Submitted:
24 May 2023
Posted:
26 May 2023
You are already at the latest version
Abstract
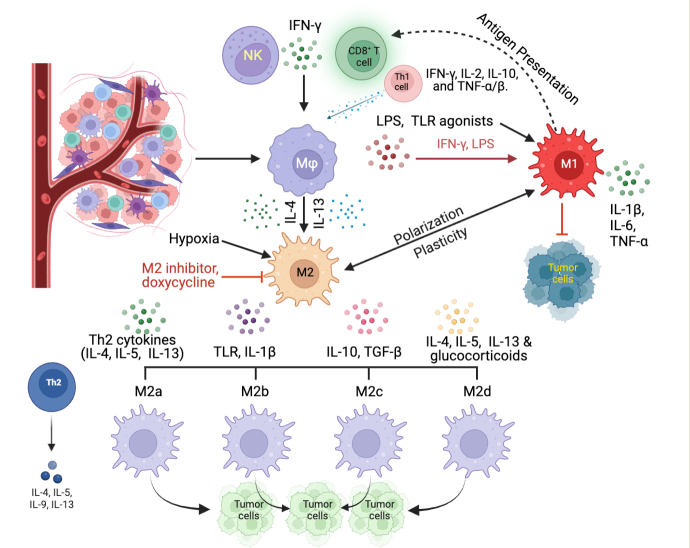
Keywords:
1. Introduction
2. TAMs in Cancer Progression
2.1. Classification of Macrophages
2.2. Pro-tumor functions of TAMs
2.3. Anti-tumor functions of TAMs
3. Regulatory Signaling Pathways of TAMs in the TME
3.1. Regulation of TAM phenotype and function
3.2. Crosstalk between TAMs and other cell types in the TME
3.3. The factors in TME affecting the polarization of TAM
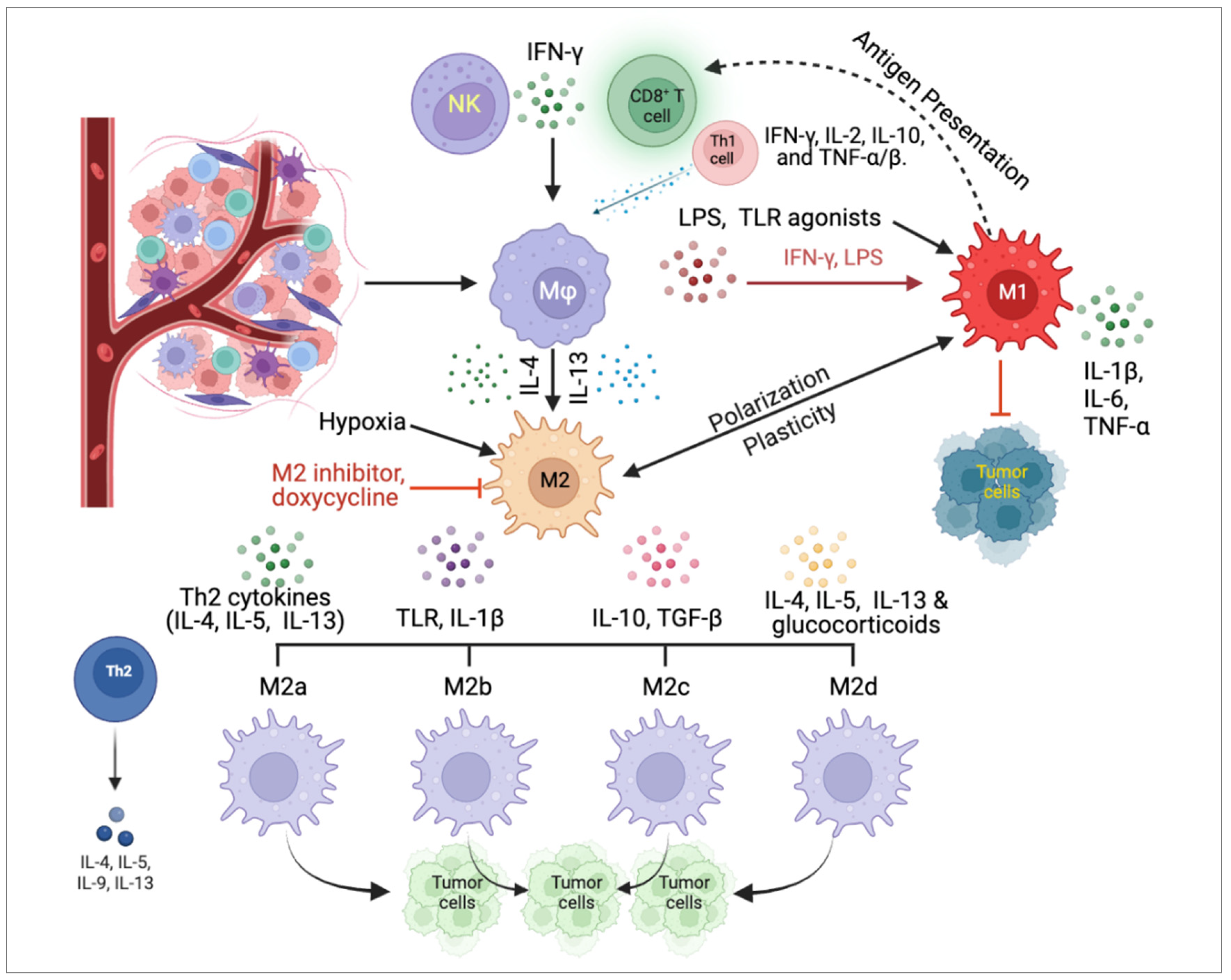
4. Challenges in Clinical Applications of TAMs
4.1. Heterogeneity and plasticity of TAMs
4.2. Limited efficacy of TAM-targeted therapies
4.3. Lack of reliable biomarkers for TAM identification and characterization
5. Standardizing TAM Isolation Protocols
6. Clinical Trials Involving TAMs
7. Conclusions
8. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Guo, N.; Zhou, Y.; Chen, J.; Wei, Q.; Han, M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B 2020, 10, 2156-2170. [CrossRef]
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, S.V. The Role of Tumor Associated Macrophages (TAMs) in Cancer Progression, Chemoresistance, Angiogenesis and Metastasis - Current Status. Curr Med Chem 2021, 28, 8203-8236. [CrossRef]
- Zhang, J.; Gao, J.; Cui, J.; Wang, Y.; Jin, Y.; Zhang, D.; Lin, D.; Lin, J. Tumor-associated macrophages in tumor progression and the role of traditional Chinese medicine in regulating TAMs to enhance antitumor effects. Front Immunol 2022, 13, 1026898. [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49-61. [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front Immunol 2020, 11, 583084. [CrossRef]
- Schupp, J.; Krebs, F.K.; Zimmer, N.; Trzeciak, E.; Schuppan, D.; Tuettenberg, A. Targeting myeloid cells in the tumor sustaining microenvironment. Cell Immunol 2019, 343, 103713. [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front Immunol 2020, 11, 1731. [CrossRef]
- Xiang, X.; Wang, J.; Lu, D.; Xu, X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther 2021, 6, 75. [CrossRef]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-Associated Macrophages: A Potential Target for Cancer Therapy. Front Oncol 2021, 11, 693517. [CrossRef]
- Li, M.; He, L.; Zhu, J.; Zhang, P.; Liang, S. Targeting tumor-associated macrophages for cancer treatment. Cell Biosci 2022, 12, 85. [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov 2022, 21, 799-820. [CrossRef]
- Liu, M.; Yang, J.; Xu, B.; Zhang, X. Tumor metastasis: Mechanistic insights and therapeutic interventions. MedComm (2020) 2021, 2, 587-617. [CrossRef]
- Kumari, N.; Choi, S.H. Tumor-associated macrophages in cancer: Recent advancements in cancer nanoimmunotherapies. J Exp Clin Cancer Res 2022, 41, 68. [CrossRef]
- Richards, D.M.; Hettinger, J.; Feuerer, M. Monocytes and macrophages in cancer: Development and functions. Cancer Microenviron 2013, 6, 179-191. [CrossRef]
- Lin, Y.; Xu, J.; Lan, H. Tumor-associated macrophages in tumor metastasis: Biological roles and clinical therapeutic applications. J Hematol Oncol 2019, 12, 76. [CrossRef]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab 2019, 30, 36-50. [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol Cancer 2023, 22, 48. [CrossRef]
- Li, C.; Xu, X.; Wei, S.; Jiang, P.; Xue, L.; Wang, J.; Senior, C. Tumor-associated macrophages: Potential therapeutic strategies and future prospects in cancer. J Immunother Cancer 2021, 9. [CrossRef]
- Petty, A.J.; Owen, D.H.; Yang, Y.; Huang, X. Targeting Tumor-Associated Macrophages in Cancer Immunotherapy. Cancers (Basel) 2021, 13. [CrossRef]
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’Brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front Oncol 2021, 11, 788365. [CrossRef]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 2017, 14, 399-416. [CrossRef]
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int J Mol Sci 2021, 22. [CrossRef]
- Udeabor, S.E.; Adisa, A.O.; Orlowska, A.; Sader, R.A.; Ghanaati, S. Tumor-associated macrophages, angiogenesis, and tumor cell migration in oral squamous cell carcinoma. Ann Afr Med 2017, 16, 181-185. [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol 2014, 5, 514. [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int J Mol Sci 2018, 19. [CrossRef]
- Liu, J.; Geng, X.; Hou, J.; Wu, G. New insights into M1/M2 macrophages: Key modulators in cancer progression. Cancer Cell Int 2021, 21, 389. [CrossRef]
- Aminin, D.; Wang, Y.M. Macrophages as a “weapon” in anticancer cellular immunotherapy. Kaohsiung J Med Sci 2021, 37, 749-758. [CrossRef]
- Reis-Sobreiro, M.; Teixeira da Mota, A.; Jardim, C.; Serre, K. Bringing Macrophages to the Frontline against Cancer: Current Immunotherapies Targeting Macrophages. Cells 2021, 10. [CrossRef]
