1. Introduction
Neuroendocrine neoplasms (NENs) of the pancreas constitute about 30% of all gastro-entero-pancreatic neuroendocrine neoplasms (GEP-NENs) and 1-2% of all pancreatic tumors [
1]. These tumours can be functional pancreatic neuroendocrine neoplasms (F-PanNENs) and non-functional pancreatic neuroendocrine neoplasms (NF-PanNENs) (60-90%). According to the 5th edition of the WHO gastrointestinal system classification (2019), these neoplasms were divided into well-differentiated neuroendocrine tumours (NETs) and poorly differentiated neuroendocrine carcinomas (NECs). Additionally, the PanNETs were classified into three subtypes based on the grade of their histological maturity; NET G1 — high grade, NET G2 — intermediate grade, and NET G3 — low grade (according to the number of figures of division and the proliferation index Ki-67).
Over one-third of patients with pancreatic NENs (PanNENs) present metastatic disease at diagnosis [
2]. The 5-year survival rate of PanNENs for the most part is associated with distant metastasis [
3]. Metastases are present mainly in the liver, however, bone metastases (BMets) are detected in less than 15% of all NENs patients [
4], and in only 4% of pancreatic NENs (PanNENs) [
4,
5]. BMets may be asymptomatic and incidentally detected, therefore both functional imaging, such as [
68Ga]Ga-somatostatin analogue (SSA) positron emission tomography (PET)/computed tomography (CT)/ [
18F]F-FDG PET/CT, and the anatomical scans, such as CT/ magnetic resonance imaging (MRI) are needed to assess disease status of PanNEN [
6]. The asymptomatic nature of BMets results in an underestimation of the real BMets incidence in NENs patients. The most common symptoms of BMets are pain, pathological fractures, and metastatic spinal cord compression. They can lead to malignant hypercalcemia and worsened quality of life [
7].
Metastatic disease is always connected with a limited prognosis [
8]. Therefore it is essential to find new markers that can predict the probability of metastasis and improve the clinical outcome with accurate treatment. Early detection of BMets and treatment, such as bisphosphonate, denosumab, as well as radiation therapy, can significantly reduce the risk of spinal cord compression and pathological fractures, mitigate the pain, and thus improve the quality of life of these patients [
9].
Some studies suggest that the presence of certain circulating biomarkers (CBMs) can be useful in the early diagnosis/detection of BMets [
9,
10]. To diagnose BMets in PanNENs patients, CBM including ferritin, cytokeratin 18 (CK18), CA19-9, CA125, AFP, CEA, and B2M were evaluated.
CK18 is a structural protein involved in regulating cell growth, apoptosis, mitosis, cancer-related signaling, motility, and many other important processes [
11]. It is widely expressed in epithelial tissues of many organs (kidneys, lungs, liver, pancreas, gastrointestinal tract, or mammary gland). Moreover, it is continuously expressed in various cancer tissues and is considered as a marker of apoptosis [
11,
12]. Progression of epithelial tumors is associated with cell apoptosis and an increase in serum CK18 level [
13].
CA125 comes under mucin family proteins and is a serum tumor marker for multiple cancers, such as ovarian, endometrial, pancreatic, or bladder. It is used to detect the recurrence of the disease, response to the treatment, and differentiate malignant and benign lesions [
14]. Recent data showed that the serum level of CA125 correlates with survival also in lung cancer [
15]. CA125 is expressed on the cell wall, unable to penetrate the blood. The membrane damage caused by a.o. inflammation may lead to the elevation of serum CA125 level [
16].
B2M is a small molecular weight protein ordinarily present on the surface of all nucleated cells, and it forms the light chain of the human leukocyte antigen [
17]. Membrane B2M performs multiple immune functions, while serum B2M is a marker of disease severity in renal injury, infections, amyloidosis, aging-related diseases, and lymphoproliferative disorders [
18].
This study aimed to assess the efficacy of various CBMs in the detection of BMets in patients with PanNENs. The early detection of BMets is crucial to prevent pathological fractures and physical disability in patients with PanNENs, and improves these patients' prognosis and quality of life. In case patients had elevated CBMs levels are found initially, the diagnostic procedure and treatment protocol should be changed: shorter intervals between clinical check-ups and imaging scans and more aggressive treatments at the earlier stage of the disease.
2. Materials and Methods
2.1. Study Participants
The study group comprised 115 patients with PanNENs, while the control group consisted of 40 healthy volunteers. The mean age (and range) of the patients in the study group was 53 (19–79) and in the control group 50 (25–78). Controls were the healthy volunteers recruited from the hospital and outpatient clinic personnel. The main inclusion criterion for the patient’s group was a confirmed histopathological diagnosis of pancreatic neuroendocrine neoplasm (PanNEN) according to the WHO’s 2019 classification and the American Joint Committee on Cancer/Union for International Cancer Control’s 2017 type and signed consent to participate in the study. All patients with PanNEN were recruited at the Department of Endocrinology and Neuroendocrine Tumors, Medical University of Silesia, ENETS Neuroendocrine Tumor Center of Excellence.
