Submitted:
24 May 2023
Posted:
25 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cerebrospinal fluid and blood collection
2.2. ELISA procedure
- TREM2 (Human TREM2 ELISA Kit, Abcam, Cambridge, United Kingdom),
- Aβ1-42 (Innotest β-amyloid1-42, Fujirebio, Gent, Belgium),
- total tau (Innotest hTau Ag, Fujirebio, Gent, Belgium), p-tau181 (Innotest Phospho-Tau(181P), Fujirebio, Gent, Belgium), and
- ASC (Human PYCARD/ASC/TMS1 Sandwich ELISA, LSBio, Seattle, Washington, United States).
3. Results
3.2. Figures, Tables, and Schemes
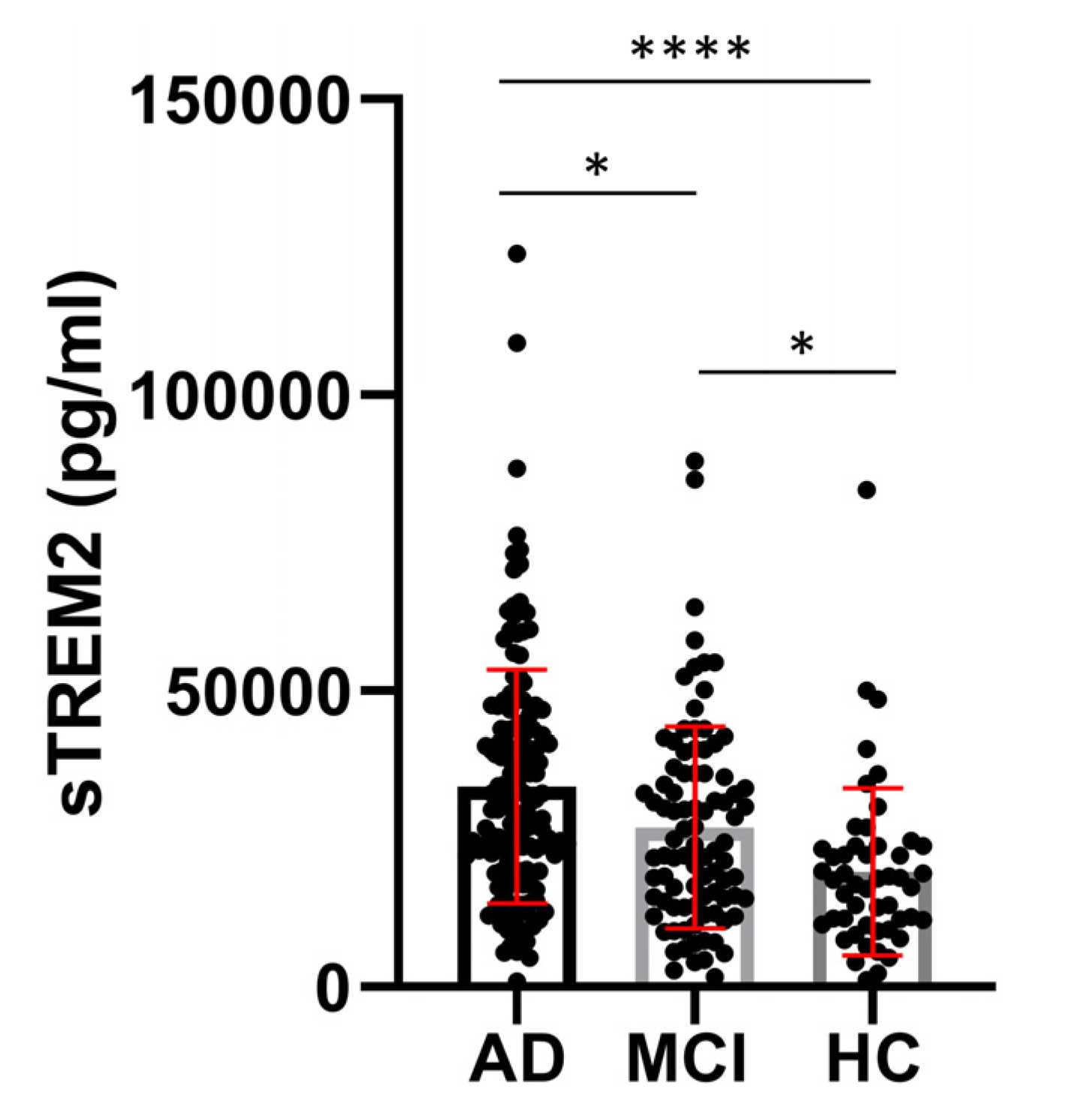
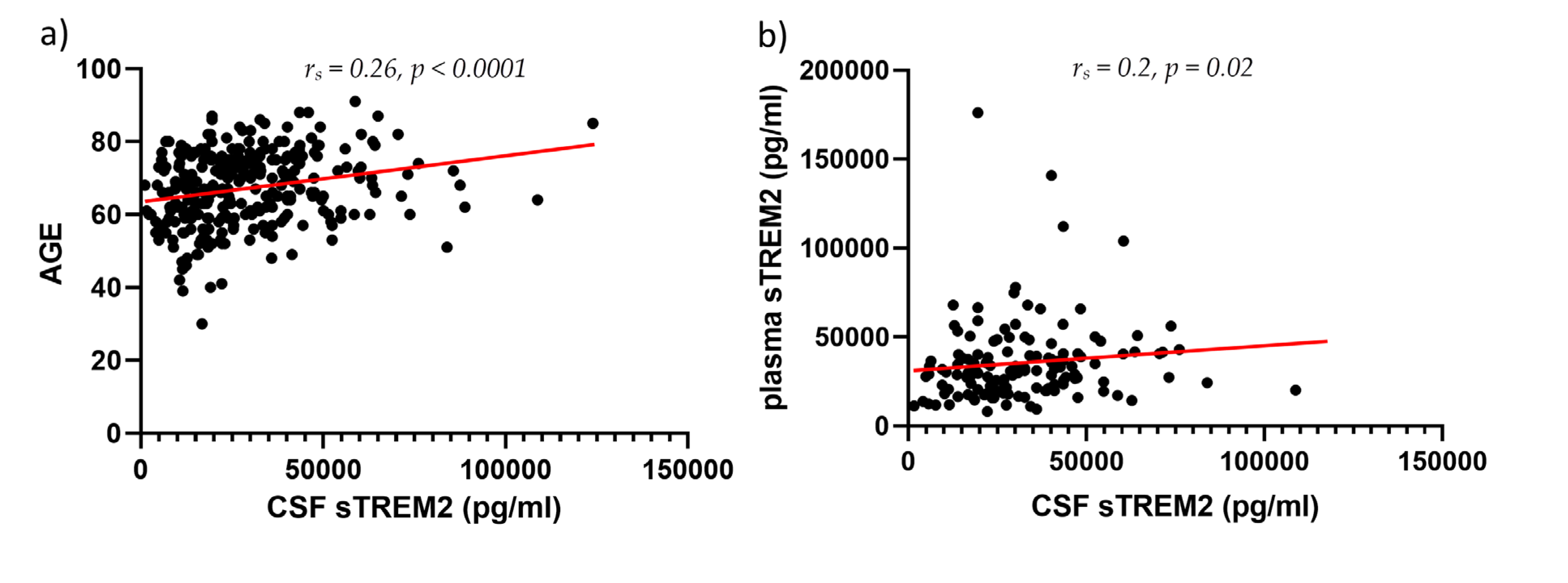
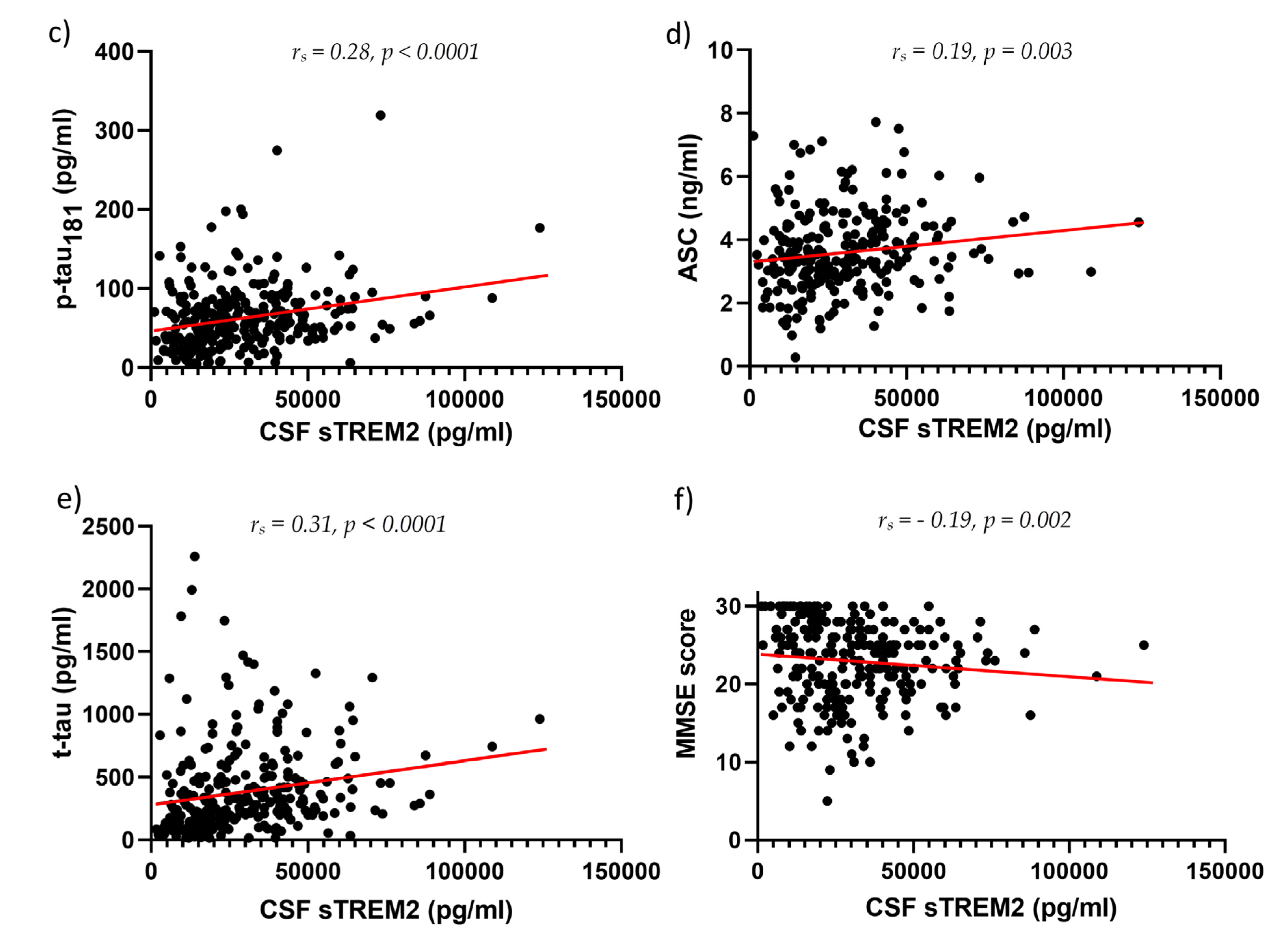
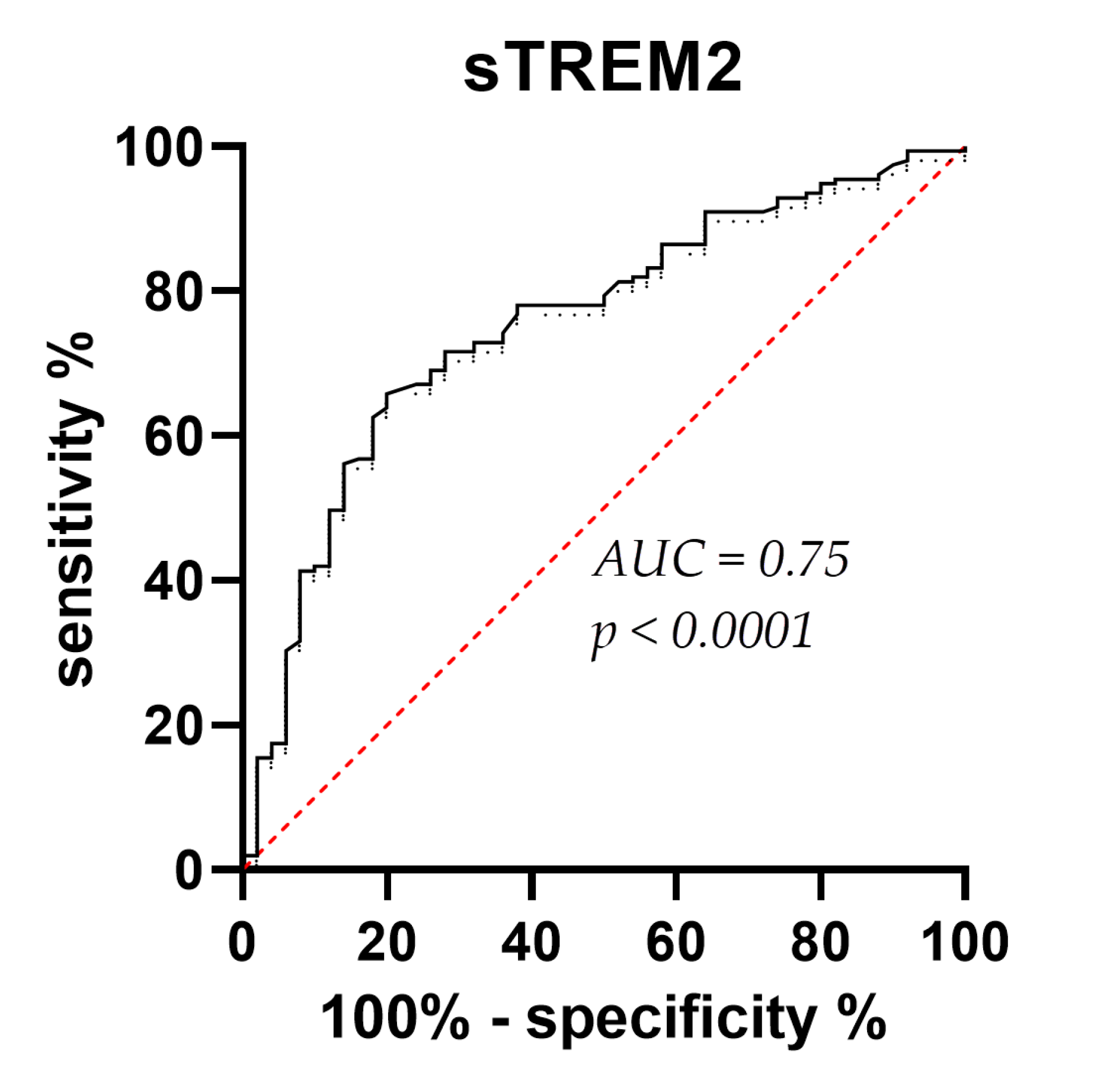
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong:, L.; Chen, X.F. The emerging roles and therapeutic potential of soluble TREM2 in Alzheimer's disease. Front Aging Neurosci. 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Jay, T.R.; von Saucken, V.E.; Landreth, G.E. TREM2 in neurodegenerative diseases. Mol Neurodegener. 2017, 12, 56. [Google Scholar] [CrossRef] [PubMed]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wang, M.; Yin, Y.; Tang, Y. The specific mechanism of TREM2 regulation of synaptic clearance in Alzheimer's disease. Front Immunol. 2022, 13, 845897. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Qin, Q.; Yang, H.C.; Wang, Y.Y.; Mi, Y.X.; Yin, Y.S.; Wang, M.; Yu, C.J.; Tang, Y. TREM2 in the pathogenesis of AD: a lipid metabolism regulator and potential metabolic therapeutic target. Mol Neurodegener. 2022, 17, 40. [Google Scholar] [CrossRef]
- Filipello, F.; You, S.F.; Mirfakhar, F.S.; Mahali, S.; Bollman, B.; Acquarone, M.; Korvatska, O.; Marsh, J.A.; Sivaraman, A.; Martinez, R.; Cantoni, C.; De Feo, L.; Ghezzi, L.; Minaya, M.A.; Renganathan, A.; Cashikar, A.G.; Satoh, J.I.; Beatty, W.; Iyer, A.K.; Cella, M.; Raskind, W.H.; Piccio, L.; Karch, C.M. Defects in lysosomal function and lipid metabolism in human microglia harboring a TREM2 loss of function mutation. Acta Neuropathol. 2023, 145, 749–772. [Google Scholar] [CrossRef]
- Šimić, G.; Španić, E.; Langer Horvat, L.; Hof, P.R. Blood-brain barrier and innate immunity in the pathogenesis of Alzheimer's disease. Prog Mol Biol Transl Sci. 2019, 168, 99–145. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; Itzkovitz, S.; Colonna, M.; Schwartz, M.; Amit, I. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef]
