Submitted:
24 May 2023
Posted:
26 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
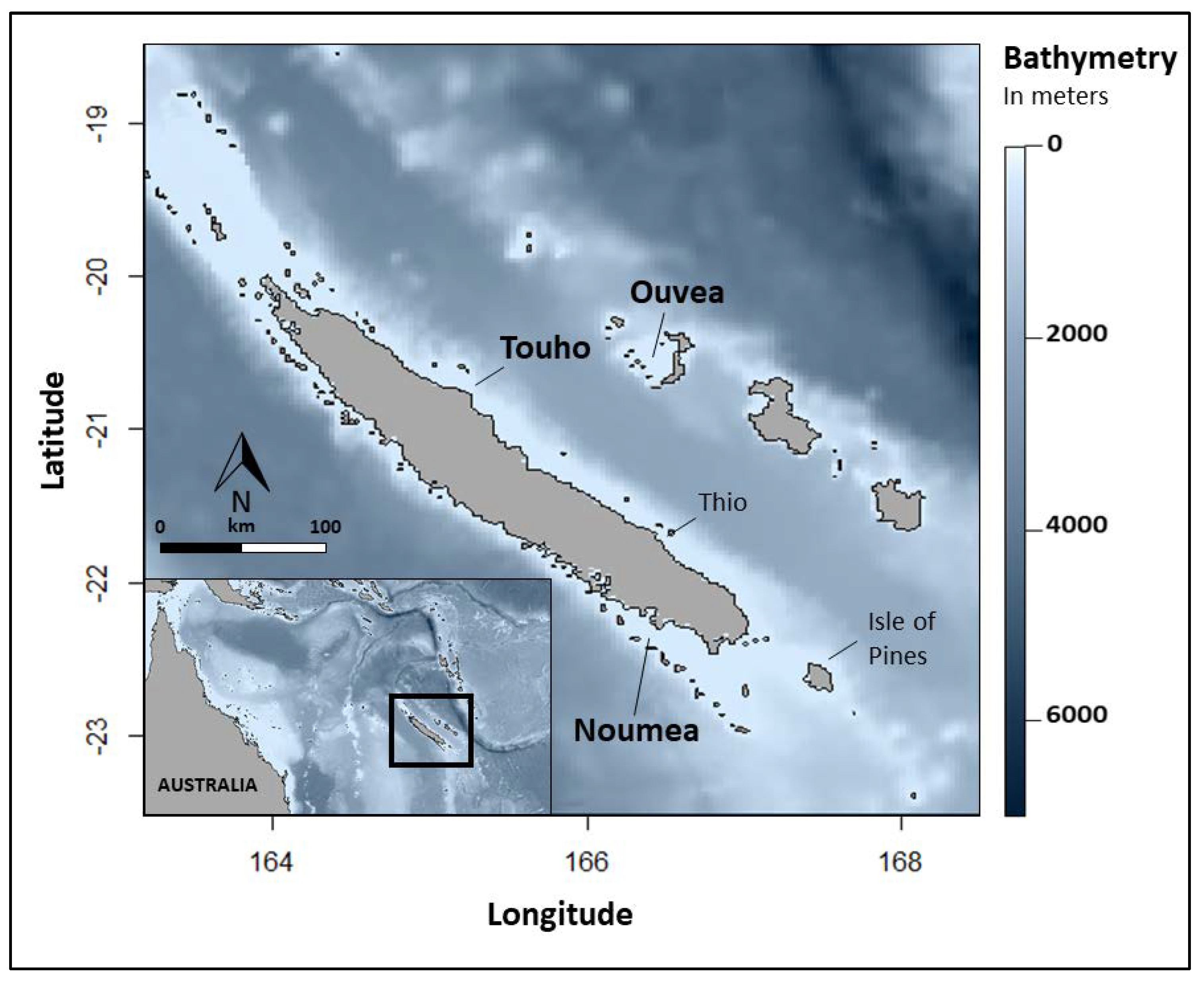
2.1. Study Sites
2.2. Tagging
2.3. Data Analysis
2.4. Statistical Analysis
2.5. Ethic Statement
3. Results
3.1. Tagging
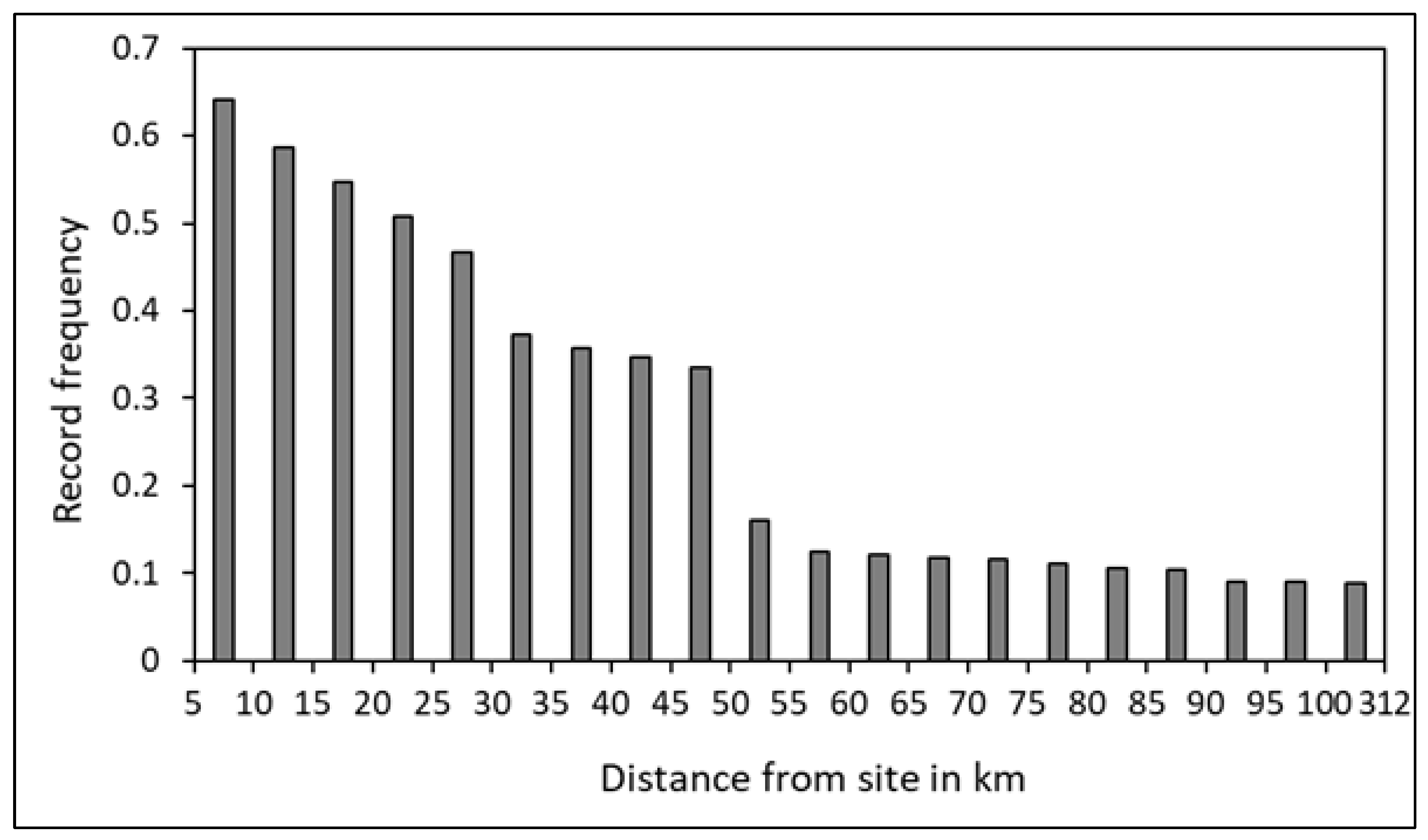
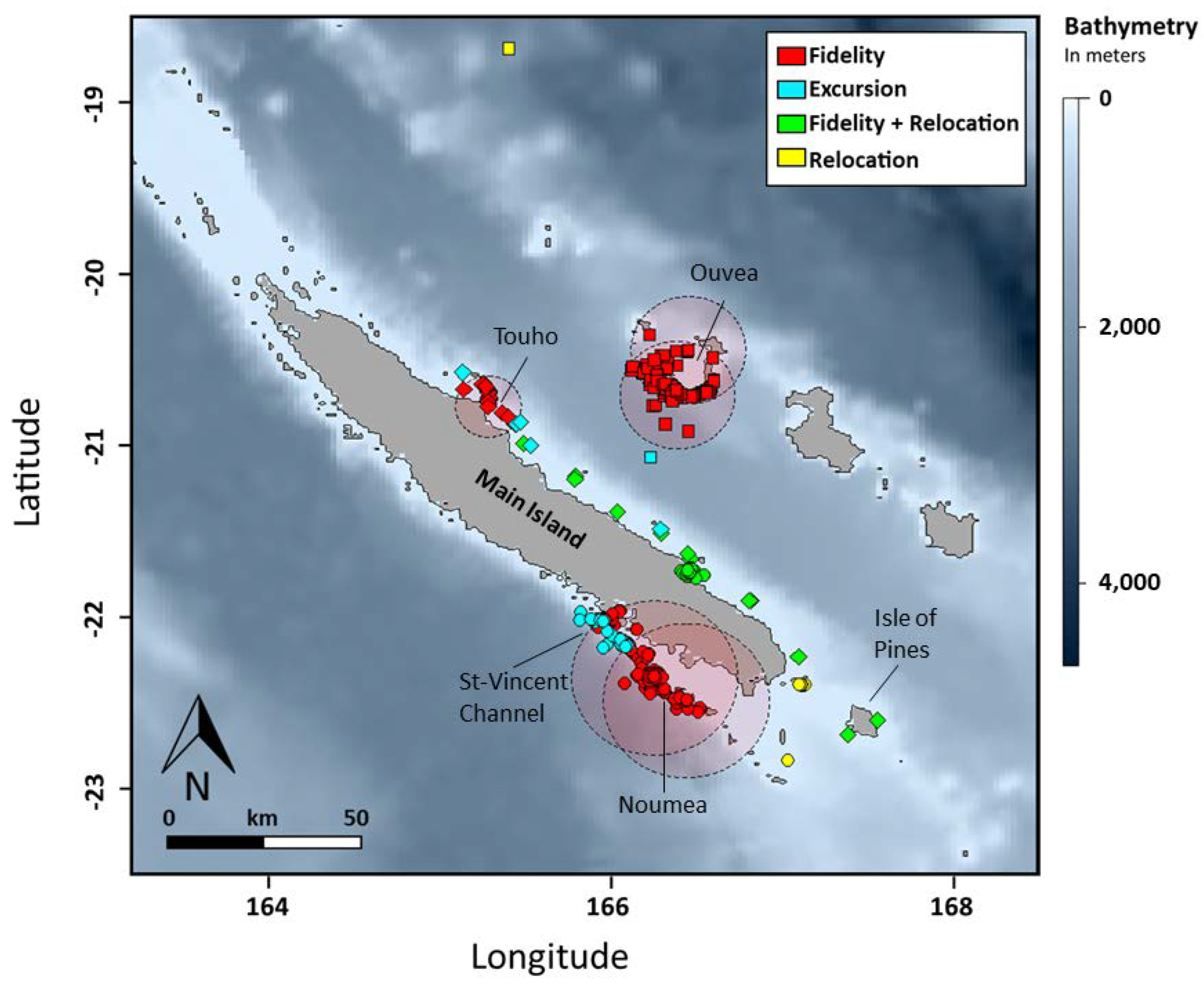
3.2. Horizontal Movements
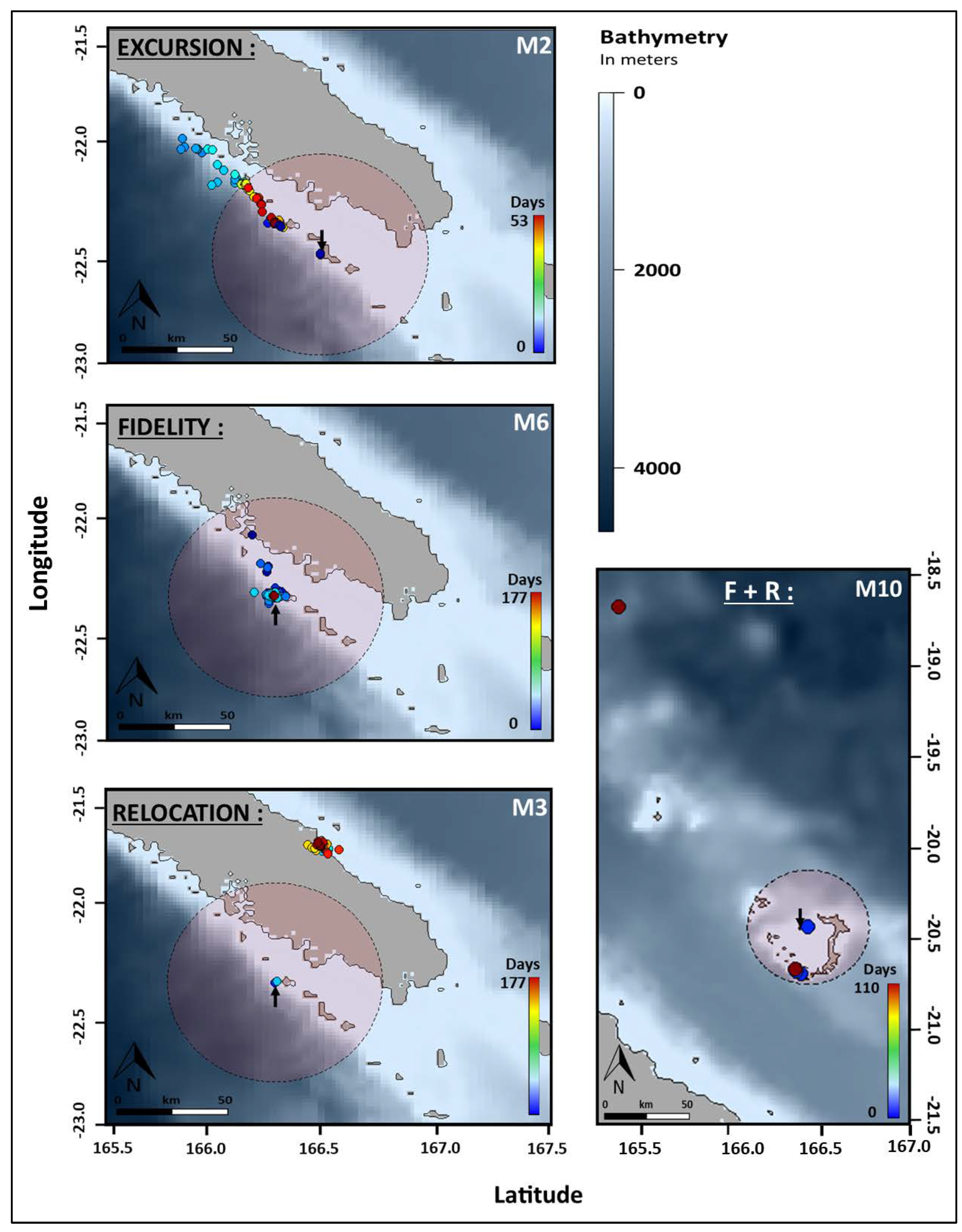
3.3. Sex
4. Discussion
Supplementary Materials
Funding
Acknowledgments
References
- Fletcher, R.; Fortin, M.-J. Spatial Ecology and Conservation Modeling; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-030-01988-4. [Google Scholar]
- Graham, R.T.; Witt, M.J.; Castellanos, D.W.; Remolina, F.; Maxwell, S.; Godley, B.J.; Hawkes, L.A. Satellite Tracking of Manta Rays Highlights Challenges to Their Conservation. PLOS ONE 2012, 7, e36834. [Google Scholar] [CrossRef]
- Palumbi, S. R. Genetic divergence, reproductive isolation, and marine speciation. Annu. Rev. Ecol. Evol. Syst. 2014, 25(1), pp. 547-572.
- Palumbi, S. R. (2003). Population genetics, demographic connectivity, and the design of marine reserves. Ecol Appl.13(sp1), 146-158.
