Submitted:
25 May 2023
Posted:
26 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Plant extract preparation
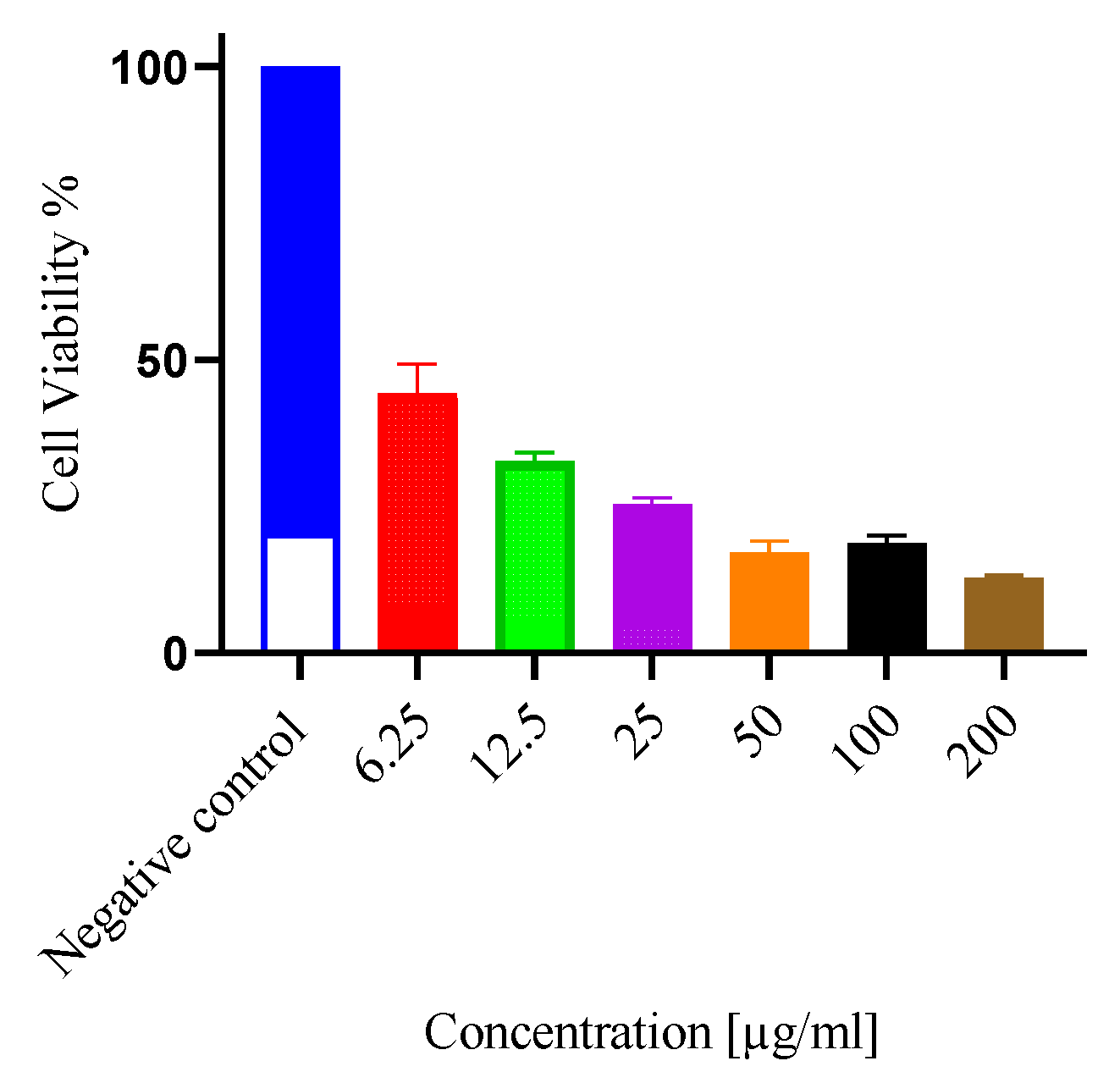
2.2. Antiproliferative & cytotoxic activity
2.3. Selectivity index(SI)
2.4. Identification and analysis of E. ingens bioactive compounds
2.4.1. Qualitative colour method
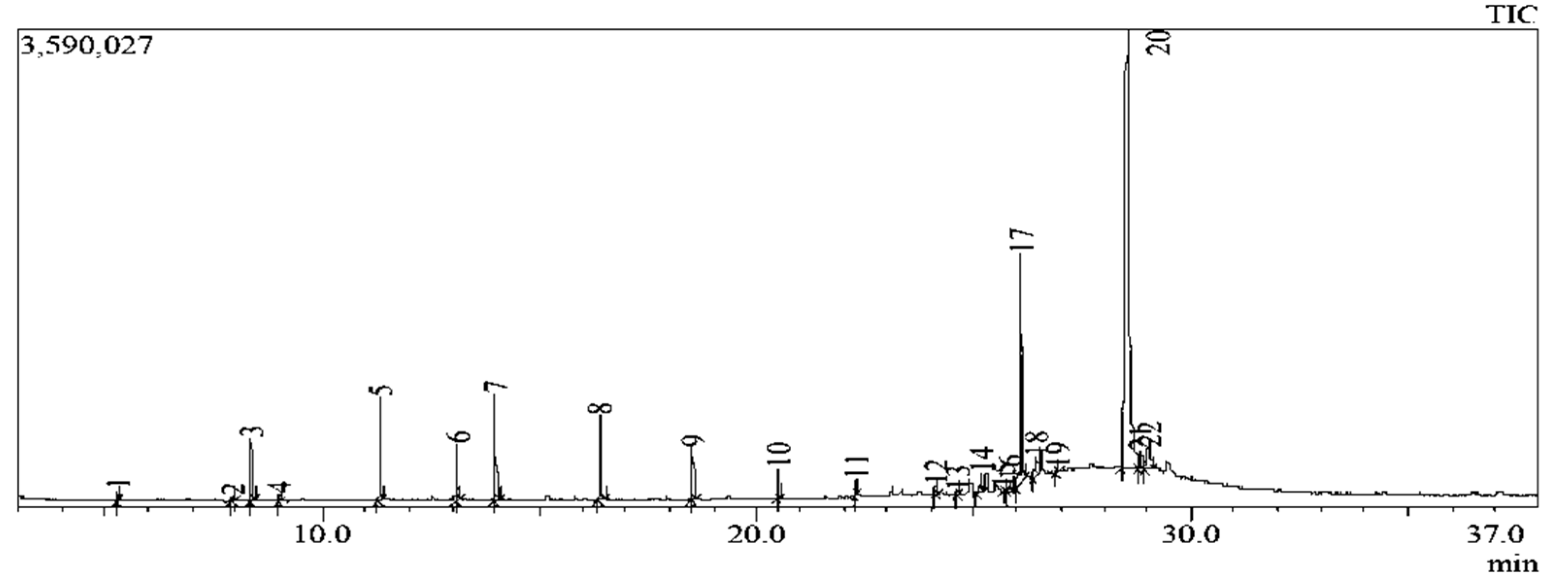
2.4.2. In-depth compounds characterisation by Gas chromatography–mass spectrometry (GC-MS)
2.5. In silico work
2.5.1. Screening for drug-like compounds in E. ingens ethyl acetate fraction
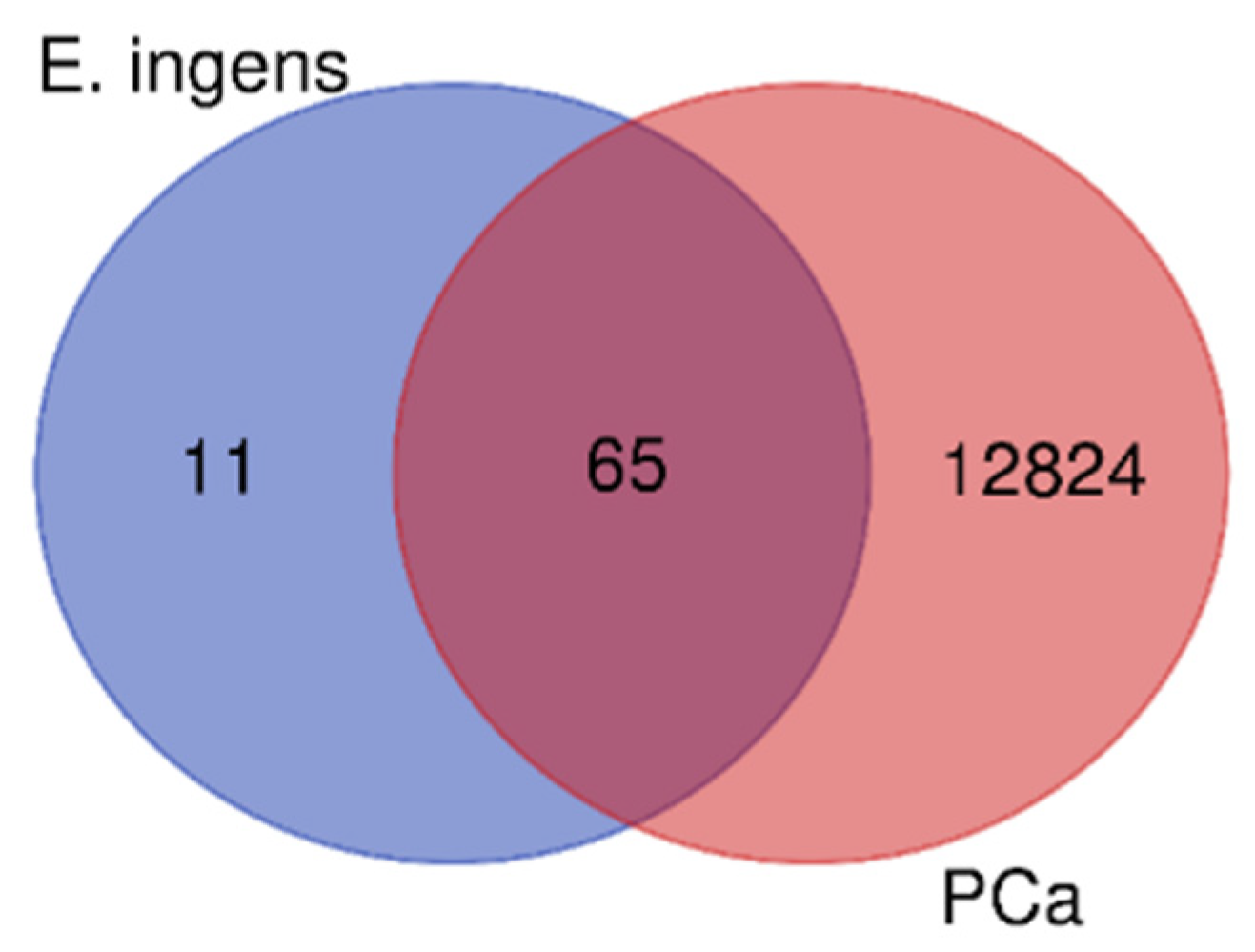
2.5.2. The targets of E. ingens prioritized drug-like compounds and therapeutic targets for prostate cancer
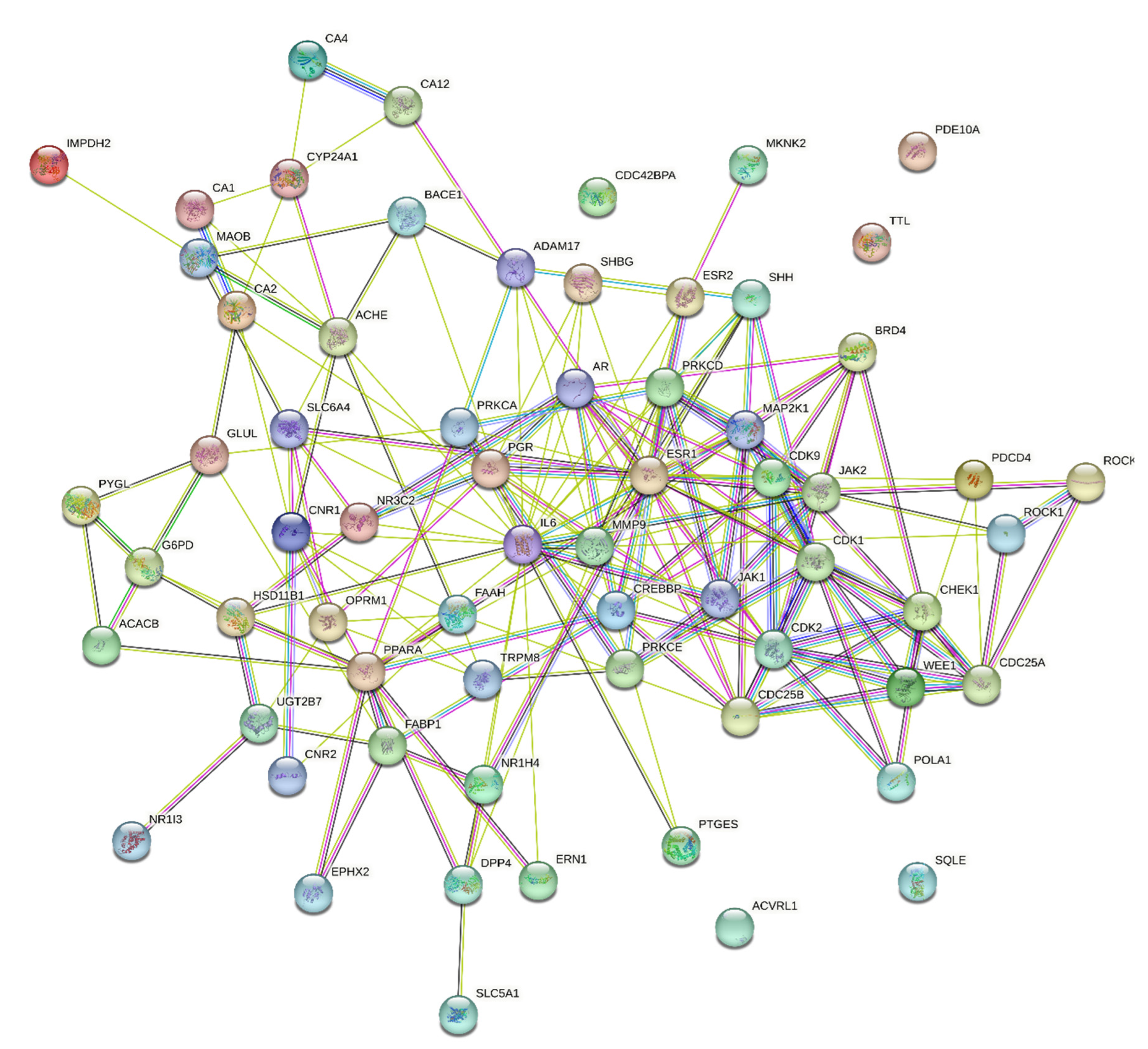
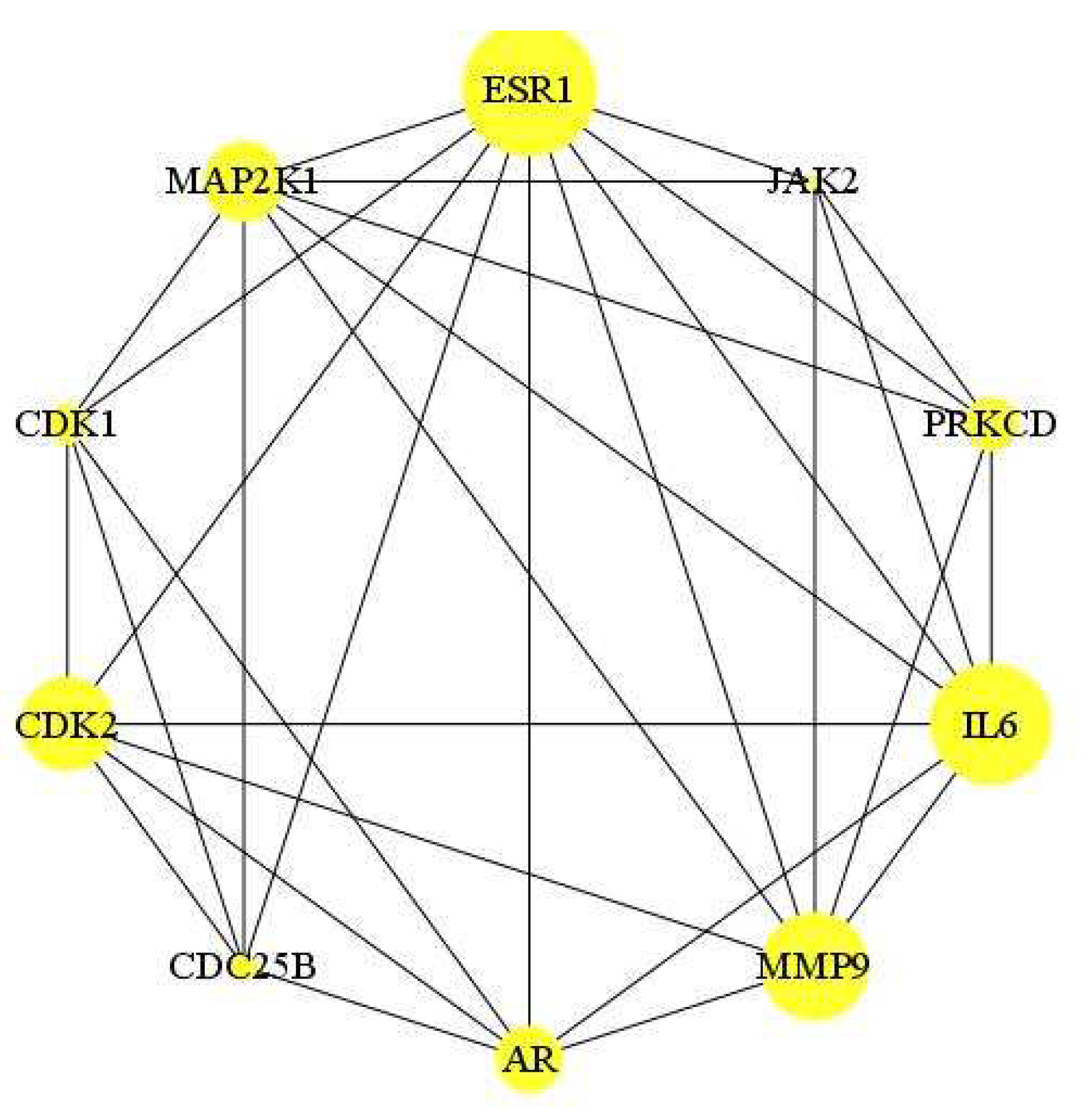
2.5.3. Compound-disease target protein–protein interaction (PPI) network
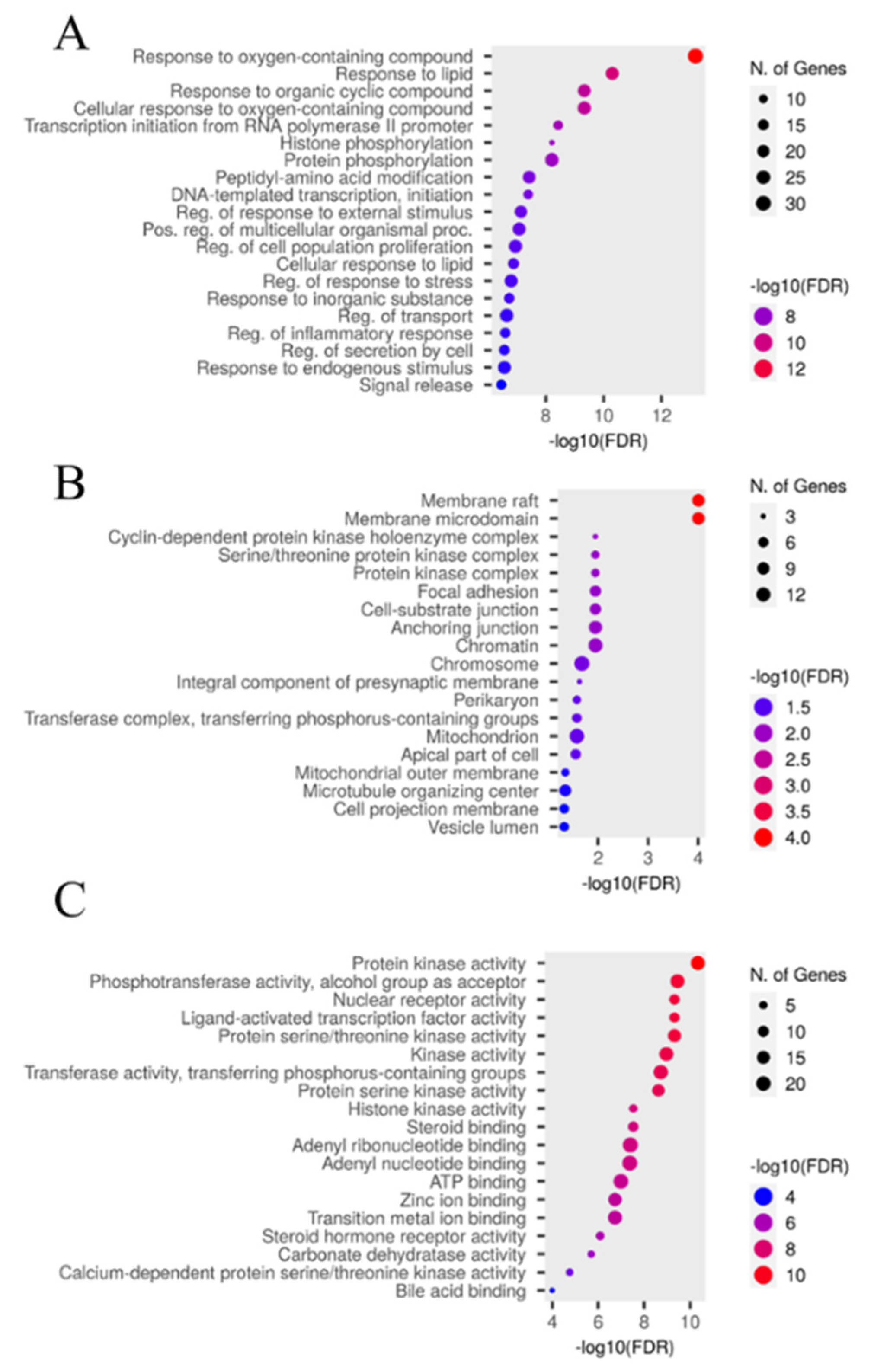
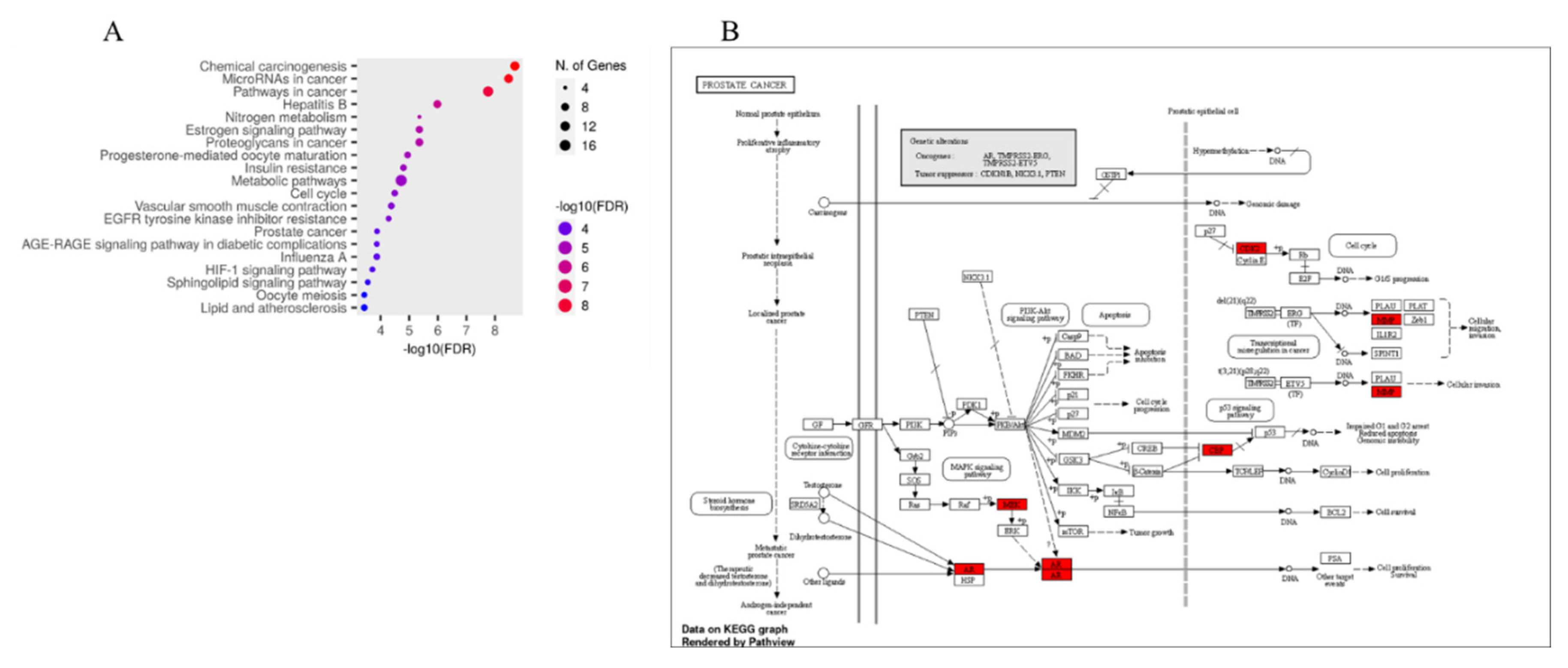
2.5.4. GO and KEGG pathway enrichment
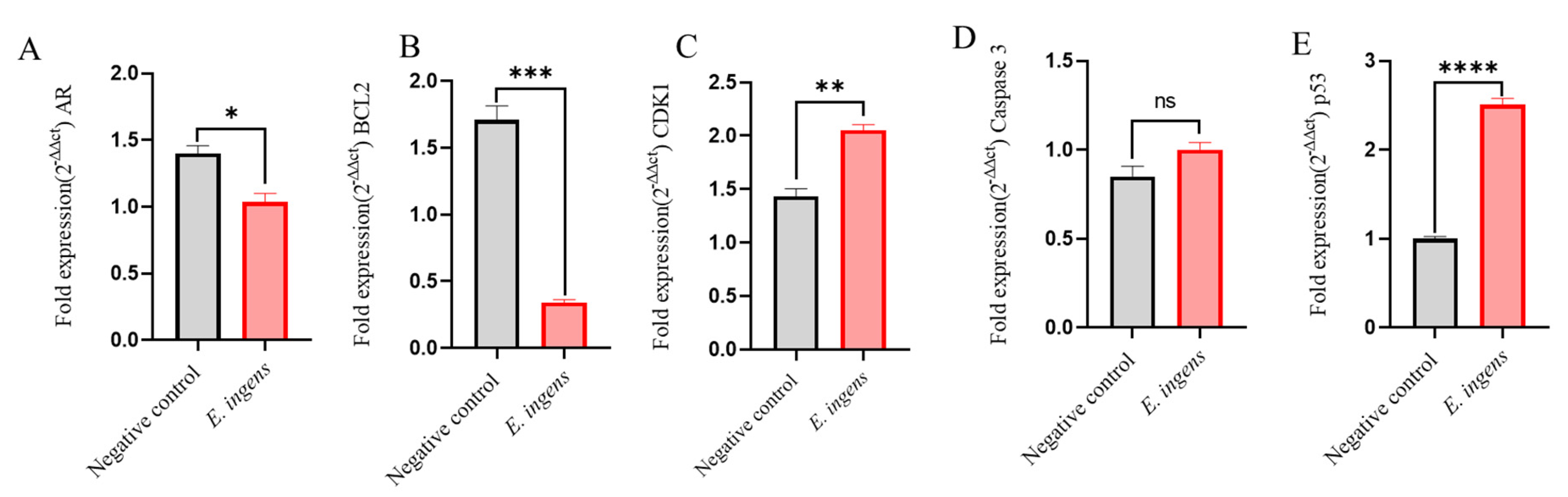
2.6. RT-qPCR results
3. Discussion
4. Materials and Methods
4.1. Plant collection and extract preparation
4.2. Cell culture
4.3. Cellular proliferation assay
4.4. Selectivity index
4.5. Identification of plant compounds
4.6. In silico work
4.6.1. Drug candidate screening test
4.6.2. Identification of candidate targets in Euphorbia ingens against Pca
4.6.3. Construction of the protein-protein interaction (PPI) network
4.6.4. Gene Ontology (GO) & Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis
4.7. Quantitative real-time polymerase chain reaction (RT-qPCR)
4.8. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Shen, M.M. Cell Types of Origin for Prostate Cancer. Curr. Opin. Cell Biol. 2015, 37, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.S.; Bhangoo, R.S.; Anderson, J.D.; Jason Shen, J.; Vargas, C.E. Prostate Cancer. Princ. Pract. Part. Ther. 2023, 383–410. [Google Scholar] [CrossRef]
- Belkahla, S.; Nahvi, I.; Biswas, S.; Nahvi, I.; Ben Amor, N. Advances and Development of Prostate Cancer, Treatment, and Strategies: A Systemic Review. Front. Cell Dev. Biol. 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Ekwan, R.; Bua, E.; Nantale, R.; Opito, R.; Abingwa, P.; Sserwanja, Q.; Kuteesa, J.; Mukunya, D. Uptake of Prostate Cancer Screening and Associated Factors among Men Aged 50 Years and above in Lira City, Uganda: A Cross-Sectional Study. BMC Public Health 2023, 23, 432. [Google Scholar] [CrossRef]
- Nowroozi, A.; Roshani, S.; Ghamari, S.-H.; Shobeiri, P.; Abbasi-Kangevari, M.; Ebrahimi, N.; Rezaei, N.; Yoosefi, M.; Malekpour, M.-R.; Rashidi, M.-M.; et al. Global and Regional Quality of Care Index for Prostate Cancer: An Analysis from the Global Burden of Disease Study 1990–2019. Arch. Public Heal. 2023, 81, 70. [Google Scholar] [CrossRef] [PubMed]
