1. Introduction
Acute Ascending Aortic Type A Dissection (aTAAD) is a life-threatening condition with an increasing annual incidence of 3–6 cases/100,000, compared to the 2.9/100,000 cases in the 2000s [
1]. It has a higher incidence in men and an overall in-hospital mortality around 60% for patients with medical management alone and 26% among those treated surgically [
2,
3]. This makes prevention a key point in the management of ascending aortic dilatation.
Based on the current American (AHA) and European (ESC) guidelines [
4,
5], prophylactic ascending aorta replacement surgery is generally indicated at an aneurysmal diameter equal or greater than 55 mm despite previous studies presenting evidence of aTAAD occurring at diameters below the recommended threshold. Already in 1996, the International Registry of Acute Aortic Dissections (IRAD) study, showed that most of the included patients with aTAAD had a diameter inferior to 55 mm [
3].
In 2007, Pape et al. reported [
6], after looking at the results of 591 aTAAD patients of the IRAD registry, that 59% of them had an AAD < 55 mm and 40% had an AAD < 50 mm. With it, they highlight the need for new methods to identify patients at risk for dissection. Moreover, in a recent study, Tozzi et al. concluded that aTAAD occurred at a computed aortic diameter below 45 mm in 87.7% of their patients and hence reinforce the concept that diameter should be a part of a patient-based decision for preventive ascending aorta replacement procedures [
7].
Since then, new proposals have emerged to stratify the risk of dissection, such as, a smaller diameter threshold, a BSA-indexed diameter [
8], or the use of the ascending aortic length [
