Introduction
Orchids are one of the largest and most diverse groups in the plant kingdom. Since their ancient origin, orchids have been undergoing rapid differentiation and speciation (Chase et al., 2001; Cribb et al., 2003). Orchids account for about 10 % of flowering plants. They have unique flower shapes and diverse lifestyles, and successfully occupy various habitat types on the earth(Roberts et al., 2008; Givnish et al., 2015; Givnish et al., 2016). There are six main stages of orchid growth and development: the seedling stage, the protocorm stage, the juvenile stage, the latent adult stage, the vegetative adult stage, and the blooming individual stage(Shefferson et al., 2020). The germination of seeds is a critical process in plant life cycle. However, considering the absence of endosperm in orchid seeds, how can they develop into mature plants? Orchids cannot successfully finish their life cycle or maintain their presence in the ecosystem without the assistance of microbes(Tsavkelova et al., 2007). Therefore, orchids are known to be associated with a wide variety of microorganisms, mainly consisting of fungi, bacteria, and viruses. In the process of seed germination, orchids create mycorrhizal associations with certain fungi. The roots of orchids are colonized by highly diverse microorganisms. It is an established fact that in the early stages of development, microorganisms can effectively promote the absorption of water and mineral elements in orchids, increase their resistance, thereby affecting seed germination, protocorm, and adult growth(Li et al., 2021b). Mycorrhiza has been extensively researched in terms of taxonomy, specificity, and interactions due to its great significance to plants. However, very little is known about the bacteria, viruses, and other types of microorganisms that are linked with orchids. Not only fungi but also bacteria play an important role in the acquisition of nutrients by orchids. The relationship that exists between bacteria and orchids presents a wealth of research opportunities. One of the most prominent trends is the investigation and preservation of orchids with the use of environmental microbes. In general, the current research on microorganisms related to orchids focuses mainly on the interaction between mycorrhizal fungi, the growth-promoting effect and application prospects of mycorrhizal fungi, as well as the structure and function of fungal and bacterial communities.
Relatively little is known about the diversity and biogeography of epiphytic orchid mycorrhiza and orchid-related bacteria and viruses(Li et al., 2021a). The complex relationship between orchids and microorganisms and the related concepts remain to be summarized. Therefore, based on the associated concepts of different orchid-microbial populations, including fungi and bacteria, this paper reviewed the latest research methods and effects of orchid-related microorganisms on orchid plants. It introduced the current research progress of orchid-related microorganisms. Finally, the problems and prospects of the research and utilization of orchid-associated microorganisms were discussed, which provided a new theoretical basis and ideas for the diverse protection and industrialization of orchid plants.
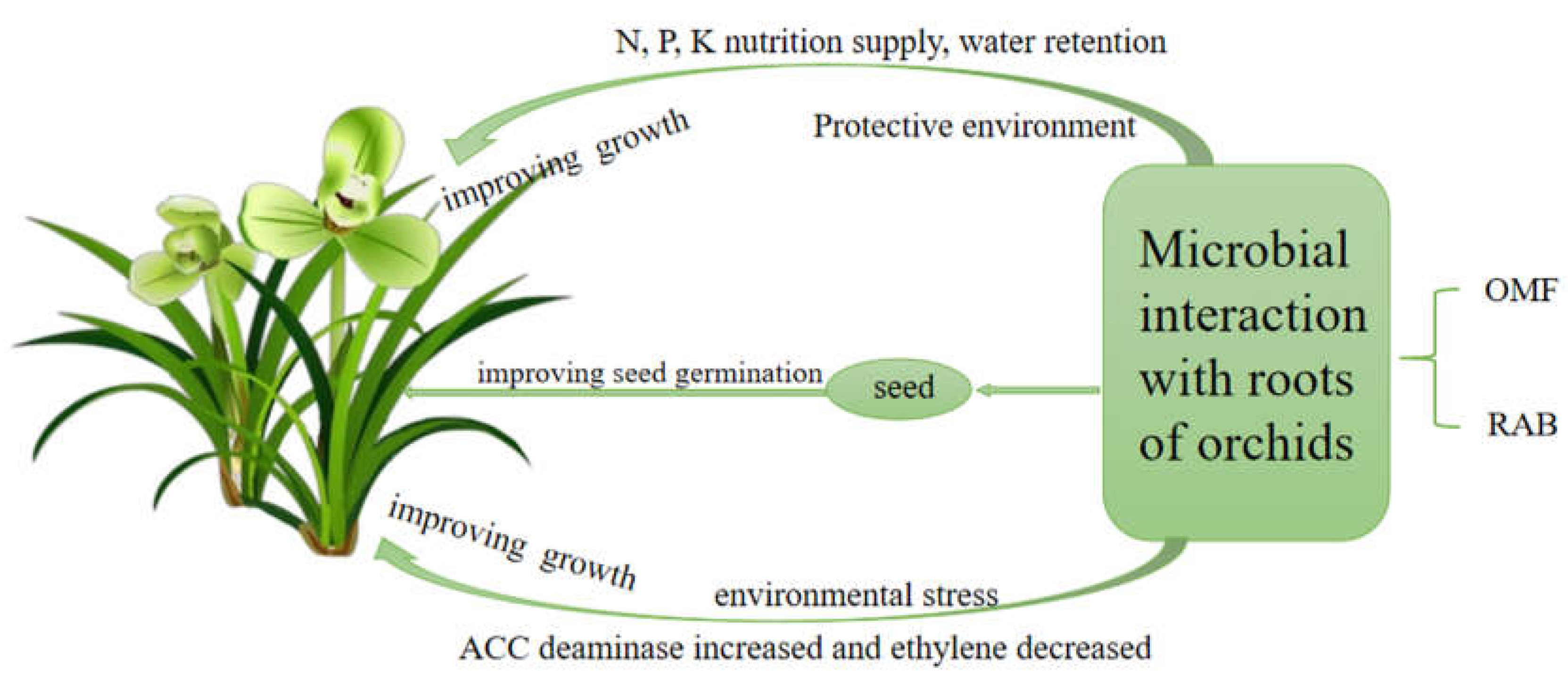
Figure 1.
