1. Introduction
Human cytomegalovirus (CMV) herpes-virus-5 is the most frequent cause of congenital infection, with a prevalence at birth of 0.64-2% [
1]. Newborn screening, confirmation of diagnosis and investigation for possible sequelae are vital in neonatology. When the treatment is appropriate, significant morbidity and mortality associated with the more complex forms of congenital infections can be reduced. CMV represents the top cause of non-hereditary mental retardation and sensorineural deafness in childhood [
2,
3,
4]. About 30-70% of women with primary CMV infection during pregnancy transmit the virus to their fetus [
5]. The fetal damage depends on the gestational age (GA) at which the fetal infection occurs. Women with prior immunity who are re-infected or have reactivation of CMV infection have a much lower fetal transmission rate of 0.5-2% [
1,
6]. Being immune therefore reduces the probability of maternal-to-fetal transmission of the virus. Non-primary infections can, however, cause serious damage to the developing fetus and have significant late sequelae in infancy and childhood [
4,
7].
A prospective study on the neonatal screening conducted in France in 2017 showed that almost half of congenital CMV infections were non-primary and that the risk of having an infected child following a non-primary infection was higher in unemployed women from lower socioeconomic groups [
8]. Approximately 10-15% of infected newborns were symptomatic at birth. The most frequent symptoms of acute infection include hepatomegaly, splenomegaly, jaundice, petechial rash, intrauterine growth retardation, and microcephaly [
9]. In terms of long-term sequelae, the infant may develop unilateral or bilateral, profound hearing loss, visual impairment and neurodevelopmental disorders, that may manifest beyond the neonatal period throughout childhood. The diagnosis of congenital CMV infection requires detection of viral nucleic acid or replicating virus in the urine (or saliva) of the newborn during the first 3 weeks of life [
6]. The detection of CMV DNA on the screening blood-spot test (Guthrie card) performed between 48 and 72 hours after birth can be a valuable method to retrospectively confirm neonatal congenital CMV infection, when the test is positive. However, the sensitivity of this method is low [
10,
11].
Neonates with signs of central nervous system infection (changes on cerebral ultrasound or abnormal hearing screening), chorioretinitis, or severe single- or multi-system disease should be treated to improve neurodevelopmental and audiological outcomes [
12]. Antiviral therapy should be started within the first month of life and continued for six months [
13]. Severely ill and symptomatic infants, and those not able to tolerate oral medications, are started on intravenous (IV) treatment with ganciclovir (6 mg/kg/dose IV twice daily), and then switched to oral valganciclovir (16 mg/kg/dose twice daily). The duration of therapy is six months in total [
14].
We describe the case of a term neonate who underwent therapeutic hypothermia (TH) due to perinatal asphyxia. After rewarming, he developed seizures secondary to intraventricular hemorrhages and was diagnosed with congenital CMV due to maternal reactivation.
2. Case report
A male newborn was born at 37 weeks GA. Maternal medical history and her pregnancy course were unremarkable. Noninvasive prenatal testing (NIPT) was normal. Maternal serology in early pregnancy (8 weeks) showed immunization for rubella, cytomegalovirus, and toxoplasmosis. First and second trimester ultrasound scans (US) were normal. At 37 weeks and 5 days GA, the US showed polyhydramnios, fetal doppler reversal, and absence of fetal movements. As the cardiotocography (CTG) tracing revealed decreased heart rate variability, an emergency cesarean section was performed. At birth, the newborn was eutrophic (birth weight 2810 g (percentile 24), length 47.5 cm (percentile 24), and head circumference 34 cm (percentile 54)), and was bradycardic, apneic, and hypotonic. Apgar scores were at 1/4/9 at 1, 5 and, 10 minutes, respectively. The newborn was ventilated with positive pressure and supplemental oxygen for 5 minutes. Blood gas analysis at 1 hour of life showed a pH of 7.12, lactate of 18.26 mmol/L, base excess -15.5mmol/L and pC02 43.3mmHg. The clinical Thompson score was 7, suggestive of mild hypoxic ischemic encephalopathy (HIE) and the modified Sarnat score was 2 (moderate HIE). The newborn fulfilled the criteria for TH and was transferred to our neonatal intensive care unit (NICU) at 3 hours of life. On arrival, laboratory examination revealed the presence of thrombocytopenia (64,000/µl) and coagulopathy (INR 2.65), therefore platelet and plasma transfusions were given before the initiation of TH (4 hours of life). Continuous full video-electroencephalogram (vEEG) monitoring showed normal electrical activity for the entire duration of TH. The blood result series are listed in
Table 1.