- Chateau, O.; Wantiez, L. Movement patterns of four coral reef fish species in a fragmented habitat in New Caledonia: implications for the design of marine protected area networks. ICES J. Mar. Sci. 2008, 66, 50–55. [Google Scholar] [CrossRef]
- Meyer, C.G.; Papastamatiou, Y.P.; Clark, T.B. Differential movement patterns and site fidelity among trophic groups of reef fishes in a Hawaiian marine protected area. Mar. Biol. 2010, 157, 1499–1511. [Google Scholar] [CrossRef]
- Foote, A.D.; Similä, T.; Víkingsson, G.A.; Stevick, P.T. Movement, site fidelity and connectivity in a top marine predator, the killer whale. Evol. Ecol. 2009, 24, 803–814. [Google Scholar] [CrossRef]
- Passadore, C.; Möller, L.; Diaz-Aguirre, F.; Parra, G.J. High site fidelity and restricted ranging patterns in southern Australian bottlenose dolphins. Ecol. Evol. 2017, 8, 242–256. [Google Scholar] [CrossRef]
- Madigan, D. J.; Brooks, E. J.; Bond, M. E.; Gelsleichter, J.; Howey, L. A.; Abercrombie, D. L. ; ..; Chapman, D. D. Diet shift and site-fidelity of oceanic whitetip sharks Carcharhinus longimanus along the Great Bahama Bank. Mar. Ecol. Prog. 2015, 529, pp. 185–197. [Google Scholar]
- Sims, D.W.; Quayle, V.A. Selective foraging behaviour of basking sharks on zooplankton in a small-scale front. Nature 1998, 393, 460–464. [Google Scholar] [CrossRef]
- Heyman, W.; Graham, R.; Kjerfve, B.; Johannes, R. Whale sharks Rhincodon typus aggregate to feed on fish spawn in Belize. Mar. Ecol. Prog. Ser. 2001, 215, 275–282. [Google Scholar] [CrossRef]
- Graham, R.T.; Roberts, C.M.; Smart, J.C. Diving behaviour of whale sharks in relation to a predictable food pulse. J. R. Soc. Interface 2005, 3, 109–116. [Google Scholar] [CrossRef]
- Anderson, R.C.; Adam, M.S.; Goes, J.I. From monsoons to mantas: seasonal distribution of Manta alfredi in the Maldives. Fish. Oceanogr. 2011, 20, 104–113. [Google Scholar] [CrossRef]
- Germanov, E.S.; Marshall, A.D. Running the Gauntlet: Regional Movement Patterns of Manta alfredi through a Complex of Parks and Fisheries. PLOS ONE 2014, 9, e110071. [Google Scholar] [CrossRef] [PubMed]
- Jaine, F.; Rohner, C.; Weeks, S.; Couturier, L.; Bennett, M.; Townsend, K.; Richardson, A. Movements and habitat use of reef manta rays off eastern Australia: offshore excursions, deep diving and eddy affinity revealed by satellite telemetry. Mar. Ecol. Prog. Ser. 2014, 510, 73–86. [Google Scholar] [CrossRef]
- Jaine, F.R.A.; Couturier, L.I.E.; Weeks, S.J.; Townsend, K.A.; Bennett, M.B.; Fiora, K.; Richardson, A.J. When Giants Turn Up: Sighting Trends, Environmental Influences and Habitat Use of the Manta Ray Manta alfredi at a Coral Reef. PLOS ONE 2012, 7, e46170. [Google Scholar] [CrossRef] [PubMed]
- Couturier, L. I.; Dudgeon, C. L.; Pollock, K. H.; Jaine, F. R. A.; Bennett, M. B.; Townsend, K. A. ;...; Richardson, A. J. Population dynamics of the reef manta ray Manta alfredi in eastern Australia. Coral Reefs, 2014, 33(2), pp. 329-342.
- Setyawan, E.; Sianipar, A. B.; Erdmann, M.; Fischer, A. M.; Haddy, J. A.; Beale, C. S. ;...; Mambrasar, R. Site fidelity and movement patterns of reef manta rays (Mobula alfredi: Mobulidae) using passive acoustic telemetry in northern Raja Ampat, Indonesia. Nat. Conserv. Res. 2018. [Google Scholar]
- O'Malley, M.P.; Townsend, K.A.; Hilton, P.; Heinrichs, S.; Stewart, J.D. Characterization of the trade in manta and devil ray gill plates in China and South-east Asia through trader surveys. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 27, 394–413. [Google Scholar] [CrossRef]
- White, W.T.; Giles, J.; Dharmadi; Potter, I. C. Data on the bycatch fishery and reproductive biology of mobulid rays (Myliobatiformes) in Indonesia. Fish. Res. 2006, 82, 65–73. [Google Scholar] [CrossRef]
- O’malley, M.P.; Lee-Brooks, K.; Medd, H.B. The Global Economic Impact of Manta Ray Watching Tourism. PLOS ONE 2013, 8, e65051. [Google Scholar] [CrossRef]
- Venables, S.; McGregor, F.; Brain, L.; van Keulen, M. Manta ray tourism management, precautionary strategies for a growing industry: a case study from the Ningaloo Marine Park, Western Australia. Pac. Conserv. Biol. 2016, 22, 295. [Google Scholar] [CrossRef]
- Ward-Paige, C.A.; Davis, B.; Worm, B. Global Population Trends and Human Use Patterns of Manta and Mobula Rays. PLOS ONE 2013, 8, e74835. [Google Scholar] [CrossRef]
- Rohner, C.; Pierce, S.; Marshall, A.; Weeks, S.; Bennett, M.; Richardson, A. Trends in sightings and environmental influences on a coastal aggregation of manta rays and whale sharks. Mar. Ecol. Prog. Ser. 2013, 482, 153–168. [Google Scholar] [CrossRef]
- Croll, D. A.; Dewar, H.; Dulvy, N. K.; Fernando, D.; Francis, M. P.; Galván-Magaña, F. ;...; White, W. T. Vulnerabilities and fisheries impacts: the uncertain future of manta and devil rays. Aquat. Conserv.: Mar. Freshw. Ecosyst. 2016, 26(3), pp. 562-575.