- Bosland, M.C.; Shittu, O.B.; Ikpi, E.E.; Akinloye, O. Potential New Approaches for Prostate Cancer Management in Resource-Limited Countries in Africa. Ann. Glob. Heal. 2023. [Google Scholar] [CrossRef]
- Thomas, T.S.; Pachynski, R.K. Treatment of Advanced Prostate Cancer. Mo. Med. 2018, 115, 156. [Google Scholar] [CrossRef]
- Shore, N.; Garcia-Horton, V.; Terasawa, E.; Ayyagari, R.; Grossman, J.P.; Adrianus, &; Waldeck, R. Safety Differences across Androgen Receptor Inhibitors in Nonmetastatic Castration-Resistant Prostate Cancer. https://doi.org/10.2217/fon-2022-1123 2023. [CrossRef]
- Okaiyeto, K.; Oguntibeju, O.O. African Herbal Medicines: Adverse Effects and Cytotoxic Potentials with Different Therapeutic Applications. Int. J. Environ. Res. Public Health 2021, 18. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Yamthe, L.R.T.; Tali, B.T.; Adetunji, C.O.; Rahavian, A.; Mudau, F.N.; Martorell, M.; Setzer, W.N.; Rodrigues, C.F.; et al. Phytochemicals in Prostate Cancer: From Bioactive Molecules to Upcoming Therapeutic Agents. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Tan, L.; Chen, M.; He, C. Pharmacological Activities and Molecular Mechanisms of Pulsatilla Saponins. Chinese Med. (United Kingdom) 2022, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.G. Diversity and Classification of Flowering Plants: Eudicots. Plant Syst. 2010, 275–448. [Google Scholar] [CrossRef]
- Jain, V.; Dudhal, A.; Chitale, R.; Bankar, P. A Survey of Some Medicinally Important Plants of the Family Euphorbiaceae from Baramati Area of Pune District of Maharashtra, India. Prasad Bank. 2021, 6, 456–462. [Google Scholar]
- Koduru, S.; Grierson, D.S.; Afolayan, A.J. Ethnobotanical Information of Medicinal Plants Used for Treatment of Cancer in the Eastern Cape Province, South Africa. Curr. Sci. 2017, 92, 906–908. [Google Scholar]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, C.H.; Nilsson, N.; Simonsen, H.T.; Rønsted, N. Global Medicinal Uses of Euphorbia L.(Euphorbiaceae). J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef]
- Kena, M.A. Antifungal Activities of Monsonia Burkeana and Euphorbia Ingens Extracts against Penicillium Digitatum, the Causal Agent of Citrus Green Mould. African Plant Prot. 2016, 19, 1–3. [Google Scholar] [CrossRef]
- Njeru, S.N.; Meshack, A.O. Potency of Extracts of Selected Plant Species from Mbeere, Embu County-Kenya against Mycobacterium Tuberculosis. J. Med. Plants Res. 2016, 10, 149–157. [Google Scholar] [CrossRef]
- Ross, M.J.; Steyn, G.J. Preliminary Results on the Ichthyocidal Properties of Euphorbia Ingens (Euphorbiaceae). African J. Aquat. Sci. 2004, 29, 265–269. [Google Scholar] [CrossRef]
- Okpako, I.O.; Ng’ong’a, F.A.; Kyama, M.C.; Njeru, S.N. Phytochemical Screening and Gas Chromatography- Mass Spectrometry Analysis of Euphorbia Ingens Organic Root Extract. J. Med. Plants Res. 2023, 17, 100–105. [Google Scholar] [CrossRef]
