Submitted:
24 May 2023
Posted:
26 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Production and Mechanism of miRNAs
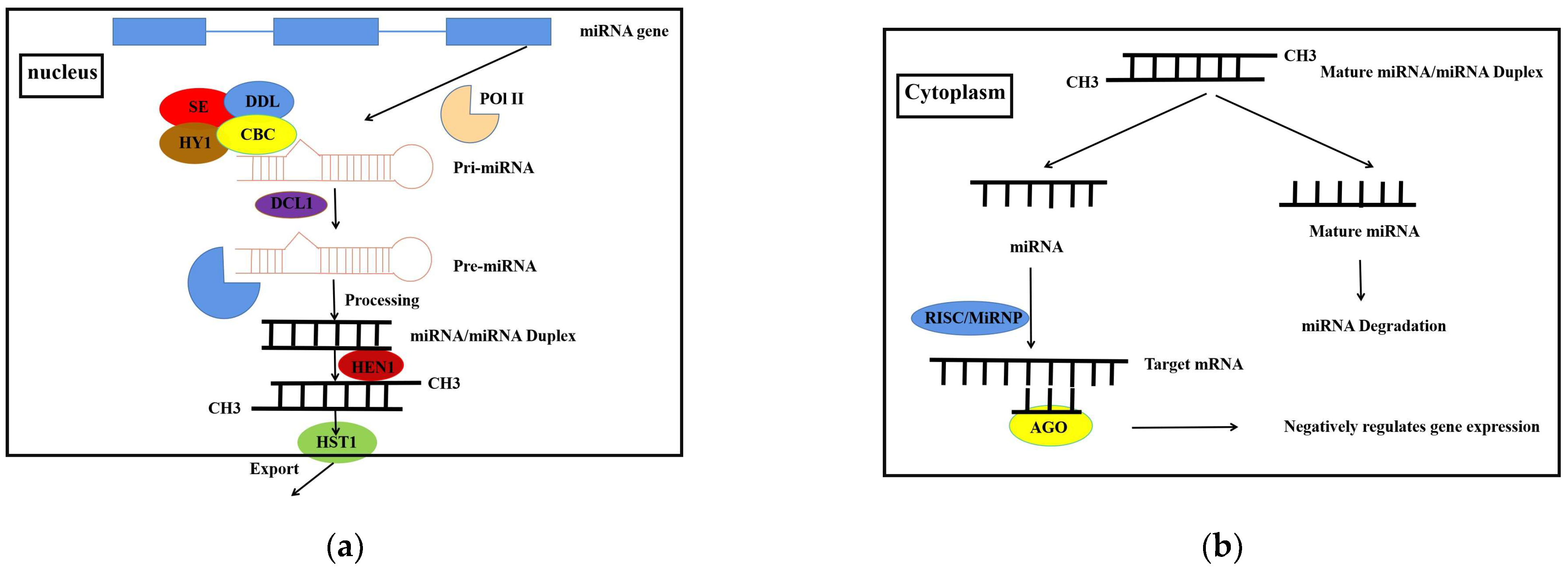
2.1. Production of miRNAs
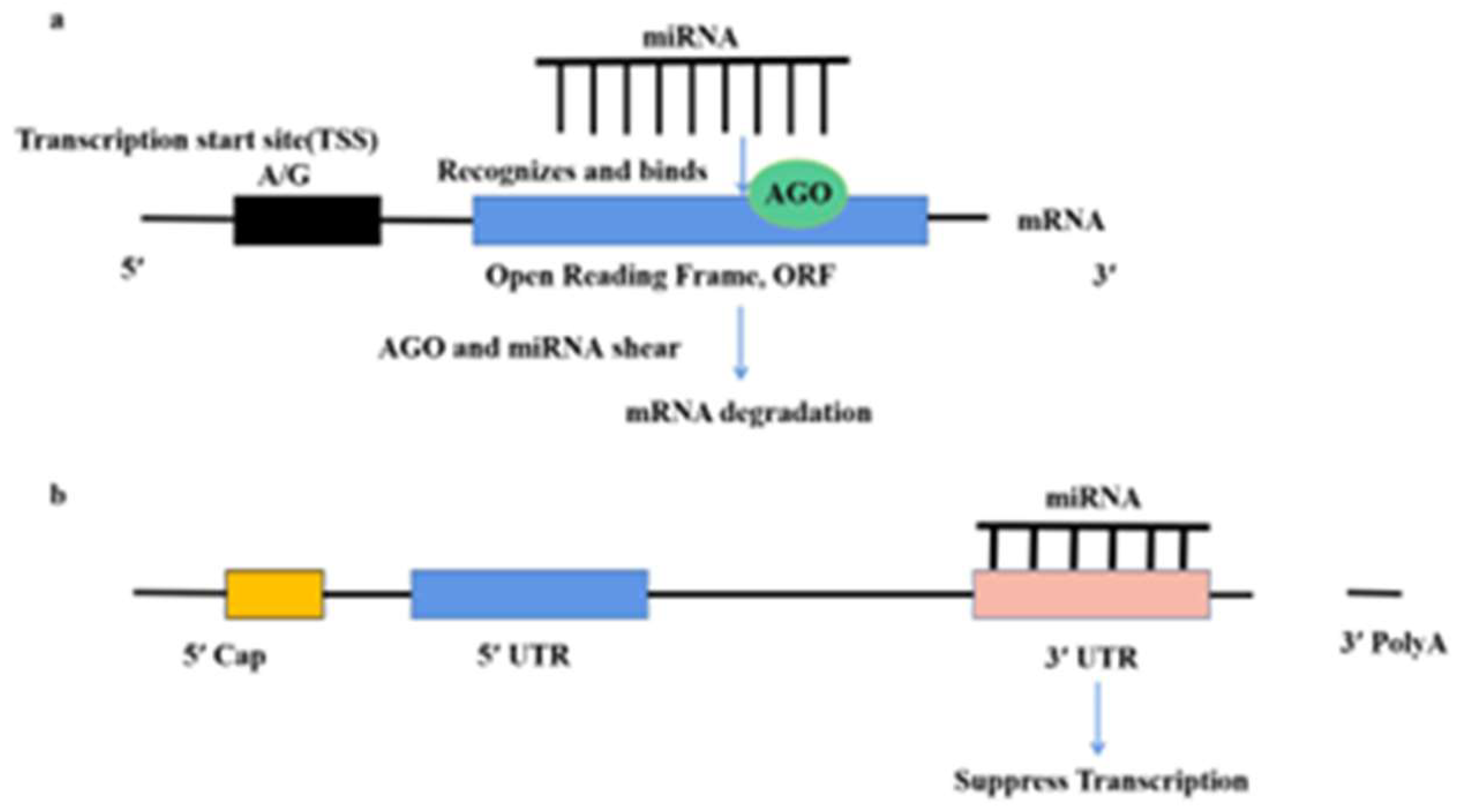
2.2. Mechanism of miRNAs
3. For miRNA and Drought Stress
4. For miRNA and Salt Stress
5. For miRNA and Temperature Stress
5.1. For miRNA and Cold Stress
5.2. For miRNA and High Temperature Stress
6. For miRNA and Heavy Metals Stress
7. For miRNA and Nutrient Stress
8. Conclusion and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laubinger S, Sachsenberg T, Zeller G, Busch W, Lohmann JU, Rätsch G, Weigel D. Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc Natl Acad Sci USA 2008, 105, 8795–800. [CrossRef] [PubMed]
- Rodriguez, A.; Griffiths-Jones, S.; Ashurst, J.L.; Bradley, A. Identification of Mammalian microRNA Host Genes and Transcription Units. Genome Res. 2004, 14, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Kim VN, Nam JW. Genomics of microRNA. Trends Genet. 2006, 22, 165–73.
- Iki, T.; Yoshikawa, M.; Meshi, T.; Ishikawa, M. Cyclophilin 40 facilitates HSP90-mediated RISC assembly in plants. EMBO J. 2011, 31, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Wu, G.; Gonzalez-Sulser, A.; Vaucheret, H.; Poethig, R.S. Nuclear processing and export of microRNAs in Arabidopsis. Proc. Natl. Acad. Sci. 2005, 102, 3691–3696. [Google Scholar] [CrossRef] [PubMed]
- Thieme CJ, Schudoma C, May P, Walther D. Give It AGO: The Search for miRNA-Argonaute Sorting Signals in Arabidopsis thaliana Indicates a Relevance of Sequence Positions Other than the 5’-Position Alone. Front Plant Sci. 2012, 3, 272.
- Liu, Q.; Wang, F.; Axtell, M.J. Analysis of Complementarity Requirements for Plant MicroRNA Targeting Using a Nicotiana benthamiana Quantitative Transient Assay. Plant Cell 2014, 26, 741–753. [Google Scholar] [CrossRef]