- Ulland, T.K.; Song, W.M.; Huang, S.C.; Ulrich, J.D.; Sergushichev, A.; Beatty, W.L.; Loboda, A.A.; Zhou, Y.; Cairns, N.J.; Kambal, A.; Loginicheva, E.; Gilfillan, S.; Cella, M.; Virgin, H.W.; Unanue, E.R.; Wang, Y.; Artyomov, M.N.; Holtzman, D.M.; Colonna, M. TREM2 maintains microglial metabolic fitness in Alzheimer's disease. Cell. 2017, 170, 649–663.e13. [Google Scholar] [CrossRef]
- Wang, Y.; Ulland, T.K.; Ulrich, J.D.; Song, W.; Tzaferis, J.A.; Hole, J.T.; Yuan, P.; Mahan, T.E.; Shi, Y.; Gilfillan, S.; Cella, M.; Grutzendler, J.; DeMattos, R.B.; Cirrito, J.R.; Holtzman, D.M.; Colonna, M. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med. 2016, 213, 667–675. [Google Scholar] [CrossRef]
- Yang, J.; Fu, Z.; Zhang, X.; Xiong, M.; Meng, L.; Zhang, Z. TREM2 ectodomain and its soluble form in Alzheimer's disease. J Neuroinflammation. 2020, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Ewers, M.; Biechele, G.; Suárez-Calvet, M.; Sacher, C.; Blume, T.; Morenas-Rodriguez, E.; Deming, Y.; Piccio, L.; Cruchaga, C.; Kleinberger, G.; Shaw, L.; Trojanowski, J.Q.; Herms, J.; Dichgans, M.; Brendel, M.; Haass, C.; Franzmeier, N. Higher CSF sTREM2 and microglia activation are associated with slower rates of β-amyloid accumulation. EMBO Mol Med. 2020, 12, e12308. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S.; Ibrahim, A.; Rhinn, H.; Ilaria Tassi, I.; Rosenthal, A.; Schwabe, T.; Colonna, M. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer's disease model. J Exp Med. 2020, 217, e20200785. [Google Scholar] [CrossRef]
- Jain, N.; Lewis, C.A.; Ulrich, J.D.; Holtzman, D.M. Chronic TREM2 activation exacerbates Aβ-associated tau seeding and spreading. J Exp Med. 2023, 220, e20220654. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Xu, Y.; Zhuo, R.; Wang, T.; Wang, K.; Huang, R.; Wang, D.; Yue Gao, Y.; Zhu, Y.; Sheng, X.; Chen, K.; Wang, N.; Zhu, L.; Can, D.; Marten, Y.; Shinohara, M.; Liu, C.C.; Du, D.; Sun, H.; Wen, L.; Xu, H.; Bu, G.; Chen, X.F. Soluble TREM2 ameliorates pathological phenotypes by modulating microglial functions in an Alzheimer's disease model. Nat Commun. 2019, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Guo, H.; Zhang, X.; Yang, Z.; Ruganzu, J.B.; Yang, Z.; Wu, X.; Bi, W.; Ji, S.; Yang, W. TREM2 inhibits tau hyperphosphorylation and neuronal apoptosis via the PI3K/Akt/GSK-3β signaling pathway in vivo and in vitro. Mol Neurobiol. 2023, 60, 2470–2485. [Google Scholar] [CrossRef]