Schematic diagram of the effect of environmental microorganisms on orchids. The combination of RAB and OMF jointly affects the germination and the subsequent growth and development of orchid seeds. In the schematic diagram, microorganisms play different roles under different environmental conditions.
Figure 1.
Schematic diagram of the effect of environmental microorganisms on orchids. The combination of RAB and OMF jointly affects the germination and the subsequent growth and development of orchid seeds. In the schematic diagram, microorganisms play different roles under different environmental conditions.
Concepts of associated microorganism in orchids
The orchid seed is one of the smallest seed types among the angiosperms. It has only one kind of embryo that is not surrounded by endosperm and is underdeveloped. During the whole process of its life cycle, especially in the early stage, because of lack of nutritional resources, it is very dependent on microorganisms. Mycorrhizae and other closely related microbes are essential for the successful completion of the life cycle of orchid plants and their continuous existence within an ecosystem(Mei et al., 2020). Orchids rely heavily on the microorganisms that inhabit their environments.
To explore the mysteries surrounding orchids from the point of view of environmental microorganisms, we first need to understand the classification of microorganisms related to orchids. Based on previous studies, the related concepts of orchids and their microorganisms are summarized as follows:
Root-associated microorganisms refer to the sum of endophytic fungi, bacteria, viruses, actinomycetes, and other microorganisms in plant roots or undergroud tissues. Root endophytic fungi refer to all fungal groups living in roots or root-like tissues in a certain period of plant life history, including mycorrhizal fungi and non-mycorrhizal endophytic fungi, and may also contain pathogenic fungi. Endophytic fungi are an indispensable key factor (McCormick et al., 2018). They can directly participate in the physiological and metabolic activities of plant roots and even the whole plant, ensuring plant growth, individual competition, and pathogen protection (Swarts and Dixon, 2009). Mycorrhizal fungi are a type of endophytic fungus that can create particular structures with higher plant roots or other root-like organs. They inhabit the surface, cortex, or epidermal cells of plant roots or root-like organs (Heijden et al., 2015), which can promote the absorption of soil nutrients by plants, improve the level of host mineral elements, and protect plants from biotic and abiotic stresses. Most plants can form mycorrhiza. Among all mycorrhizal plants, arbuscular mycorrhiza accounts for about 72 %, ectomycorrhiza accounts for about 2 %, orchid mycorrhiza accounts for about 10 %, and Ericoid mycorrhiza accounts for about 1.5 %. Mycorrhizal fungi are considered to be the main component of plant microorganisms. They are microbial communities closely related to multicellular communities of individuals and have no less impact on humans than on plant health. In this context, mycorrhizal fungi play a vital role in advancing the microbial revolution. They develop sustainable tools based on microbes, which help protect plant health and enhance industrial productivity (Genre et al., 2020).
Mycorrhizal fungi of the orchid usually refer to the fungal groups that form an endosymbiotic relationship or a one-way favorable relationship with orchid roots, corms, and underground stems, belonging to endophytic fungi. However, some orchid mycorrhizal fungi(OMF) do not belong to the true symbiotic fungi because they often use and deceive mycorrhizal fungi to provide nutrition for them, and mycorrhizal fungi do not benefit from it. In addition to orchid mycorrhizal fungi, there are many endophytic bacteria and rhizosphere bacteria, collectively known as root-associated bacteria ( RAB ), which enhance plant fitness, diversity, and plant co-existence through two-way or three-way interactions with plant hosts and mycorrhizal fungi(Kaur and Sharma, 2021). Among them, the root-associated bacteria that confer plant growth benefits are called plant growth promoter rhizobacteria (PGPR). They generally belong to Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes (Santoyo et al., 2016). In these phyla, Rhizobium, Bacillus, Pseudomonas, and Burkholderia are the dominant genera of PGPR. Proven functional contributions of root-associated bacteria include promoting germination of seeds and the subsequent growth of plants.(Kang et al., 2012; Glick et al., 2012). Although the relationship between roots and PGPR is no longer questionable for multiple growth benefits to host plants, there are few studies on the role of PGPR or other RAB in plant evolution (Siefert et al., 2019). It is becoming more apparent that the growth, variety, and efficiency of plants depend on the three-way interactions between plants, PGPR, and mycorrhizal fungus(van der Heijden et al., 2016). For example, PGPR can interact with fungi to provide nutrients for plants by enhancing mycorrhizal connections. This type of PGPR has been identified as mycorrhizal growth-promoting bacteria (MHB), which are known to stimulate mycelial expansion, enhance lateral root formation, or change host immunity to increase fungal infection (Bonfante and Anca., 2009; Kurth et al., 2013). We summarize the concepts related to orchid-associated microorganisms as follows :
Orchid mycorrhizal fungi (OMF): a fungus that establishes mycorrhizal associations with orchids. When hyphae enter orchids, they form a coiled structure called pelotons in the cells of orchid roots.
Root-associated bacteria (RAB): bacteria inhabiting the rhizosphere or roots of the host plant.
Plant rhizosphere growth-promoting bacteria (PGPR): a class of rhizosphere-related bacteria that can promote plant growth.
Mycorrhizal growth-promoting bacteria (MHB): a class of plant rhizosphere growth-promoting bacteria that promote mycorrhizal colonization by improving spore germination, hyphae, and root branching or by alleviating biotic and abiotic stresses.
Relevant research methods and commonly used databases
Orchids are a highly diverse and extensive family of flowering plants that possess significant ecological and economic importance. The emergence of sequencing technologies and transgenic techniques marks a significant advancement in orchid research, facilitating the application of molecular approaches to unravel the long-standing questions in orchid basic and applied biology(Zhang et al., 2022).
The second-generation sequencing technology-metagenomics technology, based on genomics technology, takes all DNA sequences and 16S rDNA sequences in environmental samples as research objects to study the diversity, population relationship, functional relationship, and relationship with the environment of microorganisms in the environment. Metagenomics technology avoids the shortcomings of most microorganisms that cannot be cultured and detected. Compared with traditional morphological research, this method is automated, batch, streamlined, economical, and convenient. The study of orchids by metagenomics is not limited to fungi but extends to all environmental microorganisms, including bacteria, archaea, and so on. Metagenomic technology has promoted the classification and culture of associated microorganisms in orchids, stimulated the comprehensive understanding of organisms, and facilitated the ecological research of microbial communities in orchids.