Exclusion criteria for studied subjects were: age less than 18, pregnancy, insufficiency of renal, liver, and heart. The local Ethics Committee approved this study. Information on age, sex, BMI, grade, clinical stage, and bone metastasis of the patients with PanNEN was assessed through hospital records. The characteristics of the studied groups are presented in
Table 1.
For the detection of BMets, in the majority of patients with PanNEN we performed a functional examination using [68Ga]Ga-DOTATATE PET/CT ([18F]F-FDG PET/CT was done mainly for poorly differentiated pancreatic neuroendocrine carcinoma (PanNEC).
In 8 patients with PanNEN (3 men and 5 women) BMets were confirmed: by CT in 3 cases, by [68Ga]Ga-DOTATATE PET/CT in 4 cases, and by [18F]FDG PET/CT in 1 case.
The study was conducted in accordance with the good clinical practice guidelines and the Declaration of Helsinki.
2.2. Circulating Biomarkers (CBMs) measurement
The levels of selected biomarkers in the blood serum are described below. The peripheral blood samples (5 ml) were taken from all study participants, leaving the blood to clot. Blood samples from PanNEN patients patients were taken at different disease stages: before (2 cases) or after tumour-specific treatment (6 cases). Then, the samples were spun, and next, the serum was put into boxes and kipped at -80 °C for further analysis.
ELISA or EIA was performed with commercially available kits: ELISA kits for ferritin, CY18, and B2M, but EIA kits for AFP, CA125, CA19-9, and CEA. All immunoassays were done at the Local Laboratory of the Medical University of Silesia.
2.3. Statistical Analysis
Data were presented as median and interquartile range. The comparison of CBM concentrations between study and control groups and patients with PanNEN with BMets and without BMets was performed using a nonparametric, 2-tailed Mann-Whitney U test. To investigate the diagnostic capacity of CBM in detecting BMets, receiver operating characteristic (ROC) curves were plotted, and the area under the curve (AUC), sensitivity, and specificity was calculated. The correlation coefficient between CBMs concentration, age, BMI, and Ki-67 proliferation index was calculated using the Spearman rank correlation test. The significance threshold in all tests was set at a value of ≤ 0.05. Statistical analysis was performed using Statistica v. 13.36.0 (StatSoft, Kraków, Poland) software.