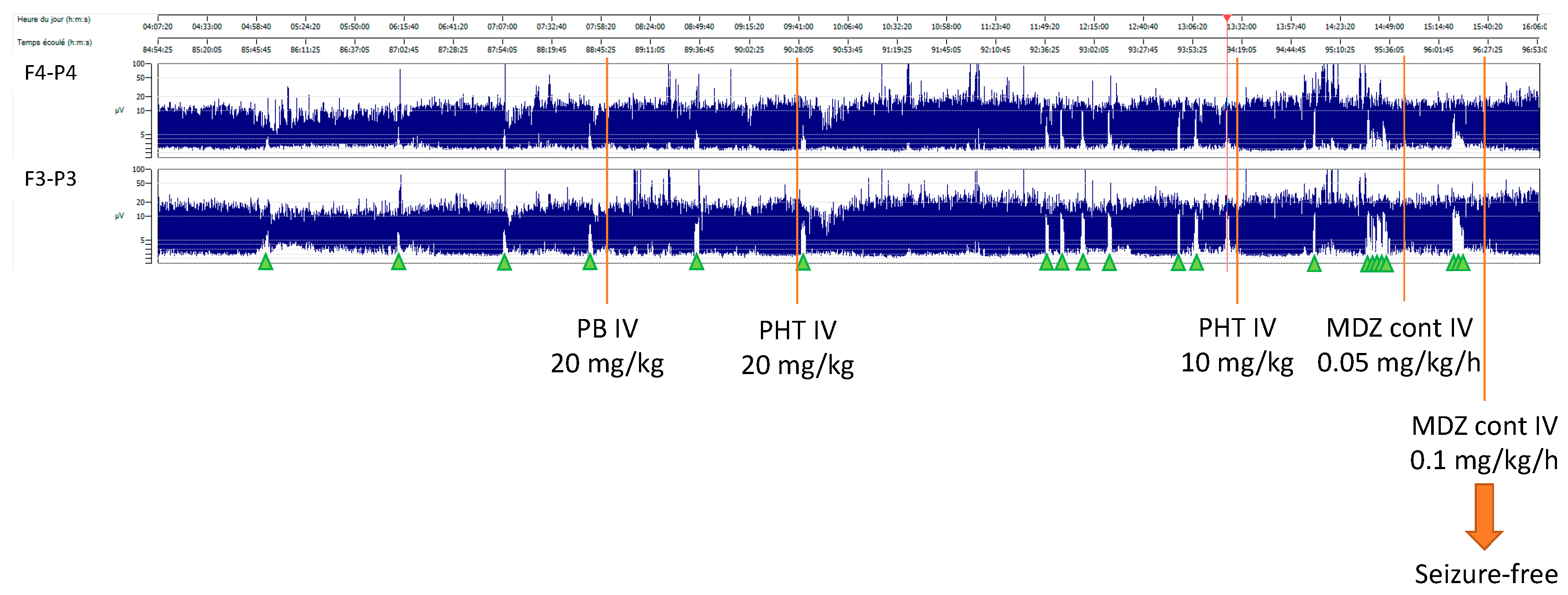
After 72 hours of TH, rewarming was started (+ 0.5°C per hour). Nine hours after the initiation of rewarming, the baby developed isolated apneic seizures. The vEEG showed left fronto-central rhythmic discharges corresponding to the episodes of apnea. A loading dose of intravenous phenobarbital (20 mg/kg) was administered but did not stop the seizures. A subsequent loading dose of phenytoin (20 mg/kg) was administered with no response. Due to the evolution into a status epilepticus, continuous intravenous midazolam was initiated and progressively increased to a maximum dose of 0.2 mcg/kg/min, followed by the resolution of seizures (
Figure 1).