- Duan, Z.; Luo, Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther 2021, 6, 127. [CrossRef]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schroder, C.P. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat Rev 2018, 70, 178-189. [CrossRef]
- Wang, H.W.; Joyce, J.A. Alternative activation of tumor-associated macrophages by IL-4: Priming for protumoral functions. Cell Cycle 2010, 9, 4824-4835. [CrossRef]
- Boutilier, A.J.; Elsawa, S.F. Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci 2021, 22. [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front Immunol 2019, 10, 792. [CrossRef]
- Wang, L.X.; Zhang, S.X.; Wu, H.J.; Rong, X.L.; Guo, J. M2b macrophage polarization and its roles in diseases. J Leukoc Biol 2019, 106, 345-358. [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186-195. [CrossRef]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol 2012, 189, 3508-3520. [CrossRef]
- Ferrante, C.J.; Pinhal-Enfield, G.; Elson, G.; Cronstein, B.N.; Hasko, G.; Outram, S.; Leibovich, S.J. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation 2013, 36, 921-931. [CrossRef]
- Klaver, D.; Thurnher, M. Control of Macrophage Inflammation by P2Y Purinergic Receptors. Cells 2021, 10. [CrossRef]
- Xu, Y.; Wang, X.; Liu, L.; Wang, J.; Wu, J.; Sun, C. Role of macrophages in tumor progression and therapy (Review). Int J Oncol 2022, 60. [CrossRef]
- Wang, X.; Wu, Y.; Gu, J.; Xu, J. Tumor-associated macrophages in lung carcinoma: From mechanism to therapy. Pathol Res Pract 2022, 229, 153747. [CrossRef]
- Lichtnekert, J.; Kawakami, T.; Parks, W.C.; Duffield, J.S. Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol 2013, 13, 555-564. [CrossRef]
- Kohno, K.; Koya-Miyata, S.; Harashima, A.; Tsukuda, T.; Katakami, M.; Ariyasu, T.; Ushio, S.; Iwaki, K. Inflammatory M1-like macrophages polarized by NK-4 undergo enhanced phenotypic switching to an anti-inflammatory M2-like phenotype upon co-culture with apoptotic cells. J Inflamm (Lond) 2021, 18, 2. [CrossRef]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit Rev Immunol 2012, 32, 463-488. [CrossRef]
- Gao, J.; Liang, Y.; Wang, L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front Immunol 2022, 13, 888713. [CrossRef]
- Feng, Y.; Ye, Z.; Song, F.; He, Y.; Liu, J. The Role of TAMs in Tumor Microenvironment and New Research Progress. Stem Cells Int 2022, 2022, 5775696. [CrossRef]
- Zhu, S.; Luo, Z.; Li, X.; Han, X.; Shi, S.; Zhang, T. Tumor-associated macrophages: Role in tumorigenesis and immunotherapy implications. J Cancer 2021, 12, 54-64. [CrossRef]
- Larionova, I.; Kazakova, E.; Gerashchenko, T.; Kzhyshkowska, J. New Angiogenic Regulators Produced by TAMs: Perspective for Targeting Tumor Angiogenesis. Cancers (Basel) 2021, 13. [CrossRef]
- Lu, C.; Liu, Y.; Ali, N.M.; Zhang, B.; Cui, X. The role of innate immune cells in the tumor microenvironment and research progress in anti-tumor therapy. Front Immunol 2022, 13, 1039260. [CrossRef]
- Afik, R.; Zigmond, E.; Vugman, M.; Klepfish, M.; Shimshoni, E.; Pasmanik-Chor, M.; Shenoy, A.; Bassat, E.; Halpern, Z.; Geiger, T.; et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med 2016, 213, 2315-2331. [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int J Mol Sci 2021, 23. [CrossRef]
- Quintero-Fabian, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argaez, V.; Lara-Riegos, J.; Ramirez-Camacho, M.A.; Alvarez-Sanchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front Oncol 2019, 9, 1370. [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun 2020, 11, 5120. [CrossRef]
- Foster, D.S.; Jones, R.E.; Ransom, R.C.; Longaker, M.T.; Norton, J.A. The evolving relationship of wound healing and tumor stroma. JCI Insight 2018, 3. [CrossRef]
- Marangio, A.; Biccari, A.; D’Angelo, E.; Sensi, F.; Spolverato, G.; Pucciarelli, S.; Agostini, M. The Study of the Extracellular Matrix in Chronic Inflammation: A Way to Prevent Cancer Initiation? Cancers (Basel) 2022, 14. [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J Biomed Sci 2019, 26, 78. [CrossRef]
- Butti, R.; Khaladkar, A.; Bhardwaj, P.; Prakasam, G. Heterotypic signaling of cancer-associated fibroblasts in shaping the cancer cell drug resistance. Cancer Drug Resist 2023, 6, 182-204. [CrossRef]
- Gao, Y.; Zhou, H.; Liu, G.; Wu, J.; Yuan, Y.; Shang, A. Tumor Microenvironment: Lactic Acid Promotes Tumor Development. J Immunol Res 2022, 2022, 3119375. [CrossRef]
- Wang, Z.H.; Peng, W.B.; Zhang, P.; Yang, X.P.; Zhou, Q. Lactate in the tumour microenvironment: From immune modulation to therapy. EBioMedicine 2021, 73, 103627. [CrossRef]
- Jing, H.; Wu, X.; Xiang, M.; Wang, C.; Novakovic, V.A.; Shi, J. Microparticle Phosphatidylserine Mediates Coagulation: Involvement in Tumor Progression and Metastasis. Cancers (Basel) 2023, 15. [CrossRef]
