Submitted:
23 May 2023
Posted:
25 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
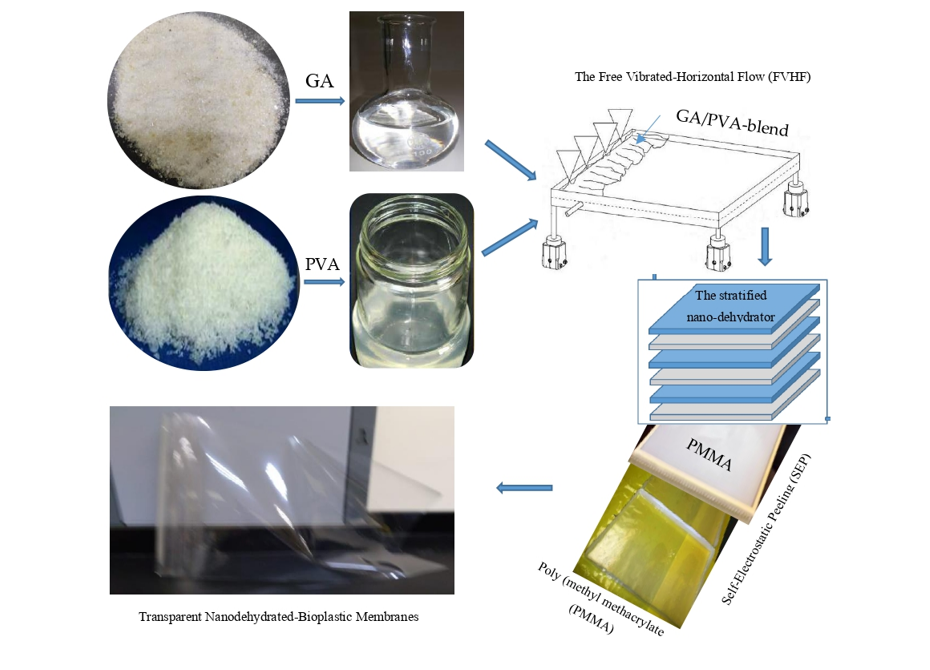
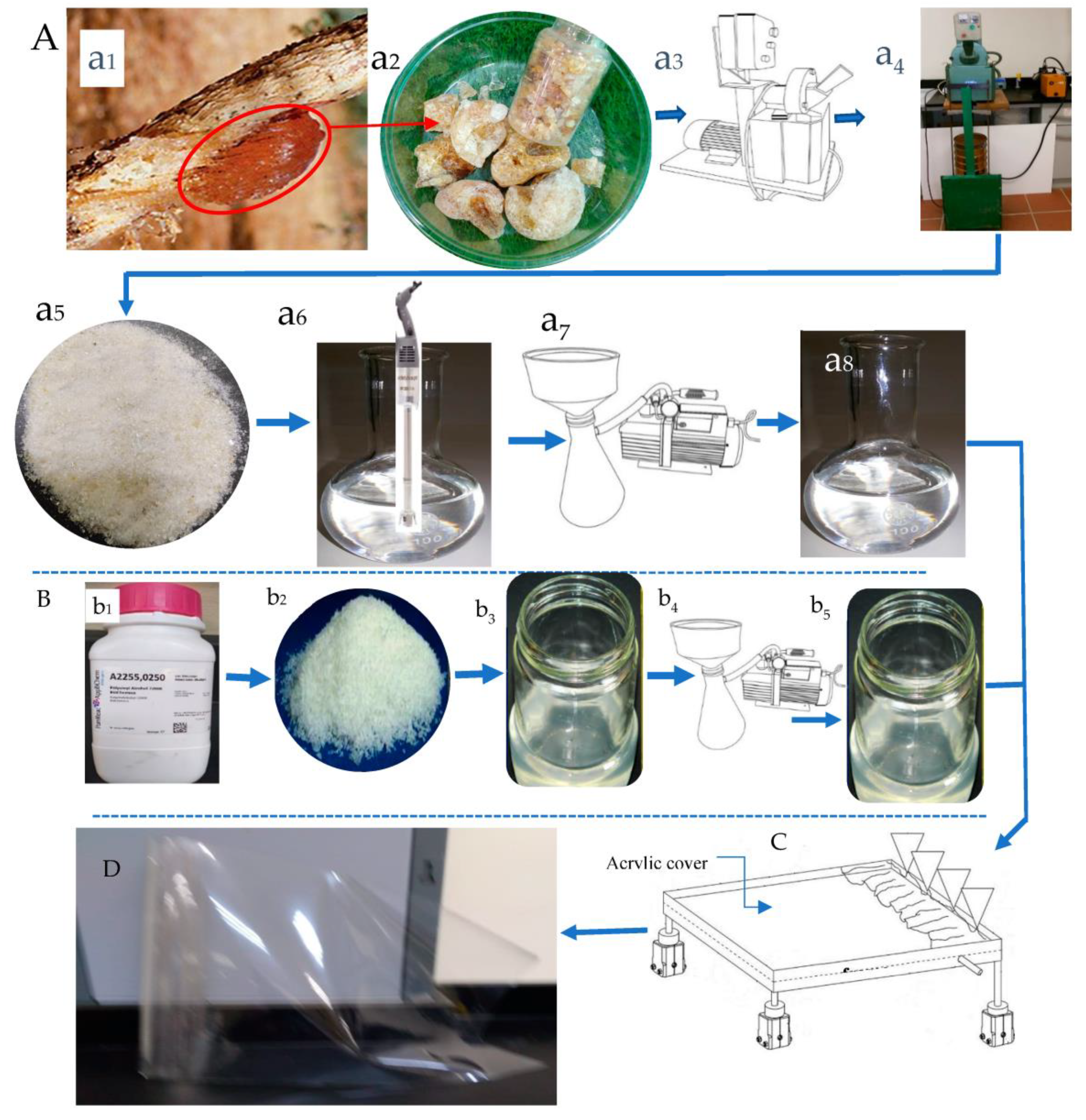
2.1. Raw Material
2.2. Preparation of the Chemical Reagents
2.2.1. GA
2.2.2. PVA
2.3. Preparation of the Bioplastic Blends
2.4. Casting the Bioplastic Blends
2.5. Drying the NDBs
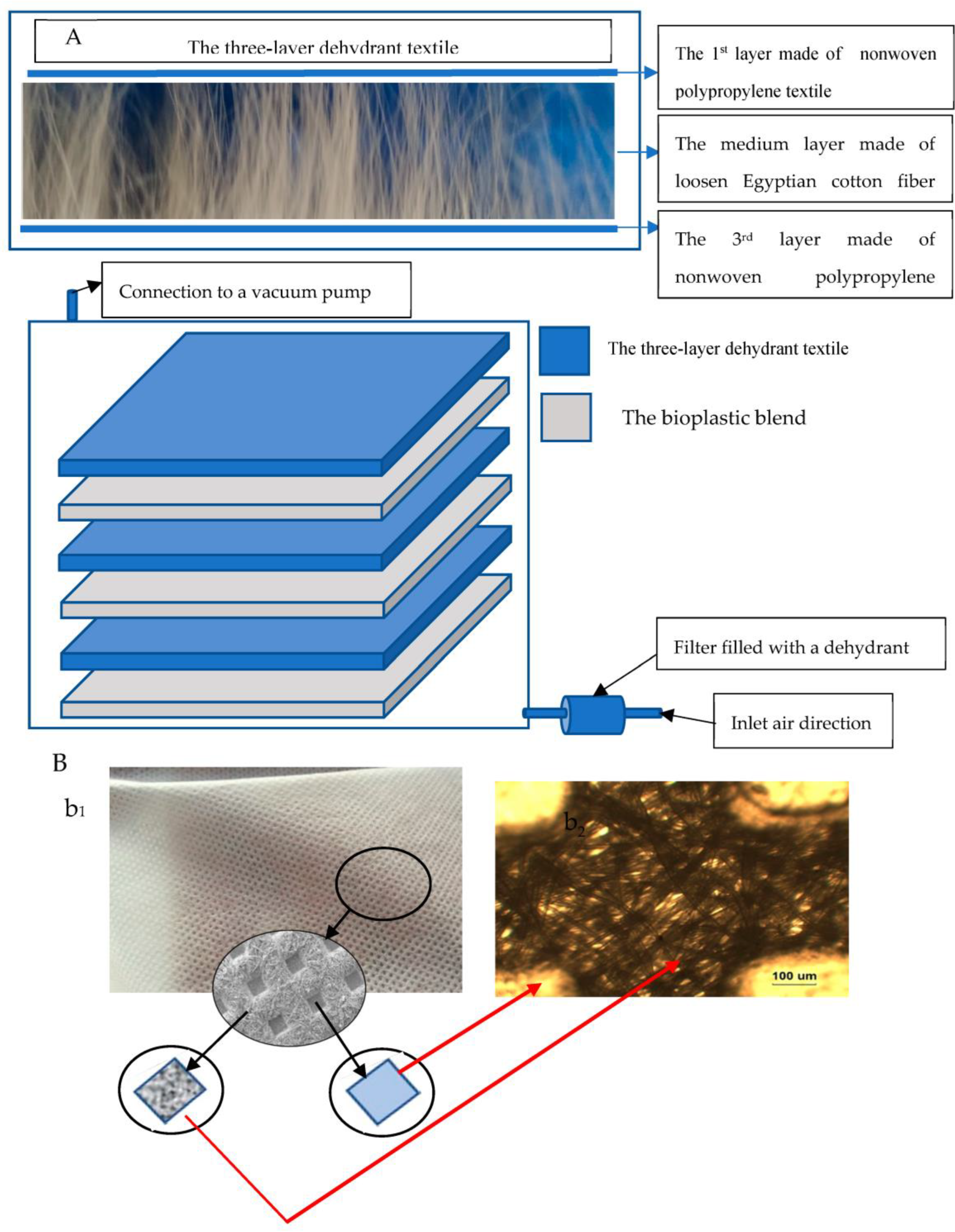
The Stratified Nano-Dehydrator (SND)
2.6. Characterization of the Bioplastic Membranes
2.6.1. FTIR
2.6.2. X-ray Diffraction (XRD)
2.6.3. Thermal Analysis
2.6.4. Surface Topography (ST) [57,93]
2.7. Microbial Biodegradation
2.7.1. Sample Preparation and Soil Burial Studies
2.7.2. Isolation and Counting of Microbial Communities
2.8. Statistical Design and Analysis
3. Results
3.1. Chemical and Physical Properties of the Bioplastic Membranes
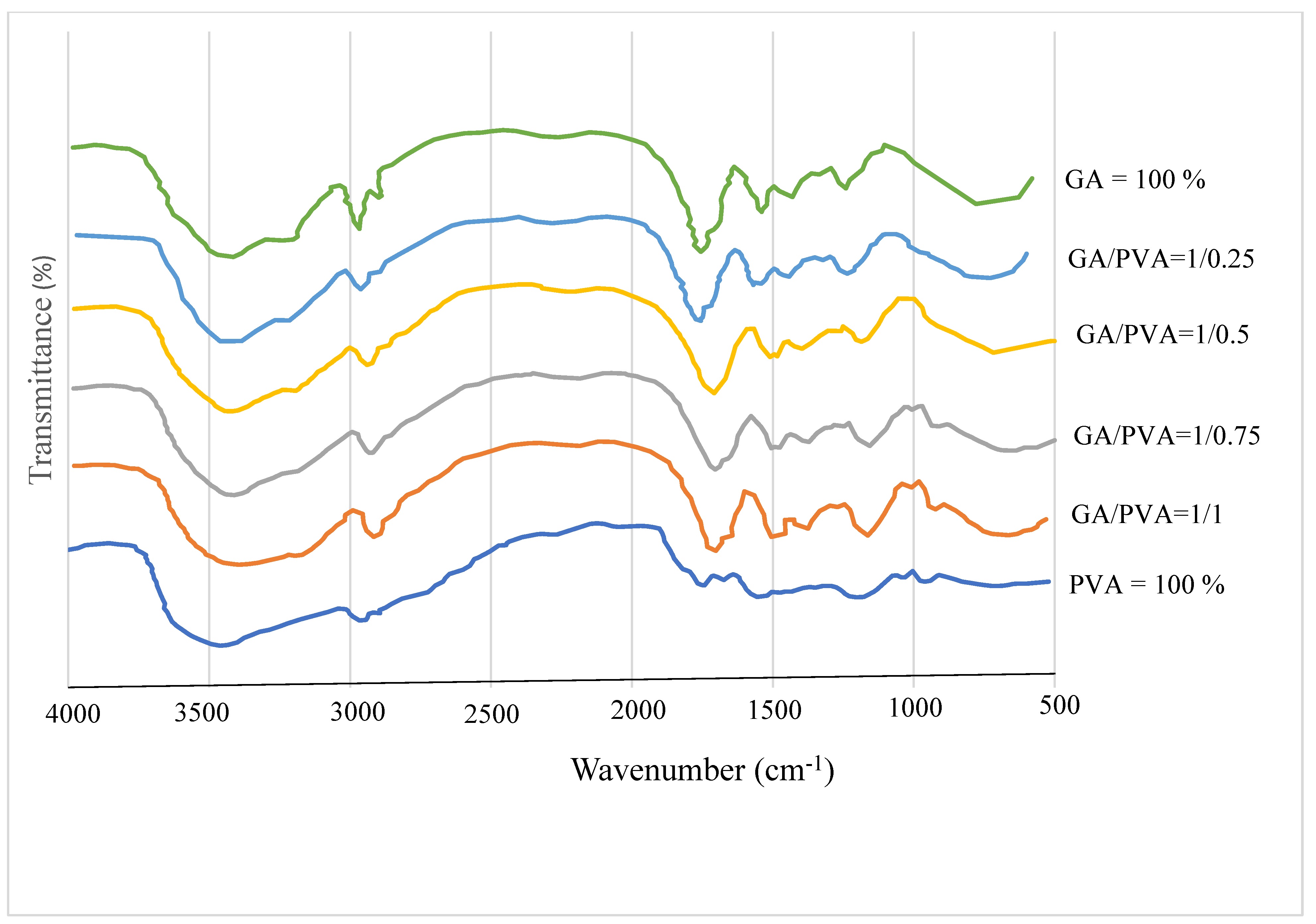
3.1.1. FTIR
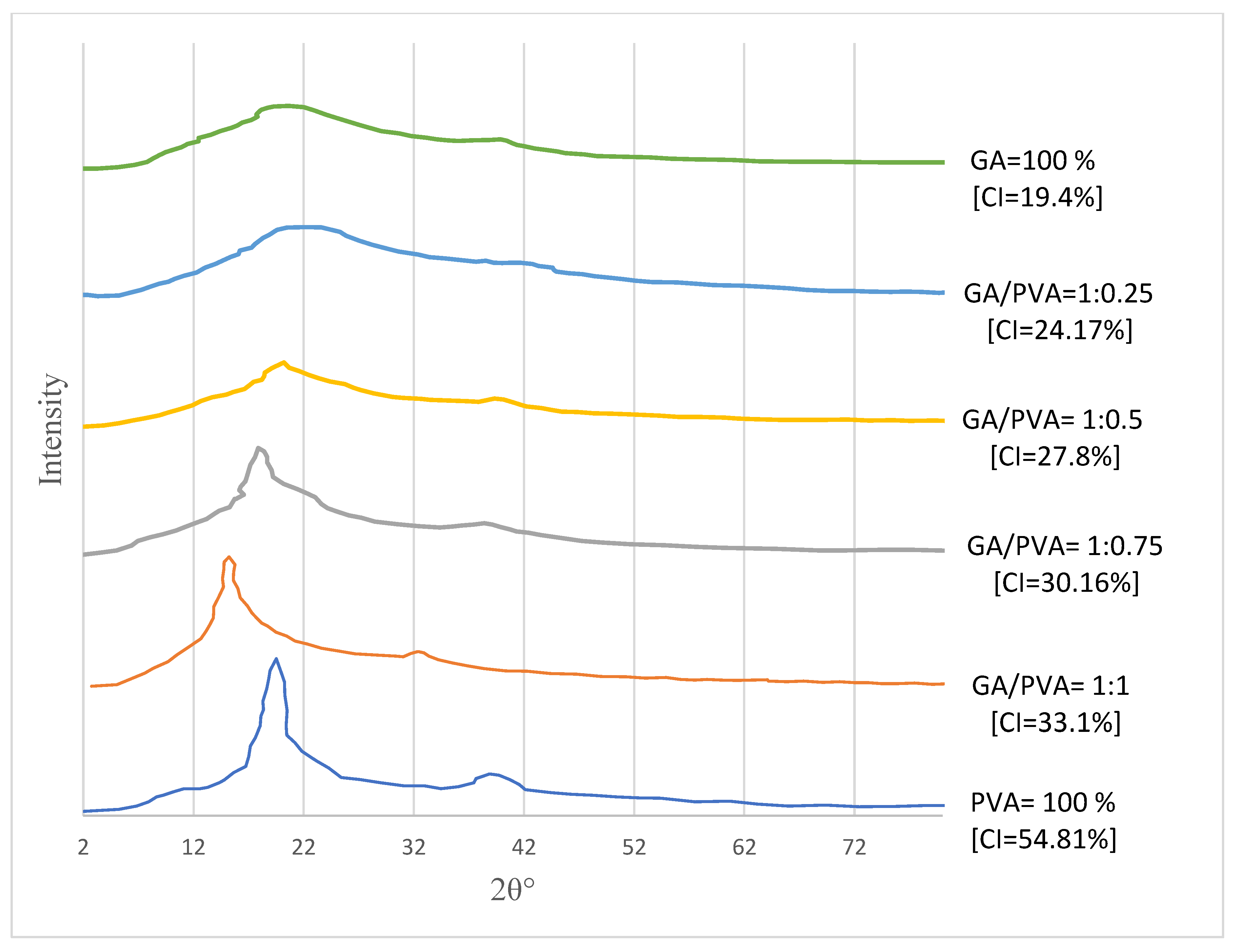
3.1.2. XRD
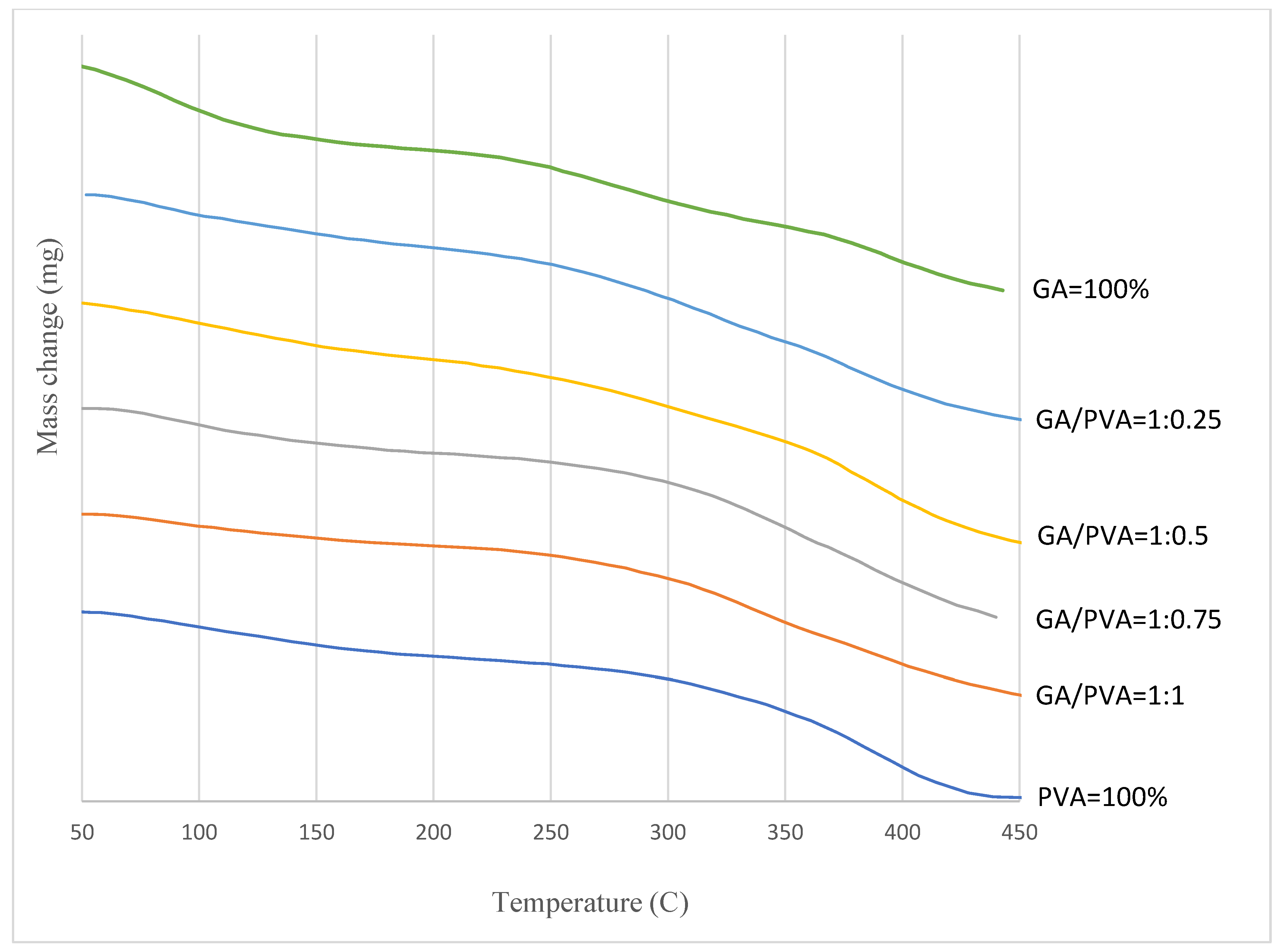
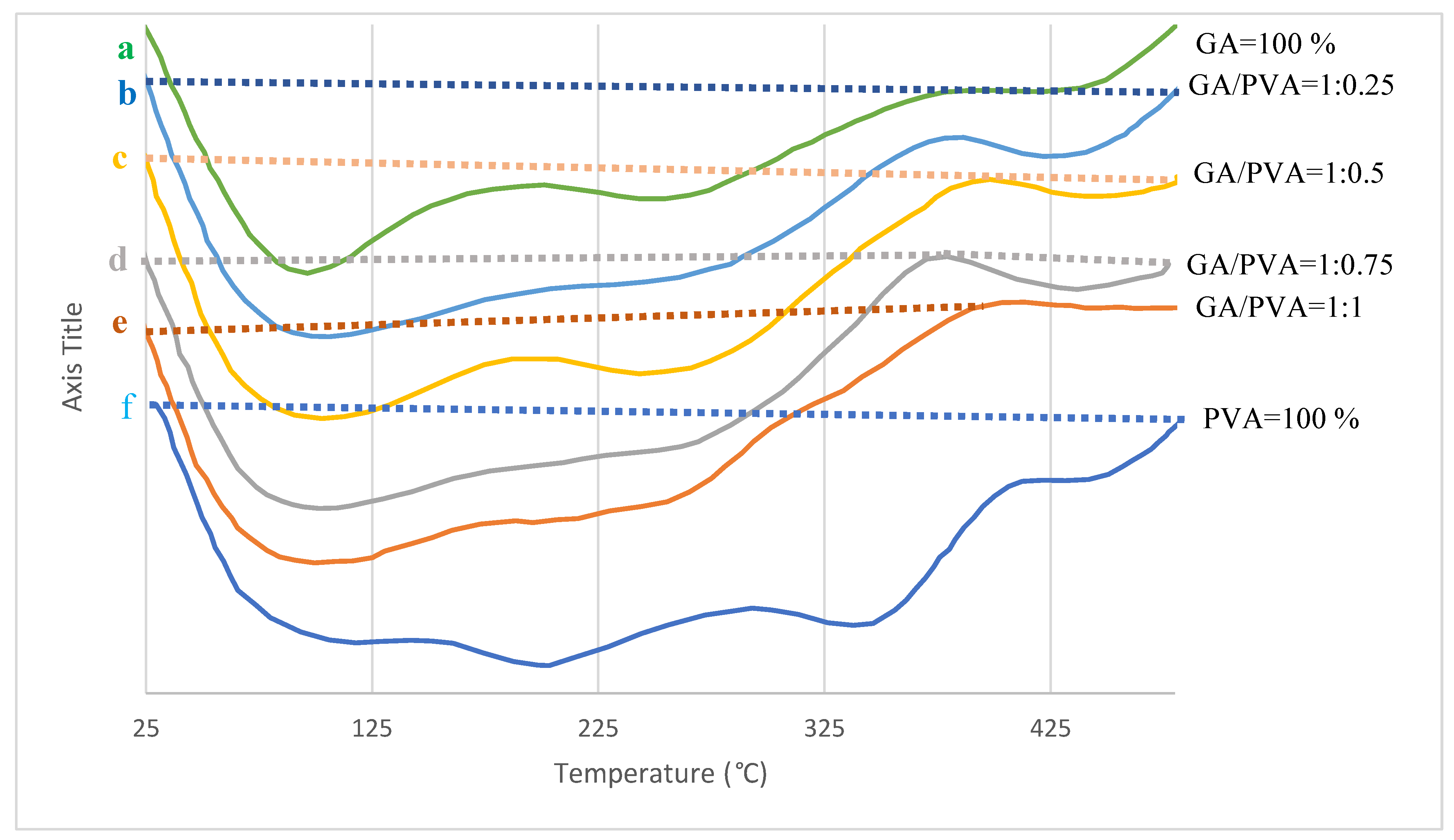
3.1.3. TGA
| T-Zones °C |
GA/PVA Ratio | |||||
|---|---|---|---|---|---|---|
| GA 100% |
1:0.25 | 1:0.5 | 1:0.75 | 1:1 | PVA 100% |
|
| 50°–100° | 13.68 Ad | 4.57 Bg | 4.85 Be | 5.02 Bef | 5.23 Bef | 5.69 Bf |
| 100°–150° | 11.11 Ae | 6.32 ABf | 6.37 ABe | 6.93 ABe | 6.47ABef | 8.98 Bef |
| 150°–200° | 4.18 BCg | 4.88 BCg | 6.46 Be | 4.26 BCef | 3.23 Cf | 8.07 A ` ef |
| 200°–250° | 6.96 Bf | 8.18 Ae | 6.18 Be | 3.7 Cf | 5.71 Bef | 3.9 Cg |
| 250°–300° | 7.48 Cf | 14.9 Ad | 7.36 Ce | 10.77 ABd | 11.62 Bd | 10.15 ABe |