- Franco-Zorrilla, J.M.; Valli, A.; Todesco, M.; Mateos, I.; Puga, M.I.; Rubio-Somoza, I.; Leyva, A.; Weigel, D.; García, J.A.; Paz-Ares, J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 2007, 39, 1033–1037. [Google Scholar] [CrossRef]
- Aukerman, M.J.; Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef]
- Brodersen, P.; Sakvarelidze-Achard, L.; Bruun-Rasmussen, M.; Dunoyer, P.; Yamamoto, Y.Y.; Sieburth, L.; Voinnet, O. Widespread Translational Inhibition by Plant miRNAs and siRNAs. Science 2008, 320, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Lanet, E.; Delannoy, E.; Sormani, R.; Floris, M.; Brodersen, P.; Crété, P.; Voinnet, O.; Robaglia, C. Biochemical Evidence for Translational Repression by Arabidopsis MicroRNAs. Plant Cell 2009, 21, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ma, Z.; Hu, L.; Huang, K.; Zhang, M.; Zhang, S.; Jiang, W.; Wu, T.; Du, X. Involvement of rice transcription factor OsERF19 in response to ABA and salt stress responses. Plant Physiol. Biochem. 2021, 167, 22–30. [Google Scholar] [CrossRef]
- Ma Z, Jin YM, Wu T, Hu L, Zhang Y, Jiang W, Du X. OsDREB2B, an AP2/ERF transcription factor, negatively regulates plant height by conferring GA metabolism in rice. Front Plant Sci. 2022, 13, 1007811.
- Singh, A.; Jain, D.; Pandey, J.; Yadav, M.; Bansal, K.C.; Singh, I.K. Deciphering the role of miRNA in reprogramming plant responses to drought stress. Crit. Rev. Biotechnol. 2022, 43, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Gruber, M.Y.; Hannoufa, A. Transcriptome analysis of microRNA156 overexpression alfalfa roots under drought stress. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Feyissa BA, Arshad M, Gruber MY, Kohalmi SE, Hannoufa A. The interplay between miR156/SPL13 and DFR/WD40-1 regulate drought tolerance in alfalfa. BMC Plant Biol. 2019, 19, 434.