- Bejarano, L.; Jordao, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov 2021, 11, 933-959. [CrossRef]
- Wang, J.; Li, D.; Cang, H.; Guo, B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med 2019, 8, 4709-4721. [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res 2019, 79, 4557-4566. [CrossRef]
- Davis, R.J.; Van Waes, C.; Allen, C.T. Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol 2016, 58, 59-70. [CrossRef]
- Yang, Y.; Li, C.; Liu, T.; Dai, X.; Bazhin, A.V. Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation. Front Immunol 2020, 11, 1371. [CrossRef]
- Zhang, Z.; Liu, S.; Zhang, B.; Qiao, L.; Zhang, Y.; Zhang, Y. T Cell Dysfunction and Exhaustion in Cancer. Front Cell Dev Biol 2020, 8, 17. [CrossRef]
- Pu, Y.; Ji, Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front Immunol 2022, 13, 874589. [CrossRef]
- Laviron, M.; Petit, M.; Weber-Delacroix, E.; Combes, A.J.; Arkal, A.R.; Barthelemy, S.; Courau, T.; Hume, D.A.; Combadiere, C.; Krummel, M.F.; et al. Tumor-associated macrophage heterogeneity is driven by tissue territories in breast cancer. Cell Rep 2022, 39, 110865. [CrossRef]
- Li, S.; Yu, J.; Huber, A.; Kryczek, I.; Wang, Z.; Jiang, L.; Li, X.; Du, W.; Li, G.; Wei, S.; et al. Metabolism drives macrophage heterogeneity in the tumor microenvironment. Cell Rep 2022, 39, 110609. [CrossRef]
- Cassetta, L.; Noy, R.; Swierczak, A.; Sugano, G.; Smith, H.; Wiechmann, L.; Pollard, J.W. Isolation of Mouse and Human Tumor-Associated Macrophages. Adv Exp Med Biol 2016, 899, 211-229. [CrossRef]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front Oncol 2019, 9, 1512. [CrossRef]
- Lecoultre, M.; Dutoit, V.; Walker, P.R. Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: A review. J Immunother Cancer 2020, 8. [CrossRef]
- Lee, C.C.; Lin, J.C.; Hwang, W.L.; Kuo, Y.J.; Chen, H.K.; Tai, S.K.; Lin, C.C.; Yang, M.H. Macrophage-secreted interleukin-35 regulates cancer cell plasticity to facilitate metastatic colonization. Nat Commun 2018, 9, 3763. [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers (Basel) 2021, 13. [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct Target Ther 2021, 6, 263. [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer vaccines as promising immuno-therapeutics: Platforms and current progress. J Hematol Oncol 2022, 15, 28. [CrossRef]
- Huang, Y.; Ge, W.; Zhou, J.; Gao, B.; Qian, X.; Wang, W. The Role of Tumor Associated Macrophages in Hepatocellular Carcinoma. J Cancer 2021, 12, 1284-1294. [CrossRef]
- Xu, W.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Targeting tumor associated macrophages in hepatocellular carcinoma. Biochem Pharmacol 2022, 199, 114990. [CrossRef]
- Beltraminelli, T.; De Palma, M. Biology and therapeutic targeting of tumour-associated macrophages. J Pathol 2020, 250, 573-592. [CrossRef]
- Ge, Z.; Ding, S. The Crosstalk Between Tumor-Associated Macrophages (TAMs) and Tumor Cells and the Corresponding Targeted Therapy. Front Oncol 2020, 10, 590941. [CrossRef]
- Hartley, G.P.; Chow, L.; Ammons, D.T.; Wheat, W.H.; Dow, S.W. Programmed Cell Death Ligand 1 (PD-L1) Signaling Regulates Macrophage Proliferation and Activation. Cancer Immunol Res 2018, 6, 1260-1273. [CrossRef]
- Zhao, W.; Hu, X.; Li, W.; Li, R.; Chen, J.; Zhou, L.; Qiang, S.; Wu, W.; Shi, S.; Dong, C. M2-Like TAMs Function Reversal Contributes to Breast Cancer Eradication by Combination Dual Immune Checkpoint Blockade and Photothermal Therapy. Small 2021, 17, e2007051. [CrossRef]
- Liu, T.; Zhu, C.; Chen, X.; Guan, G.; Zou, C.; Shen, S.; Wu, J.; Wang, Y.; Lin, Z.; Chen, L.; et al. Ferroptosis, as the most enriched programmed cell death process in glioma, induces immunosuppression and immunotherapy resistance. Neuro Oncol 2022, 24, 1113-1125. [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol 2014, 4, 64. [CrossRef]
- Wu, D.; Liu, X.; Mu, J.; Yang, J.; Wu, F.; Zhou, H. Therapeutic Approaches Targeting Proteins in Tumor-Associated Macrophages and Their Applications in Cancers. Biomolecules 2022, 12. [CrossRef]
- Cheng, Y.; Song, S.; Wu, P.; Lyu, B.; Qin, M.; Sun, Y.; Sun, A.; Mu, L.; Xu, F.; Zhang, L.; et al. Tumor Associated Macrophages and TAMs-Based Anti-Tumor Nanomedicines. Adv Healthc Mater 2021, 10, e2100590. [CrossRef]
- Zhang, S.Y.; Song, X.Y.; Li, Y.; Ye, L.L.; Zhou, Q.; Yang, W.B. Tumor-associated macrophages: A promising target for a cancer immunotherapeutic strategy. Pharmacol Res 2020, 161, 105111. [CrossRef]
- Monnier, M.; Paolini, L.; Vinatier, E.; Mantovani, A.; Delneste, Y.; Jeannin, P. Antitumor strategies targeting macrophages: The importance of considering the differences in differentiation/polarization processes between human and mouse macrophages. J Immunother Cancer 2022, 10. [CrossRef]