- Lalremruati, M.; Lalmuansangi, C.; Zosangzuali, M.; Tochhawng, L.; Trivedi, A.K.; Kumar, N.S.; Siama, Z. Mussaenda Macrophylla Wall. Exhibit Anticancer Activity against Dalton’s Lymphoma Ascites (DLA) Bearing Mice via Alterations of Redox-Homeostasis and Apoptotic Genes Expression. J. Basic Appl. Zool. 2022 831 2022, 83, 1–14. [Google Scholar] [CrossRef]
- Al Kury, L.T.; Taha, Z.; Mahmod, A.I.; Talib, W.H. Xanthium Spinosum L. Extracts Inhibit Breast Cancer in Mice by Apoptosis Induction and Immune System Modulation. Pharmaceuticals 2022, 15, 1504. [Google Scholar] [CrossRef]
- Eghianruwa, Q.; Osoniyi, O.; Wachira, S.; Maina, N.; Mbugua, R.; Imbuga, M. In Vitro Antiproliferative Studies of Extracts of the Marine Molluscs: Tympanatonus Fuscatus Var Radula (Linnaeus) and Pachymelania Aurita (Muller). Int. J. Biochem. Mol. Biol. 2019, 10, 1–8. [Google Scholar] [PubMed]
- Grover, M.; Behl, T.; Virmani, T.; Sanduja, M.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Meraya, A.M.; Bungau, S.G. Exploration of Cytotoxic Potential of Longifolene/Junipene Isolated from Chrysopogon Zizanioides. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Mohammed, R.; Tawfike, A.F.; Lithy, N.M.; Abouzid, S.F.; Amin, M.N.; Abdelmohsen, U.R.; Amin, E. Cytotoxic Activity and Metabolic Profiling of Fifteen Euphorbia Species. Metabolites 2020, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Chikwana, N.; Maina, E.N.; Gavamukulya, Y.; Bulimo, W.; Wamunyokoli, F. Antiproliferative Activity, c-Myc and FGFR1 Genes Expression Profiles and Safety of Annona Muricata Fruit Extract on Rhabdomyosarcoma and BALB/c Mice. J. Complement. Altern. Med. Res. 2021, 14, 30–46. [Google Scholar] [CrossRef]
- El-Baba, C.; Baassiri, A.; Kiriako, G.; Dia, B.; Fadlallah, S.; Moodad, S.; Darwiche, N. Terpenoids’ Anti-Cancer Effects: Focus on Autophagy. Apoptosis 2021, 26, 491–511. [Google Scholar] [CrossRef]
- Ansari, I.A.; Akhtar, M.S. Current Insights on the Role of Terpenoids as Anticancer Agents: A Perspective on Cancer Prevention and Treatment. Nat. Bio-active Compd. Chem. Pharmacol. Heal. Care Pract. 2019, 2, 53–80. [Google Scholar] [CrossRef]
- Dhyani, P.; Sati, P.; Sharma, E.; Attri, D.C.; Bahukhandi, A.; Tynybekov, B.; Szopa, A.; Sharifi-Rad, J.; Calina, D.; Suleria, H.A.R.; et al. Sesquiterpenoid Lactones as Potential Anti-Cancer Agents: An Update on Molecular Mechanisms and Recent Studies. Cancer Cell Int. 2022, 22, 1–18. [Google Scholar] [CrossRef]
- Ansari, I.A.; Akhtar, M.S. Recent Insights on the Anticancer Properties of Flavonoids: Prospective Candidates for Cancer Chemoprevention and Therapy. Nat. Bio-active Compd. Prod. Appl. 2019, 1, 425–448. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, J.; Chen, N.G.; Shi, Z.; Qiu, J.; He, C.; Chen, M. Recent Advances in Anticancer Activities and Drug Delivery Systems of Tannins. Med. Res. Rev. 2017, 37, 665–701. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Quispe, C.; Sharifi-Rad, J.; Cruz-Martins, N.; Nigam, M.; Mishra, A.P.; Konovalov, D.A.; Orobinskaya, V.; Abu-Reidah, I.M.; Zam, W. Phytosterols: From Preclinical Evidence to Potential Clinical Applications. Front. Pharmacol. 2021, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Khan, F.; Farooqui, A.; Ansari, I.A. Andrographolide Exhibits Anticancer Potential Against Human Colon Cancer Cells by Inducing Cell Cycle Arrest and Programmed Cell Death via Augmentation of Intracellular Reactive Oxygen Species Level. Nutr. Cancer 2018, 70, 787–803. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Grande, M.A.; Gorinstein, S.; Espitia-Rangel, E.; Dávila-Ortiz, G.; Martínez-Ayala, A.L. Plant Sources, Extraction Methods, and Uses of Squalene. Int. J. Agron. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Xiong, W.D.; Gong, J.; Xing, C. Ferruginol Exhibits Anticancer Effects in OVCAR-3 Human Ovary Cancer Cells by Inducing Apoptosis, Inhibition of Cancer Cell Migration and G2/M Phase Cell Cycle Arrest. Mol. Med. Rep. 2017, 16, 7013–7017. [Google Scholar] [CrossRef]
- Seenivasan, A.; Manikkam, R.; Kaari, M.; Sahu, A.K.; Said, M.; Dastager, S.G. 2,4-Di-Tert-Butylphenol (2,4-DTBP) Purified from Streptomyces Sp. KCA1 from Phyllanthus Niruri: Isolation, Characterization, Antibacterial and Anticancer Properties. J. King Saud Univ. - Sci. 2022, 34, 102088. [Google Scholar] [CrossRef]
- Tonisi, S.; Okaiyeto, K.; Hoppe, H.; Mabinya, L. V; Nwodo, U.U.; Okoh, A.I. Chemical Constituents, Antioxidant and Cytotoxicity Properties of Leonotis Leonurus Used in the Folklore Management of Neurological Disorders in the Eastern Cape, South Africa. 3 Biotech 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Thekkangil, A.; George, B.; Prakash, S.M.U.; Suchithra, T. V Mechanism of Streptomyces Albidoflavus STV1572a Derived 1-Heneicosanol as an Inhibitor against Squalene Epoxidase of Trichophyton Mentagrophytes. Microb. Pathog. 2021, 154, 104853. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, M.; Wang, H.; Li, S.; Wang, X.; Li, Y.; Wang, D.; Li, S. Network Pharmacology to Unveil the Biological Basis of Health-Strengthening Herbal Medicine in Cancer Treatment. Cancers (Basel). 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Fu, L.; Xu, P.; Wang, T.; Li, P. Network Pharmacology and Experimental Validation to Explore the Effect and Mechanism of Kanglaite Injection Against Triple-Negative Breast Cancer. Drug Des. Devel. Ther. 2023, 17, 901–917. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, J.; Li, Y.; Li, D.; Xu, L.; Hou, T. The Application of in Silico Drug-Likeness Predictions in Pharmaceutical Research. Adv. Drug Deliv. Rev. 2015, 86, 2–10. [Google Scholar] [CrossRef]