- Pereira, J.B.; Janelidze, S.; Strandberg, O.; Whelan, C.D.; Zetterberg, H.; Blennow, K.; Palmqvist, S.; Stomrud, E.; Mattsson-Carlgren, N.; Hansson, O. Microglial activation protects against accumulation of tau aggregates in nondemented individuals with underlying Alzheimer's disease pathology. Nat Aging. 2022, 2, 1138–1144. [Google Scholar] [CrossRef]
- Lee, C.Y.D.; Daggett, A.; Gu, X.; Jiang, L.L.; Langfelder, P.; Li, X.; Wang, N.; Zhao, Y.; Park, C.S.; Cooper, Y.; Ferando, I.; Mody, I.; Coppola, G.; Xu, H.; Yang, X.W. Elevated TREM2 gene dosage reprograms microglia responsivity and ameliorates pathological phenotypes in Alzheimer's disease models. Neuron. 2018, 97, 1032–1048.e5. [Google Scholar] [CrossRef]
- Cosker, K.; Mallach, A.; Limaye, J.; Piers, T.M.; Staddon, J.; Neame, S.J.; Hardy, J.; Pocock, J.M. Microglial signalling pathway deficits associated with the patient-derived R47H TREM2 variants linked to AD indicate inability to activate inflammasome. Sci Rep. 2021, 11, 13316. [Google Scholar] [CrossRef]
- Yang, S.; Yang, Y.; Wang, F.; Luo, Q.Y.; Zhang, Y.; Zheng, F.; Shu, Q.; Chen, Q.; Fang, X. TREM2 dictates antibacterial defense and viability of bone marrow-derived macrophages during bacterial infection. Am J Respir Cell Mol Biol. 2021, 65, 176–188. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, C.; Zhu, Y.; Fan, H.; Liu, Q.; Liu, Y.; Chen, K.; Wu, Y.; Liang, S.; Li, M.; Li, L.; Liu, X.; Zhang, Y.; Wu, C.; Lu, G.; Wu, M. TREM2/β-catenin attenuates NLRP3 inflammasome-mediated macrophage pyroptosis to promote bacterial clearance of pyogenic bacteria. Cell Death Dis. 2022, 13, 771. [Google Scholar] [CrossRef] [PubMed]
- Jay, T.R.; Miller, C.M.; Cheng, P.J.; Graham, L.C.; Bemiller, S.; Broihier, M.L.; Xu, G.; Margevicius, D.; Karlo, J.C.; Sousa, G.L; Cotleur, A.C.; Butovsky, O.; Bekris, L.; Staugaitis, S.M; Leverenz, J.B.; Pimplikar, S.W.; Landreth, G.E.; Howell, G.R.; Ransohoff, R.M.; Lamb, B.T. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer's disease mouse models. J Exp Med. 2015, 212, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Long, W.; Gao, M.; Jiao, F.; Chen, Z.; Liu, M.; Yu, L. TREM2 regulates high glucose-induced microglial inflammation via the NLRP3 signaling pathway. Brain Sci. 2021, 11, 896. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liu, F.; Li, H.; Wang, K.; Cao, X.; Xu, X.; Zhou, Y.; Zou, J.; Zhang, X.; Cui, X. TREM2 ameliorates anesthesia and surgery-induced cognitive impairment by regulating mitophagy and NLRP3 inflammasome in aged C57/BL6 mice. Neurotoxicology. 2022, 90, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, F.; Jiang, W.; Wang, K.; Cao, X.; Zou, J.; Zhou, Y.; Li, Z.; Liu, S.; Cui, X.; Zhang, X. TREM2 ameliorates lipopolysaccharide-induced oxidative stress response and neuroinflammation by promoting sirtuin3 in BV2 cells. Neurotox Res. 2022, 40, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Del-Aguila, J.L.; Benitez, B.A.; Li, Z.; Dube, U.; Mihindukulasuriya, K.A.; Budde, J.P.; Farias, F.H.G.; Fernández, M.V.; Ibanez, L.; Jiang, S.; Perrin, R.J.; Cairns, N.J.; Morris, J.C.; Harari, O.; Cruchaga, C. TREM2 brain transcript-specific studies in AD and TREM2 mutation carriers. Mol Neurodegener. 2019, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Filipello, F.; Goldsbury, C.; You, S.F.; Locca, A.; Karch, C.M.; Piccio, L. Soluble TREM2: Innocent bystander or active player in neurological diseases? Neurobiol Dis. 2022, 165, 105630. [Google Scholar] [CrossRef]
- Zhong, L.; Chen, X.F.; Wang, T.; Wang, Z.; Liao, C.; Wang, Z.; Huang, R.; Wang, D.; Li, X.; Wu, L.; Jia, L.; Zheng, H.; Painter, M.; Atagi, Y.; Liu, C.C.; Zhang, Y.W.; Fryer, J.D.; Xu, H.; Bu, G. Soluble TREM2 induces inflammatory responses and enhances microglial survival. J Exp Med. 2017, 214, 597–607. [Google Scholar] [CrossRef]
- Qiao, W.; Chen, Y.; Zhong, J.; Madden, B.J.; Charlesworth, C.M.; Martens, Y.A.; Liu, C.C.; Knight, J.; Ikezu, T.C.; Kurti, A.; Zhu, Y.; Meneses, A.; Rosenberg, C.L.; Kuchenbecker, L.A.; Vanmaele, L.K.; Li, F.; Chen, K. , Shue, F.; Dacquel, M.V.; Fryer, J.; Pandey, A.; Zhao, N.; Bu, G. Trem2 H157Y increases soluble TREM2 production and reduces amyloid pathology. Mol Neurodegener. 2023, 18, 8. [Google Scholar] [CrossRef]
- Gratuze, M.; Leyns, C.E.; Sauerbeck, A.D.; St-Pierre, M.K.; Xiong, M.; Kim, N.; Serrano, J.R.; Tremblay, M.È.; Kummer, T.T.; Colonna, M.; Ulrich, J.D.; Holtzman, D.M. Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J Clin Invest. 2020, 130, 4954–4968. [Google Scholar] [CrossRef]