9], but none of them have achieved enough evidence for clinical use.
In order to examine our experience and explore a novel surrogate marker (RADAR) based on the ratio between the descending and ascending aortic diameters, we have decided to conduct this study. The goal is to facilitate patient tailored decision-making rather than to apply the one-size fits all concept in ascending aortic dilatation.
2. Materials and Methods
2.1. Patient Selection
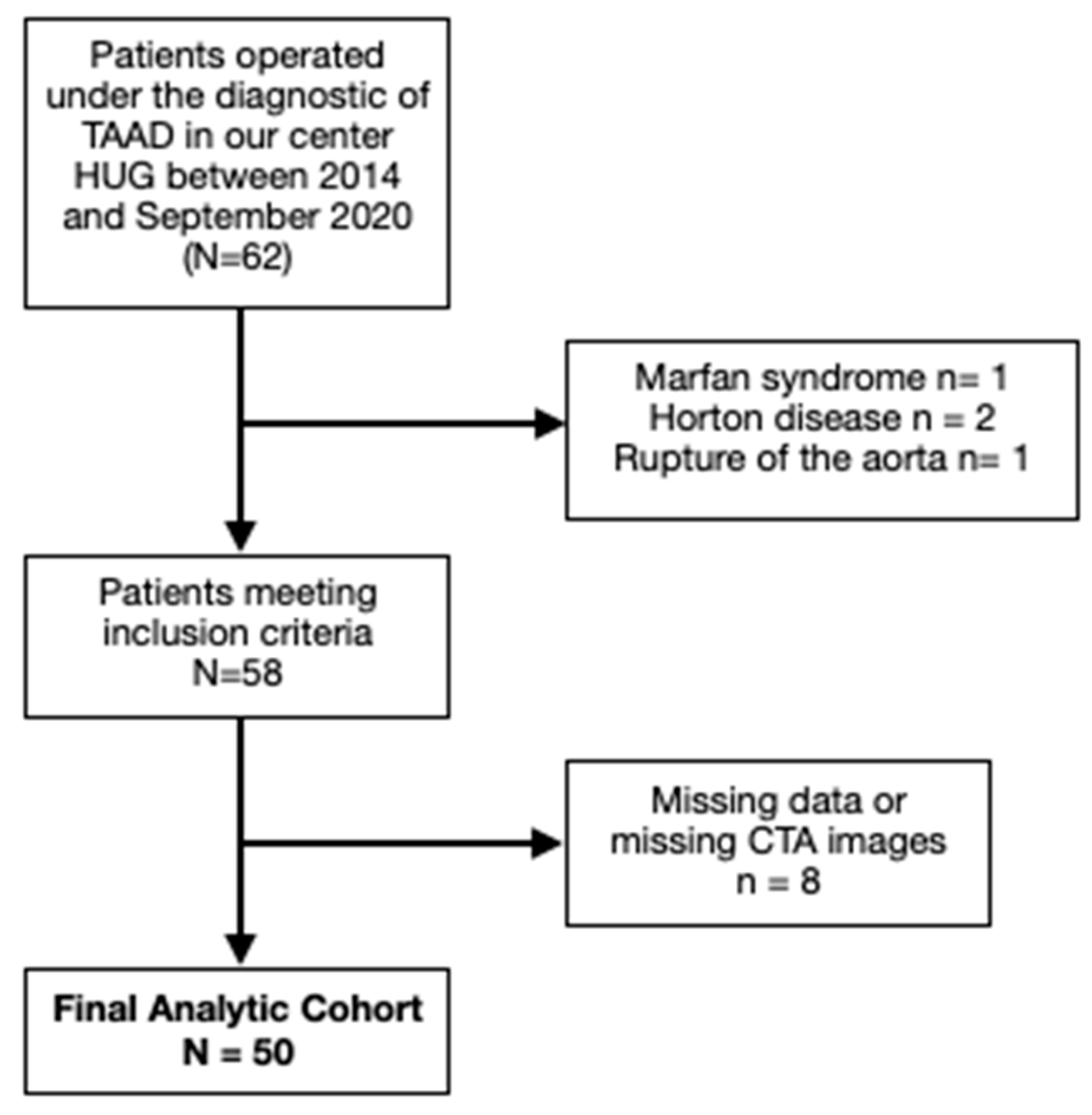
A retrospective observational study was conducted at our center with 62 patients who underwent surgery for aTAAD between January 2014 and September 2020. Patients with bicuspid aortic valve (verified with preoperative transesophageal echocardiography), connective-tissue disorders, Horton disease and traumatic or iatrogenic ascendant aortic dissection were excluded. Within this cohort, patients with non-available CT-scan images at admission or missing data were also excluded. A total of 50 patients were finally included [
Figure 1].
2.2. Data Collection and Imaging Analysis
Demographic and clinical variables, including cardiovascular risk factors, were collected retrospectively from medical charts and electronic medical records by physician review. The following parameters were recorded: age, gender, BSA, weight, height, arterial hypertension, smoking and alcohol history. Data was also extracted from preoperative TTE images, including number of aortic valve cusps and degree of aortic valve insufficiency.
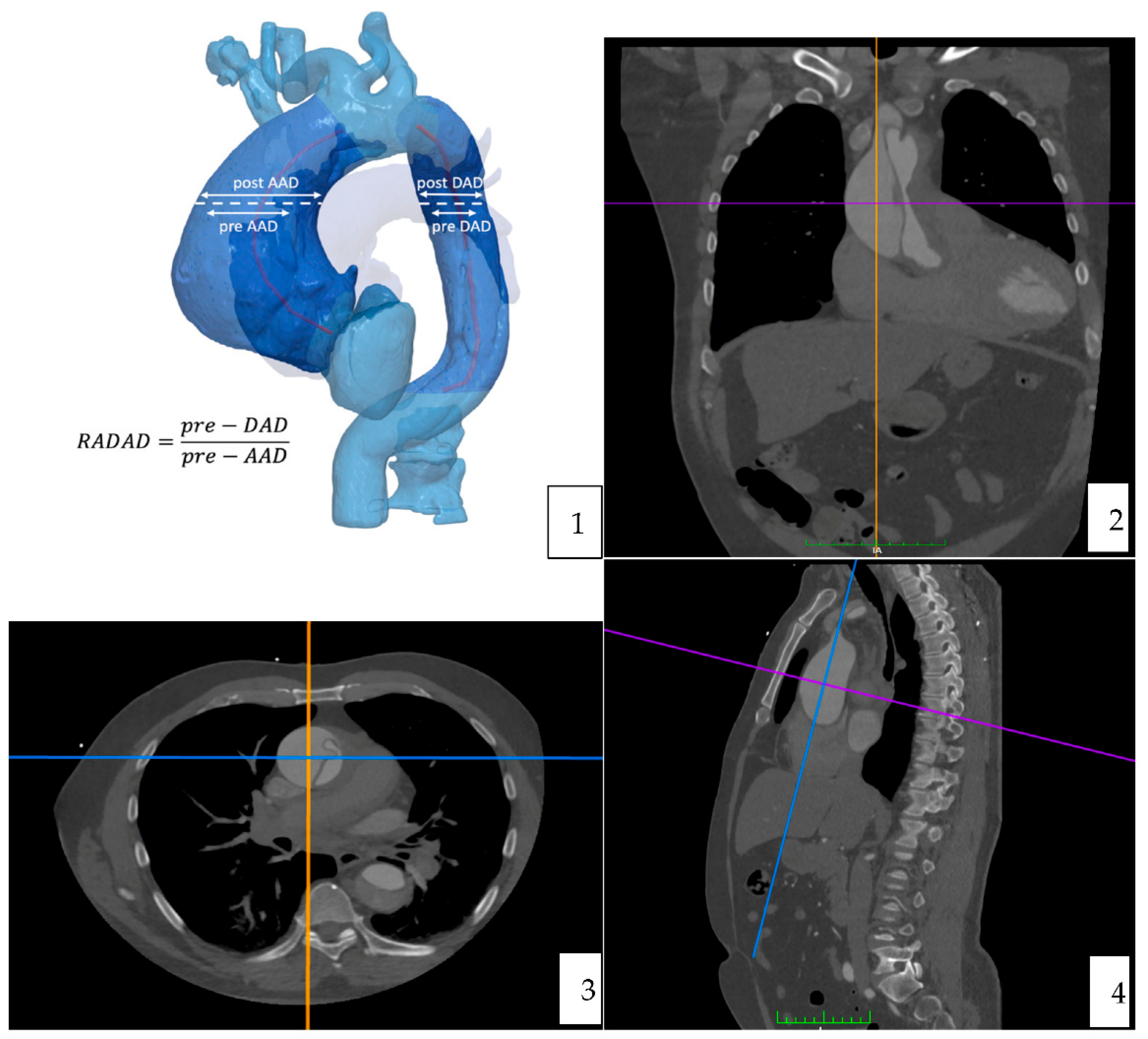