- López-Galiano, M.J.; García-Robles, I.; González-Hernández, A.I.; Camañes, G.; Vicedo, B.; Real, M.D.; Rausell, C. Expression of miR159 Is Altered in Tomato Plants Undergoing Drought Stress. Plants 2019, 8, 201. [Google Scholar] [CrossRef]
- Reyes, J.L.; Chua, N. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Xing, H.; Ke, W.; Shi, Y.; Sui, Z.; Xu, R.; Gao, L.; Guo, G.; Li, J.; et al. Positional cloning and characterization reveal the role of a miRNA precursor gene ZmLRT in the regulation of lateral root number and drought tolerance in maize. J. Integr. Plant Biol. 2022, 65, 772–790. [Google Scholar] [CrossRef]
- Kaushal, M. Microbes in Cahoots with Plants: MIST to Hit the Jackpot of Agricultural Productivity during Drought. Int. J. Mol. Sci. 2019, 20, 1769. [Google Scholar] [CrossRef] [PubMed]
- Romero-Romero JL, Inostroza-Blancheteau C, Orellana D, Aquea F, Reyes-Díaz M, Gil PM, Matte JP, Arce-Johnson P. Stomata regulation by tissue-specific expression of the Citrus sinensis MYB61 transcription factor improves water-use efficiency in Arabidopsis. Plant Physiol Biochem. 2018, 130, 54–60.
- Hoshika, Y.; Fares, S.; Pellegrini, E.; Conte, A.; Paoletti, E. Water use strategy affects avoidance of ozone stress by stomatal closure in Mediterranean trees—A modelling analysis. Plant, Cell Environ. 2019, 43, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Lertngim, N.; Ruangsiri, M.; Klinsawang, S.; Raksatikan, P.; Thunnom, B.; Siangliw, M.; Toojinda, T.; Siangliw, J.L. Photosynthetic Plasticity and Stomata Adjustment in Chromosome Segment Substitution Lines of Rice Cultivar KDML105 under Drought Stress. Plants 2022, 12, 94. [Google Scholar] [CrossRef]
- Yuan, W.; Suo, J.; Shi, B.; Zhou, C.; Bai, B.; Bian, H.; Zhu, M.; Han, N. The barley miR393 has multiple roles in regulation of seedling growth, stomatal density, and drought stress tolerance. Plant Physiol. Biochem. 2019, 142, 303–311. [Google Scholar] [CrossRef]
- Zhao J, Yuan S, Zhou M, Yuan N, Li Z, Hu Q, Bethea FG Jr, Liu H, Li S, Luo H. Transgenic creeping bentgrass overexpressing Osa-miR393a exhibits altered plant development and improved multiple stress tolerance. Plant Biotechnol J. 2019, 17, 233–251.
- Visentin I, Pagliarani C, Deva E, Caracci A, Turečková V, Novák O, Lovisolo C, Schubert A, Cardinale F. A novel strigolactone-miR156 module controls stomatal behaviour during drought recovery. Plant Cell Environ. 2020, 43, 1613–1624.
- Zhou Y, Liu W, Li X, Sun D, Xu K, Feng C, Kue Foka IC, Ketehouli T, Gao H, Wang N, Dong Y, Wang F, Li H. Integration of sRNA, degradome, transcriptome analysis and functional investigation reveals gma-miR398c negatively regulates drought tolerance via GmCSDs and GmCCS in transgenic Arabidopsis and soybean. BMC Plant Biol. 2020, 20, 190.
- Hang N, Shi T, Liu Y, Ye W, Taier G, Sun Y, Wang K, Zhang W. Overexpression of Os-microRNA408 enhances drought tolerance in perennial ryegrass. Physiol Plant. 2021, 172, 733–747.
- Wang, L. , Bai X. , Qiao Y., Si L., Yu Z., Ni C., Li T., Guo C., Xiao K. Tae-MiR9674a, a MicroRNA Member of Wheat, Confers Plant Drought and Salt Tolerance through Modulating the Stomata Movement and ROS Homeostasis. Plant Biotechnol Rep. 2022, 1, 3. [Google Scholar]
- Mir, I.R.; Rather, B.A.; Sehar, Z.; Masood, A.; Khan, N.A. Nitric oxide in co-ordination with nitrogen reverses cadmium-inhibited photosynthetic activity by interacting with ethylene synthesis, strengthening the antioxidant system, and nitrogen and sulfur assimilation in mustard (Brassica juncea L.). Sci. Hortic. 2023, 314. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, T.; Huang, K.; Jin, Y.-M.; Li, Z.; Chen, M.; Yun, S.; Zhang, H.; Yang, X.; Chen, H.; et al. A Novel AP2/ERF Transcription Factor, OsRPH1, Negatively Regulates Plant Height in Rice. Front. Plant Sci. 2020, 11, 709. [Google Scholar] [CrossRef] [PubMed]
- Pegler JL, Oultram JMJ, Grof CPL, Eamens AL. Molecular Manipulation of the miR399/PHO2 Expression Module Alters the Salt Stress Response of Arabidopsis thaliana. Plants (Basel). 2020, 10, 73.