After sequencing, different types of information can be obtained by comparing and analyzing different databases. For example, Chen et al. completed the genome sequencing assembly of
Platanthera zijinensis and
Platanthera guangdongensis and compared them with other sequenced orchid genome data. It provides a detailed analysis of the fungal heterotrophic evolution process from the initial type-partial type-complete type. It reveals the molecular mechanism of fungal heterotrophic orchid morphogenesis and nutrient acquisition ( Chen et al., 2022 ). The establishment of an orchid genome database not only provides a reference basis for genome annotation but also plays an important role in the establishment of orchid genetic basic data and the mining and application of functional genes. The establishment, update, and maintenance of various databases and analysis platforms provide abundant resources for the research of plants and microorganisms, which has important theoretical guiding significance and application value. The commonly used database information and content are shown in
Table 1.
In addition, with the expansion of the scale of biological data, the application of machine learning in biology is increasing. Ramoneda et al. have found a way to predict the environmental pH preference of bacteria by quickly looking at the genome of bacteria and using machine learning to help researchers cultivate a critical bacterial population that has never been cultivated before ( Ramoneda et al., 2023 ). This new method will also help to further reveal the interaction, nutritional relationship, and evolutionary relationship between orchids and various microorganisms and even promote the development of orchid-related industries.
Diversity of associated microorganisms in orchids
Orchid mycorrhizal fungi (OMF)
OMF exhibit a significant diversity and play a pivotal role in the orchid life cycle.Numberous studies have shown that orchids derive mineral nutrients, and occasionally even organic compounds, from interactions with orchid mycorrhizal fungi. In recent years, the molecular identification of fungi through high-throughput methods has significantly expanded our knowledge of the diversity of OMF, revealing that it is a dynamic consequence that is co-regulated by environmental filters, limits on dispersal, spatiotemporal scales, the history of biogeography, as well as the distribution, selection, and phylogenetic spectrum width of host orchids (Li et al., 2021).
As one of the main mycorrhizal types, the mycorrhiza of orchid has a unique structure of hyphae invading plant cells and winding to form hyphae, which depend on digestive hyphae for nutrition. Orchid mycorrhiza is generally formed in two ways. One is through the infection of orchid seeds. The seeds fully absorb water, expand and turn green, then differentiate and break through the seed coat. The seed coat is broken to form radicles. The mycelium invades the radicle from the petiole end, gathers in the cortical cells, and forms the mycelium group. The mycelium group is considered to be a place for nutrient transfer between symbionts, and it is also a sign of orchid mycorrhizal formation. The second is the infection of the roots of the plant. The hyphae invade the parenchyma cells of the root of the plant, forming a fine intracellular hyphae roll.
Previous research indicates that there are 4 phyla, 7 classes, 14 orders, and 50 genera of mycorrhizal fungi that are associated with orchid plants. Mycorrhizal fungi of orchid are widely distributed in the soil around the world. Studies on the species of mycorrhizal fungi and the effects of fungi on orchid seeds, seedlings, and adult plants have been extensively carried out. At the phylum level, it mainly belongs to Basidiomycetes and Ascomycota ( Jiang et al., 2011; Illyes et al., 2010). At the family level, the dominant fungi of orchid fungi are Tulasnellaceae, Ceratobasidiaceae, Sebacinaceae, Thelephoraceae, Russulaceae, etc. ( Cevallos et al., 2017; Pandey et al., 2013). At the genus level, the dominant fungi of orchids are Epulorhiza, Ceratorhiza, Ceratobasidium, Sistotrema, Tuasnella, Russula, Mycena, Tricharina and Pezia, among which Tuasnella is the most important genus of orchids (Pereira et al., 2005). The majority of orchid mycorrhizal fungi are saprophytic, meaning that they can live and reproduce on their own in the soil, even in the absence of a supply of carbohydrates. At different points in their life cycles, orchids get into intricate symbiotic partnerships with different types of fungi. Understanding the diverse nature of OMF marks only the start of an enthralling scientific expedition.
Root-associated bacteria (RAB)
In addition to plant-fungal symbiosis in OMF plants, many intra-root and rhizosphere bacteria (root-associated bacteria ) enhance plant adaptation, diversity, and co-existence between plants through bilateral or tripartite interactions with plant hosts and mycorrhizal fungi. A variety of bacteria are associated with orchids and can be found in the roots, leaves, and flowers of plants. Some bacteria are involved in nitrogen fixation, while others can help protect orchids from disease or other environmental stressors.
The initial findings regarding bacterial isolates from the roots of orchids were first documented with Australian land-based orchids. According to fatty acid analysis, these isolates mainly belong to Pseudomonas, Xanthobacterium, Arthrosillus, and Bacillus (Wilkinson et al.,1994). Similarly, orchid RAB isolates belonging to the genus Mycobacteria, Arthrossius, Bacillus, Pseudomonas, and Erythrosococcus were identified from the roots of Russian epiphytic and terrestrial orchids based on various morphological, biochemical, or molecular analyses (Tsavkelova et al., 2007). Subsequently, a study by Júnior et al., based on 16S rDNA sequencing, isolated bacterial strains from the root section of the tropical orchid Cattleya walkeriana, belonging to the genera Bacillus, Pseudomonas, Enterobacteriaceae, and Burkholderi (Galdiano Júnior et al., 2011).