3. Results
3.1. Patients with PanNENs vs. Controls
We present the demographic and clinical characteristics of the participants recruited for the study (PanNEN patients and controls) in
Table 1. One hundred and fifteen PanNEN patients comprised 43% males and 57% females, compared to the control subject, where the group of women significantly dominated (77.5%). Most patients (93%) were diagnosed with well-differentiated NET: fifty-two patients had NET G1, while forty-five patients had NET G2. Seven percent only of these patients (8/115) had bone metastasis. Bone metastases were identified at different time points, but always these were secondary bone metastases following liver or lymph node metastases. The pattern of bone metastasis and clinical characteristics of the patients with pancreatic neuroendocrine neoplasms are shown in
Table 2.
3.2. BM-PanNEN patients vs non-BM-PanNEN patients and CBMs
In the second part of this study, we established CBMs levels according to BMets presence or absence (
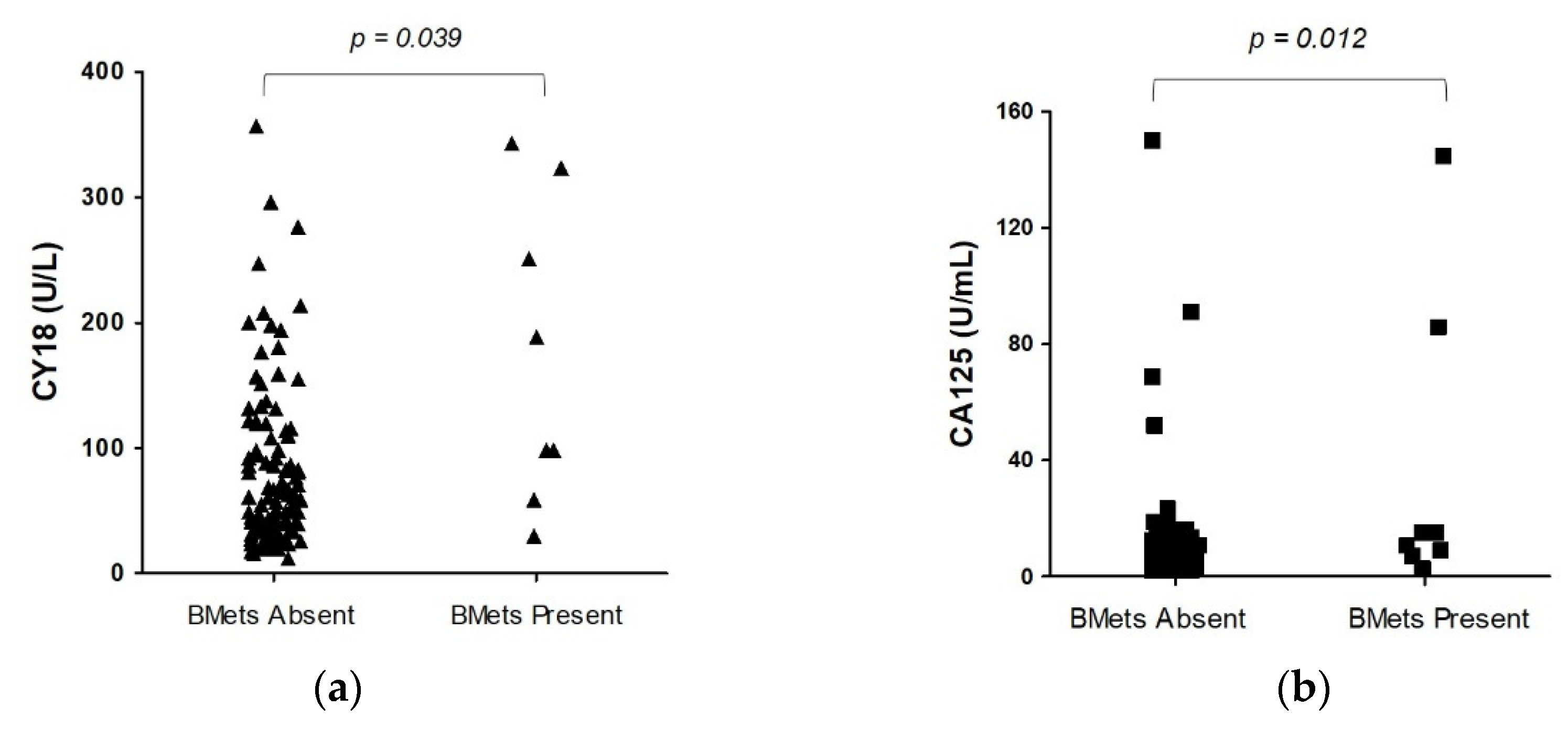
Table 2). Before CBMs measurements, all PanNEN patients displayed normal routine lab tests, including alkaline phosphatase, ALT/AST, calcium, or phosphate levels. The medians of two of all CBMs (CY18 and CA125) in BM-PanNEN patients (n = 8) were significantly increased (p < 0.05) versus non-BM-PanNEN patients (n = 107). The circulating CY18 level in BM-PanNEN patients set (174.20 U/L ± 121.14; 144 [79 – 288]) was significantly higher (p = 0.04) compared to non-BM-PanNEN patients (94.17 U/L ± 93.58; 62 [36 – 120]). The serum CA125 concentration was elevated (p = 0.01) in BM-PanNEN patients (36.29 U/mL ± 51.47; 13 [8 – 50]) compared to the non-BM-PanNEN patients (9.65 U/mL ± 18.16; 6 [3 – 9]) (
Figure 1). The concentrations of other assessed CBMs, as well as proliferative index Ki-67 and tumor size of primary, did not differ significantly between these groups (p > 0.05) (
Table 3).