- Arif, M.A.; Top, O.; Csicsely, E.; Lichtenstern, M.; Beheshti, H.; Adjabi, K.; Frank, W. DICER-LIKE1a autoregulation based on intronic microRNA processing is required for stress adaptation in Physcomitrium patens. Plant J. 2021, 109, 227–240. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, J.X.; Hu, Y.F.; Fang, C.Y.; Yu, Y.J.; Yang, J.; Zhu, B.; Ruan, Y.L.; Zhu, Z.J. Overexpression of sly-miR398b increased salt sensitivity likely via regulating antioxidant system and photosynthesis in tomato. Environ. Exp. Bot. 2021, 181, 104273. [Google Scholar] [CrossRef]
- Liu, J.N.; Ma, X.; Yan, L.; Liang, Q.; Fang, H.; Wang, C.; Dong, Y.; Chai, Z.; Zhou, R.; Bao, Y.; et al. MicroRNA and Degradome Profiling Uncover Defense Response of Fraxinus velutina Torr. to Salt Stress. Front. Plant Sci. 2022, 13, 847853. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, J.; Li, Z.; Hu, Q.; Yuan, N.; Zhou, M.; Xia, X.; Noorai, R.; Saski, C.; Li, S.; et al. MicroRNA396-mediated alteration in plant development and salinity stress response in creeping bentgrass. Hortic. Res. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Abla, M.; Sun, H.; Li, Z.; Wei, C.; Gao, F.; Zhou, Y.; Feng, J. Identification of miRNAs and Their Response to Cold Stress in Astragalus Membranaceus. Biomolecules 2019, 9, 182. [Google Scholar] [CrossRef]
- Wang, S.-T.; Sun, X.-L.; Hoshino, Y.; Yu, Y.; Jia, B.; Sun, Z.-W.; Sun, M.-Z.; Duan, X.-B.; Zhu, Y.-M. MicroRNA319 Positively Regulates Cold Tolerance by Targeting OsPCF6 and OsTCP21 in Rice (Oryza sativa L.). PLOS ONE 2014, 9, e91357. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, W. MicroRNA156 amplifies transcription factor-associated cold stress tolerance in plant cells. Mol. Genet. Genom. 2018, 294, 379–393. [Google Scholar] [CrossRef]
- Dong, Y.; Tang, M.; Huang, Z.; Song, J.; Xu, J.; Ahammed, G.J.; Yu, J.; Zhou, Y. The miR164a-NAM3 module confers cold tolerance by inducing ethylene production in tomato. Plant J. 2022, 111, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Sun M, Shen Y, Chen Y, Wang Y, Cai X, Yang J, Jia B, Dong W, Chen X, Sun X. Osa-miR1320 targets the ERF transcription factor OsERF096 to regulate cold tolerance via JA-mediated signaling. Plant Physiol. 2022, 189, 2500–2516.
- de Lima, C.F.F.; Kleine-Vehn, J.; De Smet, I.; Feraru, E. Getting to the root of belowground high temperature responses in plants. J. Exp. Bot. 2021, 72, 7404–7413. [Google Scholar] [CrossRef]
- Cohen, S.P.; E Leach, J. High temperature-induced plant disease susceptibility: more than the sum of its parts. Curr. Opin. Plant Biol. 2020, 56, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Posch, B.C.; Kariyawasam, B.C.; Bramley, H.; Coast, O.; Richards, R.A.; Reynolds, M.P.; Trethowan, R.; Atkin, O.K. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J. Exp. Bot. 2019, 70, 5051–5069. [Google Scholar] [CrossRef]
- Sadok W, Lopez JR, Smith KP. Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. Plant Cell Environ. 2021, 44, 2102–2116. [Google Scholar] [CrossRef]
- Sadura, I.; Janeczko, A. Brassinosteroids and the Tolerance of Cereals to Low and High Temperature Stress: Photosynthesis and the Physicochemical Properties of Cell Membranes. Int. J. Mol. Sci. 2021, 23, 342. [Google Scholar] [CrossRef]
- Singh, R.K.; Prasad, A.; Maurya, J.; Prasad, M. Regulation of small RNA-mediated high temperature stress responses in crop plants. Plant Cell Rep. 2021, 41, 765–773. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Wang, L.; Zhou, M.; Nian, J.; Chen, M.; Lu, X.; Liu, X.; Wang, Z.; Cen, J.; et al. SEMI-ROLLED LEAF 10 stabilizes catalase isozyme B to regulate leaf morphology and thermotolerance in rice (Oryza sativa L.). Plant Biotechnol. J. 2023, 21, 819–838. [Google Scholar] [CrossRef]
- Li, L.; Chen, G.; Yuan, M.; Guo, S.; Wang, Y.; Sun, J. CsbZIP2-miR9748-CsNPF4.4 Module Mediates High Temperature Tolerance of Cucumber Through Jasmonic Acid Pathway. Front. Plant Sci. 2022, 13, 883876. [Google Scholar] [CrossRef]
- Ahmed, W.; Xia, Y.; Zhang, H.; Li, R.; Bai, G.; Siddique, K.H.M.; Guo, P. Identification of conserved and novel miRNAs responsive to heat stress in flowering Chinese cabbage using high-throughput sequencing. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.; Arshad, M.; Hannoufa, A. Alfalfa response to heat stress is modulated by microRNA156. Physiol. Plant. 2018, 165, 830–842. [Google Scholar] [CrossRef]
- Arshad, M.; Puri, A.; Simkovich, A.J.; Renaud, J.; Gruber, M.Y.; Marsolais, F.; Hannoufa, A. Label-free quantitative proteomic analysis of alfalfa in response to microRNA156 under high temperature. BMC Genom. 2020, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Hannoufa, A. Alfalfa transcriptome profiling provides insight into miR156-mediated molecular mechanisms of heat stress tolerance. Genome 2022, 65, 315–330. [Google Scholar] [CrossRef]
- Pandey, A.K.; Zorić, L.; Sun, T.; Karanović, D.; Fang, P.; Borišev, M.; Wu, X.; Luković, J.; Xu, P. The Anatomical Basis of Heavy Metal Responses in Legumes and Their Impact on Plant–Rhizosphere Interactions. Plants 2022, 11, 2554. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2021, 74, 21–31. [Google Scholar] [CrossRef]
- Chot, E.; Reddy, M.S. Role of Ectomycorrhizal Symbiosis Behind the Host Plants Ameliorated Tolerance Against Heavy Metal Stress. Front. Microbiol. 2022, 13, 855473. [Google Scholar] [CrossRef]
- Tighe-Neira, R.; Gonzalez-Villagra, J.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Impact of nanoparticles and their ionic counterparts derived from heavy metals on the physiology of food crops. Plant Physiol. Biochem. 2022, 172, 14–23. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Gautam, S.; Landi, M.; Kandhol, N.; Araniti, F.; Ramakrishnan, M.; Satish, L.; Singh, V.P.; Sharma, P.; et al. Heavy metal induced regulation of plant biology: Recent insights. Physiol. Plant. 2022, 174, e13688. [Google Scholar] [CrossRef]
- Velusamy, K.; Periyasamy, S.; Kumar, P.S.; Rangasamy, G.; Pauline, J.M.N.; Ramaraju, P.; Mohanasundaram, S.; Vo, D.-V.N. Biosensor for heavy metals detection in wastewater: A review. Food Chem. Toxicol. 2022, 168, 113307. [Google Scholar] [CrossRef]
- Vaid, N.; Sudan, J.; Dave, S.; Mangla, H.; Pathak, H. Insight Into Microbes and Plants Ability for Bioremediation of Heavy Metals. Curr. Microbiol. 2022, 79, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Delgado, N.; Handford, M. Increasing Heavy Metal Tolerance by the Exogenous Application of Organic Acids. Int. J. Mol. Sci. 2022, 23, 5438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, H.; Jiang, H.; Wang, H.; Chen, K.; Duan, J.; Feng, S.; Wu, G. Regulation of cadmium tolerance and accumulation by miR156 in Arabidopsis. Chemosphere 2019, 242, 125168. [Google Scholar] [CrossRef]
- Kumar, R.S.; Sinha, H.; Datta, T.; Asif, M.H.; Trivedi, P.K. microRNA408 and its encoded peptide regulate sulfur assimilation and arsenic stress response in Arabidopsis. Plant Physiol. 2023, 192, 837–856. [Google Scholar] [CrossRef] [PubMed]
- Nie G, Liao Z, Zhong M, Zhou J, Cai J, Liu A, Wang X, Zhang X. MicroRNA-Mediated Responses to Chromium Stress Provide Insight Into Tolerance Characteristics of Miscanthus sinensis. Front Plant Sci. 2021, 12, 666117.