In bacterial communities that interact with plants, the bacteria colonizing the roots can influence the function of the plant host. Plants specifically enlist bacteria that are associated with their roots in response to the metabolic features of the host or the physical and chemical properties of root secretions or soil. Numerous types of root-associated bacteria have been identified as contributing to plant growth by aiding in nutrient procurement (Pii et al., 2015), hormone supply(Tsavkelova et al., 2016), protection from pathogens(Srivastava et al., 2016), and mitigation of abiotic stresses (Tiwari et al., 2016a). Overall, RAB that confer growth benefits on plant hosts are known as plant growth-promoting rhizosphere bacteria and generally belong to the bacterial phyla Proteobacteria, Actinobacteria, Firmicutes, and Bacteroidetes (Santoyo et al., 2016). Within these phyla, rhizobia, bacillus, pseudomonas, and Burkholderia species stand out as PGPR, and some of their proven functional contributions include mediating seed germination and subsequent plant growth. Although the symbiotic association between roots and PGPR yields multiple growth benefits for host plants is no longer questionable, there is little research on the role of PGPR or other RAB in plant ecology and evolution (Siefert et al., 2019). Research on RAB in plant ecology and evolution is still in its early phases. However, their functional contributions have been mostly disregarded in Orchids. With an estimated 30,000 species, orchids is an outstanding model system for studying evolution, due to its wide range of taxa and ecological strategies, including various methods of pollination and dependence on mycorrhiza. It also displays unique habits, habitats, and patterns of carbon capture.
The function of associated microorganisms on orchids
Function of fungi on orchids
Companion microbes are vital for promoting plant growth and development. In the case of mycorrhizal mutualism, fungi provide soil minerals, namely nitrogen and phosphorus, to host plants in exchange for carbon. Mycorrhizal fungi establish connections with the root systems of over 90% of plants and supply them with as much as 80% of their required mineral nutrients. Compared with arbuscular mycorrhizal fungi, orchid mycorrhizal fungi have high host specificity, which seriously hinders the breeding and conservation of orchid because it is difficult to obtain affinity fungi that can support the growth of the protocorm into seedlings. The symbiotic relationship between root and endophytic fungi is crucial for the natural seed germination and proto-bulb development of orchids (Yeh et al., 2019). As early as 1903, Bernard confirmed that under natural ecological conditions, orchid seeds could only germinate and grow if they were infected with the right fungus. Since then, studies have shown that mycorrhizal fungi are closely related to the growth and development of orchids. It is mainly manifested in the following aspects:
Function of bacteria on orchids
Enhance the stress tolerance of plants
Plants under biotic and abiotic stress induce the production of ethylene, which at a certain concentration promotes cell death and restricts plant growth (Afzal et al., 2019). Under adversity conditions, plants produce ACC (direct precursor of ethylene) in roots, which are transported to the upper ground by ACC (direct precursor of ethylene) and converted to ethylene in leaves (Liu et al., 2017). Beneficial bacteria in association with roots chelate ACC, break it down through ACC deaminase production, and utilize the resulting nitrogen and carbon by-products to lower ethylene levels, thereby enhancing plant growth during times of stress.
Research progress on microorganisms associated with orchid
Research progress on orchids and fungi
The evolutionary relationship between orchids and their mycorrhizal fungi is currently in a state of debate. Coevolution, which involves intimate interactions between two different groups of organisms, is considered a key driver of speciation, especially when such intimate interactions show a high degree of specificity(Raes et al., 2007).
Some studies suggest that highly specific relationships between orchids and fungi may promote orchid diversification and may also play a key role in the formation of certain orchid taxa in some cases that show high specificity for orchid interactions with mycorrhizal fungi (Waterman et al.,2008). Other studies suggest that there is no co-evolutionary relationship between orchids and their mycorrhizal fungi. While mycorrhizal interactions do influence the evolution of plant nutrient uptake strategies, most plants do not interact specifically with narrow-field fungal taxa (Molina et al.,1992), which makes it unlikely that plant and fungal species will co-evolve in pairs, and even specific orchid-mycorrhizal fungal relationships are unlikely to lead to co-species differentiation.
Therefore, whether there is a co-evolutionary relationship between orchids and mycorrhizal fungi is still unresolved, and the evolutionary relationship with endophytic fungi has not been systematically explored. As a highly evolved plant taxa, Orchidaceae has a rich diversity of species, the range of species selection is too small to fully clarify the evolutionary problem, and limited research methods, whether the mycorrhizal fungal taxa that have been discovered can represent the entire fungal taxa and Orchidaceae may have evolutionary relationships with orchids is still a mystery. Therefore, further research is needed to truly uncover the evolutionary relationship between orchids and mycorrhizal fungi, and endophytic fungi.
In addition, the nutritional relationship between orchids and mycorrhizal fungi has always been one of the hot spots in research. In the orchid family, about 200 species do not have chlorophyll, and their entire life history depends on their mycorrhizal fungi to provide nutrients, which can provide orchids with a variety of nutrients such as C, N, P, VITAMINS, and plant hormones such as gibberellinr (Yoder J. A et al.,2000).
Research progress on orchids and bacteria
Plant-bacteria associations have been explored for decades. However, a comprehensive understanding of the mechanism of action of plant growth-promoting bacteria remains somewhat elusive, and it can be challenging to effectively leverage these intricate mechanisms to consistently enhance plant growth in a natural setting. It is currently understood that bacteria can offer various advantages to host plants, especially in terms of facilitating growth and preserving overall well-being. Plants have the ability to "choose" their microorganisms, including those that reside within their tissues. Research has revealed that bacterial endophytes are capable of communicating and interacting with plants more efficiently than rhizosphere bacteria, especially under varying environmental conditions. As Hardoim et al. propose that "plants prefer bacteria with high ACC deaminase activity" to bring benefits to plants and that this is an evolutionary choice. At the same time, the selected bacteria encountered a protective environment with a generally stable nutrient supply, providing them with a suitable ecological niche. This mechanism makes plants more inclined to select endophytic bacteria so that bacteria and host plants can interact optimally. Also, as Hardoim et al. speculated, isolated strains of pseudomonas isolated in the experiment not only showed the highest levels of ACC deaminase activity but were also more likely to have high levels of plant growth-promoting traits than any other isolated bacterial strain(Hardoim et al.,2008).
The capability of various bacterial endophytes to enhance plant growth results from either direct or indirect mechanisms. Direct promotion of plant growth happens when bacteria assist the plant in obtaining essential nutrients or regulating its hormone levels. Nutrients that promote plant growth through bacterial access typically include nitrogen, phosphorus, and iron. Regulation of hormone levels may require plant growth to promote the bacterial synthesis of one or more plant hormones, such as auxin, cytokinin, and gibberellin.