- Conway, E.M.; Collen, D.; Carmeliet, P. Molecular Mechanisms of Blood Vessel Growth. Cardiovasc. Res. 2001, 49, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Libra, M.; Scalisi, A.; Vella, N.; Clementi, S.; Sorio, R.; Stivala, F.; Spandidos, D.A.; Mazzarino, C. Uterine Cervical Carcinoma: Role of Matrix Metalloproteinases (Review). Int. J. Oncol. 2009, 34, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Peng, C.C.; Peng, C.H.; Hsieh, C.L.; Peng, R.Y. The Aqueous Soluble Polyphenolic Fraction of Psidium Guajava Leaves Exhibits Potent Anti-Angiogenesis and Anti-Migration Actions on DU145 Cells. Evidence-based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Beinhoff, P.; Sabharwal, L.; Udhane, V.; Maranto, C.; LaViolette, P.S.; Jacobsohn, K.M.; Tsai, S.; Iczkowski, K.A.; Wang, L.; Hall, W.A.; et al. Second-Generation Jak2 Inhibitors for Advanced Prostate Cancer: Are We Ready for Clinical Development? Cancers (Basel). 2021, 13. [Google Scholar] [CrossRef]
- Jariwala, U.; Prescott, J.; Jia, L.; Barski, A.; Pregizer, S.; Cogan, J.P.; Arasheben, A.; Tilley, W.D.; Scher, H.I.; Gerald, W.L.; et al. Identification of Novel Androgen Receptor Target Genes in Prostate Cancer. Mol. Cancer 2007, 6, 39. [Google Scholar] [CrossRef]
- Chen, S.; Lu, K.; Hou, Y.; You, Z.; Shu, C.; Wei, X.; Wu, T.; Shi, N.; Zhang, G.; Wu, J.; et al. YY1 Complex in M2 Macrophage Promotes Prostate Cancer Progression by Upregulating IL-6. J. Immunother. Cancer 2023, 11, e006020. [Google Scholar] [CrossRef]
- Palicelli, A.; Croci, S.; Bisagni, A.; Zanetti, E.; De Biase, D.; Melli, B.; Sanguedolce, F.; Ragazzi, M.; Zanelli, M.; Chaux, A. What Do We Have to Know about PD-L1 Expression in Prostate Cancer? A Systematic Literature Review (Part 6): Correlation of PD-L1 Expression with the Status of Mismatch Repair System, BRCA, PTEN, and Other Genes. Biomedicines 2022, 10, 236. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Li, Y.N.; Mu, X.; Pan, Z.L.; Liu, W.B. Targeted Regulation of MiR-195 on MAP2K1 for Suppressing ADM Drug Resistance in Prostate Cancer Cells. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8599–8608. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.; Anshabo, A.T.; Portman, N.; Lim, E.; Tilley, W.; Caldon, C.E.; Wang, S. Targeting CDK2 in Cancer: Challenges and Opportunities for Therapy. Drug Discov. Today 2020, 25, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Boutros, R.; Lobjois, V.; Ducommun, B. CDC25 Phosphatases in Cancer Cells: Key Players? Good Targets? Nat. Rev. Cancer 2007, 7, 495–507. [Google Scholar] [CrossRef]
- Chen, R.Q.; Yang, Q.K.; Lu, B.W.; Yi, W.; Cantin, G.; Chen, Y.L.; Fearns, C.; Yates, J.R.; Lee, J.D. CDC25B Mediates Rapamycin-Lnduced Oncogenic Responses in Cancer Cells. Cancer Res. 2009, 69, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Makwana, V.; Rudrawar, S.; Anoopkumar-Dukie, S. Signalling Transduction of O-GlcNAcylation and PI3K/AKT/MTOR-Axis in Prostate Cancer. Biochim. Biophys. acta. Mol. basis Dis. 2021, 1867. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; Tucci, M.; Di Stefano, R.F.; Turco, F.; Scagliotti, G.V.; Di Maio, M.; Buttigliero, C. Interactions between Androgen Receptor Signaling and Other Molecular Pathways in Prostate Cancer Progression: Current and Future Clinical Implications. Crit. Rev. Oncol. Hematol. 2021, 157, 103185. [Google Scholar] [CrossRef]