| 300°–350° | 22.2 Bb | 12.62 Ede | 15.93 Dd | 22.41 Bc | 28.57 Ac | 18.08 Cd |
| 350°–400° | 18.2 Eb | 21.13 Db | 26.32 Cb | 33.3 Bb | 34.4 Bb | 44.14 Ac |
| 400°–450° | 28.6 Da | 27.68 Da | 41.43 Ca | 39.17 BCa | 46.34 Ba | 79.01 Aa |
| 450°–500° | 15.6 Ec | 18.52 Dc | 21.95 CDc | 24.66 Cc | 45.5 Ba | 58.8 Ab |
3.1.4. DTA
| GA/PVA Ratio |
Thermogram Type |
TR °C |
HC µVs/mg |
|
|---|---|---|---|---|
| a | GA = 100% | Endotherm Exotherm |
25–265 265–435 |
−1017.25 +52.39 |
| b | 1:0.25 | Endotherm | 25–475 | −2268.77 |
| c | 1.0.5 | Endotherm Exotherm |
25–397 397–480 |
−1127.7 −16.67 |
| d | 1:0.75 | Endotherm Exotherm |
25–375 375–475 |
−1276.04 −20.89 |
| e | 1:1 | Endotherm | 25–475 | −1467.19 |
| f | PVA = 100% | Endotherm | 25–475 | −2119.72 |
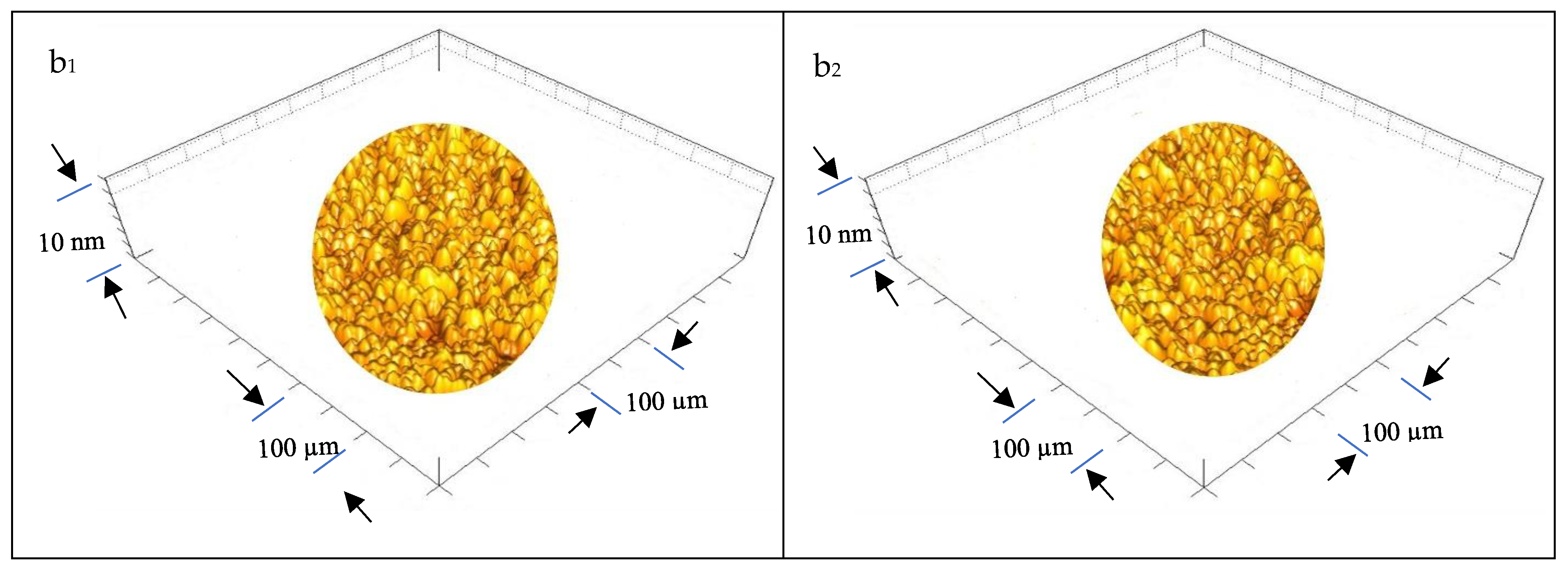
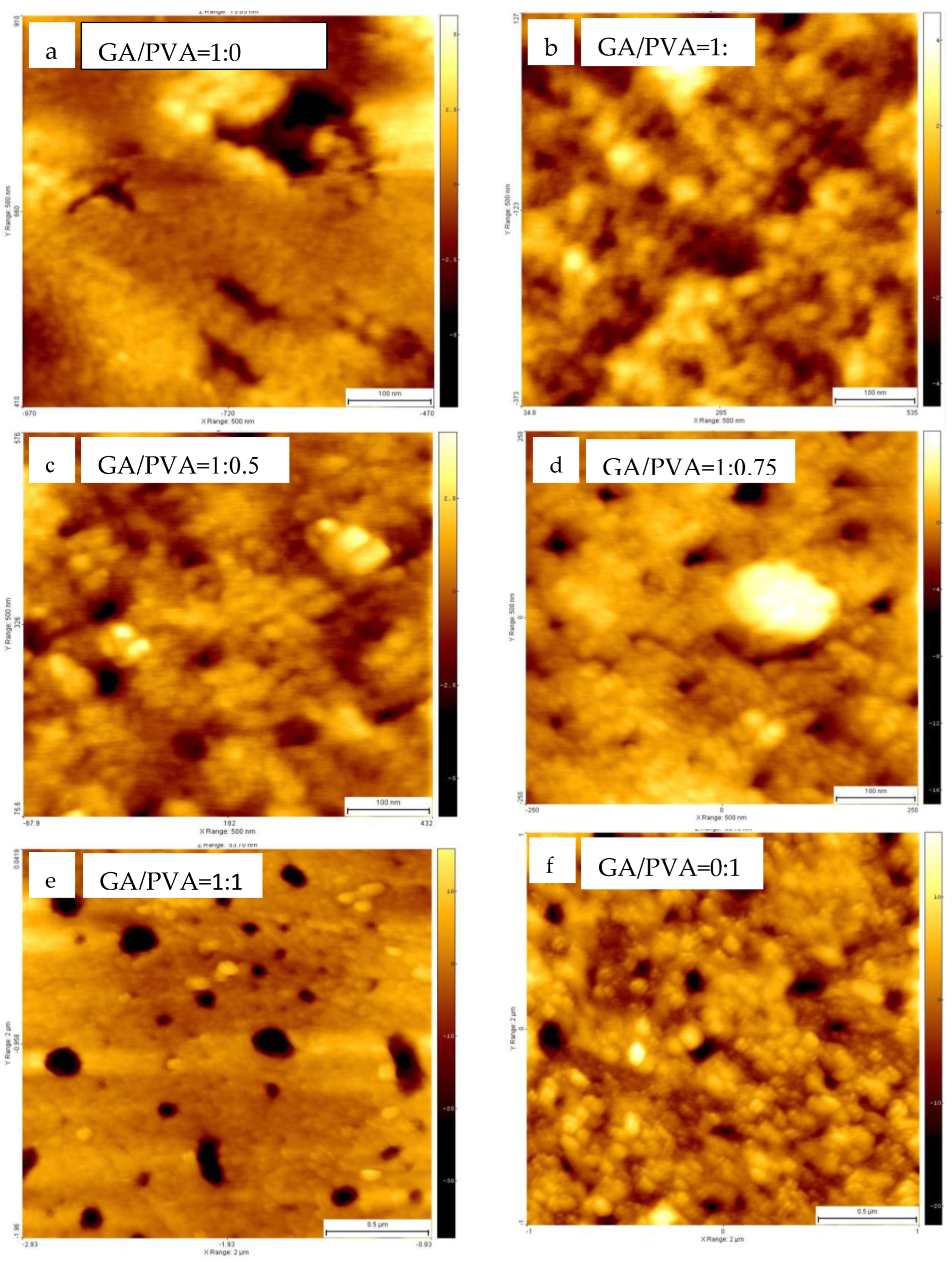
3.1.5. Ultrastructure of the Bioplastic Membrane
Surface Roughness and Nanometric Particle Size (PS)

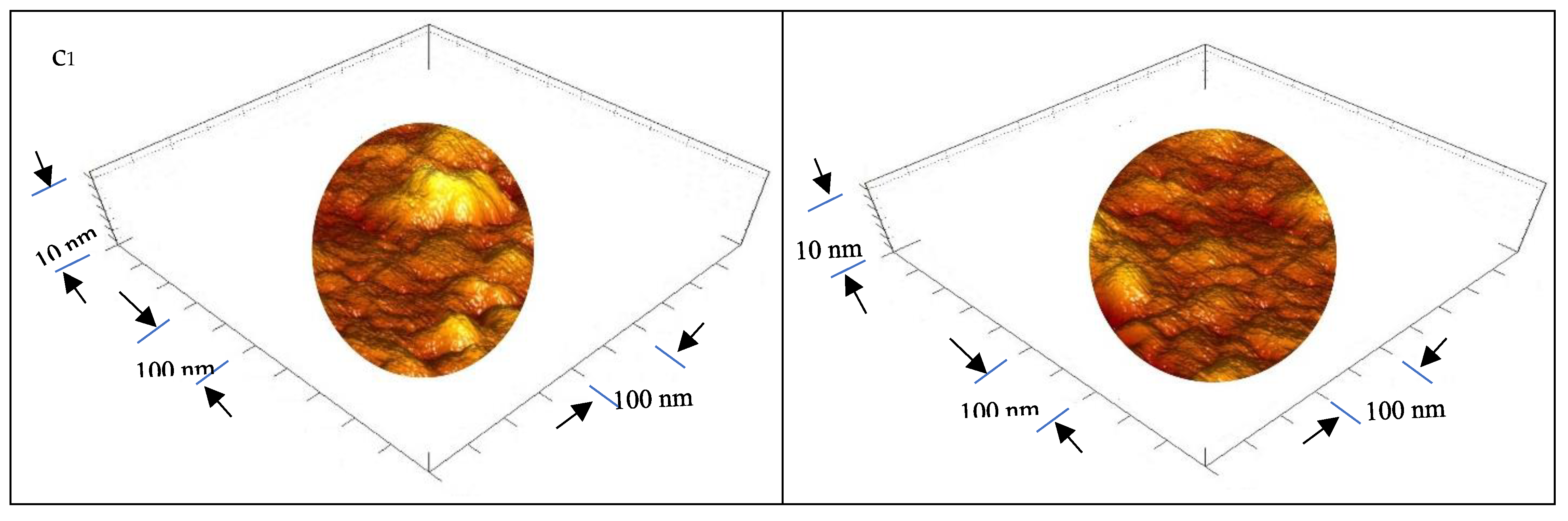
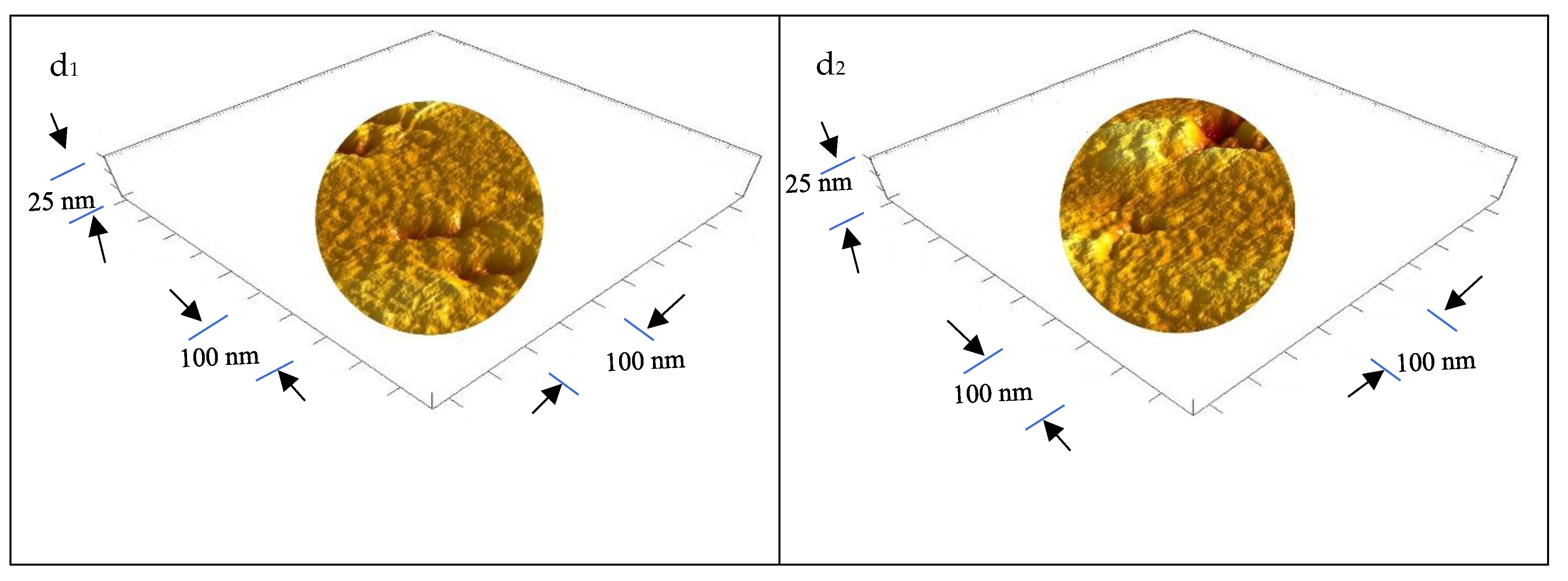
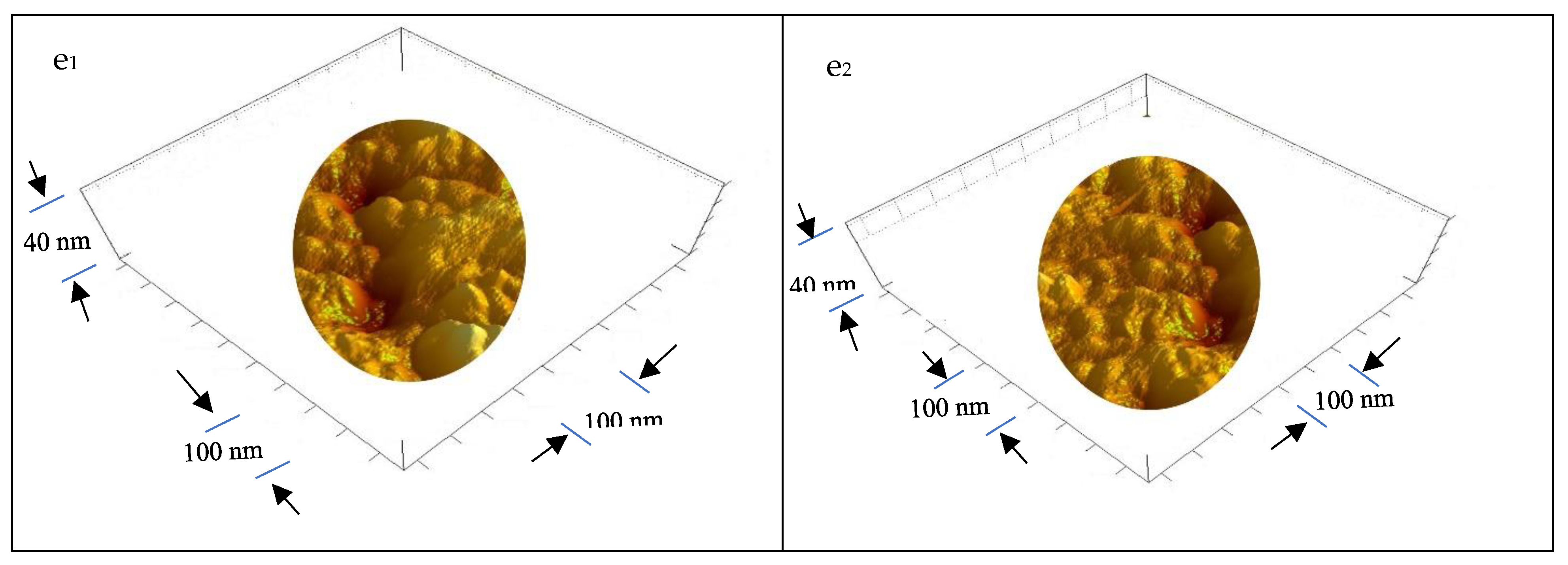
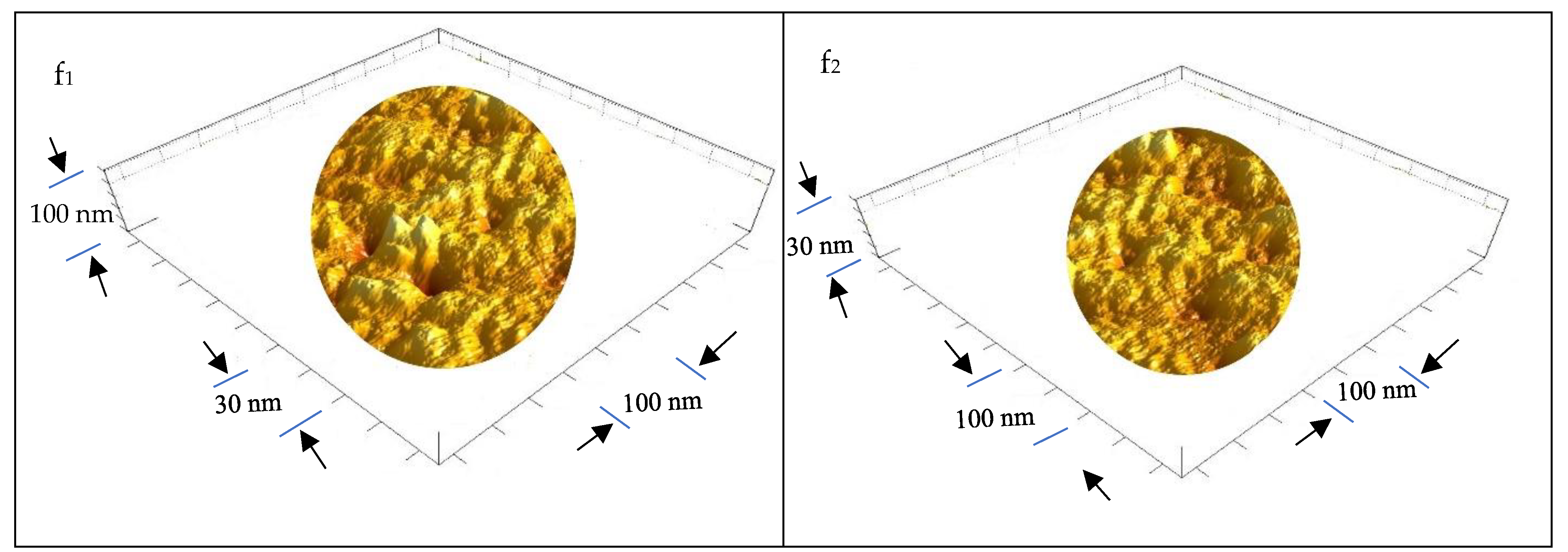
| GA/PVA Ratio |
GA Amount % |
PVA Amount % |
SPs | Particle Size nm |
Permeability | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pore Diameter nm |
Void Volume nm3 |
||||||||
| ADB | NDB | ADB | NDB | ADB | NDB | ||||
| 1/0 | 0 | 100 | Mean 1,2 Max. 3 Min. 4 SD 5 |
13.57 55.44 4.24 7.66 |
14.77 56.68 5.49 7.66 |
0.91 3.905 0.002 0.904 |
0.953 3.948 0.045 0.904 |
83.24 1397.9 0.007 160.68 |
84.29 1398.91 1.057 160.68 |
| 1:0.25 | 20 | 80 | Mean 1,2 Max. 2,3 Min. 2,4 SD |
14.17 76.94 4.24 8.93 |