- Crezee, T.; Rabold, K.; de Jong, L.; Jaeger, M.; Netea-Maier, R.T. Metabolic programming of tumor associated macrophages in the context of cancer treatment. Ann Transl Med 2020, 8, 1028. [CrossRef]
- Gunaydin, G. CAFs Interacting With TAMs in Tumor Microenvironment to Enhance Tumorigenesis and Immune Evasion. Front Oncol 2021, 11, 668349. [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med 2020, 52, 1475-1485. [CrossRef]
- Gambardella, V.; Castillo, J.; Tarazona, N.; Gimeno-Valiente, F.; Martinez-Ciarpaglini, C.; Cabeza-Segura, M.; Rosello, S.; Roda, D.; Huerta, M.; Cervantes, A.; et al. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat Rev 2020, 86, 102015. [CrossRef]
- Balazova, K.; Clevers, H.; Dost, A.F.M. The role of macrophages in non-small cell lung cancer and advancements in 3D co-cultures. Elife 2023, 12. [CrossRef]
- Bai, R.; Li, Y.; Jian, L.; Yang, Y.; Zhao, L.; Wei, M. The hypoxia-driven crosstalk between tumor and tumor-associated macrophages: Mechanisms and clinical treatment strategies. Mol Cancer 2022, 21, 177. [CrossRef]
- Raskov, H.; Orhan, A.; Gaggar, S.; Gogenur, I. Cancer-Associated Fibroblasts and Tumor-Associated Macrophages in Cancer and Cancer Immunotherapy. Front Oncol 2021, 11, 668731. [CrossRef]
- Riera-Domingo, C.; Audige, A.; Granja, S.; Cheng, W.C.; Ho, P.C.; Baltazar, F.; Stockmann, C.; Mazzone, M. Immunity, Hypoxia, and Metabolism-the Menage a Trois of Cancer: Implications for Immunotherapy. Physiol Rev 2020, 100, 1-102. [CrossRef]
- Kolesnikoff, N.; Chen, C.H.; Samuel, M.S. Interrelationships between the extracellular matrix and the immune microenvironment that govern epithelial tumour progression. Clin Sci (Lond) 2022, 136, 361-377. [CrossRef]
- Hao, N.B.; Lu, M.H.; Fan, Y.H.; Cao, Y.L.; Zhang, Z.R.; Yang, S.M. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012, 2012, 948098. [CrossRef]
- Lv, C.; Li, S.; Zhao, J.; Yang, P.; Yang, C. M1 Macrophages Enhance Survival and Invasion of Oral Squamous Cell Carcinoma by Inducing GDF15-Mediated ErbB2 Phosphorylation. ACS Omega 2022, 7, 11405-11414. [CrossRef]
- de Groot, A.E.; Myers, K.V.; Krueger, T.E.G.; Brennen, W.N.; Amend, S.R.; Pienta, K.J. Targeting interleukin 4 receptor alpha on tumor-associated macrophages reduces the pro-tumor macrophage phenotype. Neoplasia 2022, 32, 100830. [CrossRef]
- Zhang, M.Z.; Wang, X.; Wang, Y.; Niu, A.; Wang, S.; Zou, C.; Harris, R.C. IL-4/IL-13-mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int 2017, 91, 375-386. [CrossRef]
- Little, A.C.; Pathanjeli, P.; Wu, Z.; Bao, L.; Goo, L.E.; Yates, J.A.; Oliver, C.R.; Soellner, M.B.; Merajver, S.D. IL-4/IL-13 Stimulated Macrophages Enhance Breast Cancer Invasion Via Rho-GTPase Regulation of Synergistic VEGF/CCL-18 Signaling. Front Oncol 2019, 9, 456. [CrossRef]
- He, L.; Jhong, J.H.; Chen, Q.; Huang, K.Y.; Strittmatter, K.; Kreuzer, J.; DeRan, M.; Wu, X.; Lee, T.Y.; Slavov, N.; et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep 2021, 37, 109955. [CrossRef]
- Wu, L.; Kohno, M.; Murakami, J.; Zia, A.; Allen, J.; Yun, H.; Chan, M.; Baciu, C.; Liu, M.; Serre-Beinier, V.; et al. Defining and targeting tumor-associated macrophages in malignant mesothelioma. Proc Natl Acad Sci U S A 2023, 120, e2210836120. [CrossRef]
- He, Z.; Zhang, S. Tumor-Associated Macrophages and Their Functional Transformation in the Hypoxic Tumor Microenvironment. Front Immunol 2021, 12, 741305. [CrossRef]
- Zhu, L.; Zhu, X.; Wu, Y. Effects of Glucose Metabolism, Lipid Metabolism, and Glutamine Metabolism on Tumor Microenvironment and Clinical Implications. Biomolecules 2022, 12. [CrossRef]
- Bader, J.E.; Voss, K.; Rathmell, J.C. Targeting Metabolism to Improve the Tumor Microenvironment for Cancer Immunotherapy. Mol Cell 2020, 78, 1019-1033. [CrossRef]
- Deligne, C.; Midwood, K.S. Macrophages and Extracellular Matrix in Breast Cancer: Partners in Crime or Protective Allies? Front Oncol 2021, 11, 620773. [CrossRef]
- Mun, J.Y.; Leem, S.H.; Lee, J.H.; Kim, H.S. Dual Relationship Between Stromal Cells and Immune Cells in the Tumor Microenvironment. Front Immunol 2022, 13, 864739. [CrossRef]
- Pernot, S.; Evrard, S.; Khatib, A.M. The Give-and-Take Interaction Between the Tumor Microenvironment and Immune Cells Regulating Tumor Progression and Repression. Front Immunol 2022, 13, 850856. [CrossRef]
- Fang, L.; Liu, K.; Liu, C.; Wang, X.; Ma, W.; Xu, W.; Wu, J.; Sun, C. Tumor accomplice: T cell exhaustion induced by chronic inflammation. Front Immunol 2022, 13, 979116. [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol Cancer 2021, 20, 131. [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol Life Sci 2020, 77, 1745-1770. [CrossRef]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther 2021, 6, 362. [CrossRef]