- He, R.; Ou, S.; Chen, S.; Ding, S. Network Pharmacology-Based Study on the Molecular Biological Mechanism of Action for Compound Kushen Injection in Anti-Cancer Effect. Med. Sci. Monit. 2020, 26, 1–15. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Lee, J.H.; Chai, E.Z.P.; Kanchi, M.M.; Kar, S.; Arfuso, F.; Dharmarajan, A.; Kumar, A.P.; Ramar, P.S.; Looi, C.Y.; et al. Cancer Prevention and Therapy through the Modulation of Transcription Factors by Bioactive Natural Compounds. Semin. Cancer Biol. 2016, 40–41, 35–47. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, S.; Chen, Y.; Qing, Y.; Wu, R.; Ma, T.; Zhang, Z.; Wang, Y.; Li, K. Verification of Cell Cycle-Associated Cyclin-Dependent Kinases Facilitated Prostate Cancer Progression by Integrated Bioinformatic Analysis and Experimental Validation. Heliyon 2022, 8, e10081. [Google Scholar] [CrossRef]
- Gao, X.T.; Liang, J.; Wang, L.Y.; Zhang, Z.; Yuan, P.; Wang, J.; Gao, Y.; Ma, F.; Calagua, C.; Ye, H.; et al. Phosphorylation of the Androgen Receptor at Ser81 Is Co-Sustained by CDK1 and CDK9 and Leads to AR-Mediated Transactivation in Prostate Cancer. Mol. Oncol. 2021, 15, 1901–1920. [Google Scholar] [CrossRef]
- Hatano, K.; Nonomura, N. Systemic Therapies for Metastatic Castration-Resistant Prostate Cancer: An Updated Review. World J. Mens. Health 2023, 41. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, B.; Karlsen, S.J.; Saatcioglu, F. Genistein Differentially Modulates Androgen-Responsive Gene Expression and Activates JNK in LNCaP Cells. Oncol. Rep. 2008, 19, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Mohamed, H.; Shah, A.M.; Yu, S.; Akhlaq, M.; Xiao, H.; Li, S.; Naz, T.; Nosheen, S.; Bai, X. In Vitro Anticancer Potential of Berberis Lycium Royle Extracts against Human Hepatocarcinoma (HepG2) Cells. Biomed Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M.; Barbacid, M. Cell Cycle, CDKs and Cancer: A Changing Paradigm. Nat. Rev. cancer 2009, 9, 153–166. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Goda, M.S.; Nafie, M.S.; Abu-Elsaoud, A.M.; Hareeri, R.H.; Aldurdunji, M.M.; Elhady, S.S.; Badr, J.M.; Eltahawy, N.A. Comparative Assessment of the Antioxidant and Anticancer Activities of Plicosepalus Acacia and Plicosepalus Curviflorus: Metabolomic Profiling and In Silico Studies. Antioxidants 2022, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.C.; Kamarudin, M.N.A.; Naidu, R. Anticancer Mechanism of Flavonoids on High-Grade Adult-Type Diffuse Gliomas. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.C. Trease and Evans’ Pharmacognosy: Sixteenth Edition; Elsevier Inc., 2009; ISBN 9780702029332.