- Lawson, J. M.; Fordham, S. V.; O’Malley, M. P.; Davidson, L. N.; Walls, R. H.; Heupel, M. R. ; ..; Dulvy, N. K. Sympathy for the devil: a conservation strategy for devil and manta rays. PeerJ, 2017, 5, e3027. [Google Scholar]
- McGregor, F.; Richardson, A.J.; Armstrong, A.J.; Armstrong, A.O.; Dudgeon, C.L. Rapid wound healing in a reef manta ray masks the extent of vessel strike. PLOS ONE 2019, 14, e0225681. [Google Scholar] [CrossRef]
- Stewart, J. D.; Jaine, F. R. A.; Armstrong, A. J.; Armstrong, A. O.; Bennett, M. B.; Burgess, K. B. ; ..; Stevens, G. M. Research priorities to support effective manta and devil ray conservation. Front. Mar. Sci. 2018, 5, pp 314. [Google Scholar]
- Marshall, A.; Kashiwagi, T.; Bennett, M.B.; Deakos, M.; Stevens, G.; McGregor, F.; Clark, T.; Ishihara, H.; Sato, K. Mobula alfredi (amended version of 2011 assessment). The IUCN Red List of Threatened Species 2018: e.T195459A126665723. 2018 https://dx.doi.org/10.2305/IUCN.UK.2011-2.RLTS.T195459A126665723.en. Downloaded on 02 January 2021.
- Baird, R. W.; Schorr, G. S.; Webster, D. L.; Mahaffy, S. D.; McSweeney, D. J.; Hanson, M. B.; Andrews, R. D. Open-ocean movements of a satellite-tagged Blainville's beaked whale (Mesoplodon densirostris): evidence for an offshore population in Hawaii? Aquat. Mamm. 2011, 37(4), pp. 506-511.
- A Block, B.; Costa, D.P.; Boehlert, G.W.; E Kochevar, R. Revealing pelagic habitat use: the tagging of Pacific pelagics program. Oceanol. Acta 2002, 25, 255–266. [Google Scholar] [CrossRef]
- Hart, K.; Hyrenbach, K. Satellite telemetry of marine megavertebrates: the coming of age of an experimental science. Endanger. Species Res. 2009, 10, 9–20. [Google Scholar] [CrossRef]
- Mate, B.R.; Best, P.B.; Lagerquist, B.A.; Winsor, M.H. Coastal, offshore, and migratory movements of South African right whales revealed by satellite telemetry. Mar. Mammal Sci. 2010, 27, 455–476. [Google Scholar] [CrossRef]
- Phillips, E.; Horne, J.; Adams, J.; Zamon, J. Selective occupancy of a persistent yet variable coastal river plume by two seabird species. Mar. Ecol. Prog. Ser. 2018, 594, 245–261. [Google Scholar] [CrossRef]
- Hofman, M.P.G.; Hayward, M.W.; Heim, M.; Marchand, P.; Rolandsen, C.M.; Mattisson, J.; Urbano, F.; Heurich, M.; Mysterud, A.; Melzheimer, J.; et al. Right on track? Performance of satellite telemetry in terrestrial wildlife research. PLOS ONE 2019, 14, e0216223. [Google Scholar] [CrossRef]
- Haywood, J. C.; Fuller, W. J.; Godley, B. J.; Margaritoulis, D.; Shutler, J. D.; Snape, R. T. ;...; Broderick, A. C. Spatial ecology of loggerhead turtles: Insights from stable isotope markers and satellite telemetry. Divers. Distrib. 2020, 26(3), pp. 368-381.
- Meyers, M.M.; Francis, M.P.; Erdmann, M.; Constantine, R.; Sianipar, A. Movement patterns of whale sharks in Cenderawasih Bay, Indonesia, revealed through long-term satellite tagging. Pac. Conserv. Biol. 2020, 26, 353–364. [Google Scholar] [CrossRef]
- Heupel, M.R.; Simpfendorfer, C.A.; Collins, A.B.; Tyminski, J.P. Residency and movement patterns of bonnethead sharks, Sphyrna tiburo, in a large Florida estuary. Environ. Biol. Fishes 2006, 76, 47–67. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Gallagher, A.; Lazarre, D. A review of shark satellite tagging studies. J. Exp. Mar. Biol. Ecol. 2011, 398, 1–8. [Google Scholar] [CrossRef]
- Crossin, G. T.; Heupel, M. R.; Holbrook, C. M.; Hussey, N. E.; Lowerre-Barbieri, S. K.; Nguyen, V. M. ;...; Cooke, S. J. Acoustic telemetry and fisheries management. Ecol Appl. 2017, 27(4), pp. 1031-1049.
- Sims, D.W.; Southall, E.J.; Tarling, G.A.; Metcalfe, J.D. Habitat-specific normal and reverse diel vertical migration in the plankton-feeding basking shark. J. Anim. Ecol. 2005, 74, 755–761. [Google Scholar] [CrossRef]
- Andrews, K.S.; Williams, G.D.; Levin, P.S. Seasonal and Ontogenetic Changes in Movement Patterns of Sixgill Sharks. PLOS ONE 2010, 5, e12549. [Google Scholar] [CrossRef] [PubMed]
- Bonfil, R.; Meÿer, M.; Scholl, M. C.; Johnson, R.; O'Brien, S.; Oosthuizen, H. ;...; Paterson, M. Transoceanic migration, spatial dynamics, and population linkages of white sharks. Science, 2005, 310(5745), pp. 100-103.
- Brunnschweiler, J. M.; Baensch, H.; Pierce, S. J.; Sims, D. W. Deep-diving behaviour of a whale shark Rhincodon typus during long-distance movement in the western Indian Ocean. J. Fish Biol. 2009, 74(3), pp. 706-714.
- Thorrold, S.R.; Afonso, P.; Fontes, J.; Braun, C.D.; Santos, R.S.; Skomal, G.B.; Berumen, M.L. Extreme diving behaviour in devil rays links surface waters and the deep ocean. Nat. Commun. 2014, 5, 4274. [Google Scholar] [CrossRef] [PubMed]
- Lassauce, H.; Chateau, O.; Erdmann, M.V.; Wantiez, L. Diving behavior of the reef manta ray (Mobula alfredi) in New Caledonia: More frequent and deeper night-time diving to 672 meters. PLOS ONE 2020, 15, e0228815. [Google Scholar] [CrossRef] [PubMed]
- Lassauce, H.; Chateau, O.; Erdmann, M. V.; Wantiez, L. Citizen science to infer characteristics and habitat use of the population of reef manta rays (Mobula alfredi) in New Caledonia. University of New Caledonia, Noumea, New Caledonia, 2023, Manuscript in preparation.