- Zhou, M.; Zheng, S.; Liu, R.; Lu, L.; Zhang, C.; Zhang, L.; Yant, L.; Wu, Y. The genome-wide impact of cadmium on microRNA and mRNA expression in contrasting Cd responsive wheat genotypes. BMC Genom. 2019, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Bian, H.; Zeng, Z.; Hou, N.; Shi, B.; Wang, J.; Zhu, M.; Han, N. miR393-Mediated Auxin Signaling Regulation is Involved in Root Elongation Inhibition in Response to Toxic Aluminum Stress in Barley. Plant Cell Physiol. 2017, 58, 426–439. [Google Scholar] [CrossRef]
- Zinta, R.; Tiwari, J.K.; Buckseth, T.; Thakur, K.; Goutam, U.; Kumar, D.; Challam, C.; Bhatia, N.; Poonia, A.K.; Naik, S.; et al. Root system architecture for abiotic stress tolerance in potato: Lessons from plants. Front. Plant Sci. 2022, 13, 926214. [Google Scholar] [CrossRef]
- Phour, M.; Sindhu, S.S. Mitigating abiotic stress: microbiome engineering for improving agricultural production and environmental sustainability. Planta 2022, 256, 1–34. [Google Scholar] [CrossRef]
- Prasad, R. Cytokinin and Its Key Role to Enrich the Plant Nutrients and Growth Under Adverse Conditions-An Update. Front. Genet. 2022, 13, 883924. [Google Scholar] [CrossRef]
- Swain, R.; Sahoo, S.; Behera, M.; Rout, G.R. Instigating prevalent abiotic stress resilience in crop by exogenous application of phytohormones and nutrient. Front. Plant Sci. 2023, 14, 1104874. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, R.K.; Li, H.-B.; Guo, D.-J.; Sharma, A.; Verma, K.K.; Solanki, M.K.; Upadhyay, S.K.; Lakshmanan, P.; Yang, L.-T.; et al. Nitrogen fixation and phytohormone stimulation of sugarcane plant through plant growth promoting diazotrophic Pseudomonas. Biotechnol. Genet. Eng. Rev. 2023, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Jezek M, Allan AC, Jones JJ, Geilfus CM. New Phytol. 2023.
- Johnson, R.; Vishwakarma, K.; Hossen, S.; Kumar, V.; Shackira, A.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Lyzenga, W.J.; Liu, Z.; Olukayode, T.; Zhao, Y.; Kochian, L.V.; Ham, B.-K. Getting to the roots of N, P, and K uptake. J. Exp. Bot. 2023, 74, 1784–1805. [Google Scholar] [CrossRef]
- Yousuf, P.Y.; Shabir, P.A.; Hakeem, K.R. miRNAomic Approach to Plant Nitrogen Starvation. Int. J. Genom. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Vega, A.; O’brien, J.A.; A Gutiérrez, R. Nitrate and hormonal signaling crosstalk for plant growth and development. Curr. Opin. Plant Biol. 2019, 52, 155–163. [Google Scholar] [CrossRef]
- Islam, W.; Tauqeer, A.; Waheed, A.; Zeng, F. MicroRNA Mediated Plant Responses to Nutrient Stress. Int. J. Mol. Sci. 2022, 23, 2562. [Google Scholar] [CrossRef]
- Du, Q.; Wang, K.; Zou, C.; Xu, C.; Li, W.-X. The PILNCR1-miR399 Regulatory Module Is Important for Low Phosphate Tolerance in Maize. Plant Physiol. 2018, 177, 1743–1753. [Google Scholar] [CrossRef]
- Hu, B.; Wang, W.; Deng, K.; Li, H.; Zhang, Z.; Zhang, L.; Chu, C. MicroRNA399 is involved in multiple nutrient starvation responses in rice. Front. Plant Sci. 2015, 6, 188. [Google Scholar] [CrossRef]
- Thornburg TE, Liu J, Li Q, Xue H, Wang G, Li L, Fontana JE, Davis KE, Liu W, Zhang B, Zhang Z, Liu M, Pan X. Potassium Deficiency Significantly Affected Plant Growth and Development as Well as microRNA-Mediated Mechanism in Wheat (Triticum aestivum L.). Front Plant Sci. 2020, 11, 1219.