The vEEG monitoring was stopped 24 hours after the last recorded seizure. Due to the severity and atypical evolution of the neurological and general state of the newborn, a sepsis screen was performed. Lab examination showed a mildly increased CRP of 9.7 mg/dl (negative value <5 mg/dl), persistent thrombocytopenia (39,000/µl), and an ongoing mild coagulopathy (INR 1.36). Intravenous broad-spectrum antibiotic therapy was started, and a brain ultrasonography (US) was performed, showing a bilateral intraventricular hemorrhage with mild ventricular dilatation (grade III). The brain Magnetic Resonance Imaging (MRI) confirmed the bilateral ventricular hemorrhage, and the presence of ischemic-hemorrhagic spots in the frontal white matter and periventricular hyper echogenicity. The biventricular hemorrhage was initially attributed to the HIE and to the TH-related thrombocytopenia and coagulopathy, that persisted despite platelet and plasma transfusions. However, despite antibiotic and supportive therapy, the clinical evolution worsened, and on day 9 of life, an echocardiography revealed severe cardiac failure with an ejection fraction of 33% and signs suggestive of cardiomyopathy. The cardiomyopathy screening, including metabolic and infectious screening of the blood, urine, cerebrospinal fluid (CSF), and nasal secretions, resulted in a positive Polymerase Chain Reaction (PCR) for CMV DNA in the CSF, nasal-pharyngeal swab, urine, and blood.
In order to ascertain the timing of the CMV infection, the Guthrie card performed at birth was retrieved and tested for CMV DNA. The test was positive, demonstrating that the newborn had contracted intrauterine CMV infection due to maternal reactivation or re-infection. The child’s persistent thrombocytopenia, which was present also prior to cooling, was likely caused by congenital CMV infection. Due to the severity of the congenital infection, therapy with intravenous ganciclovir was initiated, and after 14 days, it was switched to oral valganciclovir for a total of 6 months. Auditory evoked potentials and fundoscopy at one month of age were normal. To date the baby is 12 months old. He had a normal developmental outcome at the 9 months follow-up but was unfortunately lost during the follow-up.
4. Discussion
cCMV infection can cause serious complications in the fetus or immunologically immature newborns, causing severe sequelae at birth and later in childhood. Term infants who are infected typically present with no symptoms and have a lower chance of long-term sequelae because of their ability to control the infection. However, premature neonates or infants with primary immune disorders of T cells or natural killer cells, are at a higher risk, and may present with acute severe, potentially life-threatening symptoms that can result in permanent, irreversible sensorineural and cognitive disorders, and neurodevelopmental delay.
The CMV virus can cause significant neurological damage to the developing fetal brain. Being a neurotropic virus, it targets all brain cells, generating a widespread inflammatory reaction that leads to cellular histopathological alterations. In cases of severe cCMV infection, histopathological analysis of the brain tissues showed that the periventricular areas, which contain abundant immature cells, are mostly affected. An efficient immune system may control this phenomenon, as in asymptomatic newborns, but sometimes within the host an overwhelming inflammatory reaction mediates the development of an immune cascade that further damages the brain tissue. Immunocompromised individuals are at higher risk for this evolution [
15].
The possibility of cCMV infection as the underlying cause of thrombocytopenia was not initially suspected in our patient. The occurrence of acute provoked seizures that appeared in the context of the cerebral hemorrhage, after the ending of rewarming, and the cardiomyopathy prompted further investigations that led to the diagnosis of cCMV. Incorporating universal neonatal screening for cCMV as a standard protocol could have expedited the diagnosis, potentially preventing the cerebral hemorrhage. Severe HIE in term infants affects primarily gray matter, especially the basal ganglia and thalamus [
16]. The brain MRI findings in our patient did not suggest perinatal asphyxia. Instead, there was involvement of the periventricular areas. The biventricular and frontal intraparenchymal hemorrhages on MRI were not specific to cCMV but were rather due to thrombocytopenia and coagulopathy.
