Submitted:
26 May 2023
Posted:
29 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant material and field trials
2.1. Location and Soil Characteristics
2.2. Experimental Treatments and Growing Conditions
2.2. Grain and semolina analyses
2.3. Gluten protein composition analysis of harvested grain from 2021 trial
2.4. Statistical analysis
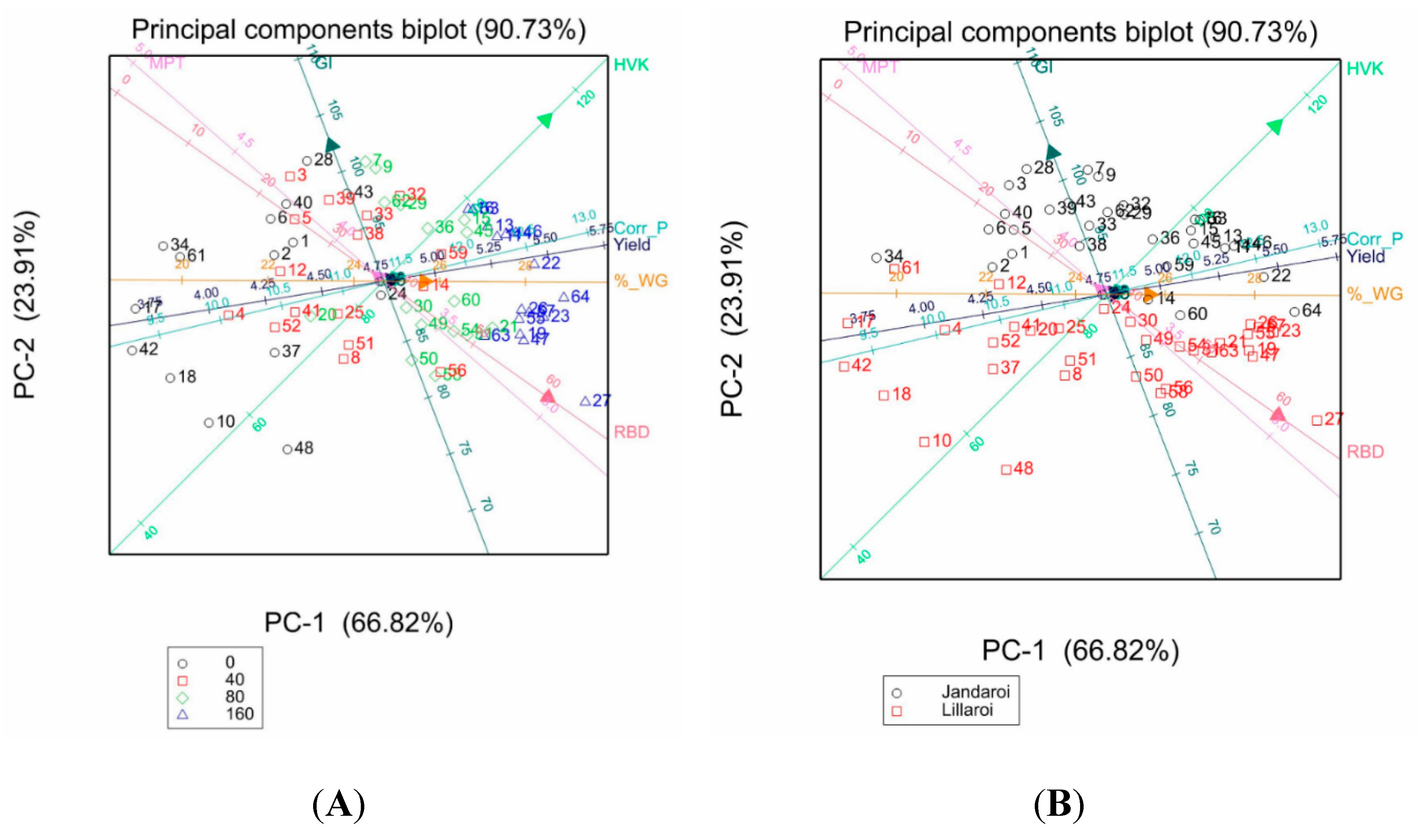