- Albanus, R.; Jain, N.; Novotny, B.; Brase, L.; Rodriguez, L.; Mansel, C.; Kipnis, M.; O'Brien, S.; Pasillas, M.P.; Lee, C.; Manis, M.; Colonna, M.; Harari, O.; Glass, C.K.; Ulrich, J.D.; Holtzman, D.M. TREM2-independent microgliosis promotes tau-mediated neurodegeneration in the presence of ApoE4. Neuron. 2023, 111, 202–219.e7. [Google Scholar] [CrossRef]
- Vautheny, A.; Duwat, C.; Aurégan, G.; Joséphine, C.; Hérard, A.S.; Jan, C.; Mitja, J.; Gipchtein, P.; Gaillard, M.C.; Buée, L.; Blum, D.; Hantraye, P.; Bonvento, G.; Brouillet, E.; Cambon, K.; Bemelmans, A.P. THY-Tau22 mouse model accumulates more tauopathy at late stage of the disease in response to microglia deactivation through TREM2 deficiency. Neurobiol Dis. 2021, 155, 105398. [Google Scholar] [CrossRef]
- Brown, G.C.; St George-Hyslop, P. Does soluble TREM2 protect against Alzheimer's disease? Front Aging Neurosci. 2022, 13, 834697. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.D.; Ulland, T.K.; Colonna, M.; Holtzman, D.M. Elucidating the role of TREM2 in Alzheimer's disease. Neuron. 2017, 94, 237–248. [Google Scholar] [CrossRef]
- Suárez-Calvet, M.; Kleinberger, G.; Araque Caballero, M.Á.; Brendel, M.; Rominger, A.; Alcolea, D.; Fortea, J.; Lleó, A.; Blesa, R.; Gispert, J.D.; Sánchez-Valle, R.; Antonell, A.; Rami, L.; Molinuevo, J.L.; Brosseron, F.; Traschütz, A.; Heneka, M.T.; Struyfs, H.; Engelborghs, S.; Sleegers, K.; Van Broeckhoven, C.; Zetterberg, H.; Nellgård, B.; Blennow, K.; Crispin, A.; Michael Ewers, M.; Haass, C. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer's disease and associate with neuronal injury markers. EMBO Mol Med. 2016, 8, 466–476. [Google Scholar] [CrossRef]
- Suárez-Calvet, M.; Morenas-Rodríguez, E.; Kleinberger, G.; Schlepckow, K.; Araque Caballero, M.A.; Franzmeier, N.; Capell, A.; Fellerer, K.; Nuscher, B.; Eren, E.; Levin, J.; Deming, Y.; Piccio, L.; Karch, C.M.; Cruchaga, C.; Shaw, L.M.; Trojanowski, J.Q.; Weiner, M.; Ewers, M.; Haass, C. Early increase of CSF sTREM2 in Alzheimer's disease is associated with tau related-neurodegeneration but not with amyloid-β pathology. Mol Neurodegener. 2019, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Z.; Tan, L.; Bi, Y.L.; Shen, X.N.; Xu, W.; Ma, Y.H.; Li, H.Q.; Dong, Q.; Yu, J.T. Dynamic changes of CSF sTREM2 in preclinical Alzheimer's disease: the CABLE study. Mol Neurodegener. 2020, 15, 25. [Google Scholar] [CrossRef]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Carrillo, M.C.