3.3. Diagnostic accuracy CY18, CA125, and B2M assays
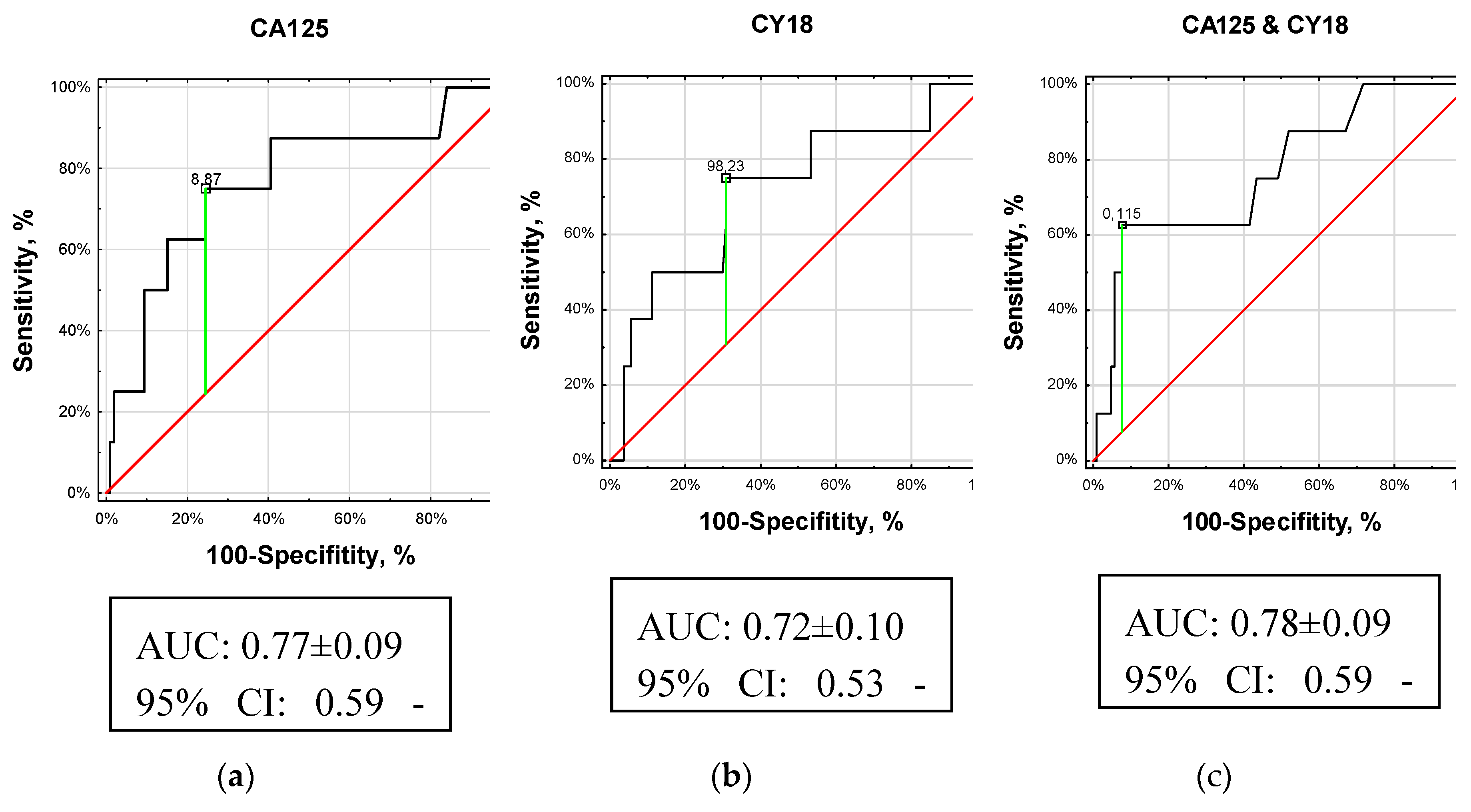
We calculated the AUC and plotted ROC curves to assess the diagnostic value of CBMs in BMets. Given these analyses, three CBMs (CA125, CY18, and B2M ) could differentiate BM-PanNEN patients from non-BM-PanNEN patients (p < 0.05). The accuracy of diagnostic in the patients with BM-PanNEN was 75% for CA125 compared to 70% for CY18 and 53% for B2M.
3.3.1. Cancer antigen 125 (CA125)
The median value of CA125 for the PanNEN patients at the time of bone metastatic disease was 13 U/mL and 6 U/l for those of the non-BMets metastatic group (
Table 2).
The AUC analyses could differentiate PanNEN patients with BMets from PanNEN without BMets (p < 0.01, AUC 0.77 ± 0.09; z score: 2.87, Youden index J: 50%). It should be noted that an AUC of 0.77 would be considered a useful biomarker of BMets (
Figure 2(a)). For the cut-off value of 8.87 U/mL for CA125, the specificity/sensitivity was 76/75%, and the accuracy was similarly 75%.
3.3.2. Cytokeratin 18 (CY18)
The median value of CY18 was 144 U/L for the PanNEN patients with BMets and 62 U/l for those of the non-BMets metastatic group (
Table 2).
The AUC analyses could differentiate PanNEN with BMets from PanNEN without BMets (p = 0.03, AUC 0.72 ± 0.10; z score: 2.22, Youden index J: 44%). It should be noted that an AUC of 0.72 would be considered a fair biomarker of BMets (
Figure 2(b)). For the cut-off value of 98.23 U/l for CY18, the accuracy, sensitivity, and specificity were 70%, 75%, and 69%, respectively.
3.3.3. Combination of CY18 and CA125 (multiROC).
Next, we combined the CA125 and CY18 serum, to construct a further ROC curve. This demonstrated that the serum CA125 and CY18 classifiers had higher accuracy for BMets with a similar AUC of 0.78 (95% CI 0.59–0.95;
Figure 2c) to CA125. Thus, the combination of CA125 and CY18 in serum was similar to individual CA125 distinguishing between PanNEN with BMets and PanNEN without BMets (
Figure 2C). The sensitivity for the cut-off value of 0.12 was calculated as 63%, and the specificity and accuracy were higher at 93%, and 90%, respectively.