There is ongoing debate regarding universal CMV testing of all newborns at birth. According to the European Expert Consensus, newborns with a maternal history of CMV infection during pregnancy, those who are symptomatic, and infants with sensorineural hearing loss should be screened for CMV infection [
14]. Studies have shown that approximately half of cCMV infections were due to maternal non-primary infection during pregnancy. Moreover, a systematic review and meta-analysis revealed that maternal infection status did not appear to have any significant impact on the occurrence of neonatal symptomatic disease, sensorineural hearing loss, and neurologic sequelae [
4]. An important prospective cohort study, which included almost 12,000 neonates who underwent universal CMV PCR urine screening for cCMV, demonstrated that 41% of neonates with cCMV were symptomatic. The early diagnosis of cCMV can lead to the early initiation of antiviral therapies and reduce neurological impairments and sequelae. In fact, in this cohort, 58% of symptomatic neonates that were treated early had normal development or mild sequelae, whereas in infants treated for symptomatic CMV not undergoing universal screening, the rate of severe neurological sequelae is 70-90%. Furthermore, 21% of infants with symptomatic cCMV had subclinical symptoms and the universal screening allowed for normal development or mild sequelae, because of early initiation of therapy [
17]. Another study that included more than 6,000 children suggests the utility of using real-time PCR assays on buccal swab samples as a universal screening approach. To avoid parental anxiety in asymptomatic cases and reduce follow-up costs, a screening algorithm was developed to allow the determination of CMV DNA load in saliva and stratify CMV-infected infants according to low and high risk of cCMV disease. The clinical follow-up of these patients is still needed to correlate the severity of cCMV disease with CMV DNA load in saliva samples, and to establish reference cut-off values [
18]. False positive tests using saliva CMV PCR assay are common; therefore, a positive saliva test should be confirmed by a urine sample [
8].
The diagnosis of cCMV infection requires the presence of viral nucleic acid or a replicating virus within the first three weeks of life. After this period, it can be difficult to assess whether the infection is postnatally acquired [
6]. The dried blood spot (Guthrie card) is a reliable method for confirming cCMV infection retrospectively [
10], but its low sensitivity limits its value as a screening test [
19].
A European Expert Consensus in 2017 advised treating only patients with cCMV infection who have evidence of central nervous system involvement, life-threatening disease, severe single-organ disease, or multiorgan involvement. Oral valganciclovir for 6 months is the treatment of choice, and intravenous ganciclovir should be used in neonates unable to tolerate oral drugs or in the initial phase of severe symptomatic newborns [
14].
5. Conclusions
cCMV may produce widespread organ disease in the central nervous, reticuloendothelial, and hematopoietic systems. The sequelae and long-term morbidity may be underrated. Congenital CMV infection, along with other viruses, including herpes simplex and enteroviruses, should therefore be considered in the differential diagnosis of neonatal sepsis unresponsive to antibiotics. This case highlights that cCMV from maternal non-primary infection can be as severe as cCMV from maternal primary infection. During pregnancy, it is important to monitor maternal serology for CMV, even in immune women, to identify reactivation or re-infection of the virus and implement screening measures to enable prompt diagnosis. The use of a universal screening program in neonates at birth could help establish early diagnosis and redirect early severity workup, in order to start early treatment to prevent severe sequelae later in life.
Author Contributions
VM and SC collected the data and wrote the first draft of the manuscript. VM, SC and KC revised extensively literature, EC was in charge of the neurological and EEG data, CA anf FP revised for important intellectual content.
Funding
This research received no external funding.
Informed Consent Statement
Written informed consent was obtained from the patient’s parents for publication of this case report and any accompanying images. As the paper we submit is a case report, no ethical committee approval was necessary.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schutze GE, Jacobs RF. Nelson Textbook of Pediatrics. 20th ed. 2015. p. 1590–1594.
- Townsend, C.L. 176 neonati con cCMV; 16 da infezione non primaria, 9/16 esiti neurologici. Clin Infect Dis 2013.