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; Nelson, P.T.; Schneider, J.A.; Thal, D.R.; Thies, B.; Trojanowski, J.Q.; Vinters, H.V.; Montine, T.J. National Institute on Aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; Nelson, P.T.; Schneider, J.A.; Thal, D.R.; Trojanowski, J.Q.; Vinters, H.V.; Hyman, B.T. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol 2012, 123, 1–11. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; Snyder, P.J.; Carrillo, M.C.; Thies, B.; Phelps, C.H. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s association workgroups on. Alzheimer’s Dement 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Heslegrave, A.; Heywood, W.; Paterson, R.; Magdalinou, N.; Svensson, J.; Johansson, P.; Öhrfelt, A.; Blennow, K.; Hardy, J.; Schott, J.; Mills, K.; Zetterberg, H. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer's disease. Mol Neurodegener. 2016, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, C.G.; Assogna, M.; Di Donna, M.G.; Bernocchi, F.; De Lucia, V.; Nuccetelli, M.; Fiorelli, D.; Loizzo, S.; Mercuri, N.B.; Koch, G.; Martorana, A.; Motta, C. Cerebrospinal fluid sTREM-2, GFAP, and β-S100 in symptomatic sporadic Alzheimer's disease: Microglial, astrocytic, and APOE contributions along the Alzheimer's disease continuum. J Alzheimers Dis. 2023, 92, 1385–1397. [Google Scholar] [CrossRef]
- Zhao, A.; Jiao, Y.; Ye, G.; Kang, W.; Tan, L.; Li, Y.; Deng, Y.; Liu, J. Alzheimer’s disease neuroimaging initiative (ADNI). Soluble TREM2 levels associate with conversion from mild cognitive impairment to Alzheimer's disease. J Clin Invest. 2022, 132, e158708. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.J.; Guo, J. Biofluid biomarkers of Alzheimer's disease: Progress, problems, and perspectives. Neurosci Bull. 2022, 38, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Salih, D.A.; Bayram, S.; Guelfi, S.; Reynolds, R.H.; Shoai, M.; Ryten, M.; Brenton, J.W.; Zhang, D.; Matarin, M.; Botia, J.A.; Shah, R.; Brookes, K.J.; Guetta-Baranes, T.; Morgan, K.; Bellou, E.; Cummings, D.M. , Escott-Price, V.; Hardy, J. Genetic variability in response to amyloid beta deposition influences Alzheimer's disease risk. Brain Commun. 2019; 1, fcz022. [Google Scholar] [CrossRef]
- Andrews, S.J.; Renton, A.E.; Fulton-Howard, B.; Podlesny-Drabiniok, A.; Marcora, E.; Goate, A.M. The complex genetic architecture of Alzheimer's disease: novel insights and future directions. EBioMedicine. 2023, 90, 104511. [Google Scholar] [CrossRef]