- Sergazy, S.; Vetrova, A.; Orhan, I.E.; Senol Deniz, F.S.; Kahraman, A.; Zhang, J.Y.; Aljofan, M. Antiproliferative and Cytotoxic Activity of Geraniaceae Plant Extracts against Five Tumor Cell Lines. Futur. Sci. OA 2022, 8. [Google Scholar] [CrossRef]
- Fadeyi, S.A.; Fadeyi, O.O.; Adejumo, A.A.; Okoro, C.; Myles, E.L. In Vitro Anticancer Screening of 24 Locally Used Nigerian Medicinal Plants. BMC Complement. Altern. Med. 2013, 13, 79. [Google Scholar] [CrossRef]
- Navid, M.; Rad, S.; Behrouz, S.; Aghajani, S.; Behrouz, M.; Zarenezhad, E.; Ghanbariasad, A. Design, Synthesis, Anticancer and in Silico Assessment of 8-Caffeinyl-Triazolylmethoxy Hybrid Conjugates. RSC Adv. 2023, 13, 3056–3070. [Google Scholar] [CrossRef]
- Patel, N.; Patel SmtSSPatel, L.N.; Patel, N.B.; Patel, L.N.; Patel, K.D.; Patel, M. V; Kalasariya, H.S. ADMET & Cytotoxicity Prediction of Red Seaweed Gracillaria Dura: An in Silico Approach. Artic. World J. Pharm. Res. 2020, 9, 991–1004. [Google Scholar] [CrossRef]
- Borse, S.; Joshi, M.; Saggam, A.; Bhat, V.; Walia, S.; Marathe, A.; Sagar, S.; Chavan-Gautam, P.; Girme, A.; Hingorani, L.; et al. Ayurveda Botanicals in COVID-19 Management: An in Silico Multi-Target Approach. PLoS One 2021, 16, e0248479. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Wu, Y.; Li, A.S.M.; Fu, X.Q.; Yu, Z.L. Network Pharmacology and Molecular Docking-Based Prediction of Active Compounds and Mechanisms of Action of Cnidii Fructus in Treating Atopic Dermatitis. BMC Complement. Med. Ther. 2022, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Hamedifar, H.; Larijani, B.; Ansari, S.; Mahdavi, M. Synthesis and Pharmacological Properties of Polysubstituted 2-Amino-4H-Pyran-3-Carbonitrile Derivatives. Mol. Divers. 2020, 24, 1385–1431. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Lin, J.; Zhang, N.; Wang, Q.; Li, N.; Yao, K. Effect of Xuefu Zhuyu Capsule on Myocardial Infarction: Network Pharmacology and Experimental Verification. Evidence-based Complement. Altern. Med. 2023, 2023. [Google Scholar] [CrossRef]
- Zhu, B.-J.; Nai, G.-Y.; Pan, T.-X.; Ma, Z.-F.; Huang, Z.-D.; Shi, Z.-Z.; Pang, Y.-H.; Li, N.; Lin, J.-X.; Ling, G.-M. To Explore the Active Constituents of Sedum Aizoon L in the Treatment of Coronary Heart Disease Based on Network Pharmacology and Molecular Docking Methodology. Ann. Transl. Med. 2022, 10, 1327–1327. [Google Scholar] [CrossRef]
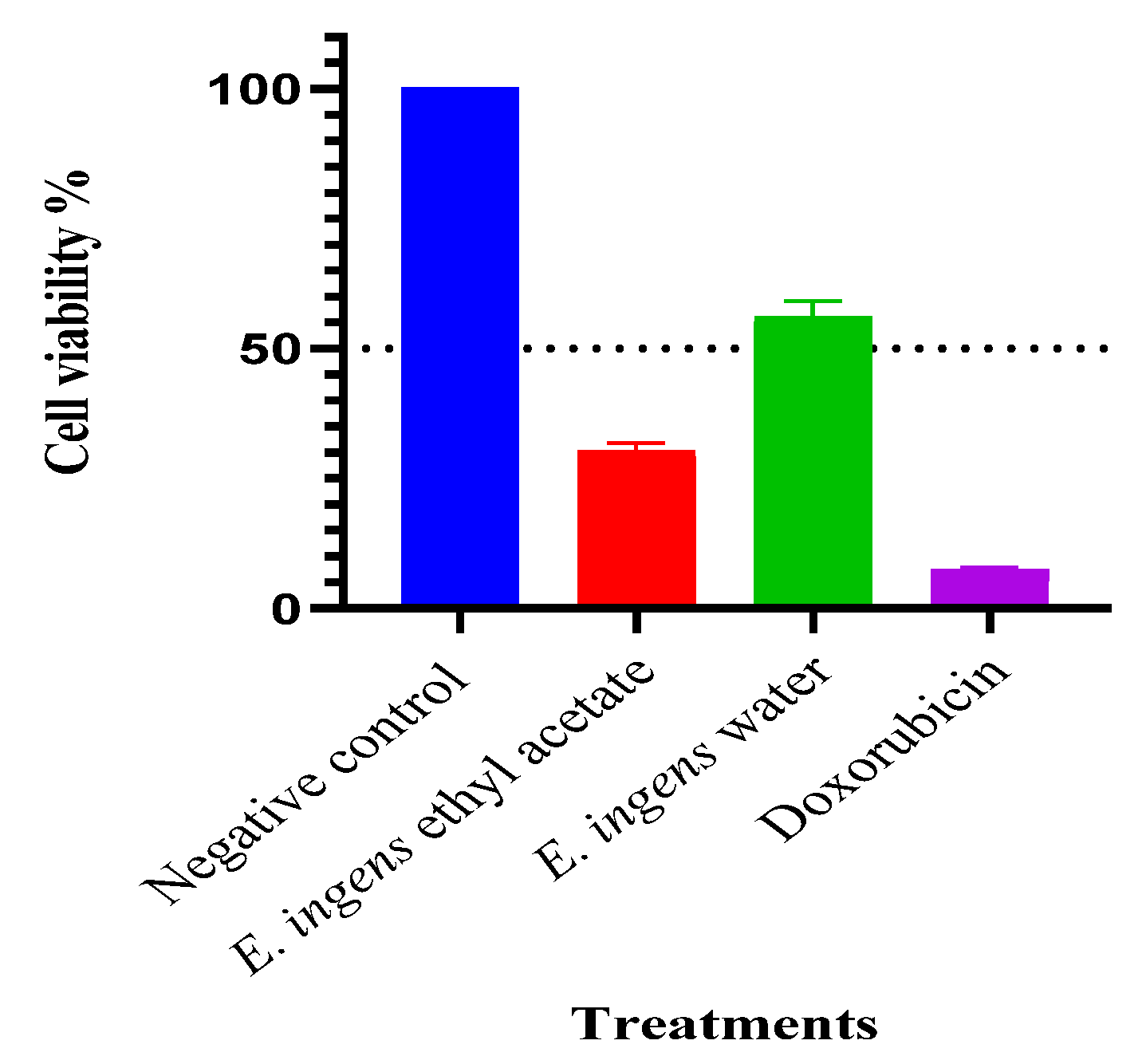

| Extract | IC50 (µg/ml) | CC50 (µg/ml) | SI |
|---|---|---|---|
| E. ingens ethyl acetate | 9.71 ± 0.40a | 80.19 ± 6.12a | 8.26 |
| Doxorubicin | 5.30 ± 0.11b | 176.10 ± 8.09b | 33.23 |
| Phytoconstituent | E. ingens ethyl acetate |
|---|---|
| Alkaloids | - |
| Phenols | ++ |
| Tannins | +++ |
| Terpenoids | +++ |
| Flavonoids | +++ |
| Saponins | +++ |
| Quinones | - |
| Sterols | +++ |
| Peak$Nr. | Rt (min) | Compound identified | Peak$Area % | MW$(g/mol) | MF | Structure Type |