- Tumino, N.; Fiore, P.F.; Pelosi, A.; Moretta, L.; Vacca, P. Myeloid derived suppressor cells in tumor microenvironment: Interaction with innate lymphoid cells. Semin Immunol 2022, 61-64, 101668. [CrossRef]
- Zou, Z.; Lin, H.; Li, M.; Lin, B. Tumor-associated macrophage polarization in the inflammatory tumor microenvironment. Front Oncol 2023, 13, 1103149. [CrossRef]
- Wang, B.; Zhao, Q.; Zhang, Y.; Liu, Z.; Zheng, Z.; Liu, S.; Meng, L.; Xin, Y.; Jiang, X. Targeting hypoxia in the tumor microenvironment: A potential strategy to improve cancer immunotherapy. J Exp Clin Cancer Res 2021, 40, 24. [CrossRef]
- He, L.; Marneros, A.G. Doxycycline inhibits polarization of macrophages to the proangiogenic M2-type and subsequent neovascularization. J Biol Chem 2014, 289, 8019-8028. [CrossRef]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front Mol Biosci 2019, 6, 160. [CrossRef]
- Jurj, A.; Ionescu, C.; Berindan-Neagoe, I.; Braicu, C. The extracellular matrix alteration, implication in modulation of drug resistance mechanism: Friends or foes? J Exp Clin Cancer Res 2022, 41, 276. [CrossRef]
- Hasan, M.N.; Capuk, O.; Patel, S.M.; Sun, D. The Role of Metabolic Plasticity of Tumor-Associated Macrophages in Shaping the Tumor Microenvironment Immunity. Cancers (Basel) 2022, 14. [CrossRef]
- Liu, J.; Cao, X. Glucose metabolism of TAMs in tumor chemoresistance and metastasis. Trends Cell Biol 2023. [CrossRef]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588-602 e510. [CrossRef]
- Lopez-Yrigoyen, M.; Cassetta, L.; Pollard, J.W. Macrophage targeting in cancer. Ann N Y Acad Sci 2021, 1499, 18-41. [CrossRef]
- Zhang, X.M.; Chen, D.G.; Li, S.C.; Zhu, B.; Li, Z.J. Embryonic Origin and Subclonal Evolution of Tumor-Associated Macrophages Imply Preventive Care for Cancer. Cells 2021, 10. [CrossRef]
- Niu, Y.; Chen, J.; Qiao, Y. Epigenetic Modifications in Tumor-Associated Macrophages: A New Perspective for an Old Foe. Front Immunol 2022, 13, 836223. [CrossRef]
- Ma, R.Y.; Black, A.; Qian, B.Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol 2022, 43, 546-563. [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal 2020, 18, 59. [CrossRef]
- Hass, R.; von der Ohe, J.; Ungefroren, H. Impact of the Tumor Microenvironment on Tumor Heterogeneity and Consequences for Cancer Cell Plasticity and Stemness. Cancers (Basel) 2020, 12. [CrossRef]
- Truxova, I.; Cibula, D.; Spisek, R.; Fucikova, J. Targeting tumor-associated macrophages for successful immunotherapy of ovarian carcinoma. J Immunother Cancer 2023, 11. [CrossRef]
- Buonfiglioli, A.; Hambardzumyan, D. Macrophages and microglia: The cerberus of glioblastoma. Acta Neuropathol Commun 2021, 9, 54. [CrossRef]
- Malfitano, A.M.; Pisanti, S.; Napolitano, F.; Di Somma, S.; Martinelli, R.; Portella, G. Tumor-Associated Macrophage Status in Cancer Treatment. Cancers (Basel) 2020, 12. [CrossRef]
- Shen, X.; Zhou, S.; Yang, Y.; Hong, T.; Xiang, Z.; Zhao, J.; Zhu, C.; Zeng, L.; Zhang, L. TAM-targeted reeducation for enhanced cancer immunotherapy: Mechanism and recent progress. Front Oncol 2022, 12, 1034842. [CrossRef]
- Wang, D.R.; Wu, X.L.; Sun, Y.L. Therapeutic targets and biomarkers of tumor immunotherapy: Response versus non-response. Signal Transduct Target Ther 2022, 7, 331. [CrossRef]
- Anderson, K.G.; Stromnes, I.M.; Greenberg, P.D. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer Cell 2017, 31, 311-325. [CrossRef]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer 2019, 18, 157. [CrossRef]
- Zhan, C.; Jin, Y.; Xu, X.; Shao, J.; Jin, C. Antitumor therapy for breast cancer: Focus on tumor-associated macrophages and nanosized drug delivery systems. Cancer Med 2023. [CrossRef]
- Cheng, K.; Cai, N.; Zhu, J.; Yang, X.; Liang, H.; Zhang, W. Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun (Lond) 2022, 42, 1112-1140. [CrossRef]
- Lasagna, A.; Muzzana, M.; Ferretti, V.V.; Klersy, C.; Pagani, A.; Cicognini, D.; Pedrazzoli, P.; Brugnatelli, S.G. The Role of Pre-treatment Inflammatory Biomarkers in the Prediction of an Early Response to Panitumumab in Metastatic Colorectal Cancer. Cureus 2022, 14, e24347. [CrossRef]
- Brady, R.V.; Thamm, D.H. Tumor-associated macrophages: Prognostic and therapeutic targets for cancer in humans and dogs. Front Immunol 2023, 14, 1176807. [CrossRef]
- Li, X.; Wang, R.; Zhang, Y.; Han, S.; Gan, Y.; Liang, Q.; Ma, X.; Rong, P.; Wang, W.; Li, W. Molecular imaging of tumor-associated macrophages in cancer immunotherapy. Ther Adv Med Oncol 2022, 14, 17588359221076194. [CrossRef]
- Hu, J.; Xu, X.; Du, Y. Targeting Tumor-Associated Macrophages for Imaging. Pharmaceutics 2022, 15. [CrossRef]
- Fernandes, B.; Feltes, P.K.; Luft, C.; Nazario, L.R.; Jeckel, C.M.M.; Antunes, I.F.; Elsinga, P.H.; de Vries, E.F.J. Potential PET tracers for imaging of tumor-associated macrophages. EJNMMI Radiopharm Chem 2022, 7, 11. [CrossRef]