Computer tomography angiography studies were analyzed using the curved multiplane reformats with the DICOM viewer OsiriX vd.12.01. Measures were manually taken, in an orthogonal plan (sagittal, axial and coronal). A reference point to do all the measures was determined at the level of the pulmonary artery bifurcation [
Figure 2] [
Figure 3].
The maximal diameter (including the false lumen) of the ascending and descending aorta was measured in two perpendicular planes (anteroposterior and medio-lateral) and the sphericity index was calculated.
The whole dissected aortic circumference length and surface were obtained. Finally, the circumference and surface of the true lumen were also recorded for both the ascending and the descending aorta.
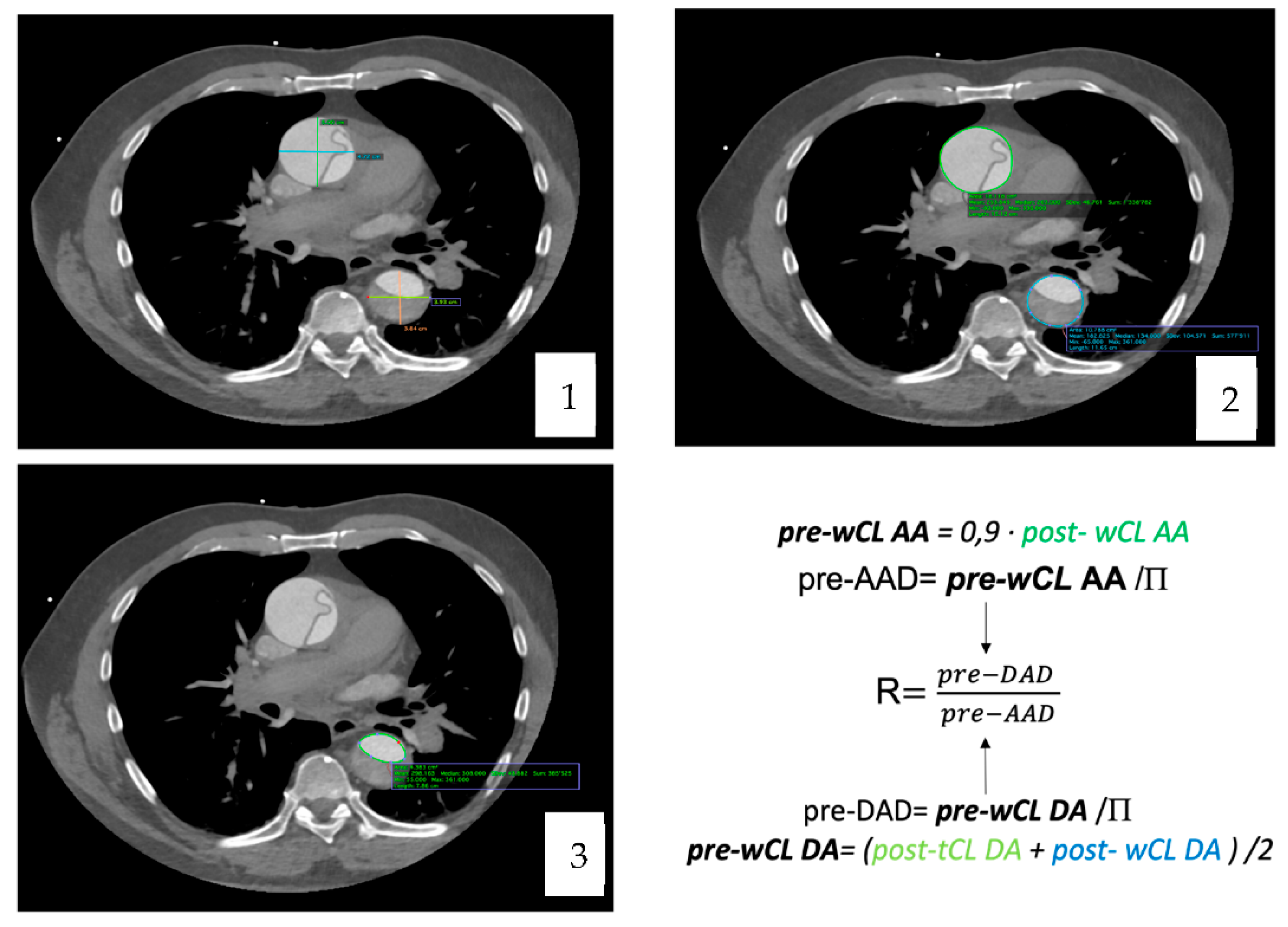
To calculate the pre-dissected AAD, we extrapolated it from the whole circumference length (wCL) [pre-dissected AAD = pre-dissected wCL/P] which was obtained with the T. Yamauchi et al. equation [
10].
As for the descending aorta, the equation applied was the one proposed for clinical use by T. Yamauchi in his article from 2018 [
11].
The diameter was then extrapolated from the perimeter with the equation [pre-DAD = pre-dissected WCL/Π]. Once the maximal pre-dissected AAD and DAD were obtained, the ratio between them was computed.
2.4. Imagining Analysis
The pre-dissected AAD and DAD obtained were compared, with normal aorta diameters, defined by gender, age and BSA, reported by Wolak et al. in their study of 2008 [
12].
They assessed the normal limits of ascending and descending aortic dimensions by non-contrast gated cardiac CT, adjusted to age, gender and BSA, in a large, low-risk population of subjects undergoing coronary artery calcium scanning.