Therefore, endophytic bacteria may have an advantage over rhizosphere bacteria because living within plant tissues increases the chance of bacteria coming into contact with plant cells. Naturally, bacteria found in the area surrounding plant roots also carry the possibility of infiltrating and inhabiting the roots themselves. The variety of bacteria that reside inside plants can be considered a smaller subset of the total population of bacteria that are associated with roots or the rhizosphere.
Conclusin
OFE and orchid plants
Scientists divide plants into three types based on the strength of their dependence on fungi for nutrition. ‘The initial fungal symbiont-the early development stage of plants’ completely depends on the carbon source provided by fungi, such as the gametophyte stage of mosses, lycophytes and ferns, and the germination stage of seeds such as orchids. In ‘Part of the fungal symbiotic type or mixed-trophic type - Plant life history’ type, at least one stage of the carbon source is provided by fungi, and part of the carbon source comes from their own photosynthetic products. ‘Complete fungal symbioses or saprophytic-plant life cycle carbon sources’ are provided by fungi(Merckx et al., 2013). The seeds of orchids do not contain endosperm, and the growth and development from seed germination to protocorm stage depend on trehalose provided by fungi. Therefore, all orchids are at least primary fungal heterotrophic; extending the expression of trehalose hydrolase to the whole life cycle is the evolutionary pathway of partial fungal heterotrophic and complete fungal heterotrophic (Li et al., 2022).
Orchids possess a unique feeding pattern that necessitates direct or indirect interactions with fungi. Of the total orchid genera, approximately 200 in 763 genera, representing less than 30%, are currently under investigation for biodiversity. Orchid fungal endophytes (OFE) are recognized to impact the ecology, growth, and structural diversity of orchids (Chen et al., 2011). Due to their small seeds and limited energy reserves, appropriate endophytic colonization is vital for orchids to reproduce in suitable habitats.
RAB and orchid plants
Symbiotic association with bacteria is a fundamental condition in plants since their terrestrialization of almost 400 mya (Martin et al., 2017).Within the bacterial community that interacts with plants, the function of the host plant is regulated by bacteria that colonize in the root zone. These root-associated bacteria (RAB) are selectively recruited by the host plant through responses to factors such as host genotype, metabolic profile, root exudates, or soil physicochemical properties. The understanding of RAB recruitment and regulation is crucial for advancing knowledge of plant-microbial interactions (Bulgarelli et al., 2013). Numerous RABs are recognized for their role in enhancing plant growth by facilitating nutrient acquisition (Pii et al., 2015), supplying hormones (Tsavkelova et al., 2016), safeguarding against pathogens (Srivastava et al., 2016), and mitigating abiotic stress (Tiwari et al., 2016b). An increasing amount of literature suggests that the community structure of rhizosphere-associated bacteria (RAB) varies across different plant taxa ( Yeoh et al., 2017 ), seasonal changes ( He et al., 2020 ), and different ecological habitats ( Mukhtar et al., 2018 ). This dynamic recruitment of RAB communities, driven by host phylogeny and microenvironmental factors, suggests their potential to regulate a wider range of ecological phenomena, such as local adaptation and ecological evolution of plants. Therefore, rhizosphere microorganisms have the ability to impart new traits to plants, and thus alter their ontogeny ( Lu et al., 2018 ). For instance, Panke-Buisse et al. showed that three genotypes of Arabidopsis thaliana and Brassica rapa exhibited delayed flowering and increased reproductive fitness when inoculated with the rhizosphere microbiome of a late-flowering ecotype of A. thaliana. This study confirmed the differences in bacterial communities in the rhizosphere of early and late flowering genotypes of A. thaliana and B. rapa by 16S rDNA sequencing. This provides some diffused evidence that by affecting the reproductive traits of plant hosts, RAB could ultimately shape their evolutionary trajectories(Panke-Buisse et al., 2015).
Evolution is influenced by reproductive barriers which can result in interspecies differentiation through reproductive isolation. Although the effects of reproductive barriers on host ontogeny, phylogeny, and evolution are not well understood, plant-soil feedback processes have been extensively studied and understood in terms of microbial effects on plant individuals, populations, and communities. The interaction between plants and microorganisms plays a crucial role in these feedback processes. Plants and microbes interact via a process described as plant-soil feedback. Plants and microorganisms have an interconnection known as plant-soil feedback. During the growth of plants, they influence the physical and chemical aspects of soil surrounding them, such as soil temperature, moisture, structure, root cell lysates, pH, and nutrients (Miki, 2012). This alteration in physicochemical properties results in changes in rhizospheric bacteria and fungal communities, subsequently impacting the host's performance and fitness (Mei et al., 2020). Microbe-mediated plant-soil feedbacks range from positive to negative, whereby either can promote species co-existence and community stabilization by affecting the fitness of key host taxa (Siefert et al., 2019). Along with promoting co-existence in plant communities, plant-soil feedback is also known to steer community succession trajectories (Zhang et al., 2018).
Outlook
Orchids are of ancient origin and belong to the most evolutionary and most advanced groups, and are still undergoing rapid divergence and speciation. Their roots are associated with a variety of microorganisms, including fungi and bacteria, which colonize their roots and play important roles. Microorganisms are the most diverse and abundant group on earth and are an indispensable component of life activities. Fungi and bacteria are essential for the growth and development of Orchidaceae and the synthesis of bioactive compounds essential for both plants and humans. Because endophytes connect microbes and plants, as well as humans, some microorganisms are a major area of research. Furthermore, learning more about the relationship between plants and microorganisms may help protect orchids through rapid reproduction.
Orchid-associated microorganisms as key factors affecting plant growth and stress require more research on these highly complex belowground interactions. So we further propose several key areas of research:
1. The rhizosphere microbiome has received great attention in the past decade or so, but the structure and function of the rhizosphere microbiome remain largely unexplored. There are still few studies on the rhizosphere microbiome of orchids, and there are still many scientific questions to be opened and solved about the interrelationship and mechanism between orchids-intraroots microbiome-rhizosphere microbiome. More metabolomics, metatranscriptomics, carbon and nitrogen isotope tracking, and other research cases are needed to fully explore the mystery.