- Chen, X.; Firulyova, M.; Manis, M.; Herz, J.; Smirnov, I.; Aladyeva, E.; Wang, C.; Bao, X.; Finn, M.B.; Hu, H.; Shchukina, I.; Kim, M.W.; Yuede, C.M.; Kipnis, J.; Artyomov, M.N.; Ulrich, J.D.; Holtzman, D.M. Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature. 2023, 615, 668–677. [Google Scholar] [CrossRef]
- Winfree, R.L.; Dumitrescu, L.; Blennow, K.; Zetterberg, H.; Gifford, K.A.; Pechman, K.R.; Jefferson, A.L.; Hohman, T.J. Alzheimer's Disease Neuroimaging Initiative. Biological correlates of elevated soluble TREM2 in cerebrospinal fluid. Neurobiol Aging. 2022, 118, 88–98. [Google Scholar] [CrossRef]
- Attems, J.; Lintner, F.; Jellinger, K.A. Amyloid beta peptide 1-42 highly correlates with capillary cerebral amyloid angiopathy and Alzheimer disease pathology. Acta Neuropathol. 2004, 107, 283–291. [Google Scholar] [CrossRef]
- Attems, J.; Jellinger, K.A. Only cerebral capillary amyloid angiopathy correlates with Alzheimer pathology--a pilot study. Acta Neuropathol. 2004, 107, 83–90. [Google Scholar] [CrossRef]
- Zaretsky, D.V.; Zaretskaia, M.V.; Molkov, Y.I. Alzheimer’s Disease Neuroimaging Initiative. Patients with Alzheimer's disease have an increased removal rate of soluble beta-amyloid-42. PLoS One. 2022, 17, e0276933. [Google Scholar] [CrossRef] [PubMed]
- Španić, E.; Langer Horvat, L.; Hof. P.R.; Šimić, G. Role of microglial cells in Alzheimer's disease tau propagation. Front Aging Neurosci. 2019, 11, 271. [Google Scholar] [CrossRef]
- Španić, E.; Langer Horvat, L.; Ilić, K.; Hof, P.R.; Šimić, G. NLRP1 Inflammasome activation in the hippocampal formation in Alzheimer's disease: Correlation with neuropathological changes and unbiasedly estimated neuronal loss. Cells. 2022, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Chen, Y.F.; Yen, R.F.; Lo, Y.L.; Yang, K.C.; Jeng, J.S.; Tsai, L.K.; Chang, C.F. Plasma soluble TREM2 is associated with white matter lesions independent of amyloid and tau. Brain. 2021, 144, 3371–3380. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Decoding Alzheimer's disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. 2013, 108, 21–43. [Google Scholar] [CrossRef]
- An, Y.; Varma, V.R.; Varma, S.; Casanova, R.; Dammer, E.; Pletnikova, O.; Chia, C.W.; Egan, J.M.; Ferrucci, L.; Troncoso, J.; Levey, A.I.; Lah, J.; Seyfried, N.T.; Legido-Quigley, C.; O'Brien, R.; Thambisetty, M. Evidence for brain glucose dysregulation in Alzheimer's disease. Alzheimers Dement. 2018, 14, 318–329. [Google Scholar] [CrossRef]
