphere. Geophys. Res. Lett. 2008, 35, L20821. [Google Scholar] [CrossRef]
- Wei, M.S.; Huang, K.H. Recycling and reuse of industrial wastes in Taiwan. Waste Manag. 2001, 21, 93–97. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chou, Y.H.; Lin, C.M.; Hsu, H.C.; Lin, K.Y.; Chiu, C.S. Perspectives on resource recycling from municipal solid waste in Taiwan. Resour. Policy 2007, 32, 69–79. [Google Scholar] [CrossRef]
- Tsai, W.T. Current practice and policy for transforming E-waste into urban mining: Case study in Taiwan. Int. J. Environ. Waste Manag. 2019, 23, 1–15. [Google Scholar] [CrossRef]
- Tsai, W.T. Recycling of waste electrical & electronic equipment (WEEE) and its toxics-containing management in Taiwan- A case study. Toxics 2020, 8, 48. [Google Scholar] [PubMed]
- Chang, B.L.; Tsai, C.J. Greenhouses gases surveillance and reduction research: A case study in a TFT-LED factory (in Chinese). Ind. Pollut. Prev. Control 2006, 25, 1–20. [Google Scholar]
- Significant New Alternatives Policy (SNAP) Program (US environmental Protection Agency). Available online: https://www.epa.gov/snap (accessed on 18 May 2023).