Also, the ratio computed between pre-dissected DAD and AAD, was compared, individually, according to gender, sex and BSA, to a reference ratio extrapolated from Wolak et al. database [
12].
Data analysis was performed by the epidemiology and statistical department at our center using the statistical software SPSS version 25. Parametric tests were used for comparison between the observed and the reference diameter and ratio values.
3. Results
A total of 50 patients were included. The mean age was 65.8 ± 11.68 years and male gender was predominant by 64%. Mean weight and high were 78.84 ± 16.56 Kgs and 171.58 ± 8.40 cm respectively. Mean BSA was 1.91 ± 0.21 Kg/m
2. Arterial hypertension was present in 62% (31) of the patients. Aortic valve insufficiency was identified via TTE or TOE in 38% (19) of patients. Smoking and alcohol drinking habits were observed in 32% (16) and 34% (17) of the sample, respectively. The total mortality (30-days) was 16% (8) with a 10% (5) intraoperative mortality [
Table 1].
Eighty-eight percent of the patients (44) had a post-AAD inferior to 55 mm. Ninety-six percent (48) of the patients had a pre-AAD smaller than the current 55 mm cut-off value. Also The mean calculated pre-AAD was 40.87 ± 7.95 mm compared to a gender and BSA matched general population reference value of 34.24 ± 1,61 (
p < 0.001). Twenty percent of the patients did not present an extension of the dissection into the descending aorta. The mean calculated pre-DAD was 26.41 ± 5.32 mm, not showing a statistically significant difference with the reference DAD value (
p-value = 0.415) [
Table 2]. The sphericity index of the ascending and descending aorta was 0.93 ± 0.06 and 0.91 ± 0.09 respectively.