- Lassauce, H.; Dudgeon, C.; Armstrong, A.; Wantiez, L.; Carroll, E. Evidence of fine-scale genetic structure for reef manta rays Mobula alfredi in New Caledonia. Endanger. Species Res. 2022, 47, 249–264. [Google Scholar] [CrossRef]
- Andréfouët, S.; Cabioch, G.; Flamand, B.; Pelletier, B. A reappraisal of the diversity of geomorphological and genetic processes of New Caledonian coral reefs: a synthesis from optical remote sensing, coring and acoustic multibeam observations. Coral Reefs 2009, 28, 691–707. [Google Scholar] [CrossRef]
- Van Canneyt, O.; Dorémus, G.; Laran, S.; Ridoux, V.; Watremez, P.; Distribution et abondance de la mégafaune marine dans le sud-ouest du Pacifique. 2015 (Scientific campaign report). Available online: https://www.observatoire-pelagis.cnrs.fr/wp-content/uploads/2021/05/9-RAPPORT_REMMOA_SOP_INTER_2015.pdf (accessed on 22 May 2020).
- Freitas, C.; Lydersen, C.; Fedak, M.A.; Kovacs, K.M. A simple new algorithm to filter marine mammal Argos locations. Mar. Mammal Sci. 2007, 24, 315–325. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 7 February 2021).
- Pante, E.; Simon-Bouhet, B. marmap: A Package for Importing, Plotting and Analyzing Bathymetric and Topographic Data in R. PLOS ONE 2013, 8, e73051. [Google Scholar] [CrossRef]
- Armstrong, A. J.; Armstrong, A. O.; McGregor, F.; Richardson, A. J.; Bennett, M. B.; Townsend, K. A. ; ..; Dudgeon, C. L. Satellite tagging and photographic identification reveal connectivity between two UNESCO World Heritage Areas for reef manta rays. Front. Mar. Sci. 2020, 7, pp 725. [Google Scholar]
- Braun, C.D.; Skomal, G.B.; Thorrold, S.R.; Berumen, M.L. Movements of the reef manta ray (Manta alfredi) in the Red Sea using satellite and acoustic telemetry. Mar. Biol. 2015, 162, 2351–2362. [Google Scholar] [CrossRef]
- Kessel, S. T.; Elamin, N. A.; Yurkowski, D. J.; Chekchak, T.; Walter, R. P.; Klaus, R. ;...; Hussey, N. E. Conservation of reef manta rays (Manta alfredi) in a UNESCO World Heritage Site: Large-scale island development or sustainable tourism? PLoS One, 2017, 12(10), e0185419. [Google Scholar]
- Peel, L.R.; Stevens, G.M.W.; Daly, R.; Daly, C.A.K.; Collin, S.P.; Nogués, J.; Meekan, M.G. Regional Movements of Reef Manta Rays (Mobula alfredi) in Seychelles Waters. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Chapple, T. K.; Curnick, D. J.; Carlisle, A. B.; Castleton, M.; Jacoby, D. M. ; ..; Block, B. A. Individual variation in residency and regional movements of reef manta rays Mobula alfredi in a large marine protected area. Mar. Ecol. Prog. Ser. 2020, 639, pp. 137–153. [Google Scholar]
- Dewar, H.; Mous, P.; Domeier, M.; Muljadi, A.; Pet, J.; Whitty, J. Movements and site fidelity of the giant manta ray, Manta birostris, in the Komodo Marine Park, Indonesia. Mar. Biol. 2008, 155, 121–133. [Google Scholar] [CrossRef]
- Couturier, L.I.E.; Jaine, F.R.A.; Townsend, K.A.; Weeks, S.J.; Richardson, A.J.; Bennett, M.B. Distribution, site affinity and regional movements of the manta ray, Manta alfredi (Krefft, 1868), along the east coast of Australia. Mar. Freshw. Res. 2011, 62, 628–637. [Google Scholar] [CrossRef]
- Couturier, L.; Newman, P.; Jaine, F.; Bennett, M.; Venables, W.; Cagua, E.; Townsend, K.; Weeks, S.; Richardson, A. Variation in occupancy and habitat use of Mobula alfredi at a major aggregation site. Mar. Ecol. Prog. Ser. 2018, 599, 125–145. [Google Scholar] [CrossRef]
- Armstrong, A. O.; Armstrong, A. J.; Jaine, F. R.; Couturier, L. I.; Fiora, K.; Uribe-Palomino, J. ;...; Richardson, A. J. Prey density threshold and tidal influence on reef manta ray foraging at an aggregation site on the Great Barrier Reef. PLoS One, 2016, 11(5), e0153393.