- George, S.; Georgouli, M.; Sanz-Moreno, V. Protocol to drive human monocyte-to-macrophage polarization in vitro using tumor conditioned media. STAR Protoc 2022, 3, 101666. [CrossRef]
- Madsen, D.H.; Jurgensen, H.J.; Siersbaek, M.S.; Kuczek, D.E.; Grey Cloud, L.; Liu, S.; Behrendt, N.; Grontved, L.; Weigert, R.; Bugge, T.H. Tumor-Associated Macrophages Derived from Circulating Inflammatory Monocytes Degrade Collagen through Cellular Uptake. Cell Rep 2017, 21, 3662-3671. [CrossRef]
- Haque, A.; Moriyama, M.; Kubota, K.; Ishiguro, N.; Sakamoto, M.; Chinju, A.; Mochizuki, K.; Sakamoto, T.; Kaneko, N.; Munemura, R.; et al. CD206(+) tumor-associated macrophages promote proliferation and invasion in oral squamous cell carcinoma via EGF production. Sci Rep 2019, 9, 14611. [CrossRef]
- Modak, M.; Mattes, A.K.; Reiss, D.; Skronska-Wasek, W.; Langlois, R.; Sabarth, N.; Konopitzky, R.; Ramirez, F.; Lehr, K.; Mayr, T.; et al. CD206+ tumor-associated macrophages cross-present tumor antigen and drive antitumor immunity. JCI Insight 2022, 7. [CrossRef]
- Bennett, S.; Breit, S.N. Variables in the isolation and culture of human monocytes that are of particular relevance to studies of HIV. J Leukoc Biol 1994, 56, 236-240. [CrossRef]
- Kubala, M.H.; DeClerck, Y.A. Conditional Knockdown of Gene Expression in Cancer Cell Lines to Study the Recruitment of Monocytes/Macrophages to the Tumor Microenvironment. J Vis Exp 2017. [CrossRef]
- Zhang, X.; Zeng, Y.; Qu, Q.; Zhu, J.; Liu, Z.; Ning, W.; Zeng, H.; Zhang, N.; Du, W.; Chen, C.; et al. PD-L1 induced by IFN-gamma from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer. Int J Clin Oncol 2017, 22, 1026-1033. [CrossRef]
- Massalha, H.; Bahar Halpern, K.; Abu-Gazala, S.; Jana, T.; Massasa, E.E.; Moor, A.E.; Buchauer, L.; Rozenberg, M.; Pikarsky, E.; Amit, I.; et al. A single cell atlas of the human liver tumor microenvironment. Mol Syst Biol 2020, 16, e9682. [CrossRef]
- Subramanian, S.; Busch, C.J.; Molawi, K.; Geirsdottir, L.; Maurizio, J.; Vargas Aguilar, S.; Belahbib, H.; Gimenez, G.; Yuda, R.A.A.; Burkon, M.; et al. Long-term culture-expanded alveolar macrophages restore their full epigenetic identity after transfer in vivo. Nat Immunol 2022, 23, 458-468. [CrossRef]
- Tsubaki, T.; Kadonosono, T.; Sakurai, S.; Shiozawa, T.; Goto, T.; Sakai, S.; Kuchimaru, T.; Sakamoto, T.; Watanabe, H.; Kondoh, G.; et al. Novel adherent CD11b(+) Gr-1(+) tumor-infiltrating cells initiate an immunosuppressive tumor microenvironment. Oncotarget 2018, 9, 11209-11226. [CrossRef]
- Wang, X.; Shen, Y.; MengLv, L.; Zhang, X.; Yang, J.; Wang, F.; Yang, J. Thalidomide suppresses breast cancer tumor growth by inhibiting tumor-associated macrophage accumulation in breast tumor-bearing mice. Eur J Pharm Sci 2020, 151, 105302. [CrossRef]
- Clark, N.M.; Bos, P.D. Tumor-Associated Macrophage Isolation and In Vivo Analysis of Their Tumor-Promoting Activity. Methods Mol Biol 2019, 1884, 151-160. [CrossRef]
- Rodrigues, J.; Heinrich, M.A.; Teixeira, L.M.; Prakash, J. 3D In Vitro Model (R)evolution: Unveiling Tumor-Stroma Interactions. Trends Cancer 2021, 7, 249-264. [CrossRef]
- Barca, C.; Foray, C.; Hermann, S.; Herrlinger, U.; Remory, I.; Laoui, D.; Schafers, M.; Grauer, O.M.; Zinnhardt, B.; Jacobs, A.H. The Colony Stimulating Factor-1 Receptor (CSF-1R)-Mediated Regulation of Microglia/Macrophages as a Target for Neurological Disorders (Glioma, Stroke). Front Immunol 2021, 12, 787307. [CrossRef]
- Zhu, M.; Bai, L.; Liu, X.; Peng, S.; Xie, Y.; Bai, H.; Yu, H.; Wang, X.; Yuan, P.; Ma, R.; et al. Silence of a dependence receptor CSF1R in colorectal cancer cells activates tumor-associated macrophages. J Immunother Cancer 2022, 10. [CrossRef]
- Benner, B.; Good, L.; Quiroga, D.; Schultz, T.E.; Kassem, M.; Carson, W.E.; Cherian, M.A.; Sardesai, S.; Wesolowski, R. Pexidartinib, a Novel Small Molecule CSF-1R Inhibitor in Use for Tenosynovial Giant Cell Tumor: A Systematic Review of Pre-Clinical and Clinical Development. Drug Des Devel Ther 2020, 14, 1693-1704. [CrossRef]
- Johnson, M.; Dudek, A.Z.; Sukari, A.; Call, J.; Kunk, P.R.; Lewis, K.; Gainor, J.F.; Sarantopoulos, J.; Lee, P.; Golden, A.; et al. ARRY-382 in Combination with Pembrolizumab in Patients with Advanced Solid Tumors: Results from a Phase 1b/2 Study. Clin Cancer Res 2022, 28, 2517-2526. [CrossRef]
- Belli, C.; Antonarelli, G.; Repetto, M.; Boscolo Bielo, L.; Crimini, E.; Curigliano, G. Targeting Cellular Components of the Tumor Microenvironment in Solid Malignancies. Cancers (Basel) 2022, 14. [CrossRef]
- Yan, S.; Wan, G. Tumor-associated macrophages in immunotherapy. FEBS J 2021, 288, 6174-6186. [CrossRef]