15.42 78.19 5.49 8.93 |
0.553 3.54 0.001 0.457 |
0.606 3.593 0.055 0.457 |
105.74 1374.8 0.005 156.17 |
106.74 1375.47 1.008 156.17 |
| 1:0.5 | 66.7 | 33.3 | Mean 1,2 Max. 2,3 Min. 2,4 SD 5 |
15.15 67.01 4.24 8.51 |
16.4 68.26 5.49 8.51 |
0.608 2.38 0.001 0.469 |
0.671 2.443 0.064 0.469 |
120.66 8009 0.002 309.6 |
121.87 8010.22 1.219 309.6 |
| 1:0.75 | 57.1 | 42.9 | Mean 1,2 Max. 2,3 Min. 2,4 SD 5 |
17.01 72.32 4.24 9.26 |
18.07 73.38 5.3 9.26 |
0.714 3.608 0.007 0.615 |
0.788 3.683 0.082 0.615 |
226.98 8411.8 0.007 631.41 |
228.18 8412.98 1.215 631.41 |
| 1:1 | 50 | 50 | Mean 1,2 Max. 2,3 Min. 2,4 SD 5 |
18.42 89.75 4.24 12.69 |
19.58 90.91 5.4 12.69 |
1.145 4.75 0.019 2.342 |
1.23 4.839 0.093 1.002 |
460.18 8411.8 0.007 1062.04 |
461.5 8413.1 1.34 1062.04 |
| 0/1 | 100 | 0 | Mean 1,2 Max. 2,3 Min. 2,4 SD 5 |
20.34 89.75 4.24 14.58 |
21.35 90.76 5.25 14.58 |
1.485 14.851 0.019 2.342 |
1.58 14.946 0.114 2.342 |
548.95 9315 0.001 1198.36 |
552.41 9318.46 3.46 1198.36 |
Permeability and Void Volume (PD and VV)
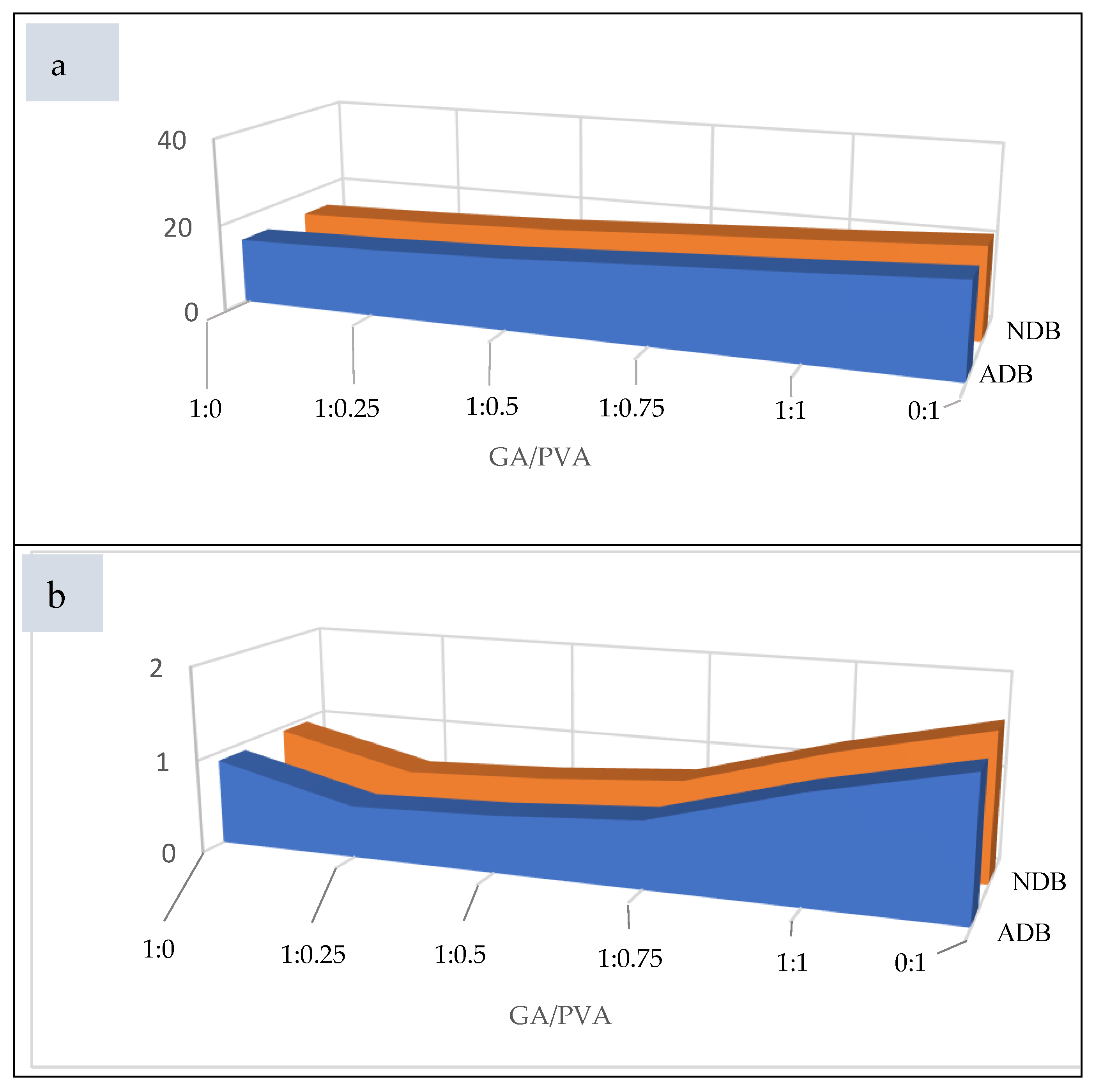
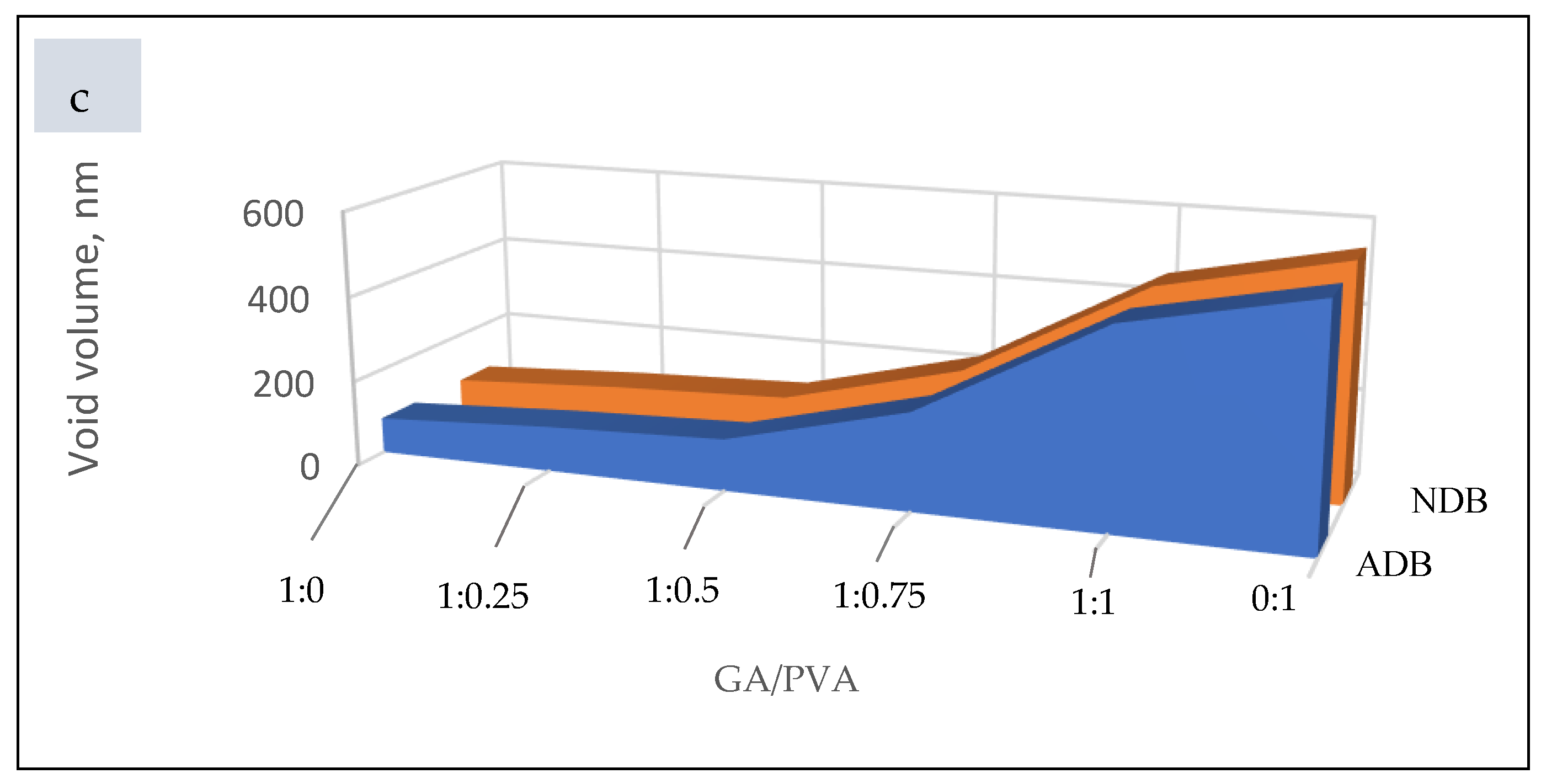
3.1.6. Bacterial and Fungal Biodegradation
4. Discussion
4.1. Scientific Illustration of the Ease of Peeling of the Bioplastic Membranes from the Acrylic Substrate
- Acrylic is a powerful static generator in terms of electrostatic charge. When its surface is wiped back and forth, positive and negative surficial charges arise that draw and hold microscopic particles. Surficial charge variations have the potential to cause agglomerated particles to discharge in an unanticipated manner, endangering contamination-sensitive materials [120]. PMMA is positioned close to the middle of this empirical series for the surface potential and is regarded as a tribo-positive electron-donating material [112,120].