- Fontana JE, Wang G, Sun R, Xue H, Li Q, Liu J, Davis KE, Thornburg TE, Zhang B, Zhang Z, Pan X. Impact of potassium deficiency on cotton growth, development and potential microRNA-mediated mechanism. Plant Physiol Biochem. 2020, 153, 72–80.
- Ye, Z.; Zeng, J.; Long, L.; Ye, L.; Zhang, G. Identification of microRNAs in response to low potassium stress in the shoots of Tibetan wild barley and cultivated. Curr. Plant Biol. 2020, 25, 100193. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, H.; Hamera, S.; Chen, X.; Fang, R. miR444a has multiple functions in the rice nitrate-signaling pathway. Plant J. 2014, 78, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Prusty, S.; Sahoo, R.K.; Nayak, S.; Poosapati, S.; Swain, D.M. Proteomic and Genomic Studies of Micronutrient Deficiency and Toxicity in Plants. Plants 2022, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Ninkuu, V.; Liu, Z.; Sun, X. Genetic regulation of nitrogen use efficiency in Gossypium spp. Plant, Cell Environ. 2023, 46, 1749–1773. [Google Scholar] [CrossRef]
- Huang, S.; Wang, P.; Yamaji, N.; Ma, J.F. Plant Nutrition for Human Nutrition: Hints from Rice Research and Future Perspectives. Mol. Plant 2020, 13, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.S.; Smart, S.M.; Cybulski, J.D.; McMahon, K.W.; Marcks, B.; Nowakowski, C. Insights from Fossil-Bound Nitrogen Isotopes in Diatoms, Foraminifera, and Corals. Annu. Rev. Mar. Sci. 2023, 15, 407–430. [Google Scholar] [CrossRef]
- Helliwell, K.E. Emerging trends in nitrogen and phosphorus signalling in photosynthetic eukaryotes. Trends Plant Sci. 2022, 28, 344–358. [Google Scholar] [CrossRef]
- Kong, W.W.; Yang, Z.M. Identification of iron-deficiency responsive microRNA genes and cis-elements in Arabidopsis. Plant Physiol. Biochem. 2010, 48, 153–159. [Google Scholar] [CrossRef]
- Valdés-López, O.; Yang, S.S.; Aparicio-Fabre, R.; Graham, P.H.; Reyes, J.L.; Vance, C.P.; Hernández, G. MicroRNA expression profile in common bean (Phaseolus vulgaris) under nutrient deficiency stresses and manganese toxicity. New Phytol. 2010, 187, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Kayihan, D.S.; Kayihan, C.; Özden Çiftçi, Y. Moderate level of toxic boron causes differential regulation of micrornas related to jasmonate and ethylene metabolisms in arabidopsis thaliana. Turk. J. Botany. 2019, 43, 167–172. [Google Scholar] [CrossRef]
- Ozhuner, E.; Eldem, V.; Ipek, A.; Okay, S.; Sakcali, S.; Zhang, B.; Boke, H.; Unver, T. Boron Stress Responsive MicroRNAs and Their Targets in Barley. PLOS ONE 2013, 8, e59543. [Google Scholar] [CrossRef]
- Giacomelli, J.I.; Weigel, D.; Chan, R.L.; Manavella, P.A. Role of recently evolved miRNA regulation of sunflower HaWRKY6 in response to temperature damage. New Phytol. 2012, 195, 766–773. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, J.; Wang, Z.; Wen, Y.; Wang, J.; He, W.; Liu, B.; Si, H.; Wang, D. Identification of Novel and Conserved MicroRNAs Related to Drought Stress in Potato by Deep Sequencing. PLOS ONE 2014, 9, e95489. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-P.; Montgomery, T.A.; Fahlgren, N.; Kasschau, K.D.; Nonogaki, H.; Carrington, J.C. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 2007, 52, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Boualem, A.; Laporte, P.; Jovanovic, M.; Laffont, C.; Plet, J.; Combier, J.; Niebel, A.; Crespi, M.; Frugier, F. MicroRNA166 controls root and nodule development in Medicago truncatula. Plant J. 2008, 54, 876–887. [Google Scholar] [CrossRef]
- Trindade, I.; Capitão, C.; Dalmay, T.; Fevereiro, M.P.; dos Santos, D.M. miR398 and miR408 are up-regulated in response to water deficit in Medicago truncatula. Planta 2009, 231, 705–716. [Google Scholar] [CrossRef]
- Li, W.-X.; Oono, Y.; Zhu, J.; He, X.-J.; Wu, J.-M.; Iida, K.; Lu, X.-Y.; Cui, X.; Jin, H.; Zhu, J.-K. The Arabidopsis NFYA5 Transcription Factor Is Regulated Transcriptionally and Posttranscriptionally to Promote Drought Resistance. Plant Cell 2008, 20, 2238–2251. [Google Scholar] [CrossRef]
- Jagadeeswaran, G.; Li, Y.; Sunkar, R. Redox signaling mediates the expression of a sulfate-deprivation-inducible microRNA395 in Arabidopsis. Plant J. 2013, 77, 85–96. [Google Scholar] [CrossRef]
- E Barrera-Figueroa, B.; Gao, L.; Diop, N.N.; Wu, Z.; Ehlers, J.D.; A Roberts, P.; Close, T.J.; Zhu, J.-K.; Liu, R. Identification and comparative analysis of drought-associated microRNAs in two cowpea genotypes. BMC Plant Biol. 2011, 11, 127–127. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R.; Zhu, J.-K. Novel and Stress-Regulated MicroRNAs and Other Small RNAs from Arabidopsis[W]. Plant Cell 2004, 16, 2001–2019. [Google Scholar] [CrossRef]
- Liu, H.-H.; Tian, X.; Li, Y.-J.; Wu, C.-A.; Zheng, C.-C. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 2008, 14, 836–843. [Google Scholar] [CrossRef]
- Shamimuzzaman; Vodkin, L. Identification of soybean seed developmental stage-specific and tissue-specific miRNA targets by degradome sequencing. BMC Genom. 2012, 13, 310–310. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Tao, Y.; Zhu, C. Emerging roles of microRNAs in the mediation of drought stress response in plants. J. Exp. Bot. 2013, 64, 3077–3086. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Yan, J.; Wang, K.; Luo, H.; Zhang, W. MiR319 mediated salt tolerance by ethylene. Plant Biotechnol. J. 2019, 17, 2370–2383. [Google Scholar] [CrossRef]
- He F, Xu C, Fu X, Shen Y, Guo L, Leng M, Luo K. The MicroRNA390/TRANS-ACTING SHORT INTERFERING RNA3 Module Mediates Lateral Root Growth under Salt Stress via the Auxin Pathway. Plant Physiol. 2018, 177, 775–791.