- McCoy, E.; Burce, R.; David, D.; Aca, E. Q.; Hardy, J.; Labaja, J. ; ..; Araujo, G. Long-term photo-identification reveals the population dynamics and strong site fidelity of adult whale sharks to the coastal waters of Donsol, Philippines. Front. Mar. Sci. 2018, 5, pp 271. [Google Scholar]
- Setyawan, E.; Erdmann, M. V.; Lewis, S. A.; Mambrasar, R.; Hasan, A. W.; Templeton, S. ; ..; Cerutti-Pereyra, F. Natural history of manta rays in the Bird's Head Seascape, Indonesia, with an analysis of the demography and spatial ecology of Mobula alfredi (Elasmobranchii: Mobulidae). J. Ocean Sci. Found. 2020, 36, pp. 49–83. [Google Scholar]
- Torréton, J.-P.; Rochelle-Newall, E.; Pringault, O.; Jacquet, S.; Faure, V.; Briand, E. Variability of primary and bacterial production in a coral reef lagoon (New Caledonia). Mar. Pollut. Bull. 2010, 61, 335–348. [Google Scholar] [CrossRef]
- Le Borgne, R.; Douillet, P.; Fichez, R.; Torréton, J.-P. Hydrography and plankton temporal variabilities at different time scales in the southwest lagoon of New Caledonia: A review. Mar. Pollut. Bull. 2010, 61, 297–308. [Google Scholar] [CrossRef]
- McCauley, D. J.; DeSalles, P. A.; Young, H. S.; Papastamatiou, Y. P.; Caselle, J. E.; Deakos, M. H. ;...; Micheli, F. Reliance of mobile species on sensitive habitats: a case study of manta rays (Manta alfredi) and lagoons. Mar. Biol. 2014, 161(9), pp. 1987-1998. [Google Scholar]
- Heupel, M.R.; Carlson, J.; Simpfendorfer, C. Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 2007, 337, 287–297. [Google Scholar] [CrossRef]
- Papastamatiou, Y.P.; Lowe, C.G.; Caselle, J.E.; Friedlander, A.M. Scale-dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll. Ecology 2009, 90, 996–1008. [Google Scholar] [CrossRef] [PubMed]
- Dale, J.J.; Wallsgrove, N.J.; Popp, B.N.; Holland, K.N. Nursery habitat use and foraging ecology of the brown stingray Dasyatis lata determined from stomach contents, bulk and amino acid stable isotopes. Mar. Ecol. Prog. Ser. 2011, 433, 221–236. [Google Scholar] [CrossRef]
- Barr, Y.; Abelson, A. Feeding–Cleaning Trade-Off: Manta Ray “Decision-Making” as a Conservation Tool. Front. Mar. Sci. 2019, 6, pp 88. [Google Scholar] [CrossRef]
- Stevens, G. M. W. Conservation and population ecology of manta rays in the Maldives, Doctoral dissertation, University of York, 2016.
- Perryman, R.J.Y.; Venables, S.K.; Tapilatu, R.F.; Marshall, A.D.; Brown, C.; Franks, D.W. Social preferences and network structure in a population of reef manta rays. Behav. Ecol. Sociobiol. 2019, 73, 114. [Google Scholar] [CrossRef]
- Stewart, J.D.; Hoyos-Padilla, E.M.; Kumli, K.R.; Rubin, R.D. Deep-water feeding and behavioral plasticity in Manta birostris revealed by archival tags and submersible observations. Zoology 2016, 119, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Thums, M.; Meekan, M.; Stevens, J.; Wilson, S.; Polovina, J. Evidence for behavioural thermoregulation by the world's largest fish. J. R. Soc. Interface, 2013, 10(78), 20120477.
- Speed, C. W.; Meekan, M. G.; Field, I. C.; McMahon, C. R.; Harcourt, R. G.; Stevens, J. D. ; ..; Bradshaw, C. J. A. Reef shark movements relative to a coastal marine protected area. Reg. Stud. Mar. Sci. 2016, 3, pp. 58–66. [Google Scholar]
- Laran, S.; Doremus, G.; Mannocci, L.; Van Canneyt, O.; Watremez, P.; Cadinouche, A. ;...; Razafindrakoto, Y. Progress of the REMMOA aerial surveys conducted in the French EEZ and adjacent waters: contrasted cetacean habitats in the southwest Indian Ocean. Report, 2016. [Google Scholar]
- Lassauce, H.; Chateau, O. ; M. V.; Wantiez, L. Spatial ecology of the population of reef manta rays (Mobula alfredi) in New Caledonia using satellite telemetry: 2-Vertical behaviour. University of New Caledonia, Noumea, New Caledonia, 2023. [Google Scholar]
- Harris, J. L.; McGregor, P. K.; Oates, Y.; Stevens, G. M. Gone with the wind: Seasonal distribution and habitat use by the reef manta ray (Mobula alfredi) in the Maldives, implications for conservation. Aquatic Conservation: Aquat. Conserv.: Mar. Freshw. Ecosyst. 2020, 30(8), pp. 1649-1664.
- Clark, T. B. Abundance, home range, and movement patterns of manta rays (Manta alfredi, M. birostris) in Hawaiʻi. Doctoral dissertation, University of Hawaii at Manoa, Honolulu, December 2010.
- Deakos, M. H.; Baker, J. D.; Bejder, L. Characteristics of a manta ray Manta alfredi population off Maui, Hawaii, and implications for management. Mar. Ecol. Prog. Ser. 2011, 429, pp. 245–260. [Google Scholar] [CrossRef]
- Kitchen-Wheeler, A.-M.; Ari, C.; Edwards, A.J. Population estimates of Alfred mantas (Manta alfredi) in central Maldives atolls: North Male, Ari and Baa. Environ. Biol. Fishes 2011, 93, 557–575. [Google Scholar] [CrossRef]
- Carpentier, A. S.; Berthe, C.; Ender, I.; Jaine, F. R.; Mourier, J.; Stevens, G. ;...; Clua, E. Preliminary insights into the population characteristics and distribution of reef (Mobula alfredi) and oceanic (M. birostris) manta rays in French Polynesia. Coral Reefs, 2019, 38(6), pp. 1197-1210.