The CA125 AUC and CY18 AUC > 0.7 (black curves) indicate they are fair biomarkers for PanNENs with BMets. A maximum AUC = 1 identifies an ideal (perfect) differentiation between these groups. The diagonal red line (AUC = 0.5) corresponds to chance discrimination.
The individual CA125 AUC and combination AUC of CA125 and CY18 were upper than 0.75, which may be clinically helpful biomarkers for distinguishing between PanNEN with BMets and PanNEN without BMets.
3.3.4. Beta-2 microglobulin (B2M)
The median values of B2M for the PanNEN patients with BMets and for those of the non-BMets metastatic group were not significantly different (p > 0.05) (
Table 2).
Although the AUC analyses could differentiate PanNEN with BMets from PanNEN without BMets (p = 0.02), an AUC of 0.67 would be considered a poor biomarker of BMets. Youden index J was 50%. The sensitivity and specificity for the cut-off value 1.16 mg/L were calculated as 100 and 50 %, respectively; accuracy was 53%.
4. Discussion
The most important factor influencing NEN patients’ prognosis is metastasis [
3,
8]. Most frequently, metastases are located in the liver but can also be found in other organs such as the lungs, brain, or bones [
4,
5]. The presence of metastasis is always connected with poor prognosis and worse outcomes. Some studies showed that patients with BMets have shorter survival compared to patients with metastasis in other locations [
4,
9].
We tried to find effective biomarkers that may be useful in the detection of BMets in patients with PanNEN. We analyzed potentially valuable proteins such as ferritin, CA19-9, CA125, AFP, CEA, CK18, and B2M. We revealed that levels of three of their (CA125, CY18, and B2M) were significantly higher in patients with metastatic bone disease than those without BMets.
Serum cytokeratins (CKs) levels are low in healthy individuals. During the process of carcinogenesis, which includes proteolytic degradation in dying cells, abnormal mitosis, and apoptosis, the fragments of CKs are released into the blood, and their level is raised [
13,
19]. As a result, they can be useful as tumor markers and help to predict tumor progression and metastasis formation [
19]. Therefore, our study tried to find the correlation between serum CK18 levels and the probability of BMets in patients with PanNEN. Cytokeratin 18 exhibits overexpression in many types of cancer originating from epithelial organs [
20,
21,
22]. A paper by Menz A. et al. confirmed that issue in adenocarcinomas of the lung, pancreas, small bowel, prostate, and cervix uteri [
20].
Other investigators showed a higher expression of CK18 in Paget’s tumor cells (skin lesions and lymph node metastases). Furthermore, soluble CK18 forms were significantly higher in patients with metastasis compared to non-metastatic disease [
23,
24].
On the other hand, some studies showed a negative correlation between CK18 concentration and disease advancement (the lower CK18 concentrations were related to lymph node metastasis and poor survival in patients with breast cancer) [
25]. A study by Yin B. et al. revealed a negative correlation between serum CK18 level and tumor aggressiveness in prostate cancer [
26].
To our knowledge, serum CK18 levels in PanNEN patients with BMets were not studied. Our study noted a difference in CK 18 serum levels in patients with BMets and without them. Patients with BMets had a higher level of CK18, so it seems to be clinically useful as a diagnostic factor of bone lesions.
We also tried to find the correlation between the CA125 level and the incidence of BMets in patients with PanNEN. Increased CA125 levels can be connected with many malignancies localized in the ovary, breast, liver, lung, pancreas, gastrointestinal tract, uterine, cervix, and endometrium [
27]. Its level can also be elevated in healthy individuals such as women in the follicular phase of the menstrual cycle, during pregnancy [
28], and in non-malignant conditions such as endometriosis, ovarian cysts, pelvic inflammatory disease, cirrhosis, hepatitis, ascites or heart failure [
28,
29,
30]. CA125 has been used so far as a marker of ovarian cancer. It has limited sensitivity in detecting ovarian cancer, but it's helpful in monitoring response to treatment and detecting residual or recurrent disease after therapy. Its level also correlates with staging and tumor size [
31,
32,
33]. Zhang M. et al. proved that CA125 is significantly elevated not only in ovarian cancer but also in lung and pancreatic cancer and decreased in rectal cancer [
34]. In our study, the level of CA125 was significantly higher in patients with BM-PanNEN versus non-BM-PanNEN.