- Bai, Q.; Wang, X.; Chen, X.; Shi, G.; Liu, Z.; Guo, C.; Xiao, K. Wheat miRNA TaemiR408 Acts as an Essential Mediator in Plant Tolerance to Pi Deprivation and Salt Stress via Modulating Stress-Associated Physiological Processes. Front. Plant Sci. 2018, 9, 499. [Google Scholar] [CrossRef]
- Guo, X.; Niu, J.; Cao, X. Heterologous Expression of Salvia miltiorrhiza MicroRNA408 Enhances Tolerance to Salt Stress in Nicotiana benthamiana. Int. J. Mol. Sci. 2018, 19, 3985. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Chen, D.; Cheng, Y.; Zhang, X.; Song, L.; Hu, M.; Dong, J.; Shen, F. MicroRNA414c affects salt tolerance of cotton by regulating reactive oxygen species metabolism under salinity stress. RNA Biol. 2019, 16, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Aslam M, Sugita K, Qin Y, Rahman A. Aux/IAA14 Regulates microRNA-Mediated Cold Stress Response in Arabidopsis Roots. Int J Mol Sci. 2020, 21, 8441.
- Huo, C.; Zhang, B.; Wang, R. Research progress on plant noncoding RNAs in response to low-temperature stress. Plant Signal. Behav. 2021, 17, 2004035. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhang, N.; Wang, Q.; Fu, Y.; Wang, F.; Su, Y.; Xue, B.; Zhou, L.; Liao, H. The Effect of Low Temperature Stress on the Leaves and MicroRNA Expression of Potato Seedlings. Front. Ecol. Evol. 2021, 9. [Google Scholar] [CrossRef]
- Stief, A.; Altmann, S.; Hoffmann, K.; Pant, B.D.; Scheible, W.-R.; Bäurle, I. Arabidopsis miR156 Regulates Tolerance to Recurring Environmental Stress through SPL Transcription Factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef] [PubMed]
- Zhang M, An P, Li H, Wang X, Zhou J, Dong P, Zhao Y, Wang Q, Li C. The miRNA-Mediated Post-Transcriptional Regulation of Maize in Response to High Temperature. Int J Mol Sci. 2019, 20, 1754.
- Gong, J.; Li, D.; Li, H.; Zhou, H.; Xu, J. Identification of manganese-responsive microRNAs in Arabidopsis by small RNA sequencing. Czech J. Genet. Plant Breed. 2019, 55, 76–82. [Google Scholar] [CrossRef]
- da Silva, R.G.; Rosa-Santos, T.M.; França, S.d.C.; Kottapalli, P.; Kottapalli, K.R.; Zingaretti, S.M. Microtranscriptome analysis of sugarcane cultivars in response to aluminum stress. PLOS ONE 2019, 14, e0217806. [Google Scholar] [CrossRef]
- Shi, D.-Q.; Zhang, Y.; Ma, J.-H.; Li, Y.-L.; Xu, J. Identification of Zinc Deficiency-Responsive MicroRNAs in Brassica juncea Roots by Small RNA Sequencing. J. Integr. Agric. 2013, 12, 2036–2044. [Google Scholar] [CrossRef]
- Halder, K.; Chaudhuri, A.; Abdin, M.Z.; Datta, A. Tweaking the Small Non-Coding RNAs to Improve Desirable Traits in Plant. Int. J. Mol. Sci. 2023, 24, 3143. [Google Scholar] [CrossRef]
- Yan, G.; Hua, Y.; Jin, H.; Huang, Q.; Zhou, G.; Xu, Y.; He, Y.; Zhu, Z. Sly-miR398 Participates in Cadmium Stress Acclimation by Regulating Antioxidant System and Cadmium Transport in Tomato (Solanum lycopersicum). Int J Mol Sci. 2023, 24, 1953. [Google Scholar] [CrossRef] [PubMed]
| Abiotic Stress Type | miRNA | Expression | Species | Target Genes | Reference |
|---|---|---|---|---|---|
| Drought | MicroRNA-157 | Upregulated | Arabidopsis thaliana | SPB Transcription factor | [94] |
| Drought | MicroRNA-159 | Upregulated | Arabidopsis thaliana | MYB and TCP Transcription factors | [95] |
| Drought | MicroRNA-160 | Downregulated | Arabidopsis thaliana | ARF10, ARF16, ARF17 | [96] |
| Drought | MicroRNA-166 | Upregulated | Medicago truncatula | HD-ZIPIII Transcription factors | [97,98] |
| Drought | MicroRNA-167 | Upregulated | Arabidopsis thaliana | ARF6,ARF8 | [94] |
| Drought | MicroRNA-168 | Upregulated | Arabidopsis thaliana | ARGONAUTE, MAPK | [94] |
| Drought | MicroRNA-169 | Downregulated | Arabidopsis thaliana | NF-YA transcription factor, SIMRP1 | [99] |
| Drought | MicroRNA-171 | Upregulated | Arabidopsis thaliana | GRAS transcription factor | [94] |
| Drought | MicroRNA-319 | Upregulated | Arabidopsis thaliana | TCP Family | [100] |
| Drought | MicroRNA-390 | Upregulated | Vigna unguiculata | ARF Family | [101] |