- Armstrong, A.O.; Armstrong, A.J.; Bennett, M.B.; Richardson, A.J.; Townsend, K.A.; Dudgeon, C.L. Photographic identification and citizen science combine to reveal long distance movements of individual reef manta rays Mobula alfredi along Australia’s east coast. Mar. Biodivers. Rec. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Venables, S.; van Duinkerken, D.; Rohner, C.; Marshall, A. Habitat use and movement patterns of reef manta rays Mobula alfredi in southern Mozambique. Mar. Ecol. Prog. Ser. 2020, 634, 99–114. [Google Scholar] [CrossRef]
- Chapman, D.D.; Feldheim, K.A.; Papastamatiou, Y.P.; Hueter, R.E. There and Back Again: A Review of Residency and Return Migrations in Sharks, with Implications for Population Structure and Management. Annu. Rev. Mar. Sci. 2015, 7, 547–570. [Google Scholar] [CrossRef]
- Marshall, A.D.; Pierce, S.J.; Bennett, M.B. Morphological measurements of manta rays (Manta birostris) with a description of a foetus from the east coast of Southern Africa. Zootaxa 2008, 1717, 24–30. [Google Scholar] [CrossRef]
- Germanov, E. S.; Bejder, L.; Chabanne, D. B.; Dharmadi, D.; Hendrawan, I. G.; Marshall, A. D. ; ..; Loneragan, N. R. Contrasting habitat use and population dynamics of reef manta rays within the Nusa Penida marine protected area, Indonesia. Front. Mar. Sci. 2019, 6, pp 215. [Google Scholar]
| Movement Patterns | Pattern explanation | Noumea | Ouvea | Touho | TOTAL | |||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||
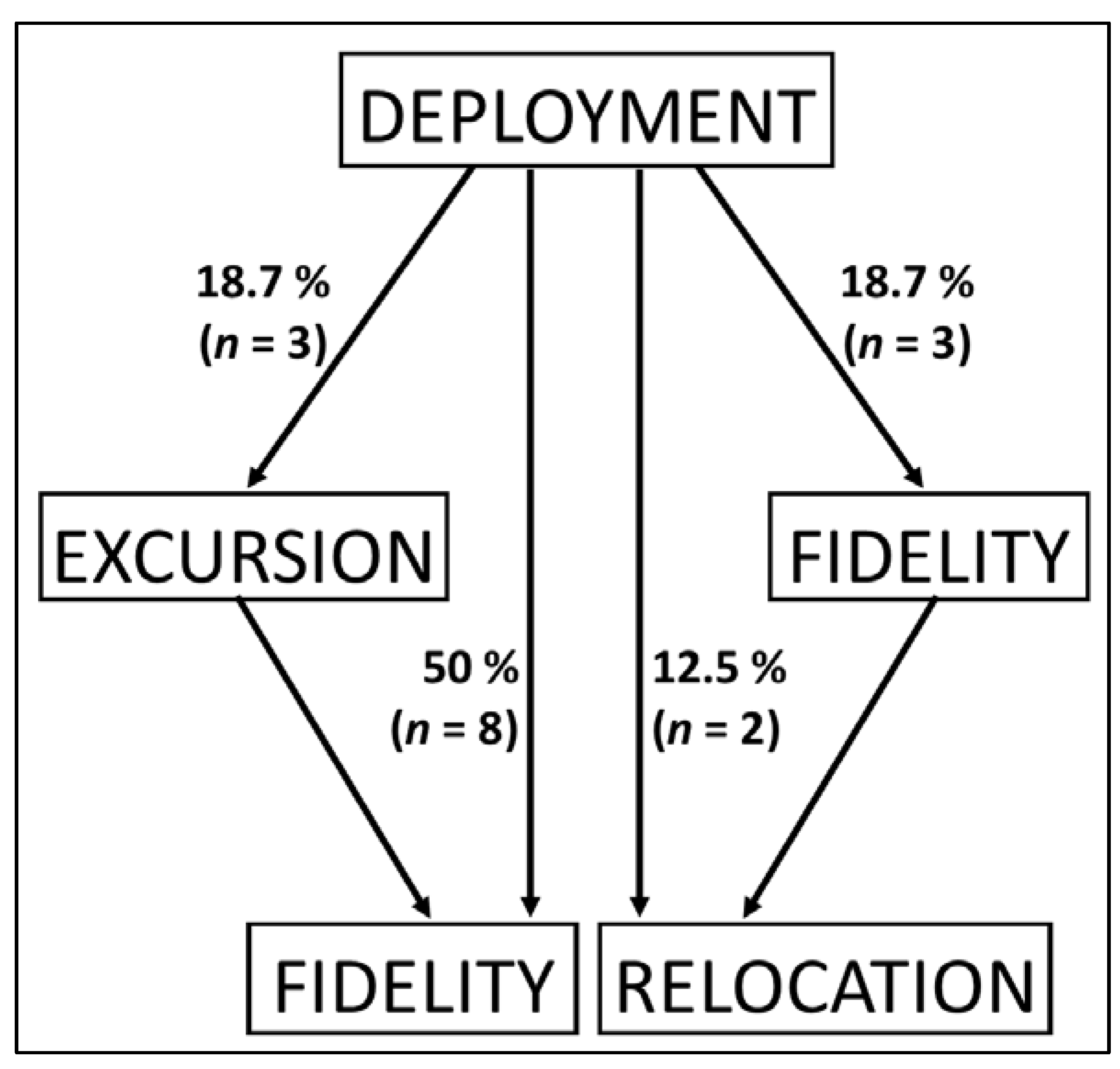
| FIDELITY | 100 % records in the HR | 1 | 1 | 0 | 3 | 1 | 1 | 7 |
| EXCURSION | ≥ 1 record outside the home range followed by ≥ 1 record within the HR | 0 | 2 | 1 | 0 | 1 | 1 | 5 |
| FIDELITY + RELOCATION | ≥ 50 % records in the HR followed by ≥ 1 record outside at tag release | 1 | 0 | 1 | 0 | 0 | 0 | 2 |
| RELOCATION | ≥ 50 % records outside the HR until tag release | 0 | 1 | 0 | 0 | 1 | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