2. In the microbial research of orchids, the number of samples is still insufficient, which can neither fully explain the relationship between all orchids and microorganisms, nor can it represent the relationship between orchids and microorganisms, and it is still necessary to continue to improve the microbial information of various species and habitats in future studies.
3. Microbial species database and gene function database still need to be vigorously built and improved. The current research on microorganisms is still very limited and proportionally estimated, and there are still trillions of microbial species that have not been discovered. At the same time, the microbial gene function database is also quite imperfect. Especially, the species and gene function databases of endogenous microorganisms shall be vigorously improved. All this makes it challenging for researchers to explore the species composition and gene function of microorganisms in the roots and rhizosphere microorganisms of orchids.
4. Ranaceae contains five subfamilies. Previous phylogenetic studies addressed relationships in five subfamilies, most families and some subfamilies. However, some and many subfamilies were not sampled in previous studies, and the relationship between them is unclear. These families and subgroups with unclear relationships also include some epiphytic ones, largely limiting the study of the evolution of orchids and epiphytes.
5. The nutritional relationship between Orchidaceae and their mycorrhizal fungi has not been determined, and more cases and deeper studies are needed to support it. Compared with the symbiotic study of legumes with arbuscular mycorrhizal fungi or nitrogen-fixing rhizobia, the symbiotic mechanism of orchid mycorrhizae is still very unclear.
6. Whether or not a co-evolutionary relationship between orchids and mycorrhizal fungi is obscure, while the evolutionary correlation with endophytes is not systematically explored.
7. It has been shown that plants selectively recruit root-associated bacteria in response to host metabolic characteristics or the physicochemical properties of root exudates or soil. Whether orchid species also selectively recruit specific microbial taxa is unknown, and the molecular pathways or cellular mechanisms of which are poorly understood.
Acknowledgments
This work was supported by The Forestry Peak Discipline Construction Project of Fujian Agriculture and Forestry University (72202200205).
Conflicts of Interests
The authors declare that they have no conflict of interests
References
- Arkhipova, T. N. , Veselov, S. U., Melentiev, A. I., Martynenko, E. V., and Kudoyarova, G. R. (2005). Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272: 201–209. [CrossRef]
- Afzal, I. , Shinwari, Z. K., Sikandar, S., and Shahzad, S. (2019). Plant beneficial endophytic bacteria: mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 221:36–49. [CrossRef]
- Baack E, Melo MC, Rieseberg LH, Ortiz-Barrientos D. (2015). The origins of reproductive isolation in plants. New Phytologist 207: 968–984. [CrossRef]
- Bonfante P, Anca I-A. (2009). Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu Rev Microbiol 63: 363–383. [CrossRef]
- Bulgarelli D, Schlaeppi K, Spaepen S, van Themaat EVL, Schulze-Lefert P.(2013). Structure and Functions of the Bacterial Microbiota of Plants. In SS Merchant, ed, Annual Review of Plant Biology, Vol 64. Annual Reviews, Palo Alto, pp 807–838. [CrossRef]
- Cevallos S, Sanchez-Rodriguez A, Decock C, Declerck S, Pablo Suarez J.(2017). Are there keystone mycorrhizal fungi associated to tropical epiphytic orchids? Mycorrhiza 27: 225–232.
- Chase M, W. (2001)The origin and biogeography of Orchidaceae. In Pridgeon, A.M., Cribb, P.J., Chase M W and Rasmussen F. Genera orchidacearum, Vol. II: Orchidoideae (part I).
- Chen R, Lin X, Shi Y. (2003). Research advances of orchid mycorrhiza. Ying Yong Yu Huan Jing Sheng Wu Xue Bao 9: 97–101.
- Chen J, Hu KX, Hou XQ, Guo SX. (2011). Endophytic fungi assemblages from 10 Dendrobium medicinal plants (Orchidaceae). World J Microbiol Biotechnol 27(5):1009–1016. [CrossRef]
- Chen, YY. , Li, C., Hsiao, YY. et al. (2022). OrchidBase 5.0: updates of the orchid genome knowledgebase. BMC Plant Biol 22: 557. [CrossRef]
- Cooper L, Meier A, Laporte M-A, Elser JL, Mungall C, Sinn BT, Cavaliere D, Carbon S, Dunn NA, Smith B, et al. (2018). The Planteome database: an integrated resource for reference ontologies, plant genomics and phenomics. Nucleic acids research 46: D1168–D1180. [CrossRef]
- Cribb P J, Kell S P, Dixon K W. (2003). Orchid conservation: a global perspective. In: Dixon K W, Kell S P, Barrett R L, editors. Orchid conservation. Kota Kinabalu, Sabah: Natural History Publications.
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. (2006). Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72: 5069–5072. [CrossRef]
- Galdiano Júnior R, Pedrinho E, Castellane TC, Lemos E. (2011). Auxin-producing bacteria isolated from the roots of Cattleya walkeriana, an endangered Brazilian orchid, and their role in acclimatization. Revista Brasileira de Ciência do Solo 35: 729–737.
- Gao C, Wu C, Zhang Q, Zhao X, Wu M, Chen R, Zhao Y, Li Z. (2020). Characterization of Chloroplast Genomes From Two Salvia Medicinal Plants and Gene Transfer Among Their Mitochondrial and Chloroplast Genomes. Front Genet 11: 574962. [CrossRef]
- Genre A, Lanfranco L, Perotto S, Bonfante P. (2020). Unique and common traits in mycorrhizal symbioses. Nat Rev Microbiol 18: 649–660. [CrossRef]
- Glick, BR. (2012). Plant growth-promoting bacteria: mechanisms and applications. Scientifica (Cairo) 2012: 963401. [CrossRef]
- Givnish T J, Spalink D, Ames M, et al. (2015). Orchid phylogenomics and multiple drivers of their extraordinary diversification. Proceedings of the Royal Society B Biological Sciences 282(1814): 2108-2111. [CrossRef]
- Givnish T J, Spalink D, Ames M, et al. (2016). Orchid historical biogeography, diversification, Antarctica and the paradox of orchid dispersal. Journal of Biogeography 43(10): 1905-1916. [CrossRef]
- Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, et al. (2012). Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40: D1178-1186. [CrossRef]
- Hardoim, P. R. , van Overbeek, L. S., & Elsas, J. D. (2008). Properties of bacterial endophytes and their proposed role in plant growth. Trends in microbiology 16(10): 463–471.