4.2. Chemical and Physical Properties of the Bioplastic Membranes
4.2.1. FTIR
4.2.2. XRD
4.2.3. TGA
4.2.4. DTA
4.2.5. Surface Roughness and Nanometric Particle Size
4.2.6. Membrane Permeability
4.2.7. Microbial Biodegradation
5. Conclusions and Future Perspectives
- Great success was achieved for the fabrication of bioplastic membranes from gum arabic mixed with polyvinyl alcohol by applying a novel casting method, termed static vibrated-free horizontal flow (VFHF), producing free air bubble sheets.
- The novel nanodehydration technique gave the best solution for drying the bioplastic sheets.
- This is the first time that an acrylic (poly(methyl methacrylate)) panel used as an ideal template surface has had an electrostatically charged hydrophobic surface. As a result, peeling off its template surface is made simpler.
- The novel technique invented and applied in the current investigation can be used for any water-based biopolymeric-based products.
- The most important properties of the nanodehydrated bioplastic membranes were studied using Fourier transform infrared spectroscopy, X-ray powder diffraction, thermogravimetric analysis, differential thermal analysis, and atomic force microscopy, to ensure that the novel techniques did not distort the product quality.
- The TBM retained its parent properties, including chemical functional groups, crystallinity index, mass loss, thermal stability, ultrastructure features (surface roughness and permeability), and its ability for microbial biodegradation.
- The addition of polyvinyl alcohol to the gum arabic enhanced the formation of sheets and their properties, including the crystallinity index and thermal stability, except for mass loss upon elevated temperatures.
- There is no statistical difference between the TBM membranes and ordinary air-dried bioplastic membranes in terms of their particle size and permeability, indicating that the novel procedures used did not distort the parent properties examined, as well as their ability for biodegradation.
- The biodegradation of the TBM material was confirmed significantly by the reduction in weight for all of the six TBM samples, and degradation was found to start within 30 and 60 days.
- Pseudomonas sp., Bacillus sp., and Micrococcus sp. were the most commonly isolated bacterial strains that appeared in different samples, while Rhizobus sp., Penicillium sp., and Fusarium sp. were the most commonly isolated fungus strains that appeared in different samples. Pure GA was the most commonly biodegraded sample among the other bioplastic samples.
- The microbial communities in all of the buried bioplastic sheets, including the control sample, were different in number and species, and the species of bacteria and fungi differed according to the type of buried sheet.
- The microbiological survey revealed that all of the six bioplastic sheets are able to be degraded, contrary to petroleum-based sheets.
6. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviation | Definition |
| ADB | Air-dried bioplastic membrane |
| ACS | The American Chemical Society |
| AFM | Atomic force microscopy |
| CI | Crystallinity index |
| DSC | Differential scanning calorimetry |
| DTA | Differential thermal analysis |
| FTIR | Fourier transform infrared spectroscopy |
| VFHF | Vibrated-free horizontal flow |
| GA | Gum arabic |
| HC | Heat change in µVs/mg |
| TBM | Transparent bioplastic membrane |
| NPS | Nanometric particle size |
| PubChem | An open chemistry database managed by the National Institutes of Health (NHI) |
| PVA | Polyvinyl alcohol |
| SD | Standard deviation |
| SEP | Self-electrostatic peeling |
| SP | Statistical parameters |
| SR | Surface roughness |
| PD | Pore diameter |
| PS | Particle size |
| TGA | Thermogravimetric analysis |
| TR | Temperature range (°C) |
| XRD | X-ray diffraction |
| VV | Void volume |
References
- Mergaert, J.; Anderson, C.; Wouters, A.; Swings, J.; Kersters, K. Biodegradation of polyhydroxyalkanoates. FEMS Microbiol. Lett. 1992, 9, 317–321. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar]
- Boyandin, A.N.; Prudnikova, S.V.; Filipenko, M.L.; Khrapov, E.A.; Vasil’ev, A.D.; Volova, T.G. Biodegradation of polyhydroxyalkanoates by soil microbial communities of different structures and detection of PHA degrading microorganisms. Appl. Biochem. Microbiol. 2012, 48, 28–36. [Google Scholar] [CrossRef]
- Merugu, R. Studies on PHB (Polyhydroxy butyrate) degradation by some species of Aspergillus. Studies. 2012, 4, 1111–1113. [Google Scholar]
- Gautam, N.; Kaur, I. Soil burial biodegradation studies of starch grafted polyethylene and identification of Rhizobium meliloti therefrom. J. Environ. Chem. Ecotoxicol. 2013, 5, 147–158. [Google Scholar]
- Karamanlioglu, M. Environmental degradation of the compostable plastic packaging material poly (lactic) acid and its impact on fungal communities in compost. PhD thesis, Manchester University, UK. 2013, 198.
- Badreldin H.A.; Al-Husseni, I.; Beegam, S.; Al-Shukaili, A.; Nemmar, A.; Schierling, S.; Queisser, N.; Schupp, N. Effect of gum Arabic on oxidative stress and inflammation in adenine–induced chronic renal failure in rats. PLoS One. 2013, 8, e55242.
- Feddersen, R.L.; Thorp, S.N. Sodium carboxymethyl cellulose. In Whistler, R.L.; Bemiller, J.N. (Eds.), Industrial gums, polysaccharides and their derivatives. Academic Press. New York. 1993, 537–578.
- Verbeken, D.; Dierckx, S.; Dewettinck, K. Exudate gums: occurrence, production, and applications. Appl. Microbiol. Biotechnol. 2003, 63, 10–21. [Google Scholar]
- Williams, P.A.; Phillips, G.O. Handbook of Hydrocolloids. Williams, P. A., Phillips, G. O., Eds.; CRC Press, Cambridge, Elsevier. 2000, 155-168.
- Anonymousa. FAO Rome. Food and Nutrition. Food and Agriculture Organization, Rome. 1990, 49.
- Buffo, R.A.; Reineccius, G.A.; Oehlert, G.W. Factors affecting the emulsifying and rheological properties of gum acacia in beverage emulsions. Food Hydrocoll. 2001, 15, 53–66. [Google Scholar] [CrossRef]
- Almuslet, N.A.; Hassan, E.A.; Al-Sherbini, A.A.M.; Muhgoub, M.G.A. Diode laser (532 nm) induced grafting of polyacrylamide onto gum Arabic. J. Phys. Sci. 2012, 23, 43–53. [Google Scholar]
- Anonymousb. Network, Kenya. Production and marketing of gum Arabic. Nairobi, Kenya: for natural gums and resins in Africa (NGARA). 2016.
- Krempel, M.; Griffin, K.; Khouryieh, H. Hydrocolloids as emulsifiers and stabilizers in beverage preservation. The science of beverages. Grumezescu, A.M.; Holban, A.M. (Eds). Preservatives and preservation approaches in beverages, Academic Press. 2019, 15, 427–465. [Google Scholar]
- Rinsky, L.H.; Rinsky, G. The Pastry chef's companion: A comprehensive resource guide for the baking and pastry professional. Chichester: John Wiley & Sons. 2009, 1, 134.
- Maqbool, M.; Ali, A.; Alderson, P.G.; Zahid, N. Exploring the new applications of gum Arabic obtained from acacia species to preserve fresh fruits and vegetables. II International Symposium on underutilized plant species: Crops for the future - beyond food security, Kuala Lumpur, Malaysia. 2013, 2, 2406-6168.
- McEachran, Rich. Gum Arabic: the invisible ingredient in soft drink supply chains. The Guardian. 2013. Available online: www.theguardian.com (accessed on 16 February 2023).
- Anderson, D.M.W.; Farquhar, J. G. K. Gum exudates from the genus Prosopis. Int. Tree Crops J. 1982, 2, 15–24. [Google Scholar] [CrossRef]
- Anderson, D.M.W.; McNab, C.G.A.; Anderson, C.G.; Brown, P.M.; Pringuer, M.A. Studies of uronic acid materials, Part 58: Gum exudates from the genus Sterculia (gum karaya). Int. Tree Crops J. 1983, 2, 147–154. [Google Scholar]
- Suliman, S.M.; Hamdouk, M.I.; Elfaki, M.B. 2000. Gum Arabic fibre as a supplement to low protein diet in chronic renal failure patients. Sudan Association of Physicians, 17th Conference, Friendship Hall, Khartoum, Sudan, March 21–23.
- Gamal el-din, A.M.; Mostafa, A.M.; Al-Shabanah, O.A.; Al-Bekairi, A.M.; Nagi, M.N. Protective effect of Arabic gum against acetaminophen-induced hepatotoxicity in mice. Pharmacol. Res. 2003, 48, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, I.B.; Awad, A.I.; Elderbi, M.A.; Shadad, S.A. Effect of gum Arabic on the absorption of a single oral dose of amoxicillin in healthy Sudanese volunteers. The Journal of J. Antimicrob. Chemother. 2004, 54, 577–8. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Ali, K.E.; Fadlalla, A.; Khalid, K.E. The effects of gum Arabic oral treatment on the metabolic profile of chronic renal failure patients under regular haemodialysis in central Sudan. Nat. Prod. Res. 2008, 22, 12–21. [Google Scholar] [CrossRef]
- Hills, S. Gum Arabic caloric value lowered. 2008. Available online: www.foodnavigator-usa.com (accessed on 24 January 2023).
- Ali, B.H.; Ziada, A.; Blunden, G. Biological effects of gum Arabic: a review of some recent research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.E.; Ayed, I.A.M.; El Badwi, S.M.A. 2013. Effect of gum Arabic on nephrotoxicity induced by Aristolochia bracteolata in rats. Scholars Acad. J. Biosci. 2013, 1, 377–380. [Google Scholar]
- Luo, Y.; Zhang, Y.; Pan, K.; Critzer, F.; Davidson, P.M.; Zhong, Q. Self-emulsification of alkaline-dissolved clove bud oil by whey protein, gum Arabic, lecithin, and their combinations. J. Agric. Food. Chem. 2014, 62, 4417–4424. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Williams, P.A.; Senan, C. Synthesis, characterization and emulsification properties of dodecenyl succinic anhydride derivatives of gum Arabic. Food Hydrocoll. 2014, 37, 143–148. [Google Scholar] [CrossRef]
- Lawrence, R.; Jeyakumar, E.; Gupta, A. Antibacterial activity of Acacia Arabica (Bark) extract against selected multi drug resistant pathogenic bacteria. Int. J. Curr. Microbiol. Appl. Sci. 2015, 1, 213–222. [Google Scholar]
- Hadavi, M. et al. Novel calcified gum Arabic porous nano-composite scaffold for bone tissue regeneration. Biochem. Biophys. Res. Commun, 2017; 488, 671–678. [Google Scholar]
- Salih, N.K. Applications of gum Arabic in medical and health benefits. In gum Arabic. Academic Press. 2018, 269–281. [Google Scholar]
- Kraaijpoel, D.; Herenius, C. Het kunstschilderboek-handboek voor materialen en technieken, Cantecleer. 2007, 183.
- Banerjee, S.S.; Chen, D.-H. Magnetic nanoparticles grafted with cyclodextrin for hydrophobic drug delivery. Chem. Mater. 2007, 19, 6345–6349. [Google Scholar] [CrossRef]
- Wilson Jr., O. C.; Blair, E.; Kennedy, S.; Rivera, G.; Mehl, P. Surface modification of magnetic nanoparticles with oleylamine and gum Arabic. Mater. Sci. Eng. C. 2008, 28, 438–442. [Google Scholar] [CrossRef]
- Kattumuri, V.; Katti, K.; Bhaskaran, S.; Boote, E.J.; Casteel, S.W.; Fent, G.M.; Robert-son, D.J.; Chandrasekhar, M.; Kannan, R.; Katti, K.V. Gum Arabic as a photochemical construct for the stabilization of gold nanoparticles: in vivo pharmacokinetics and X-ray-contrast-imaging studies. Small. 2007, 3, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.K.; Reddy, A.L.M.; Ramaprabhu, S. Exfoliated single-walled carbonnnanotube-based hydrogen sensor. Sens. Actuators B. 2008, 130, 653–660. [Google Scholar] [CrossRef]
- Park, C.; Lim, K.H.; Kwon, D.; Yoon, T.H. Biocompatible quantum dot nanocolloids stabilized by gum Arabic. Bull. Kor. Chem. Soc. 2008, 29, 1277–1279. [Google Scholar]
- Razzak, M.T.; Darwis, D. Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing. Radiat. Phys. Chem. 2001, 62, 107–113. [Google Scholar] [CrossRef]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Dos Reis, E.F.; Campos, F.S.; Lage, A.P.; Leite, R.C.; Heneine, L.G.; Vasconcelosc, W.L.; Portela Lobato, Z.I.; Mansur, H.S. Synthesis and characterization of Poly (Vinyl Alcohol) Hydrogels and Hybrids for rMPB70 Protein Adsorption. Mat. Res. 2006, 9, 185–191. [Google Scholar] [CrossRef]
- Nair, N.R.; Nampoothiri, K.M.; Pandey, A. Preparation of poly (L-lactide) blends and biodegradation by Lentzea waywayandensis. Biotechnol. Lett. 2012, 34, 2031–2035. [Google Scholar] [CrossRef]
- Liu, M.; Guo, B.; Du, M.; Jia, D. Drying induced aggregation of halloysite nanotubes in polyvinyl alcohol/halloysite nanotubes solution and its effect on properties of composite film. Appl. Phys. A. 2007, 88, 391–395. [Google Scholar] [CrossRef]
- Masti, S.P.; Chougale, R.B. Influence of Poly (Vinylpyrrolidone) on Binary Blend Films Made from Poly(Vinyl Alcohol)/Chitosan. Inter. Res. J. Env. Sci, 2014; 3, 11–13. [Google Scholar]
- Limpan, N.; Prodpran, T.; Benjakul, S.; Prasarpran, S. Influences of degree of hydrolysis and molecular weight of poly (vinyl alcohol), PVA on properties of fish myofibrillar protein/PVA blend films. Food Hydrocoll. 2012, 29, 226–233. [Google Scholar] [CrossRef]
- Qiu, K.; Netravali, A.N. Fabrication and characterization of biodegradable composites based on microfibrillated cellulose and polyvinyl alcohol. Compos. Sci. Technol. 2012, 72, 1588–1594. [Google Scholar] [CrossRef]
- Qiu, K.; Netravali, A.N. A Composting study of membrane-like polyvinyl alcohol based resins and nanocomposites. J Polym Environ. 2013a, 21, 658–674. [Google Scholar] [CrossRef]
- Qiu, K.; Netravali, A.N. Halloysite nanotube reinforced biodegradable nanocomposites using noncrosslinked and malonic acid crosslinked polyvinyl alcohol. Polym. Compos. 2013b, 34, 799–809. [Google Scholar] [CrossRef]
- Mudigoudra, B.S.; Masti, S.P.; Chougale, R.B. Thermal behavior of poly (vinyl alcohol). Poly (vinyl pyrrolidone)/chitosan ternary polymer blend films. Res. J. Recent Sci. 2012; 1, 83–86. [Google Scholar]
- Onyari, J. M.; Mulaa, F.; Muia, J.; Shiundu, P. Biodegradability of poly (lactic acid), preparation and characterization of PLA/gum Arabic blends. J Polym Environ. 2008, 16, 205–212. [Google Scholar] [CrossRef]
- Cozic, C.; Picton, L.; Garda, M.R.; Marlhoux, F.; Le Cerf, D. Analysis of arabic gum: Study of degradation and water desorption processes. Food Hydrocoll. 2009, 23, 1930–1934. [Google Scholar] [CrossRef]
- Tiwari, A.; Terada, D. ; Kobayash. Polyvinyl modified guar-gum bioplastics for packaging applications. Handbook of Bioplastics and Biocomposites Engineering Applications. 2011, 24, 177.