Another correlation we observed in our study is the relation between B2M level and the incidence of BMets in patients with PanNEN. B2M is involved in many important biological processes like regulation of survival, proliferation, and apoptosis [
35,
36]. It also stimulates the growth and progression of several cancers or metastasis in cancer cells. Prizment A. et al. pointed out that higher serum B2M is associated with increased colorectal cancer risk. The authors also suggested a significant association between serum B2M and mortality from total, lung, and hematological cancers [
37]. The elevated level of B2M is supposed to be a strong indicator of poor prognosis and reduced survival. In prostate cancer, studies found that advanced prostate cancer is connected with an increase in serum levels of B2M [
38,
39].
Our study also tried to find a relationship between other biomarkers (ferritin, CA 19-9, AFP, CEA) and the incidence of BMets in patients with PanNEN. The differences between these groups were not statistically significant, so it is possible that in PanNEN these biomarkers have no utility for BMets detection. The useful CBMs for patients with BM-PanNENs detection were Ca125, CY18, and B2M. They seem to have the diagnostic capacity as fair single biomarkers for the detection of BMets. But, the given CBMs measurements performances can not be considered to be adequate for clinical decision-making. However, more studies on larger groups are required because of the small proportion of patients with BMets.
Our study has demonstrated a serum panel of biomarkers (CA125 and CY18) to differentiate PanNEN patients with BMets from PanNEN patients without BMets with good metrics (AUC of 0.78). Indeed, significantly elevated concentrations of these biomarkers in patients with PanNEN may be useful for confirming the clinical suspicion of BMets in cases of diagnostic dilemma (difficulties in CT/MRI scan interpretation).
The use of these markers in clinical practice in our view could be helpful in the interpretation of unclear bone lesions or in screening for further diagnostic workup.
5. Conclusions
The valuable CBMs for BMets detection in patients with PanNEN were CA125, CY18, and B2M. CA125 and CY18 seem to have the diagnostic capacity as fair single biomarkers for the detection of BMets. Other CBMs assessed have not differentiated BM-PanNEN patients from non-BM-PanNEN patients.
6. Study limitations
First, the total sample amount from BM-PanNEN patients was relatively small because PanNEN is rare. Thus, we could not determine a predictive and prognostic value of CBMs for BMets.
Second, the majority of the PanNEN patients were treated before the first presentation of BMets.
Author Contributions
Conceptualization, V.R., M.W. and K.J.; methodology, V.R.; software, V.R.; validation, V.R.; formal analysis, V.R.; investigation, V.R., and M.V.; resources, V.R.; data curation, V.R., and M.W.; writing—original draft preparation, V.R., M.W. and K.J.; writing—review and editing, V.R., K.J. and B.K.K; visualization, V.R.; supervision, V.R.; project administration, V.R.; funding acquisition, V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Silesia in Katowice, grant number: KNW-1-175/N/7/K.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Medical University of Silesia (protocol number KNW/0022/KB1/103/17).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data are available upon any reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kos-Kudła, B.; Rosiek, V.; Borowska, M.; Bednarczuk, T.; Bolanowski, M.; Chmielik, E.; Ćwikła, J.B.; Foltyn, W.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Pancreatic neuroendocrine neoplasms - update of the diagnostic and therapeutic guidelines (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol. 2022, 73, 491–548. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O'Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas. 2020, 49, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Komek, H.; Ansal Balci, T.; Can, C. Efficacy of Galium-68 DOTATATE PET/CT in the Detection of Metastasis Rate of Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors. Asia Ocean J Nucl Med Biol. 2019, 7, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The epidemiology of metastases in neuroendocrine tumors. Int J Cancer. 2016, 139, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Hermans, B.C.M.; de Vos-Geelen, J.; Derks, J.L.; Latten, L.; Liem, I.H.; van der Zwan, J.M.; Speel, E.M.; Dercksen, M.W.; Dingemans, A.C. Unique Metastatic Patterns in Neuroendocrine Neoplasms of Different Primary Origin. Neuroendocrinology. 2021, 111, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.I.; Pizanias, M.; Uri, I.; Thomas, D.; Page, T.; Kolomodi, D.; Low, C.S.; Adesanya, O.; Tsoli, M.; Gross, D.J.; et al. The prognosis and management of neuroendocrine neoplasms-related metastatic bone disease: lessons from clinical practice. Endocrine. 2019, 64, 690–701. [Google Scholar] [CrossRef]