The ratio of computed pre-ADD and pre-DAD was 0.66 ± 0.13. The reference ratio from a population-based study
12 was 0.74 ± 0.016. Their mean difference in ratios was −0.088 (95% confidence interval −0.124 to −0.051,
p < 0.001) showing a significant lower average compared with reference values. The ascending aorta diameter was, in average, 1,5 larger than the descending aorta diameter. As illustrated in Table 3 and
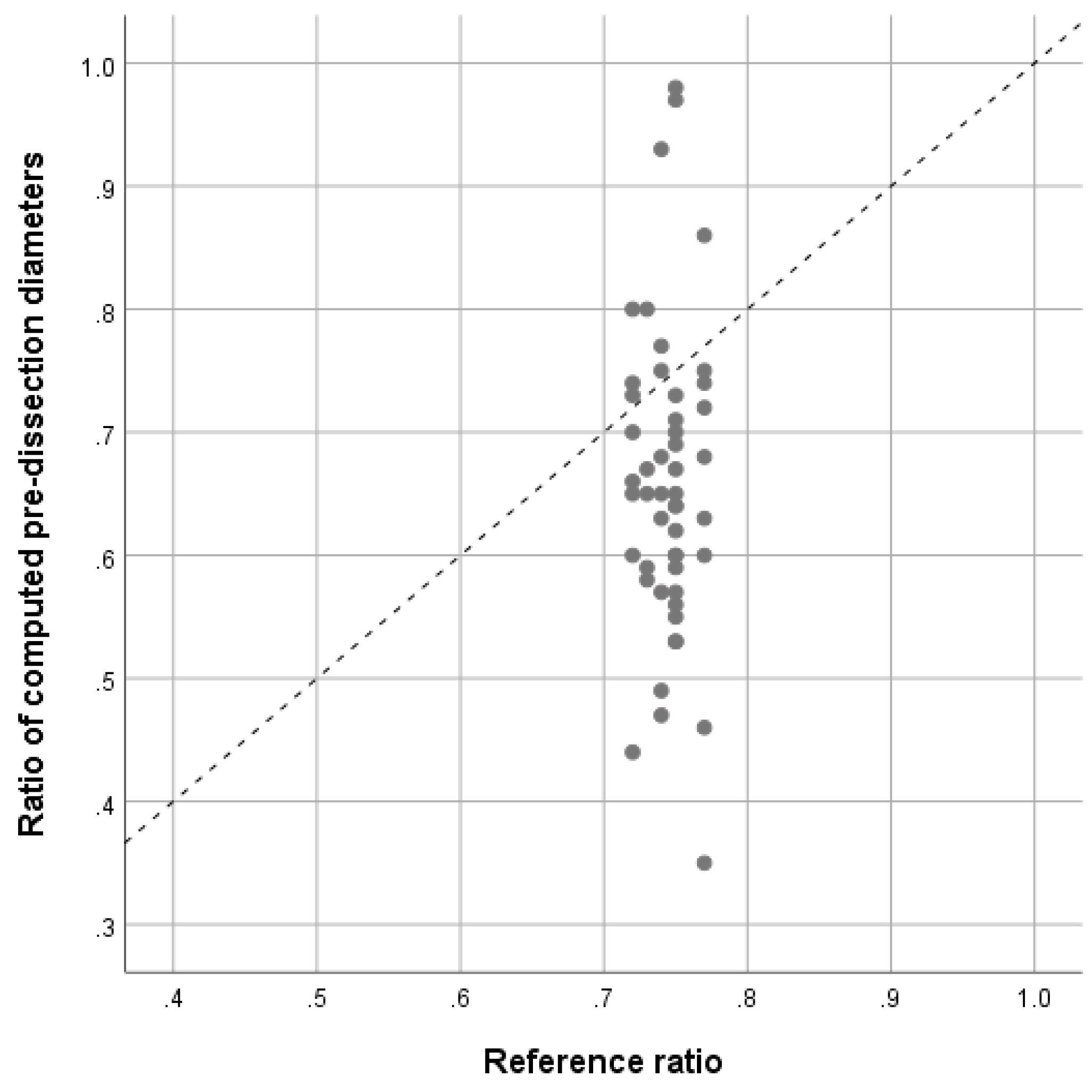
Figure 3. The corresponding scatter-plot illustrates the dispersion of calculated pre-dissection values [
Figure 4].