- Cho, S.; Lee, H.; Seo, J. Impact of genetic risk factors for Alzheimer's disease on brain glucose metabolism. Mol Neurobiol. 2021, 58, 2608–2619. [Google Scholar] [CrossRef]
- Knezović, A.; Osmanović-Barilar, J.; Ćurlin, M.; Hof, P.R.; Šimić, G.; Riederer, P.; Šalković-Petrišić, M. Staging of cognitive deficits and neuropathological and ultrastructural changes in streptozotocin-induced rat model of Alzheimer's disease. J Neural Transm. 2015, 122, 577–592. [Google Scholar] [CrossRef]
- Choi, H.; Choi, Y.; Lee, E.J.; Kim, H.; Lee, Y.; Kwon, S.; Hwang, D.W.; Lee, D.S. Hippocampal glucose uptake as a surrogate of metabolic change of microglia in Alzheimer's disease. J Neuroinflammation. 2021, 18, 190. [Google Scholar] [CrossRef]
- Piccio, L.; Buonsanti, C.; Cella, M.; Tassi, I.; Schmidt, R.E.; Fenoglio, C.; Rinker, J. 2nd.; Naismith, R.T.; Panina-Bordignon, P.; Passini, N.; Galimberti, D.; Scarpini, E.; Colonna, M.; Cross, A.H. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain. 2008, 131, 3081–3091. [Google Scholar] [CrossRef]
- Piccio, L.; Deming, Y.; Del-Águila, J.L.; Ghezzi, L.; Holtzman, D.M.; Fagan, A.M.; Fenoglio, C.; Galimberti, D.; Borroni, B.; Cruchaga, C. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 2016, 131, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, E.H.; Kim, H.J.; Jo, S.; Lee, S.; Seo, S.W.; Park, H.H.; Koh, S.H.; Lee, J.H. The relationship of soluble TREM2 to other biomarkers of sporadic Alzheimer's disease. Sci Rep. 2021, 11, 13050. [Google Scholar] [CrossRef] [PubMed]
| Group | Age (years) mean ± SD (n) |
MMSE (points) mean ± SD (n) |
sTREM2 (pg/ml) mean ± SD (n) |
ASC (ng/ml) mean ± SD (n) |
Aβ1-42 (pg/ml) mean ± SD (n) |
p-tau181 (pg/ml) mean ± SD (n) |
t-tau (pg/ml) mean ± SD (n) |
|---|---|---|---|---|---|---|---|
| AD | 70.58 ± 8.670 (154) | 20.30 ± 4.395 (142) | 33854 ± 19631 (155) | 3.817± 1.348 (126) | 549.5 ± 303.9 (152) | 77.06 ± 45.25 (147) |
522.5 ± 400.9 (146) |
| MCI | 66.42 ± 8.832 (90) | 25.64 ± 2.903 (74) | 26940 ± 17043 (90) | 3.432 ± 1.121 (78) | 665.9 ± 374.8 (86) |
53.32 ± 29,90 (87) |
257.5 ± 177,1 (86) |
| HC | 58.80 ± 10.68 (49) | 29.06 ± 2.199 (32) | 18527 ± 13566 (50) | 3.369 ± 1.272 (39) | 552.8 ± 521.7 (49) |
35.18 ± 22.70 (49) |
222.7 ± 284,0 (48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