- Garcia-Torralba, E.; Spada, F.; Lim, K.H.J.; Jacobs, T.; Barriuso, J.; Mansoor, W.; McNamara, M.G.; Hubner, R.A.; Manoharan, P.; Fazio, N.; et al. Knowns and unknowns of bone metastases in patients with neuroendocrine neoplasms: A systematic review and meta-analysis. Cancer Treat Rev. 2021, 94, 102168. [Google Scholar] [CrossRef]
- Rizzo, F.M.; Vesely, C.; Childs, A.; Marafioti, T.; Khan, M.S.; Mandair, D.; Cives, M.; Ensell,L. ; Lowe, H.; Akarca, A.U.; et al. Circulating tumour cells and their association with bone metastases in patients with neuroendocrine tumours. Br J Cancer. 2019, 120, 294–300. [Google Scholar] [CrossRef]
- Kavecansky, J.; Wei, L.; Caronia, L.; Ramirez, M.T.; Bloomston, M.; Shah, M.H. Bone metastases in well-to-moderately differentiated neuroendocrine tumors: a single institutional review from the Ohio State University Medical Center. Pancreas. 2015, 44, 198–203. [Google Scholar] [CrossRef]
- Cives, M.; Quaresmini, D.; Rizzo, F.M.; Felici, C.; D'Oronzo, S.; Simone, V.; Silvestris, F. Osteotropism of neuroendocrine tumors: role of the CXCL12/ CXCR4 pathway in promoting EMT in vitro. Oncotarget. 2017, 8, 22534–22549. [Google Scholar] [CrossRef]
- Wang, P.B.; Chen, Y.; Ding, G.R.; Du, H.W.; Fan, H.Y. Keratin 18 induces proliferation, migration, and invasion in gastric cancer via the MAPK signalling pathway. Clin Exp Pharmacol Physiol. 2021, 48, 147–156. [Google Scholar] [CrossRef]
- Singh Bhangu, J.; Macher-Beer, A.; Schimek, V.; Garmroudi, B.; Tamandl, D.; Unger, L.W.; Bachleitner-Hofmann, T.; Oehler, R. Circulating caspase-cleaved cytokeratin 18 correlates with tumour burden and response to therapy in patients with colorectal cancer liver metastasis. Clin Chim Acta. 2023, 538, 53–59. [Google Scholar]
- Eguchi, A.; Iwasa, M.; Tamai, Y.; Yamada, M.; Okuno, K.; Shigefuku, R.; Yoshikawa, K.; Tempaku, M.; Sakaguchi, K.; Tanaka, H.; Sugimoto, K.; Kobayashi, Y.; Yamaguchi, T.; Nakagawa, H. The prognostic potential of fragmented CK18 serum levels in HCC patients reflecting disease progression and overall hepatocyte damage. Front Oncol. 2022, 12, 993705. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Higashi, M.; Sugiyama, H.; Morozumi, M.; Momose, S.; Tamaru, J.I. Cancer antigen 125 expression enhances the gemcitabine/cisplatin-resistant tumor microenvironment in bladder cancer. Am J Pathol. 2022, 22, 00419–9. [Google Scholar] [CrossRef] [PubMed]
- Saad, H.M.; Tourky, G.F.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Khattab, A.M.; Elmasry, S.A.; Alsayegh, A.A.; Hakami, Z.H.; Alsulimani, A.; Sabatier, J.M.; Eid, M.W.; Shaheen, H.M.; Mohammed, A.A.; Batiha, G.E.; De Waard, M. The Potential Role of MUC16 (CA125) Biomarker in Lung Cancer: A Magic Biomarker but with Adversity. Diagnostics (Basel). 2022, 12, 2985. [Google Scholar] [CrossRef]
- Jacobs, I.; Bast, R.C. Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, J.A.; Greipp, P.R.; O'Fallon, W.M.; Kyle, R.A. Serum beta 2-microglobulin. Mayo Clin Proc. 1986, 61, 752–3. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Wei, J. Beta2-microglobulin(B2M) in cancer immunotherapies: Biological function, resistance and remedy. Cancer Lett. 2021, 517, 96–104. [Google Scholar] [CrossRef]
- Linder, S. Cytokeratin markers come of age. Tumour Biol. 2007, 28, 189–95. [Google Scholar] [CrossRef]
- Menz, A.; Weitbrecht, T.; Gorbokon, N.; Büscheck, F.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; Weidemann, S.; et al. Diagnostic and prognostic impact of cytokeratin 18 expression in human tumors: a tissue microarray study on 11,952 tumors. Mol Med. 2021, 27, 16. [Google Scholar] [CrossRef]
- Weng, Y.R.; Cui, Y.; Fang, J.Y. Biological functions of cytokeratin 18 in cancer. Mol Cancer Res. 2012, 10, 485–93. [Google Scholar] [CrossRef] [PubMed]