4. Discussion
Preventing aTAAD is still a major challenge. As the literature [
6,
7] and our results highlight, the currently recommended 55 mm threshold is insufficient to prevent aTAAD. We are facing a prevention paradox in which, thanks to operating patients with an AAD larger than 55 mm, we barely see aTAAD in patients that overpass this cutoff. But there is still a dissection risk gradient under the 55 mm threshold that we shouldn’t neglect, and hence new anatomic and radiologic criteria should be investigated. Eleftriades et al. [
13] hypothesized the relative risk of aortic dissection at sub-surgical diameters and concluded that the risk under 45 mm is very low but over that value, the risk increases by 6.3 and recommended vigilance. They also called for further research for surgical decision-making.
Alternative risk stratification tools and clinical surrogate markers have been proposed in the past few years like for example the ascending aorta surface or the elongation of ascending aorta [
9,
13,
15].
Masri A et al. [
8] described an increased mortality in those patients with an aortic cross-sectional area/height ratio above 10 cm
2/m obtained using the new 3-dimensional technology. On another hand, Krüger et al. [
9,
16,
17] found that an increase on the ascending aorta length on CTA was associated with a higher risk of aortic dissection. Based on their findings, the authors of the TAIPAN study in 2017 proposed a prophylactic aortic replacement for those patients with an ascending aortic length superior or equal to 12 cm and ascending aortic diameter between 45 and 54 mm.
9 This idea was also supported by Jinlin Wuu et al. [
18] in a more recent study of 2019 in which they concluded that an elongation between 11.5 and 12 cm increase the probability of aortic adverse events (rupture, dissection and death). Even though, evidence is yet not strong enough to clinically use those new landmarks.
It is widely acknowledged that the aortic diameter has an important interindividual variability, and hence a more patient tailored approach is mandatory to assess the individual risk of dissection. Retrospective review of the CT scans of the patients we have operated for aTAAD showed that most of the patients presented with overall AAD below the recommended cut-off value, but also had a strikingly large discrepancy between the AAD and the DAD.
The aorta remodels with growth, but the correlation between the ascending and descending aorta should remain stable. We therefore hypothesize that, an AAD remarkable larger than the DAD translates for an abnormal remodeling and might increase the risk of dissection.
Our results are based in on a sample of patients with remodeled aortas due to the acute dissection, and hence the diameter observed in a post-dissection aorta does not correspond to the pre-dissection diameter. As Nakashima [
19] described the base for aortic dissection is the medial weakness caused by the structural abnormalities of the elastic fibers. An idea that was also supported by Roberts et al. [
20] who evidence a loss of the medial elastic fibers that compose the media in dissected aortas. For that reason, we must use modelled or calculated pre-dissection values.
Different authors have tried to estimate the pre-dissection diameter. Mansour et al. [
21] concluded, after analyzing the AAD of 3400 patients’ prior and post-dissection, that the pre-AAD is 7.65 mm smaller than the observed diameter after dissection. Moreover, a 7 mm smaller pre-AAA was used by Tozzi et al. to extrapolate the pre-AAD [
7]. Due to the high interindividual variability, we considered the equations proposed by Yamauchi et al. to calculate the pre-AAD and pre-DAD more accurate [
10,
11]. Even though, using the equations we deducted a mean of 5,81 mm from the post-AAD.
Finally, based on the calculated before dissection AAD and DAA, there is a significant difference (p-value < 0.001) between the pre-dissection DAA/AAD and AAD/DAD ratios in our patients and the reference ratio from a population-based study. The main ratio AAD/DAD that we obtained (1.58 ± 0.33) implies an ascending aorta diameter 1.5 times greater than the descending aorta diameter compared to the calculated reference value from Wolak et al. database (1.34 ± 0.03).
4.1. Limitations
Despite showing a statistically significant difference between the ratios (p-value < 0.05) our results are based on a small, mono-centric, patients’ sample (n = 50), with observed diameters from already dissected ascending aortas. Furthermore, they are compared to a calculated reference value extrapolated from a population with normal aorta diameters instead of a population with aneurysmatic aortas, which would be a more interesting control.
Moreover, looking at the plot-chart [
Figure 4], there is an important dispersion in the sample, with outliers by ratios close to 1 and ratios smaller than 0.4. This variability can be explained by, first, the fact that, once dissected, the aorta is remodeled entirely and hence, even using approved equations, the pre-dissected diameters can be over-estimated (ratios > 0.4) and under-estimated (ratios close to 1). It might also be explained by the variability of the extent of the dissection regarding the involvement of the descending aorta. In our sample, 20% of the patients had isolated ascending aorta dissection without descending progression.