- Simonazzi, G. 205 neonati, 30 da madri con infezione non primaria certa. 2/30 esiti gravi. Fetal Diagn Ther 2018. [Google Scholar]
- Maltezou, P.-G.; Kourlaba, G.; Kourkouni, E.; Luck, S.; Blázquez-Gamero, D.; Ville, Y.; Lilleri, D.; Dimopoulou, D.; Karalexi, M.; Papaevangelou, V. Maternal type of CMV infection and sequelae in infants with congenital CMV: Systematic review and meta-analysis. J. Clin. Virol. 2020, 129, 104518. [Google Scholar] [CrossRef] [PubMed]
- Britt, W.J. Maternal Immunity and the Natural History of Congenital Human Cytomegalovirus Infection. Viruses 2018, 10, 405. [Google Scholar] [CrossRef]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Demmler-Harrison, G.J.; Miller, J.A. ; On behalf of the Houston Congenital Cytomegalovirus Longitudinal Study Group Maternal cytomegalovirus immune status and hearing loss outcomes in congenital cytomegalovirus-infected offspring. PLOS ONE 2020, 15, e0240172. [Google Scholar] [CrossRef]
- Leruez-Ville, M.; Magny, J.-F.; Couderc, S.; Pichon, C.; Parodi, M.; Bussières, L.; Guilleminot, T.; Ghout, I.; Ville, Y. Risk Factors for Congenital Cytomegalovirus Infection Following Primary and Nonprimary Maternal Infection. Clin. Infect. Dis. 2017, 65, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Pitfalls in the Serological Evaluation of Maternal Cytomegalovirus Infection as a Potential Cause of Fetal and Neonatal Involvements: A Narrative Literature Review. J. Clin. Med. 2022, 11, 5006. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, L.; Alberti, L.; Pariani, E.; Barbi, M.; Binda, S. Diagnosing congenital Cytomegalovirus infection: don’t get rid of dried blood spots. BMC Infect. Dis. 2020, 20, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dollard, S.C.; Dreon, M.; Hernandez-Alvarado, N.; Amin, M.M.; Wong, P.; Lanzieri, T.M.; Osterholm, E.A.; Sidebottom, A.; Rosendahl, S.; McCann, M.T.; et al. Sensitivity of Dried Blood Spot Testing for Detection of Congenital Cytomegalovirus Infection. JAMA Pediatr. 2021, 175, e205441–e205441. [Google Scholar] [CrossRef] [PubMed]
- Michelle Barton, A. Michael Forrester JM, Société canadienne de pédiatrie, comité des maladies infectieuses et d’immunisation (Ontario). Mise à jour sur l’infection congénitale à cytomégalovirus : la prévention prénatale, le diagnostic néonatal et la prise en charge. Paediatr Child Health 2020. [Google Scholar]
- Kimberlin, D.W.; Jester, P.M.; Sánchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; Estrada, B.; Jacobs, R.F.; et al. Valganciclovir for Symptomatic Congenital Cytomegalovirus Disease. New Engl. J. Med. 2015, 372, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Luck SE, Wieringa JW, Blázquez-Gamero D, Henneke P, Schuster K, Butler K, et al. Congenital cytomegalovirus a European expert consensus statement on diagnosis and management. Pediatr Infect Dis J. 2017;36(12):1205–13.
- Krstanović, F.; Britt, W.J.; Jonjić, S.; Brizić, I. Cytomegalovirus Infection and Inflammation in Developing Brain. Viruses 2021, 13, 1078. [Google Scholar] [CrossRef] [PubMed]
- Cabaj A, Bekiesińska-Figatowska M, Madzik J. MRI patterns of hypoxic-ischemic brain injury in preterm and full term infants—Classical and less common MR findings. Polish J Radiol. 2012;77(3):71–6.
- Yamada, H.; Tanimura, K.; Fukushima, S.; Fujioka, K.; Deguchi, M.; Sasagawa, Y.; Tairaku, S.; Funakoshi, T.; Morioka, I. A cohort study of the universal neonatal urine screening for congenital cytomegalovirus infection. J. Infect. Chemother. 2020, 26, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Nagel, A.; Dimitrakopoulou, E.; Teig, N.; Kern, P.; Lücke, T.; Michna, D.; Korn, K.; Steininger, P.; Shahada, K.; Neumann, K.; et al. Characterization of a universal screening approach for congenital CMV infection based on a highly-sensitive, quantitative, multiplex real-time PCR assay. PLOS ONE 2020, 15, e0227143. [Google Scholar] [CrossRef] [PubMed]
- Boppana, S.B.; Ross, S.A.; Novak, Z.; Shimamura, M.; Tolan, R.W., Jr.; Palmer, A.L.; Ahmed, A.; Michaels, M.G.; Sánchez, P.J.; Bernstein, D.I.; et al. Dried Blood Spot Real-time Polymerase Chain Reaction Assays to Screen Newborns for Congenital Cytomegalovirus Infection. JAMA 2010, 303, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).