- Genard, G.; Lucas, S.; Michiels, C. Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front Immunol 2017, 8, 828. [CrossRef]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Holland, E.C.; Stephan, M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun 2019, 10, 3974. [CrossRef]
- Rodriguez-Garcia, A.; Palazon, A.; Noguera-Ortega, E.; Powell, D.J., Jr.; Guedan, S. CAR-T Cells Hit the Tumor Microenvironment: Strategies to Overcome Tumor Escape. Front Immunol 2020, 11, 1109. [CrossRef]
- Sanchez-Paulete, A.R.; Mateus-Tique, J.; Mollaoglu, G.; Nielsen, S.R.; Marks, A.; Lakshmi, A.; Khan, J.A.; Wilk, C.M.; Pia, L.; Baccarini, A.; et al. Targeting Macrophages with CAR T Cells Delays Solid Tumor Progression and Enhances Antitumor Immunity. Cancer Immunol Res 2022, 10, 1354-1369. [CrossRef]
- Guerra, E.; Di Pietro, R.; Basile, M.; Trerotola, M.; Alberti, S. Cancer-Homing CAR-T Cells and Endogenous Immune Population Dynamics. Int J Mol Sci 2021, 23. [CrossRef]
- Anfray, C.; Ummarino, A.; Andon, F.T.; Allavena, P. Current Strategies to Target Tumor-Associated-Macrophages to Improve Anti-Tumor Immune Responses. Cells 2019, 9. [CrossRef]
- Machiels, J.P.; Gomez-Roca, C.; Michot, J.M.; Zamarin, D.; Mitchell, T.; Catala, G.; Eberst, L.; Jacob, W.; Jegg, A.M.; Cannarile, M.A.; et al. Phase Ib study of anti-CSF-1R antibody emactuzumab in combination with CD40 agonist selicrelumab in advanced solid tumor patients. J Immunother Cancer 2020, 8. [CrossRef]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol 2021, 14, 45. [CrossRef]
- Przystal, J.M.; Becker, H.; Canjuga, D.; Tsiami, F.; Anderle, N.; Keller, A.L.; Pohl, A.; Ries, C.H.; Schmittnaegel, M.; Korinetska, N.; et al. Targeting CSF1R Alone or in Combination with PD1 in Experimental Glioma. Cancers (Basel) 2021, 13. [CrossRef]
- Amer, H.T.; Stein, U.; El Tayebi, H.M. The Monocyte, a Maestro in the Tumor Microenvironment (TME) of Breast Cancer. Cancers (Basel) 2022, 14. [CrossRef]
| Mφ Types | M1 Mφ | M2 Mφ | |||
|---|---|---|---|---|---|
| M2a Mφ | M2b Mφ | M2c Mφ | M2d Mφ | ||
| Stimuli | IFN-γ and/ or LPS |
IL-4 and/ or IL-13 |
immune complexes and/or TLR agonists | IL-10 and/or TGF-β | Th2 cytokines glucocorticoids |
| Function | Pro-inflammatory | Anti-inflammatory | Pro-inflammatory | Anti-inflammatory | Pro-angiogenic |
| Cytokine profile | Produce IL-12, IL-23, and iNOS |
Produce IL-10 and arginase 1 |
Produce IL-1β, IL-6, and TNF-α |
Produce IL-10 and TGF-β |
Produce IL-4, IL-10, and TGF-β |
| Cell response |
Promote Th1 cell response |
Promote Th2 cell response |
Promote Th2 cell response |
Promote Th2 cell response |
Promote Th2 cell response |
| Function | Inhibit tumor growth | Promote tumor growth | Promote angiogenesis |
Promote angiogenesis |
Promote tumor growth |
| Chemokine profile | CCL3, CCL4, CXCL9, CXCL10, CXCL11 | CCL17, CCL18, CCL22, CCL24 | |||
| Surface markers | CD80, CD86, MHC II | CD206, CD163, MRC1, IL-10 receptor | |||
| Row | NCT Number | Phases | Study title | Conditions | Completion Date |
|---|---|---|---|---|---|
| 1 | NCT00690261 | N/A | The Impact of M1/M2 TAM Polarization on Cancer Progression and Prognosis Prediction | Tumor, Lung Cancer | August, 2010 |
| 2 | NCT01551251 | N/A | Tumor-Associated Macrophage in Advanced Non-small Cell Lung Cancer | Advanced Non-small Cell Lung Cancer | December, 2010 |
| 3 | NCT05053750 | Early Phase 1 | A Pilot Study of Weekly Paclitaxel, Bevacizumab, and Tumor-Associated Macrophage Targeted Therapy (Zoledronic Acid) in Women with Recurrent, Platinum-resistant, Epithelial Ovarian, Fallopian Tube or Primary Peritoneal Cancer | Epithelial Ovarian, Fallopian Tube, Primary Peritoneal Cancer | March 31, 2023 |
| 4 | NCT01770353 | Phase 1 | MM-398 (Nanoliposomal Irinotecan, Nal-IRI) to Determine Tumor Drug Levels and to Evaluate the Feasibility of Ferumoxytol Magnetic Resonance Imaging to Measure Tumor-Associated Macrophages and to Predict Patient Response to Treatment | Solid Tumors, ER/PR Positive Breast Cancer, Triple Negative Breast Cancer, Metastatic Breast Cancer, Metastatic Breast Cancer with Active Brain Metastasis | Oct. 2, 2018 |
| 5 | NCT03888638 | N/A | The Role of Tumor-associated Macrophages in Colorectal Liver Metastases | Colorectal Liver Metastases, Colorectal Cancer, Liver Metastases, Immunotherapy | March 1, 2019 |
| 6 | NCT01493817 | N/A | Biomarkers in Samples from Younger Patients with Wilms Tumor | Wilms Tumor and Other Childhood Kidney Tumors | Completed |
| 7 | NCT02472275 | Phase 1 | PLX3397, Radiation Therapy, and Antihormone Therapy in Treating Patients with Intermediate- or High-Risk Prostate Cancer | Stage I Prostate Adenocarcinoma, Stage II Prostate Adenocarcinoma, Stage III Prostate Adenocarcinoma | August 5, 2019 |
| 8 | NCT04776980 | Early Phase 1 | Multimodality MRI and Liquid Biopsy in GBM | Multiforme Glioblastoma, Brain Tumor, Adult Glioblastoma, Recurrent Brain Tumor, Primary Brain Tumor | June 22, 2022 |