- Huang. Y.; Yang. L.; Lin, Y.; Chang, X.; Wu, H.; Chen, Y. Prognostic value of non-invasive serum Cytokeratin 18 detection in gastrointestinal cancer: a meta-analysis. J Cancer. 2019, 10, 4814–4823. [Google Scholar] [CrossRef] [PubMed]
- Urano-Takaoka, M.; Sumida, H.; Miyagawa, T.; Awaji, K.; Nagai, K.; Omatsu, J.; Miyake, T.; Sato, S. Serum Cytokeratin 18 as a Metastatic and Therapeutic Marker for Extramammary Paget's Disease. Acta Derm Venereol. 2022, 102, adv00636. [Google Scholar] [CrossRef] [PubMed]
- Tas, F.; Karabulut, S.; Yildiz, I.; Duranyildiz, D. Clinical significance of serum M30 and M65 levels in patients with breast cancer. Biomed Pharmacother. 2014, 68, 1135–40. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wang, C.; Fu, N.; Liu, L.; Zhu, D.; Wei, Z.; Zhang, H.; Xing, J.; Wang, Y. Downregulation of cytokeratin 18 enhances BCRP-mediated multidrug resistance through induction of epithelial-mesenchymal transition and predicts poor prognosis in breast cancer. Oncol Rep. 2019, 41, 3015–3026. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, M.; Zeng, Y.; Li, Y.; Zhang, C.; Getzenberg, R.H.; Song, Y. Downregulation of cytokeratin 18 is associated with paclitaxel-resistance and tumor aggressiveness in prostate cancer. Int J Oncol. 2016, 48, 1730–6, Erratum in: Int J Oncol. 2016, 49, 848. Getzenberg, Robert H [added]. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.L.; Hollingworth, J.; Reynolds, T.M. The role of CA 125 in clinical practice. J Clin Pathol. 2005, 58, 308–12. [Google Scholar] [CrossRef]
- Bast, R.C. , Jr, Xu, F.J.; Yu, Y.H.; Barnhill, S.; Zhang, Z.; Mills, G.B. CA 125: the past and the future. Int J Biol Markers. 1998, 13:179-87. [CrossRef] [PubMed]
- Falcão, F.; de Oliveira, F.R.A.; da Silva, M.C.F.C.; Sobral Filho D,C. Carbohydrate antigen 125: a promising tool for risk stratification in heart diseases. Biomark Med. 2018, 12, 367–381. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Xiubin, Z.; Wei, H.; Chenghao, G. Cancer antigen-125 and ICAM-1 are together responsible for ascites in liver cirrhosis. Clin Lab. 2014, 60, 653–8. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, X.; Liu, X.; Zhu, S. Prognostic Value of Elevated Pre-treatment Serum CA-125 in Epithelial Ovarian Cancer: A Meta-Analysis. Front Oncol. 2022, 12, 868061. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Djedovic, V.; Kjaer, S.K.; Jensen, A.; Glasspool, R.; Roxburgh, P.; DeFazio, A.; Johnatty, S.E.; Webb, P.M.; Modugno, F.; et al. CA-125 Levels Are Predictive of Survival in Low-Grade Serous Ovarian Cancer-A Multicenter Analysis. Cancers (Basel), 2022, 14, 1954. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.N.; Koh, E.Y.; Jang, J.Y.; Kim, C.W. Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening. Obstet Gynecol Sci. 2022, 65, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Fu, J.; Zhang, L. Serum CA125 levels are decreased in rectal cancer but increased in fibrosis-associated diseases and in most types of cancers. Prog Mol Biol Transl Sci. 2019, 162, 241–252. [Google Scholar] [CrossRef]
- Li, L.; Dong, M.; Wang, X.G. The Implication and Significance of Beta 2 Microglobulin: A Conservative Multifunctional Regulator. Chin Med J (Engl). 2016, 129, 448–55. [Google Scholar] [CrossRef] [PubMed]
- Althubiti, M.; Elzubier, M.; Alotaibi, G.S.; Althubaiti, M.A.; Alsadi, H.H.; Alhazmi, Z.A.; Alghamdi, F.; El-Readi, M.Z.; Almaimani. R.; Babakr, A. Beta 2 microglobulin correlates with oxidative stress in elderly. Exp Gerontol. 2021, 150, 111359. [Google Scholar] [CrossRef]
- Prizment, A.E.; Linabery, A.M.; Lutsey, P.L.; Selvin, E.; Nelson, H.H.; Folsom. A.R.; Church, T.R.; Drake, C.G.; Platz, E.A.; Joshu, C. Circulating Beta-2 Microglobulin and Risk of Cancer: The Atherosclerosis Risk in Communities Study (ARIC). Cancer Epidemiol Biomarkers Prev. 2016, 25, 657–64. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, L.; Ji, P.Y.; Zhao, G.G.; Zhong, G.P.; Wang, Z.P. Correlation of serum β2-microglobulin levels with prostate-specific antigen, Gleason score, clinical stage, tumor metastasis and therapy efficacy in prostate cancer. Arch Med Res. 2013, 44, 259–65. [Google Scholar] [CrossRef]
- Mink, S.R.; Hodge. A.; Agus. D.B.; Jain. A.; Gross. M.E. Beta-2-microglobulin expression correlates with high-grade prostate cancer and specific defects in androgen signaling. Prostate. 2010, 70, 1201–10. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).