|---|---|---|---|---|---|---|
| 5.302 | 1-decene | 0.29 | 140 | C10H20 | Alkene | |
| 7.952 | Bicyclo[3.1.1]heptan-3-ol | 0.23 | 152 | C10H16O | Terpenoid | |
| 8.381 | 1-dodecene | 2.11 | 168 | C12H24 | Alkene | |
| 9.034 | Bicyclo[3.1.1]hept-3-en-2-one | 0.25 | 150 | C10H14O | Terpenoid | |
| 11.339 | 1-tridecene | 3.64 | 182 | C13H26 | Alkene | |
| 13.123 | 2,4-di-tert-butylphenol | 2.03 | 206 | C14H22O | Phenol | |
| 14.003 | 1-octadecene | 4.05 | 252 | C18H36 | Alkene | |
| 14.003 | 1-octadecene | 4.05 | 252 | C18H36 | Alkene | |
| 18.543 | 1-heneicosanol | 1.86 | 312 | C21H44O | Fatty alcohol | |
| 18.543 | 1-heneicosanol | 1.86 | 312 | C21H44O | Fatty alcohol | |
| 18.543 | 1-heneicosanol | 1.86 | 312 | C21H44O | Fatty alcohol | |
| 24.128 | Octadecyl trifluoroacetate | 0.37 | 366 | C20H37F3O2 | Fatty Acid | |
| 24.639 | Prasterone | 0.14 | 288 | C19H28O2 | Sterol | |
| 25.177 | Andrographolide | 1.22 | 350 | C20H30O5 | Diterpenoid | |
| 25.695 | Ferruginol | 0.13 | 286 | C20H30O | Diterpenoid | |
| 25.834 | (1R,7S,E)-7-isopropyl-4,10-dimethylenecyclodec-5-enol | 0.63 | 220 | C15H24O | Sesquiterpenoid | |
| 26.079 | 2-bornanol | 16.75 | 348 | C16H20N4O5 | Terpenoid | |
| 26.435 | 11-oxoandrosterone | 1.53 | 376 | C22H36O3Si | Sterol | |
| 26.933 | Squalene | 0.20 | 410 | C30H50 | Triterpenoid | |
| 28.535 | 6-pentylidene-4,5-secoandrostane-4,17.beta.-diol | 55.39 | 362 | C24H42O2 | Sterol | |
| 28.827 | 17.beta.-hydroxy-6.alpha.-pentyl-4-nor-3,5-secoandrostan-3-oic acid, methyl ester | 1.78 | 378 | C24H42O3 | Fatty acid methyl ester | |
| 28.827 | 17.beta.-hydroxy-6.alpha.-pentyl-4-nor-3,5-secoandrostan-3-oic acid, methyl ester | 1.78 | 378 | C24H42O3 |
| S/N | Compounds | MW $(g/mol) | HD | HA | Mol$LogP | Lipinski’s $Rule $(Violation) | BBB | CYP2D6 | CYP3A4 | TPSA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-dodecene | 168.32 | 0 | 0 | 5.25 | Yes; 1 | No | No | No | 0 | |
| 1-heneicosanol | 312.6 | 1 | 1 | 5.62 | Yes; 1 | No | No | No | 20.23 | |
| 1-octadecene | 252.5 | 0 | 0 | 6.77 | Yes; 1 | No | No | No | 0 | |
| 1-tridecene | 182.35 | 0 | 0 | 5.52 | Yes; 1 | No | No | No | 0 | |
| Octadecyl trifluoroacetate | 366.5 | 0 | 5 | 5.47 | Yes; 1 | No | No | No | 26.30 | |
| Andrographolide | 350.4 | 3 | 5 | 1.98 | Yes; 0 | No | No | No | 86.99 | |
| Squalene | 410.7 | 0 | 0 | 3.59 | Yes; 0 | No | No | No | 0 |
| Genes | Primers |
|---|---|
| AR | Forward- GCTTTATCAGGGAGAACAGCCT$Reverse- TGCAGCTCTCTCGCAATCTG |
| BCL2 | Forward- GGCCTCAGGGAACAGAATGAT$Reverse- TCCTGTTGCTTTCGTTTCTTTC |
| CDK1 | Forward- GAACACCACTTGTCCCTCTAAGAT$Reverse- CTGCTTAGTTCAGAGAAAAGTGC |
| Caspase-3 | Forward- CAAAGAGGAAGCACCAGAACCC$Reverse- GGACTTGGGAAGCATAAGCGA |
| P53 | Forward- CTTCGAGATGTTCCGAGAGC$Reverse- GACCATGAAGGCAGGATGAG |
| β –Actin | Forward- GCCAACTTGTCCTTACCCAGA$Reverse- AGGAACAGAGACCTGACCCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