| 9 | NCT04168528 | Phase 1, Phase 2 | Phase I/IIa Study of 68GaNOTA-Anti-MMR-VHH2 for PET/CT | Malignant Solid Tumor, Breast Cancer, Head and Neck Cancer, Melanoma (Skin) | April 2023 |
| 10 | NCT04663126 | Early Phase 1 | Feasibility of IV Tc-99m-tilmanocept for Imaging of M2-typeTAMs in Metastatic Melanoma | Melanoma | December 2022 |
| 11 | NCT03397238 | N/A | Myeloid Cell Reprogramming in Thyroid Carcinoma | Thyroid Cancer | January 5, 2021 |
| 12 | NCT01316822 | Phase 1 | A Study of ARRY-382 in Patients with Selected Advanced or Metastatic Cancers | Metastatic Cancer | October 2012 |
| 13 | NCT00979277 | N/A | Transcriptional and Molecular Characterization of Tumor-Associated Monocytes/Macrophages in Human Cancers | Tumor, Cancer | Unknown |
| Rank | NCT Number | Phases | Study Title | Conditions | Completion Date |
|---|---|---|---|---|---|
| 1 | NCT04648254 | Phase 1 | Oral Axl/Mer/CSF1R Selective Tyrosine Kinase Inhibitor in Patients with Advanced Solid Tumor | Solid Tumor, Advanced Cancer, Metastatic Cancer | 11/18/2023 |
| 2 | NCT05438420 | Phase 1/ Phase 2 | Oral Axl/Mer/CSF1R Selective Tyrosine Kinase Inhibitor Q702 in Combination with Pembrolizumab in Patients with Selected Advanced Solid | Esophageal Cancer, Gastric Cancer, Hepatocellular Cancer, Cervical Cancer | 6/30/2026 |
| 3 | NCT05438420 | Phase 1 /Phase 2 | Study of NMS-03592088 in Patients with Relapsed or Refractory AML or CMML | Acute Myeloid Leukemia (AML), Chronic Myelomonocytic Leukemia (CMML) |
9/30/2023 |
| 4 | NCT03993873 | Phase 1/ Phase 2 | Study of TPX-0022 in Patients with Advanced NSCLC, Gastric Cancer, or Solid Tumors Harboring Genetic Alterations in MET | Advanced Solid Tumor, Metastatic Solid Tumors, MET Gene Alterations | 11/1/2023 |
| 5 | NCT04848116 | Phase 2 | Neoadjuvant Targeting of Myeloid Cell Populations in Combination with Nivolumab in Head & Neck Ca | Head and Neck Squamous Cell Carcinoma |
4/1/2026 |
| 6 | NCT05020743 | Phase 1/ Phase 2 | Study of DCC-3014 in Patients with Advanced Tumors and Tenosynovial Giant Cell Tumor | Advanced Malignant Neoplasm, Pigmented Villonodular Synovitis, Giant Cell Tumor of Tendon Sheath, Tenosynovial Giant Cell | 6/1/2024 |
| 7 | NCT05020743 | Phase 1 | Phase Ib/II Study of Chiauranib in Patients with Small Cell Lung Cancer | Small Cell Lung Cancer | 12/30/2022 |
| 8 | NCT05494580 | Phase 1/ Phase 2 | Pamiparib Plus Surufatinib in Patients with Platinum-resistant Ovarian Cancer | Ovarian Cancer, Platinum-resistant Ovarian Cancer, Fallopian Tube Carcinosarcoma, Primary Peritoneal Cancer | 8/10/2025 |
| 9 | NCT05627427 | Phase 2 | Multi-cohort Study of Surufatinib Plus Sintilimab in Metastatic NEN and Pancreatic Carcinoma Who Failed Standard Chemotherapy | Neuroendocrine Tumor Grade 3, Neuroendocrine Carcinoma, Pancreatic Carcinoma | 12/31/2024 |
| 10 | NCT05627427 | N/A | Mass Balance Study of [14C] Chiauranib | Small Cell Lung Cancer (SCLC) | 6/30/2023 |
| 11 | NCT04830813 | Phase 3 | Phase 3 Clinical Study of Chiauranib Capsule in Patients with Small-cell Lung Cancer | Small Cell Lung Cancer (SCLC) | 12/31/2024 |
| 12 | NCT05273099 | N/A | Molecular Biomarkers Predicting Early Development of Endometrial Carcinoma | Cancer of Endometrium | 12/1/2023 |
| 13 | NCT05273099 | N/A | Early Rebiopsy to Identify Biomarkers of Tumor Cell Survival Following EGFR, ALK, ROS1 or BRAF TKI Therapy | NSCLC TNM Stage 4, NSCLC, EGFR Gene Mutation, ALK Gene Mutation, ROSE Cluster 1|BRAF V600E | 9/30/2027 |
| 14 | NCT04622865 | Phase 2 | APUR: Testing the Use of FDA Approved Drugs That Target a Specific Abnormality in a Tumor Gene in People with Advanced Stage Cancer | Non-Hodgkin Lymphoma, Multiple Myeloma, Advanced Solid Tumors | 12/31/2025 |
| 15 | NCT04622865 | Phase 2 | Biomarker Driven Trial of VEGFR2 Inhibitor in Advanced Sarcoma | Sarcoma | 8/25/2023 |
| 16 | NCT02171104 | Phase 2 | A Study Evaluating the Activity of Anti-cancer Treatments Targeting Tumor Molecular Alterations/Characteristics in Advanced / Metastatic Tumors. | Malignant Solid Tumor | 11/1/2026 |
| 17 | NCT02171104 | Phase 2 | Canadian Profiling and Targeted Agent Utilization Trial (CAPTUR) | Non-Hodgkin Lymphoma, Multiple Myeloma, Advanced Solid Tumors | 1/31/2027 |
| 18 | NCT02029001 | Phase 2 | Adapting Treatment to the Tumor Molecular Alterations for Patients with Advanced Solid Tumors | Malignant Solid Neoplasms | 10/1/2026 |
| 19 | NCT02029001 | Phase 3 | Molecular Profiling of Advanced Soft-tissue Sarcomas | Soft Tissue Sarcoma | 10/1/2025 |
| 20 | NCT02029001 | Phase 1 | A Phase I Trial of Simmitinib in Advanced Solid Tumors | Advanced Solid Tumor | 3/31/2025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