5. Conclusions
The ascending aorta maximal diameter is an insufficient standalone criterion to assess the dissection risk. Due to the limitations of our study, we cannot confirm the ratio between ascending and descending aorta diameters being an independent risk factor for dissection. Even though, a cut-off value of an ascending aortic diameter of 1.5 times the descending aortic diameter might be a patient tailored surrogate marker for an increased dissection risk. RADAR combined with other parameters such as elongation of the aorta, body surface indexed diameters, ulceration, presence of a bicuspid valve might highly enhance guidance for dilated ascending aortas with diameter of less than 55 mm as a new risk stratification score. Further clinical investigation needs to be undertaken to ascertain our results.
Author Contributions
Conceptualization, Prof Huber and Dre. Lopez Perez. Methodology Prof Huber, Dre Lopez Perez, Prof. Perneger, Dr Reymond, software Prof Perneger Dr Reymond, validation Prof Huber, Dr Cikirkcioglu, Prof Perneger, formal analysis, Prof Perneger, Dre Lopez Perez; writing-original draft preparation, Dre Lopez Perez; writing-review and editing, Prof Huber, Prof Perneger, investigation, Dre Lopez Perez, resources, Prof Huber, Prof. Perneger, Dr Reymond, data curation, Prof Perneger, Dre Lopez Perez; X.; writing—original draft preparation, Dre. Lopez Perez; writing—review and editing, Porf. Huber, Dr Cikirikcioglu; visualization, Prof Huber, Dre Loepz Perez Dr Reymond; supervision, Prof Huber, Dr Cikirikcioglu, Dr Van Steenberghe, Dr Sologashivli, Dr Murith.; project administration, Prof Huber; funding acquisition, Prof Huber. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Geneva Ethics committee, authorization number 2020-03029, with written informed consent waived by the ethics committee.
Informed Consent Statement
Patient consent was waived due to the non-human testing research, but mainly patient’s data usage and the relevance of the disease and high morbi-mortality it implies.
Data Availability Statement
The data underlying this article is available and will be shared on reasonable request to the corresponding author.
Acknowledgments
I gratefully acknowledge the cardiovascular surgery team of HUG for their help in conducting this study and their work and the work of the statistical assistance provided by the Clinical Research Center at HUG.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| aTAAD |
Acute Type A Aortic Dissection |
| AA |
Ascending Aorta |
| AAD |
Ascending Aorta Diameter |
| BSA |
Body Surface Area in cm2
|
| CTA |
Computer Tomography Angiography |
| DA |
Descending Aorta |
| DAD |
Descending Aorta Diameter |
| Pre-AAD |
Pre-dissected Ascending Aorta Diameter |
| Pre-DAD |
Pre-dissected Descending Aorta Diameter |
| Post-wCL |
Post-dissected whole Circumference Length |
| Post-tCL |
Post-dissected true lumen Circumference Length |
| RADAR |
Ratio between ascending and descending aorta diameter (radium2) |
| TAAD |
Type A Aortic Dissection |
| TTE |
Transthoracic Echocardiogram |
| wCL |
Whole circumference length |
References
- Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, et al. Epidemiology and clinicopathology of aortic dissection: A population- based longitudinal study over 27 years. American College of Chest Physicians; 2000;117:1271–8. [CrossRef]
- Jassar AS, Sundt TM. How should we manage type A aortic dissection?. Gen. Thorac. Cardiovasc. Surg. Springer Tokyo; 2019;137–45. [CrossRef]
- Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): New insights into an old disease. J Am Med Assoc 2000;283:897–903. [CrossRef]
- Hiratzka LF, Bakris GL, Beckman J a., Bersin RM, Carr VF, Casey DE, et al. Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, Ameri J. Am Coll Cardiol. 2010. Apr 6;121(13):e266-369. [CrossRef]
- Erbel R, Aboyans V, Boileau C, Bossone E, Di Bartolomeo R, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases. Eur. Heart J. Oxford University Press; 2014. p. 2873–926. [CrossRef]