- Nakashima, T.; Xu, C.; Bin, Y.; Matsuo, M. Morphology and mechanical properties of poly (vinyl alcohol) and starch blends prepared by gelation/crystallization from solutions. Colloid. Polym. Sci. 2001, 279, 646–654. [Google Scholar] [CrossRef]
- Huang, X.; Netravali, A. Biodegradable green composites made using bamboo micro/nano-fibrils and chemically modified soy protein resin. Compos. Sci. Technol. 2009, 69, 1009–1015. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Nguyen, N.H.; Ševců, A.; Černík, M. Fabrication, characterization, and antibacterial properties of electrospun membrane composed of gum karaya, polyvinyl alcohol, and silver nanoparticles. J. Nanomater. 2015a, 271, 32–38. [Google Scholar]
- Padil, V.V.T.; Cernik, M. ; Tanger. Tree gum based electrospun nanofibre sheets: process optimization, characterization and environmental application. Nanocon 2014, 6th International Conference, Proceedings. 2015b.
- Hindi, S.S.; Albureikan, M.O.I. System, apparatus, and methods for manufacturing biodegradable biopolymeric materials. US patent no. 11548192, Issue date: 01, 10, 2023. [Google Scholar]
- Volova, T.G; Boyandin, A.N.; Vasil’ev, A.D.; Karpov, V.A.; Kozhevnikov, I.V.; Prudnikova, S.V.; Gitel’Zon, I.I. Biodegradation of polyhydroxyalkanoates (PHAs) in the South China Sea and identification of PHA-degrading bacteria. Microbiol. 2011, 80, 252. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Du, G. C.; Hua, Z. Z.; Zhu, Y. Biodegradation of polyvinyl alcohol by a mixed microbial culture. Enzyme Microb. Technol. 2007, 40, 1686–1691. [Google Scholar] [CrossRef]
- Corti, A.; Solaro, R.; Chiellini, E. Biodegradation of poly (vinyl alcohol) in selected mixed microbial culture and relevant culture filtrate. Polym. Degrad. Stab. 2002, 75, 447–458. [Google Scholar] [CrossRef]
- Rong, D. ; Usui, K.; Morohoshi, T., et al. Symbiotic degradation of polyvinyl alcohol by Novosphingobium sp. and Xanthobacter flavus. J. Environ. Biotechnol. 2009, 9, 131–134. [Google Scholar]
- Abd Alla, F.A.A. The Effects of microbiological biodegradation on gum Arabic structure and molecular mass. PhD dissertation, Sudan University of Science and Technology. 2012.
- Wail, F.; Sabir, A.; Jacob, K. I. Novel reverse osmosis membranes composed of modified PVA/gum Arabic conjugates: Biofouling mitigation and chlorine resistance enhancement. Carbohydr. Polym. 2017, 155, 28–39. [Google Scholar]
- Solomon, M.M. , et al. Gum Arabic-silver nanoparticles composite as a green anticorrosive formulation for steel corrosion in strong acid media. Carbohydr. Polym. 2018, 181, 43–55. [Google Scholar] [CrossRef]
- Tahsiri, Z. et al. Gum Arabic improves the mechanical properties of wild almond protein film. Carbohydr. Polym. 2019, 114994. [Google Scholar] [CrossRef]
- Ling, M. et al. Dual-functional gum Arabic binder for silicon anodes in lithium ion batteries. Nano Energy. 2015, 12, 178–185. [Google Scholar] [CrossRef]
- Silvestri, D.; Mikšíček, J.; Wacławek, S. ; Torres-Mendieta; R., Padil, V.V.; Černík, M. Production of electrospun nanofibers based on graphene oxide/gum Arabic. Int. J. Biol. Macromol. 2019; 124, 396–402. [Google Scholar]
- Lubambo, A.F.; de Freitas, R.A.; Sierakowski, M.R.; Lucyszyn, N.; Sassaki, G.L.; Serafim, B. M.; Saul, C. K. Electrospinning of commercial guar-gum: Effects of purification and filtration. Carbohydr. Polym. 2013, 93, 484–491. [Google Scholar] [CrossRef]
- Tieguhong, J.C.; Ndoye, O. Development of trade and marketing of non-wood forest products for poverty alleviation Africa. Paper presented at the workshop on lessons learnt on SFM in Africa. Uppsala, Sweden. 18-22 October, 2004.
- Anonymousc. Policy note: export marketing of gum arabic from Sudan. Washington, D.C. World Bank Group. Available online: http://documents.worldbank.org/curated/en/736741468334873447/Policy-note-export-marketing-of-gum-arabic-from-Sudan (accessed on 02 March 2023).
- ElKhawad, H.; ElBagher, M.A. Gum Arabic processing and marketing in the Sudan. MSc Thesis, in Chemical Engineering, Khartoum, Sudan. 2008. [Google Scholar]
- Naili, D. and Wenzhi, D. Technology for producing gum Arabic powder. Chinese patent, application no. CN 101143995A, Publication date: 03-19-2008.
- Chikamai, B.N.; Banks, W.B.; Anderson, D.M.W.; Weiping, W. Processing of gum Arabic and some new opportunities. Food Hydrocoll. 1996, 10, 309–316. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Trumbore, S. E. Persistence of soil organic matter as an ecosystem property. Nature. 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Abdalla, I.G.E. Enzymatic degradation and analysis of gum Arabic. PhD thesis, UOFK. 2015.
- Freedman, Z.; Zak, D.R. Soil bacterial communities are shaped by temporal and environmental filtering: evidence from a long-term chronosequence. Environ. Microbiol. 2015, 17, 3208–3218. [Google Scholar] [CrossRef]
- Turner, S.; Mikutta, R.; Meyer-Stüve, S.; Guggenberger, G.; Schaarschmidt, F.; Lazar, C. S.; Schippers, A. Microbial community dynamics in soil depth profiles over 120,000 years of ecosystem development. Front. Microbiol. 2017, 8, 874. [Google Scholar] [CrossRef]
- Adam, F.A.; Abdellah, A.M.; Abdel-Magid, H.M.; Osman, M.E.; Al-Aassaf, S.; Phillips, G.O. Effect of some isolated bacterial species on the physicochemical aspects and main components of gum arabic (Acacia senegal var. senegal). Int. J. Dev. Res. 2018; 8, 18436–18442. [Google Scholar]
- Wu, H.F.; Yue, L.Z.; Jiang, S.L.; Lu, Y.Q.; Wu, Y.X.; Wan, Z.Y. Biodegradation of polyvinyl alcohol by different dominant degrading bacterial strains in a baffled anaerobic bioreactor. Int. J. Dev. Res. 2019, 79, 2005–2012. [Google Scholar] [CrossRef]
- Ibrahim, M.; Krejčík, M.; Havlíček, K.; Petrík, S.; Eldessouki, M. Evaluation of chemical and physical properties of biodegradable gum Arabic/PVA/Ag nanofibrous membranes as a potential wrapping material. J. Eng. Fibers Fabr. 2020, 15, 1558925020946451. [Google Scholar] [CrossRef]
- Hao, J.; Chai, Y.N.; Lopes, L.D.; Ordóñez, R.A.; Wright, E.E.; Archontoulis, S.; Schachtman, D.P. The effects of soil depth on the structure of microbial communities in agricultural soils in Iowa, USA. Appl Environ Microbiol. 2021, 87, e02673–20. [Google Scholar] [CrossRef]
- Sichert, A.; Cordero, O.X. Polysaccharide-bacteria interactions from the lens of evolutionary ecology. Front Microbiol. 2021, 12, 705082. [Google Scholar] [CrossRef]
- Polman, E.M.; Gruter, G. J. M.; Parsons, J. R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Science of the Total Environment. 2021, 753, 141953. [Google Scholar] [CrossRef]
- Villa-Rivera, M.G.; Cano-Camacho, H.; López-Romero, E.; Zavala-Páramo, M.G. The role of arabinogalactan type II degradation in plant-microbe interactions. Frontiers in microbiology. 2021, 12, 730543–content. [Google Scholar] [CrossRef]
- Sasaki, Y.; Uchimura, Y.; Kitahara, K.; Fujita, K. Characterization of a GH36 α-D-galactosidase associated with assimilation of gum Arabic in Bifidobacterium longum subsp. longum JCM7052. J. of appl. glycosci. 2021, 68, 47–52. [Google Scholar] [CrossRef]
- Chien, H.-L.; Tsai, Y.-T.; Tseng, W.-S.; Wu, J.-A.; Kuo, S.-L.; Chang, S.-L.; Huang, S.-J.; Liu, C.-T. Biodegradation of PBSA films by Elite Aspergillus isolates and farmland soil. Polym. 2022, 14, 1320. [Google Scholar] [CrossRef]
- Sasaki, Y.; Komeno, M.; Ishiwata, A.; Horigome, A.; Odamaki, T.; Xiao, J. Z.; Fujita, K. Mechanism of cooperative degradation of gum arabic arabinogalactan protein by bifidobacterium longum surface enzymes. Appl Environ Microbiol. 2022, 88, e02187-21. [Google Scholar] [CrossRef]
- Santos-Beneit, F.; Chen, L.M.; Bordel, S.; Frutos de la Flor, R.; García-Depraect, O.; Lebrero, R.; Rodriguez-Vega, S.; Muñoz, R.; Börner, R.A.; Börner, T. Screening enzymes that can depolymerize commercial biodegradable polymers: Heterologous expression of Fusarium solani cutinase in Escherichia coli. Microorganisms. 2023, 11, 328. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, D.; Zhang, J. Novel polyvinyl alcohol/styrene butadiene rubber latex/carboxymethyl cellulose nanocomposites reinforced with modified halloysite nanotubes. J. Nanomater. 2013, 2013: 1-8.