- Lee, S.J.; Ryu, I.S.; Jeon, S.G.; Moon, S.-H. Emission sources and mitigation of fluorinated Non-CO2 greenhouse gas in registered CDM projects. Greenhouse Gases Sci. Technol. 2017, 7, 589–601. [Google Scholar] [CrossRef]
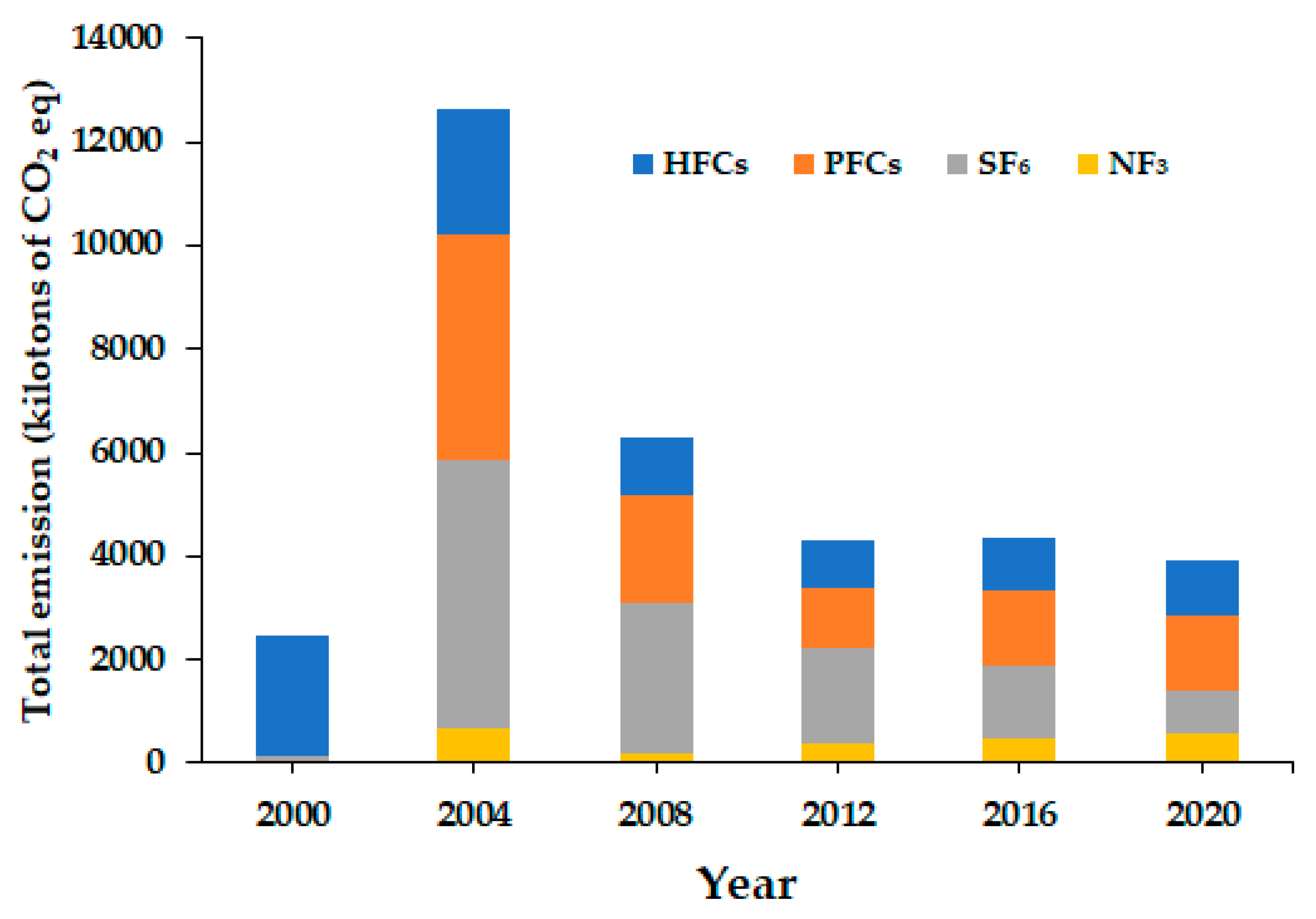
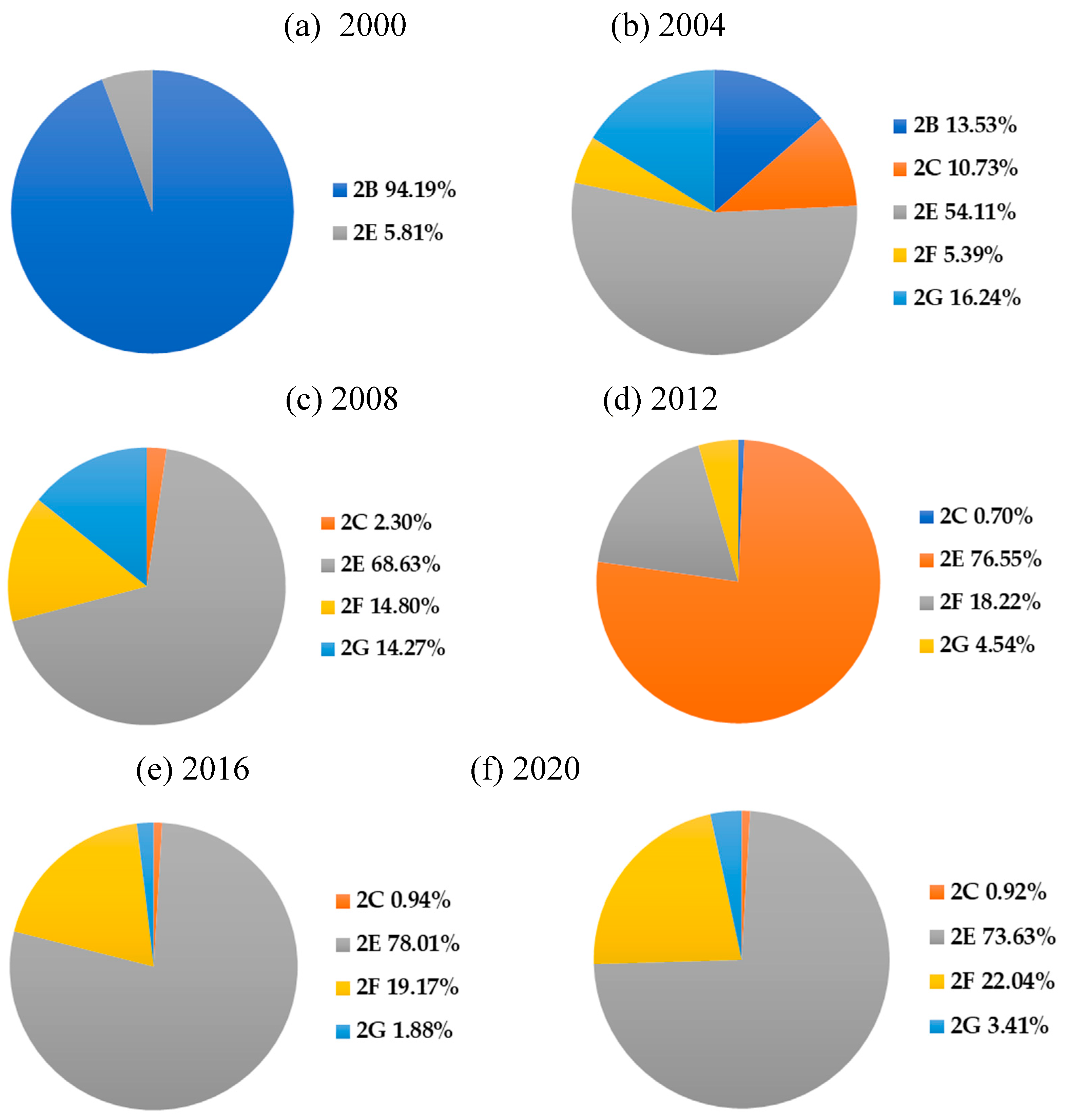
| Year | HFCs | PFCs | SF6 | NF3 | Total |
| 2000 | 2,319 | 13 | 120 | 10 | 2,462 |
| 2001 | 2,619 | 2,939 | 746 | 235 | 6,538 |
| 2002 | 2,216 | 4,143 | 3,914 | 398 | 10,671 |
| 2003 | 2,397 | 4,198 | 4,385 | 540 | 11,520 |
| 2004 | 2,451 | 4,341 | 5,193 | 659 | 12,643 |
| 2005 | 1,098 | 3,470 | 4,951 | 765 | 10,284 |
| 2006 | 1,015 | 3,664 | 3,858 | 688 | 9,225 |
| 2007 | 1,122 | 3,372 | 3,381 | 798 | 8,673 |
| 2008 | 1,074 | 2,082 | 2,912 | 204 | 6,273 |
| 2009 | 1,081 | 1,560 | 2,452 | 577 | 5,607 |
| 2010 | 971 | 1,770 | 2,218 | 258 | 5,217 |
| 2011 | 1,053 | 1,781 | 1,918 | 420 | 5,172 |
| 2012 | 907 | 1,141 | 1,852 | 388 | 4,288 |
| 2013 | 1,019 | 1,345 | 1,997 | 773 | 5,134 |
| 2014 | 1,048 | 1,556 | 1,730 | 667 | 5,001 |
| 2015 | 1,020 | 1,347 | 1,523 | 662 | 4,552 |
| 2016 | 1,026 | 1,441 | 1,418 | 472 | 4,356 |
| 2017 | 1,023 | 1,409 | 1,416 | 440 | 4,298 |
| 2018 | 1,013 | 1,536 | 1,302 | 509 | 4,360 |
| 2019 | 1,027 | 1,420 | 935 | 473 | 3,855 |