- Hoffman, M. T. , Gunatilaka, M. K., Wijeratne, K., Gunatilaka, L., and Arnold, A. E. (2013). Endohyphal bacterium enhances production of Indole-3-Acetic Acid by a foliar fungal endophyte. PLoS One 8:e73132. [CrossRef]
- He R, Zeng J, Zhao D, Huang R, Yu Z, Wu QL.(2020). Contrasting Patterns in Diversity and Community Assembly of Phragmites australis Root-Associated Bacterial Communities from Different Seasons. Appl Environ Microbiol 86: e00379-20. [CrossRef]
- van der Heijden MG, Bruin S de, Luckerhoff L, van Logtestijn RS, Schlaeppi K. (2016). A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J 10: 389–399. [CrossRef]
- Heijden MGA, Martin FM, Selosse M, Sanders IR. (2015). Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205: 1406–1423. [CrossRef]
- Illyes Z, Ouanphanivanh N, Rudnoy S, Orczan AK, Bratek Z. (2010). The Most Recent Results on Orchid Mycorrhizal Fungi in Hungary. Acta Biol Hung 61: 68–76.
- Jiang W, Yang G, Zhang C, Fu C. (2011). Species composition and molecular analysis of symbiotic fungi in roots of Changnienia amoena (Orchidaceae). Afr J Microbiol Res 5: 222–228.
- Kang S-M, Khan AL, Hussain J, Ali L, Kamran M, Waqas M, Lee I-J. (2012). Rhizonin A from Burkholderia sp. KCTC11096 and its growth promoting role in lettuce seed germination. Molecules 17: 7980–7988. [CrossRef]
- Kaur J, Sharma J. (2021). Orchid Root Associated Bacteria: Linchpins or Accessories? Front Plant Sci 12: 661966.
- Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, et al. (2013). Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22: 5271–5277. [CrossRef]
- Kurth F, Zeitler K, Feldhahn L, Neu TR, Weber T, Krištůfek V, Wubet T, Herrmann S, Buscot F, Tarkka MT. (2013). Detection and quantification of a mycorrhization helper bacterium and a mycorrhizal fungus in plant-soil microcosms at different levels of complexity. BMC Microbiol 13: 205. [CrossRef]
- Li T, Wu S, Yang W, Selosse M-A, Gao J. (2021a). How Mycorrhizal Associations Influence Orchid Distribution and Population Dynamics. Frontiers in Plant Science 12. [CrossRef]
- Li T, Yang W, Wu S, Selosse M-A, Gao J. (2021b). Progress and Prospects of Mycorrhizal Fungal Diversity in Orchids. Front Plant Sci 12: 646325. [CrossRef]
- Li, MH. , Liu, KW., Li, Z. et al. (2022). Genomes of leafy and leafless Platanthera orchids illuminate the evolution of mycoheterotrophy. Nat. Plants 8:373–388. [CrossRef]
- Liu, F. , Xing, S., Ma, H., Du, Z., and Ma, B. (2013). Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladus orientalis container seedlings. Appl. Microbiol. Biotechnol 97: 9155-9164. [CrossRef]
- Liu, H. , Carvalhais, L. C., Crawford, M., Singh, E., Dennis, P. G., Pieterse, C. M., et al. (2017). Inner plant values: diversity, colonization and benefits from endophytic bacteria. Front. Microbiol 8:2552. [CrossRef]
- Lu T, Ke M, Lavoie M, Jin Y, Fan X, Zhang Z, Fu Z, Sun L, Gillings M, Peñuelas J, et al. (2018). Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 6: 231. [CrossRef]
- Maidak BL, Cole JR, Lilburn TG, Parker CT, Saxman PR, Farris RJ, Garrity GM, Olsen GJ, Schmidt TM, Tiedje JM. (2001). The RDP-II (Ribosomal Database Project). Nucleic Acids Res 29: 173–174.
- Martin FM, Uroz S, Barker DG. (2017). Ancestral alliances: Plant mutualistic symbioses with fungi and bacteria. Science 356: eaad4501. [CrossRef]
- McCormick MK, Whigham DF, Canchani-Viruet A. (2018). Mycorrhizal fungi affect orchid distribution and population dynamics. New Phytologist 219: 1207–121. [CrossRef]
- Merckx, V. (2013). Mycoheterotrophy, the Biology of Plants Living on Fungi.Springer.
- Mei Z, Lin M, Xiong H, Xiang X, Zhou Z, Liang L. (2020). Root-associated microbiomes of Gymnadenia conopsea under the combined effect of plant geographical location and developmental stage.
- Ming, L. (2001). Studies and applications on mycorrhiza of Paphiopedilum armeniacum. Joural of Biology.
- Miki, T. (2012). Microbe-mediated plant-soil feedback and its roles in a chnaging world. Ecol. Res. 27: 509–520.
- Molina R, Massicotte H, Trappe J M. (1992). Specificity phenomena in mycorrhizal symbioses community-ecological consequences and practical implications. Chapman and Hall, New York, USA.
- Mukhtar S, Mirza BS, Mehnaz S, Mirza MS, Mclean J, Malik KA.(2018). Impact of soil salinity on the microbial structure of halophyte rhizosphere microbiome. World J Microbiol Biotechnol 34: 136. [CrossRef]
- Pandey M, Sharma J, Taylor DL, Yadon VL. (2013). A narrowly endemic photosynthetic orchid is non-specific in its mycorrhizal associations. Mol Ecol 22: 2341–2354. [CrossRef]
- Panke-Buisse K, Poole AC, Goodrich JK, Ley RE, Kao-Kniffin J. (2015). Selection on soil microbiomes reveals reproducible impacts on plant function. ISME J 9: 980–989. [CrossRef]
- Pereira OL, Kasuya MCM, Borges AC, de Araujo EF. (2005). Morphological and molecular characterization of mycorrhizal fungi isolated from neotropical orchids in Brazil. Can J Bot-Rev Can Bot 83: 54–65. [CrossRef]
- Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C. (2015). Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol Fertil Soils 51: 403–415. [CrossRef]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41: D590-596.