| Drought | MicroRNA-393 | Upregulated | Arabidopsis thaliana | (TIR1, AFB2, AFB3) (ARF5, EPF1, SPCH) | [102,103] |
| Drought | MicroRNA-396 | Upregulated | Arabidopsis thaliana | GRL transcription factor | [94] |
| Drought | MicroRNA-397 | Downregulated | Oryza sativa | Laccase genes | [104] |
| Drought | MicroRNA-398 | Upregulated | Medicago truncatula | Superoxide dismutase | [98] |
| Drought | MicroRNA-398c | Downregulated | Soybean | GmCSD1a/b,GmCSD2a/b/c,GmCCS | [28] |
| Drought | MicroRNA-408 | Upregulated | Arabidopsis thaliana | Chemocyanin precursor, kinases | [94] |
| Drought | MicroRNA-474 | Upregulated | Zea mays | PDH, PPR | [105] |
| Drought | MicroRNA-528 | Downregulated | Zea mays | POD | [105] |
| Drought | MicroRNA-811 | Downregulated | Catharanthus roseus | MYB transcription factor | [106] |
| Drought | MicroRNA-814 | Downregulated | Phaseolus vulgaris | Hydroxyproline-rich glycoprotein | [106] |
| Drought | MicroRNA-835 | Downregulated | Ricinus communis | Aquaporin | [106] |
| Drought | MicroRNA-4398 | Downregulated | Solanum tuberosum | WRKY transcription factor | [106] |
| Salt | MicroRNA-319b | Upregulated | Switchgrass | PvPCF5 | [107] |
| Salt | MicroRNA-390 | Downregulated | Poplar | ARF3.1, ARF3.2,ARF4 | [108] |
| Salt | MicroRNA-390a | Downregulated | Creeping bentgrass | AsTIR1, AsAFB2 | [26] |
| Salt | MicroRNA-396c | Upregulated | Creeping bentgrass | GRF | [37] |
| Salt | MicroRNA-408 | Upregulated | Wheat | TaCP,TaMP,TaBCP,TaFP,TaKRP,TaABP | [109] |
| Salt | MicroRNA-408 | Upregulated | Salvia miltiorrhiza | NbSOD, NbPOD, NbCAT | [110] |
| Salt | MicroRNA-414c | Downregulated | Cotton | GhFSD1 | [111] |
| Cold | MicroRNA-160 | Downregulated | Maize | [112] | |
| Cold | MicroRNA-319 | Downregulated | Rice | PCF6/TCP21 | [113] |
| Cold | MicroRNA-319 | Downregulated | Maize | [112] | |
| Cold | MicroRNA-408a | Upregulated | Maize | [112] | |
| Cold | MicroRNA-528 | Upregulated | Maize | [112] | |
| Cold | MicroRNA-5125 | Upregulated | Potato | ABF8011 | [114] |
| Cold | MicroRNA-10881 | Upregulated | Potato | GA3ox123158 | [114] |
| High temperature | MicroRNA-156 | Downregulated | Arabidopsis thaliana | SPL transcription factor | [115] |
| High temperature | MicroRNA-159 | Downregulated | Maize | MYB transcription factor | [116] |
| High temperature | MicroRNA-164 | Downregulated | Maize | NAC transcription factor | [116] |
| High temperature | MicroRNA-166 | Downregulated | Maize | HD zip | [116] |
| High temperature | MicroRNA-169 | Downregulated | Maize | SBP | [116] |
| High temperature | MicroRNA-172 | Downregulated | Maize | AP2/ERF | [116] |
| High temperature | MicroRNA-396 | Downregulated | Maize | GRF, | [116] |
| High temperature | MicroRNA-5381 | Downregulated | Maize | SAC2 | [116] |
| Heavy metals-Cd | MicroRNA-167 | Zea mays | [117] | ||
| Heavy metals-Cd | MicroRNA-393 | Zea mays | [117] | ||
| Heavy metals-Cu | MicroRNA-398 | Grape | VvCSD1 and VvCSD2 | [63] | |
| Heavy metals-Al | MicroRNA-160 | Sugarcance | [117] | ||
| Heavy metals-Al | MicroRNA-162 | Sugarcance | [117] | ||
| Heavy metals-Al | MicroRNA-164 | Sugarcance | [117] | ||
| Heavy metals-Al | MicroRNA-166 | Sugarcance | [117] | ||
| Heavy metals-Al | MicroRNA-167 | Sugarcance | [117] | ||
| Nutrients-Zn | MicroRNA-158 | Upregulated | Brassica juncea | FUT1 | [118] |
| Nutrients-K | MicroRNA-169 | Triticum aestivum | Pentose pathway | [119] | |
| Nutrients-N | MicroRNA-169 | Downregulated | Arabidopsis thaliana | HAP2 | [81] |
| Nutrients-B | MicroRNA-319 | Upregulated | Riticum aestivum | MYB transcription factor | [92] |
| Nutrients-K | MicroRNA-319 | Downregulated | Hordeum vulgare | TCP | [92] |
| Nutrients-K | MicroRNA-396 | Downregulated | Hordeum vulgare | GRF | [83] |
| Nutrients-P | MicroRNA-399 | Downregulated | Arabidopsis thaliana | Ubiquitin conjugase E2 | [117] |
| Nutrients-Mn | MicroRNA-781 | Upregulated | Arabidopsis thaliana | MCM2 | [117] |
| Nutrients-Mn | MicroRNA-826 | Upregulated | Arabidopsis thaliana | Alkenylhydroxalkylproducing 2 | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