| 2020 | 1,053 | 1,447 | 842 | 564 | 3,906 |
| Year | Emission source 2 | Total | ||||
| 2 B | 2 C | 2 E | 2 F | 2 G | ||
| 2000 | 2,319 | 0 | 143 | 0 | 0 | 2,462 |
| 2001 | 2,567 | 0 | 3,971 | 0 | 0 | 6,538 |
| 2002 | 2,157 | 1,027 | 5,544 | 0 | 1,943 | 10,671 |
| 2003 | 1,937 | 1,027 | 6,212 | 401 | 1,943 | 11,520 |
| 2004 | 1,710 | 1,357 | 6,841 | 682 | 2,053 | 12,643 |
| 2005 | 0 | 1,063 | 6,722 | 996 | 1,503 | 10,284 |
| 2006 | 0 | 770 | 6,789 | 896 | 770 | 9,225 |
| 2007 | 0 | 440 | 6,358 | 922 | 953 | 8,673 |
| 2008 | 0 | 144 | 4,305 | 929 | 895 | 6,273 |
| 2009 | 0 | 235 | 3,857 | 812 | 703 | 5,607 |
| 2010 | 0 | 57 | 4,152 | 770 | 238 | 5,217 |
| 2011 | 0 | 50 | 3,989 | 881 | 252 | 5,172 |
| 2012 | 0 | 30 | 3,280 | 783 | 195 | 4,288 |
| 2013 | 0 | 38 | 4,124 | 812 | 160 | 5,134 |
| 2014 | 0 | 33 | 3,995 | 828 | 146 | 5,001 |
| 2015 | 0 | 43 | 3,530 | 851 | 128 | 4,552 |
| 2016 | 0 | 41 | 3,398 | 835 | 82 | 4,356 |
| 2017 | 0 | 59 | 3,329 | 821 | 79 | 4,298 |
| 2018 | 0 | 81 | 3,319 | 811 | 149 | 4,360 |
| 2019 | 0 | 43 | 2,856 | 846 | 110 | 3,855 |
| 2020 | 0 | 36 | 2,876 | 861 | 133 | 3,906 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