- Chiellini, E.; Corti, A.; Solaro, R. Biodegradation of poly (vinyl alcohol) based blown films under different environmental conditions. Polym. Degrad. Stab. 1999, 64, 305–312. [Google Scholar] [CrossRef]
- Jayasekara, R.; Harding, I.; Bowater, I.; Christie, G.B.; Lonergan, G.T. Biodegradation by composting of surface modified starch and PVA blended films. J. Polym. Environ. 2003, 11, 49–56. [Google Scholar] [CrossRef]
- Matsumura, S.; Tanaka, T. Novel malonate-type copolymers containing vinyl alcohol blocks as biodegradable segments and their builder performance in detergent formulations. J. Appl. Polym. Sci. 1994, 2, 89–97. [Google Scholar] [CrossRef]
- Hindi, S.S.; Albureikan, M. Othman.I.; Al-ghamdy, A.A.; Alhummiany, H.; Ansari, M.S. Synthesis and characterization of gum Arabic based bioplastic membranes. J. Nanosci. Nanotechnol. Res. 2017a, 4, 32–42. [Google Scholar]
- Hindi, S.S.; Albureikan, M.O.I.; Attieh, A. Al-ghamdy, A.A.; Alhummiany, H.; Al-Sharabi, S.M. Effect of potassium dichromate on properties and biodegradation of gum Arabic based bioplastic membranes. Nanosci. Nanotechnol. Res. 2017b, 4, 49–58. [Google Scholar] [CrossRef]
- Hindi, S.S. Some crystallographic properties of cellulose I as affected by cellulosic resource, smoothing, and computation methods. Int. J. Innov. Res. Sci. 2017a, 6, 732–752. [Google Scholar]
- Hindi, S.S. Suitability of date palm leaflets for sulphated cellulose nanocrystals synthesis. J. Nanosci. Nanotechnol. Res. 2017b, 4, 7–16. [Google Scholar] [CrossRef]
- Hindi, S.S. 2017e. Nanocrystalline cellulose: Synthesis from pruning waste of Zizyphus spina christi and characterization. J. Nanosci. Nanotechnol, 2017c; 4, 106–114. [Google Scholar]
- Fortunati, E.; Puglia, D.; Monti, M.; Peponi, L.; Santulli, C.; Kenny, J.M.; Torre, L. Extraction of cellulose nanocrystals from Phormium tenax fibres. J. Polym. Environ. 2013, 21, 319–328. [Google Scholar] [CrossRef]
- Al-Solaimani, S.G. Chemical properties of soils and underground water of Hada Al-Sham Research Station, Kingdom of Saudi Arabia. J. Environ. Sci. Ain Shams University. 2003a, 6, 257–284. [Google Scholar]
- Al-Solaimani, S.G.; Al-Toukhy, A.; Al-Zahrani, S. Mineral characteristics, classification and evaluation of soils of Hada Al-Sham Res. Station, Kingdom of Saudi Arabia. J. Environ. Sci., Ain Shams University. 2003b. 6, 285- 322.
- Mostafa, H.M.; Sourell, H.; Bockisch, F.J. Mechanical properties of some bioplastics under different soil types used as biodegradable drip tubes. Agric. Eng. Int.: CIGR J, 2010; 12, 12–21. [Google Scholar]
- El-Nakhlawy, F.S. Principles of statistics, biostatistical experimental design and analysis. KAU Pub. Center. KSA. 2008. [Google Scholar]
- Sismanoglu, T. et al. Preparation and characterization of antibacterial Senegalia (acacia) Senegal / iron - silica bio-nanocomposites, Appl. Surf. Sci. 2015. 354, 250-255.
- Bouaziz, F.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Chaabouni, S.E. Antioxidant properties of water-soluble gum from flaxseed hulls. Antioxidants. 2016, 5, 26. [Google Scholar] [CrossRef]
- Anicuta, S.-G.; Dobre, L.; Stroescu, M.; Jipa, I. Fourier transform infrared (FTIR) spectroscopy for characterization of antimicrobial films containing chitosan. Analele UniversităŃii din Oradea Fascicula: Ecotoxicologie, Zootehnie si Tehnologii de Industrie Alimentară. 2010, 1234-1240.
- Rathna, G.V.N.; Jog, J. P.; Gaikwad, A.B. Development of non-woven nanofibers of egg albumen-poly (vinyl alcohol) blends: influence of solution properties on morphology of nanofibers. Polym. J. 2011, 43, 654–661. [Google Scholar] [CrossRef]
- Mori, T.; Sakimoto, M.; Kagi, T.; Sakai, T. Isolation and characterization of a stra108-in of Bacillus megaterium that degrades poly (vinyl alcohol). Biosci. Biotechnol. Biochem. 1996, 60, 330–332. [Google Scholar] [CrossRef]
- Patil, R.; Bagde, U.S. Enrichment and isolation of microbial strains degrading bioplastic polyvinyl alcohol and time course study of their degradation potential. African J. Biotechnol. 2015, 14, 2216–2226. [Google Scholar]
- Jecu, L.; Gheorghe, A.; Rosu, A.; Raut, I.; Grosu, E. ; Ghiurea, Marius. Ability of fungal strains to degrade PVA based materials. J Polym Environ, 2010; 18, 284–290. [Google Scholar]
- Kawai, F.; Hu, X. Biochemistry of microbial polyvinyl alcohol degradation. Appl. Microbiol. Biotechnol. 2009, 84, 227–237. [Google Scholar] [CrossRef]
- Cadmus, M.C.; Jackson, L.K.; Burton, K.A.; Plattner, R.D.; Slodki, M.E. Biodegradation of xanthan gum by Bacillus sp. Appl. Environ. Microbiol. 1982, 44, 5–11. [Google Scholar] [CrossRef]
- Busoloa, T.; Urab, D.P.; Kima, S.K.; Marzecc, M.M.; Bernasik, A.; Stachewicz, U.; Kar-Narayan, S. Surface potential tailoring of PMMA fibers by electrospinning for enhanced triboelectric performance. Nano Energy. 2019, 57, 500–506. [Google Scholar] [CrossRef]
- Anonymousd. National Center for Biotechnology Information. PubChem Compound Summary for CID 11199, polyvinyl alcohol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Vinyl-alcohol. 2023. (accessed on March 11, 2023.
- Anonymouse. National Center for Biotechnology Information. PubChem Substance Record for SID 50019090, 2-Methyl-2-propenoic acid methyl ester homopolymer, Source: LeadScope. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/50019090 (accessed on 12 March 2023).
- Anonymousf. PubChem, compound summary for CID 24847856, Galactoarabinan; 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Galactoarabinan. (accessed on 08 March 2023).
- Anonymousg. National Center for Biotechnology Information. PubChem, compound Summary for CID 439212, glycoprotein. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Glycoprotein (accessed on 13 March 2023).
- Anonymoush. National Center for Biotechnology Information. PubChem, substance record for SID 405234199, a plant arabinogalactan-[protein], Source: BioCyc. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/405234199. (accessed on 22 March 2023).
- Schmidt, D.; Coburn, C.; DeKoven, B. et al. Water-based non-stick hydrophobic coatings. Nature. 1994, 368, 39–41. [Google Scholar] [CrossRef]
- Hild, F. Surface energy of plastics. 2009. Available online: https://www.tstar.com/blog/bid/33845/surface-energy-of-plastics (accessed on 22 March 2023).
- Zi, Y.; Wang, Z.L. Nanogenerators: An emerging technology towards nanoenergy. 2017, APL Materials 5, 074103.
- Randall, R.C.; Phillips, G.O.; Williams, P.A. The role of the proteinaceous component on the emulsifying properties of gum arabic. Food hydrocoll. 1988, 2, 131–140. [Google Scholar] [CrossRef]
- Randall, R.C.; Phillips, G.O.; Williams, P.A. Fractionation and characterization of gum from Acacia senegal. Food hydrocoll. 1989, 3, 65–75. [Google Scholar] [CrossRef]
- Azzaoui, K.; Hammouti, B.; Lamhamdi, A.; Mejdoubi, E.; Berrabah, M. The gum Arabic in the southern region of Morocco. Mor. J. Chem. 2014, 3, Mor-J. [Google Scholar]
- Elsabee, M.Z.; Naguib, H. F.; Morsi, R.E. Chitosan based nanofibers, review. Mater. Sci. Eng. C. 2012, 32, 1711–1726. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.C.; Ajji, A. A fundamental study of chitosan/PEO electrospinning. Polym. J. 2011, 52, 4813–4824. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Bogatyrov, V.; Ostolska, I.; Szewczuk-Karpisz, K.; Terpiłowski, K.; Nosal-Wiercińska, A. Impact of poly(vinyl alcohol) adsorption on the surface characteristics of mixed oxide Mn x O y –SiO2. Adsorption. 2016, 22, 417–423. [Google Scholar] [CrossRef]
- Chibowski, S. , Paszkiewicz, M., Krupa, M. Investigation of the influence of the polyvinyl alcohol adsorption on the electrical properties of Al2O3–solution interface, thickness of the adsorption layers of PVA. Powder Technol, 2000; 107, 251–255. [Google Scholar]
- Nuraje, N.; Khan, W.S.; Lei, Y.; Ceylan, M.; Asmatulu, R. Superhydrophobic electrospum nanofibers. J. Mater. Chem. A.1. 2013, 1929–1946. [Google Scholar] [CrossRef]
- Osti, G.B.F.; Wolf, F.G.; Philippi, P.C. Spreading of liquid drops on acrylic surfaces. 20th International Congress of Mechanical Engineering. November 15-20, 2009, Gramado, RS, Brazil.
- Johnson, R.E. , Dettre, R.H. Wetting of low-energy surfaces. Marcel Dekker: New York, 1993.
- Hild, F. Surface energy of plastics. 2023. https://www.tstar.com/blog/bid/33845/surface-energy-of-plastics.
- Ibekwe, C.A.; Oyatogun, G.M.; Esan, T.A.; Oluwasegun, K.M. Synthesis and characterization of chitosan/gum Arabic nanoparticles for bone regeneration. Am. J. Mater. Sci. Eng. 2017, 5, 28–36. [Google Scholar]
- Mir, M.B.; Haripriya, S. Assessment of physical and structural characteristics of almond gum. Int. J. Biol. Macromol. 2016, 93, 476–482. [Google Scholar]
- Stuart, B.H. Infrared spectroscopy: Fundamentals and applications, first ed., John Wiley & Sons Ltd, West Sussex, 2004.
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. Mater. Chem. Phys. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Yoshimi, Y., Yaguchi, K., Kaneko, S., Tsumuraya, Y., and Kotake, T. Properties of two fungal endo-beta-1,3-galactanases and their synergistic action with an exo-beta-1,3-galactanase in degrading arabinogalactan-proteins. Carbohydr. Res. 2017, 45, 26–35. [CrossRef] [PubMed]
- Yoshimi, Y., Hara, K., Yoshimura, M., Tanaka, N., Higaki, T., Tsumuraya, Y., et al. Expression of a fungal exo-beta-1,3-galactanase in Arabidopsis reveals a role of type II arabinogalactans in the regulation of cell shape. J. Exp. Bot. 2020, 71, 5414–5424. [CrossRef] [PubMed]
| AG/PVA Ratio |
After 30 Days | After 60 Days | ||
|---|---|---|---|---|
| Bacteria CFU/mL |
Fungi CFU/mL |
Bacteria CFU/mL |
Fungi CFU/mL |
|
| GA = 100% | 2.8 × 106 [0.032] 1 |
1.77 × 103 [0.008] |
6.69 × 106 [0.086] |
4.32 × 103 [0.077] |
| 1:0.25 | 2.6 × 106 [0.07] |
1.8 × 103 [0.042] |
5.86 × 106 [0.074] |
3.8 × 103 [0.093] |
| 1:0.5 | 2.52 × 106 [0.028] |
1.88 × 103 [0.094] |
5.7 × 106 [0.064] |
4.21 × 103 [0.086] |
| 1:0.75 | 2.5 × 106 [0.031] |
1.93 × 103 [0.095] |
5.67 × 106 [0.095] |
4.02 × 103 [0.086] |
| 1:1 | 2.17 × 106 [0.088] |
1.9 × 103 [0.012] |
6.14 × 106 [0.088] |
3.79 × 103 [0.044] |
| PVA = 100% | 1.93 × 106 [0.008] |
2.1 × 103 [0.083] |
6.12 × 106 [0.093] |
4.83 × 103 [0.046] |
| Soil control sample | Bacteria: 2.28 × 105 CFU/mL [0.058] Fungi: 1.88 × 103 CFU/mL [0.022] |
|||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