- Pape LA, Tsai TT, Isselbacher EM, Oh JK, O’Gara PT, Evangelista A, et al. Aortic diameter ≥5.5 cm is not a good predictor of type A aortic dissection: Observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation; 2007;116:1120–7. [CrossRef]
- Tozzi P, Gunga Z, Niclauss L, Delay D, Roumy A, Pfister R, et al. Type A aortic dissection in aneurysms having modelled pre-dissection maximum diameter below 45 mm: should we implement current guidelines to improve the survival benefit of prophylactic surgery? Eur J Cardio-Thoracic Surg. Oxford University Press; 2021;59:473–8. [CrossRef]
- Masri A, Kalahasti V, Svensson LG, Roselli EE, Johnston D, Hammer D, et al. Aortic Cross-Sectional Area/Height Ratio and Outcomes in Patients with a Trileaflet Aortic Valve and a Dilated Aorta. Circulation. Lippincott Williams and Wilkins; 2016;134:1724–37. [CrossRef]
- Krüger T, Forkavets O, Veseli K, Lausberg H, Vöhringer L, Schneider W, et al. Ascending aortic elongation and the risk of dissection. Eur J Cardio-thoracic Surg. European Association for Cardio-Thoracic Surgery; 2016;50:241–7. [CrossRef]
- Yamauchi T, Masai T, Takano H, Shirakawa Y, Toda K, Sawa Y. et al. Equations Estimating the Predissected Diameter of the Ascending Aorta for Acute Type A Aortic Dissection. Ann Thorac Surg. Ann Thorac Surg; 2019;108:481–90. [CrossRef]
- Yamauchi T, Masai T, Takano H, Shirakawa Y, Toda K, Sawa Y. et al. Equations for estimating the predissected diameter of the descending aorta from computed tomographic images at the onset of aortic dissection. J Am Heart Assoc. American Heart Association Inc.; 2018;7. [CrossRef]
- Wolak A, Gransar H, Thomson LEJ, Friedman JD, Hachamovitch R, Gutstein A, et al. Aortic Size Assessment by Noncontrast Cardiac Computed Tomography: Normal Limits by Age, Gender, and Body Surface Area. JACC Cardiovasc Imaging. Elsevier; 2008;1:200–9. [CrossRef]
- Elefteriades JA, Paruchuri V, Salhab KF, Kuzmik G, Gubernikoff G, Fang H, et al. Aortic Size Distribution in the General Population: Explaining the Size Paradox in Aortic Dissection. Cardiol. 2015;131:265–72. [CrossRef]
- Eagle KA, Bhave NM. Ascending Aortic Length and Dissection Risk: In the Long Run. J. Am. Coll. Cardiol. Elsevier USA; 2019;1895–6. [CrossRef]
- Masri A, Kalahasti V, Svensson LG, Alashi A, Schoenhagen P, Roselli EE, et al. Aortic Cross-Sectional Area/Height Ratio and Outcomes in Patients with Bicuspid Aortic Valve and a Dilated Ascending Aorta. Circ Cardiovasc Imaging. Lippincott Williams and Wilkins; 2017;10. [CrossRef]
- Krüger T, Oikonomou A, Schibilsky D, Lescan M, Bregel K, Vöhringer L, et al. Aortic elongation and the risk for dissection: the Tübingen Aortic Pathoanatomy (TAIPAN) project†. Eur J Cardio-Thoracic Surg. European Association for Cardio-Thoracic Surgery; 2017;51:1119–26. [CrossRef]
- Krüger T, Sandoval Boburg R, Lescan M, Oikonomou A, Schneider W, Vöhringer L, et al. Aortic elongation in aortic aneurysm and dissection: the Tübingen Aortic Pathoanatomy (TAIPAN) project†. Eur J Cardio-Thoracic Surg. European Association for Cardio-Thoracic Surgery; 2018;54:26–33. [CrossRef]
- Wu J, Zafar MA, Li Y, Saeyeldin A, Huang Y, Zhao R, et al. Ascending Aortic Length and Risk of Aortic Adverse Events: The Neglected Dimension. J Am Coll Cardiol. Elsevier USA; 2019;74:1883–94. [CrossRef]
- Nakashima Y. Pathogenesis of Aortic Dissection: Elastic Fiber Abnormalities and Aortic Medial Weakness. Ann Vasc Dis. International Academic Publishing Co. Ltd.; 2010;3:28. [CrossRef]
- Roberts WC, Vowels TJ, Kitchens BL, Ko JM, Filardo G, Henry AC, et al. Aortic medial elastic fiber loss in acute ascending aortic dissection. Am J Cardiol. Am J Cardiol; 2011;108:1639–44. [CrossRef]
- Mansour AM, Peterss S, Zafar MA, Rizzo JA, Fang H, Charilaou P, et al. Prevention of Aortic Dissection Suggests a Diameter Shift to a Lower Aortic Size Threshold for Intervention. Cardiol. S. Karger AG; 2018;139:139–46. . [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).