- Rajkumar, M., Ae, N., and Freitas, H. (2009). Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 77: 153–160. [CrossRef]
- Ramoneda J, Stallard-Olivera E, Hoffert M, et al. (2023). Building a genome-based understanding of bacterial pH preferences. Science advances 9(17):eadf8998. [CrossRef]
- Roberts D L, Dixon K W. (2008) Orchids [J]. Current Biology 18(8): 325-329.
- Santoyo G, Moreno-Hagelsieb G, Orozco-Mosqueda M del C, Glick BR. (2016). Plant growth-promoting bacterial endophytes. Microbiol Res 183: 92–99. [CrossRef]
- Shefferson RP, Jacquemyn H, Kull T, Hutchings MJ. (2020). The demography of terrestrial orchids: life history, population dynamics and conservation. Bot J Linnean Soc 192: 315–332. [CrossRef]
- Siefert A, Zillig KW, Friesen ML, Strauss SY. (2019). Mutualists Stabilize the Co-existence of Congeneric Legumes. Am Nat 193: 200–212.
- Srivastava S, Bist V, Srivastava S, Singh PC, Trivedi PK, Asif MH, Chauhan PS, Nautiyal CS. (2016) .Unraveling Aspects of Bacillus amyloliquefaciens Mediated Enhanced Production of Rice under Biotic Stress of Rhizoctonia solani. Front Plant Sci 7: 587. [CrossRef]
- Swarts ND, Dixon KW. (2009). Terrestrial orchid conservation in the age of extinction. Annals of Botany 104: 543–556. [CrossRef]
- Tello-Ruiz MK, Jaiswal P, Ware D.(2022). Gramene: A Resource for Comparative Analysis of Plants Genomes and Pathways. Methods Mol Biol 2443: 101–131.
- Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M. (2004). MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37: 914–939. [CrossRef]
- Tiwari S, Lata C, Chauhan PS, Nautiyal CS. (2016a). Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem 99: 108–117. [CrossRef]
- Tiwari S, Lata C, Chauhan PS, Nautiyal CS. (2016b). Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol Biochem 99: 108–117. [CrossRef]
- Toumatia, O. Compant, S., Yekkour, A., Goudjal, Y., Sabaou, N., Mathieu, F., et al. (2016). Biocontrol and plant growth promoting properties of Streptomyces mutabilis strain IA1 isolated from a Saharan soil on wheat seedlings and visualization of its niches of colonization. S. Afr. J. Bot. 105: 234–239. [CrossRef]
- Tsavkelova EA, Cherdyntseva TA, Botina SG, Netrusov AI. (2007). Bacteria associated with orchid roots and microbial production of auxin. Microbiol Res 162: 69–76. [CrossRef]
- Tsavkelova EA, Egorova MA, Leontieva MR, Malakho SG, Kolomeitseva GL, Netrusov AI.(2016).Dendrobium nobile Lindl. seed germination in co- cultures with diverse associated bacteria. Plant Growth Regul 80: 79–91.
- Wilkinson, K. G. , Dixon, K. W., Sivasithamparam, K., and Ghisalberti, E. L. (1994). Effect of IAA on symbiotic germination of an Australian orchid and its production by orchid-associated bacteria. Plant Soil 159:291–295. [CrossRef]
- Waterman R J, Bidartondo M I. (2008). Deception above, deception below: linking pollination and mycorrhizal biology of orchids. Journal of Experimental Botany 59(5): 1085-1096. [CrossRef]
- Xiong M, Zhao Z, Arnold J, Yu F. (2011). Next-Generation Sequencing. BioMed Research International 2010: e370710.
- Xu Z-X, Zhu X-M, Yin H, Li B, Chen X-J, Fan X-L, Li N-Q, Selosse M-A, Gao J-Y, Han J-J.(2023). Symbiosis between Dendrobium catenatum protocorms and Serendipita indica involves the plant hypoxia response pathway. Plant Physiol kiad198. [CrossRef]
- Yeoh YK, Dennis PG, Paungfoo-Lonhienne C, Weber L, Brackin R, Ragan MA, Schmidt S, Hugenholtz P. (2017). Evolutionary conservation of a core root microbiome across plant phyla along a tropical soil chronosequence. Nat Commun 8: 215. [CrossRef]
- Yeh C-M, Chung K, Liang C-K, Tsai W-C. (2019). New Insights into the Symbiotic Relationship between Orchids and Fungi. Applied Sciences 9: 585. [CrossRef]
- Ye B, Wu Y, Zhai X, et al. (2020). Beneficial Effects of Endophytic Fungi from the Anoectochilus and Ludisia Species on the Growth and Secondary Metabolism of Anoectochilus roxburghii. ACS Omega 5(7):3487-3497. [CrossRef]
- Yoder J., A. , Zettler L. W., Stewart S L. (2000). Water requirements of terrestrial and epiphytic orchid seeds and seedlings, and evidence for water uptake by means of mycotrophy. Plant Sci 156: 145-150. [CrossRef]
- Zhang S, Yang Y, Li J, Qin J, Zhang W, Huang W, Hu H. (2018). Physiological diversity of orchids. Plant Diversity 40: 196–208. [CrossRef]
- Zhang, D., Zhao, X. W., Li, Y. Y., Ke, S. J., Yin, W. L., Lan, S., & Liu, Z. J. (2022). Advances and prospects of orchid research and industrialization. Horticulture research, 9, uhac220. [CrossRef]
- Zhou X, Su Z. (2007). EasyGO: Gene Ontology-based annotation and functional enrichment analysis tool for agronomical species. BMC Genomics 8: 246. [CrossRef]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. (2004). GENEVESTIGATOR. Arabidopsis Microarray Database and Analysis Toolbox. Plant Physiol 136: 2621–2632. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).