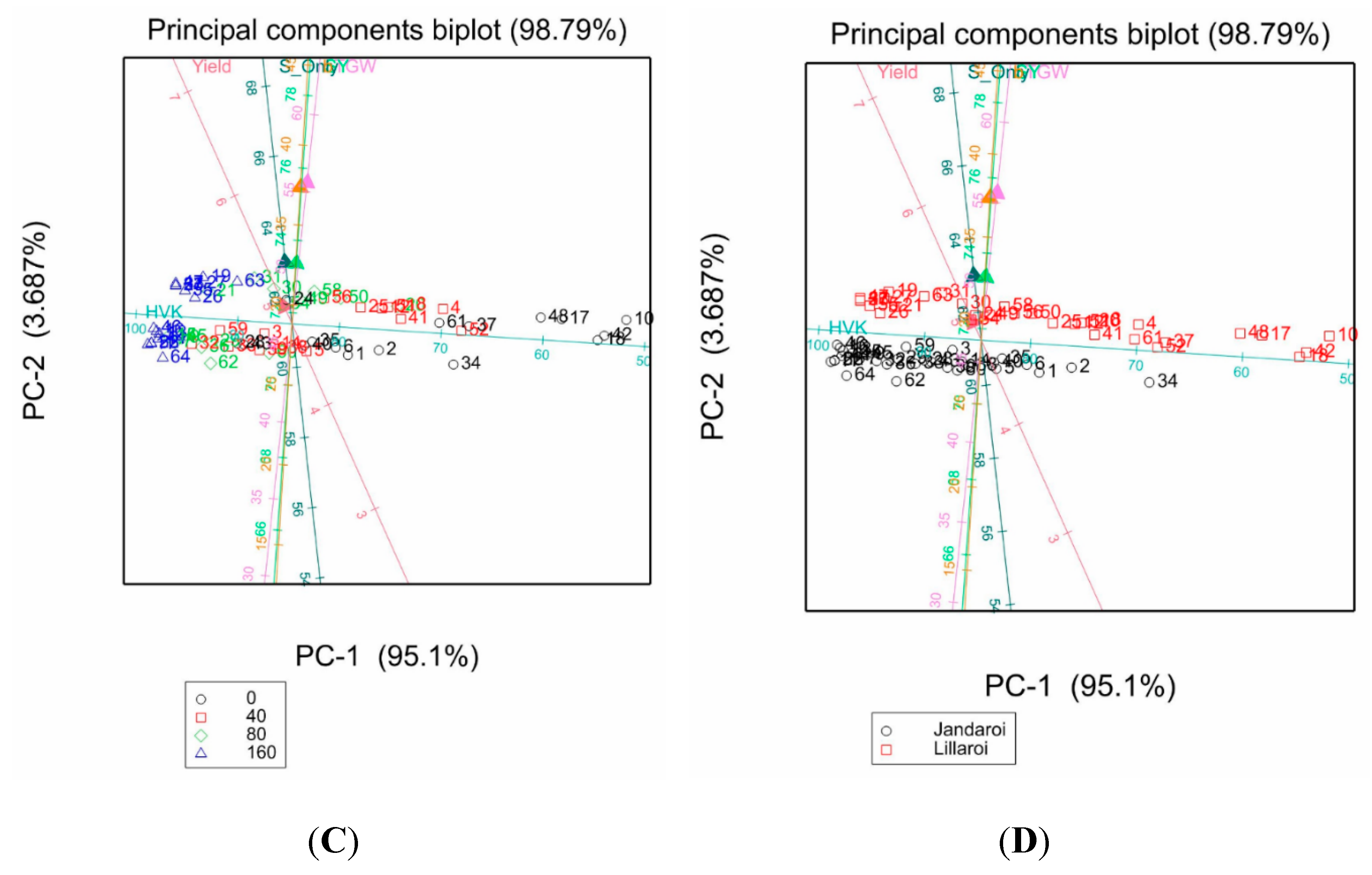
3. Results
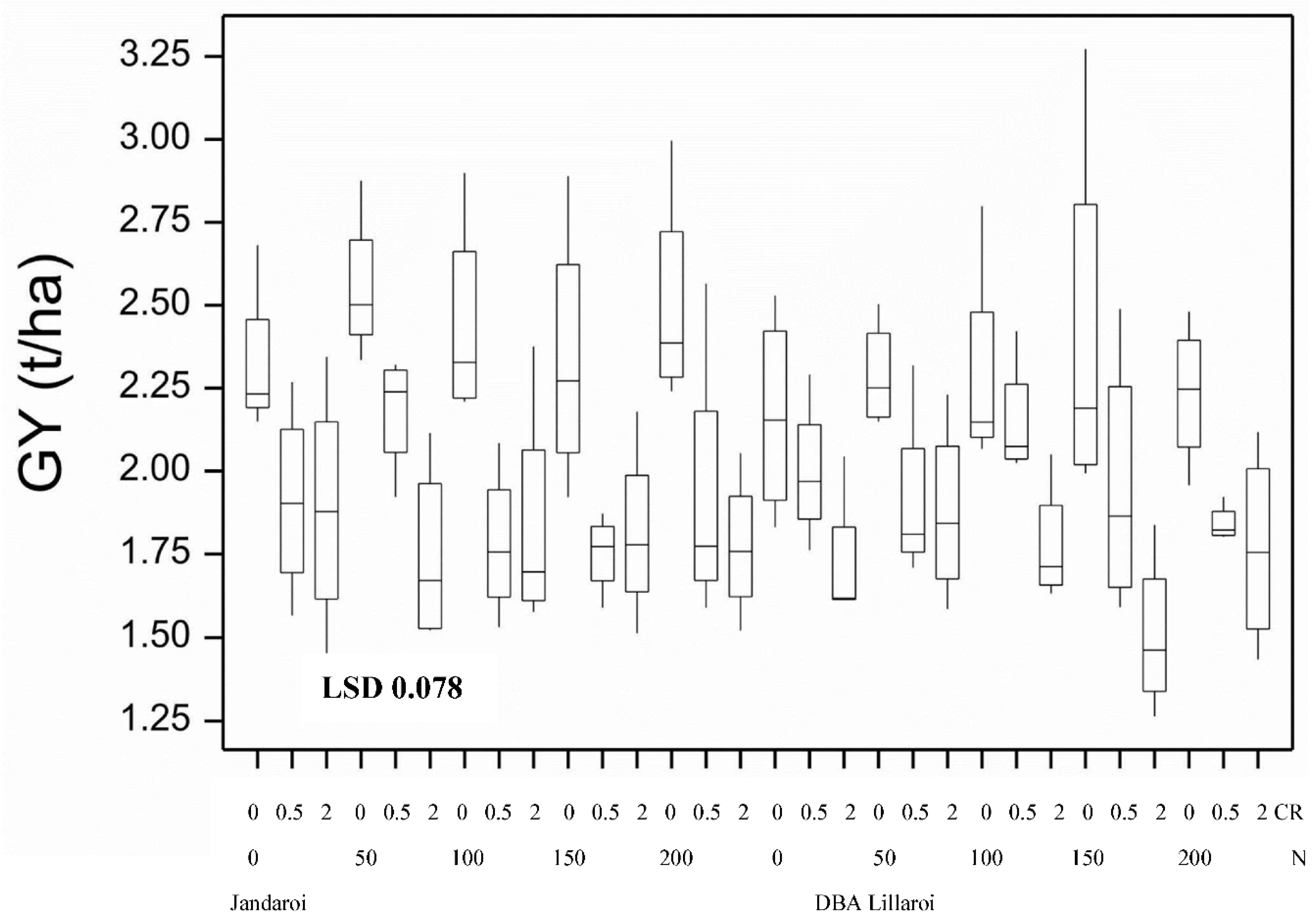
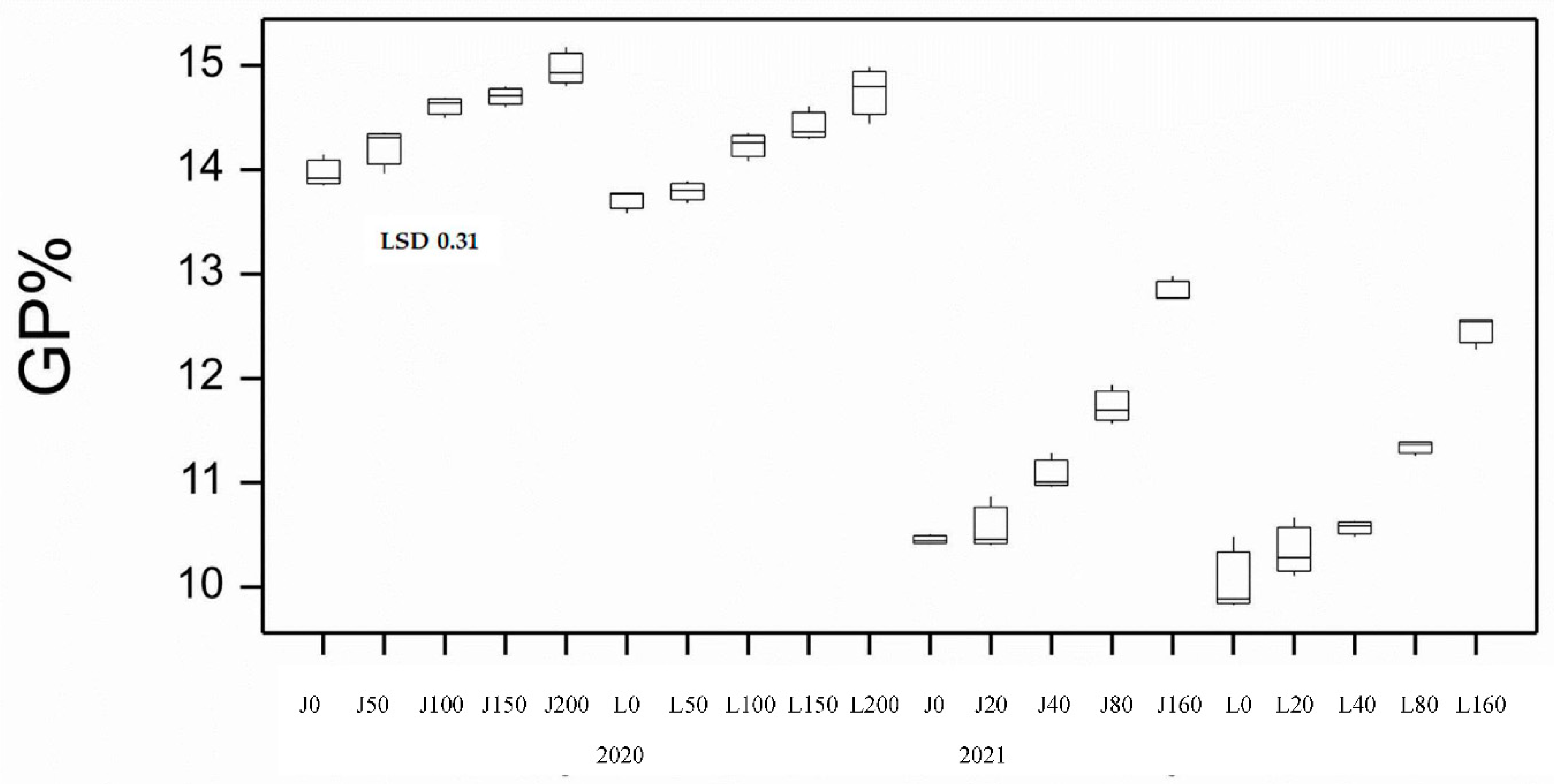
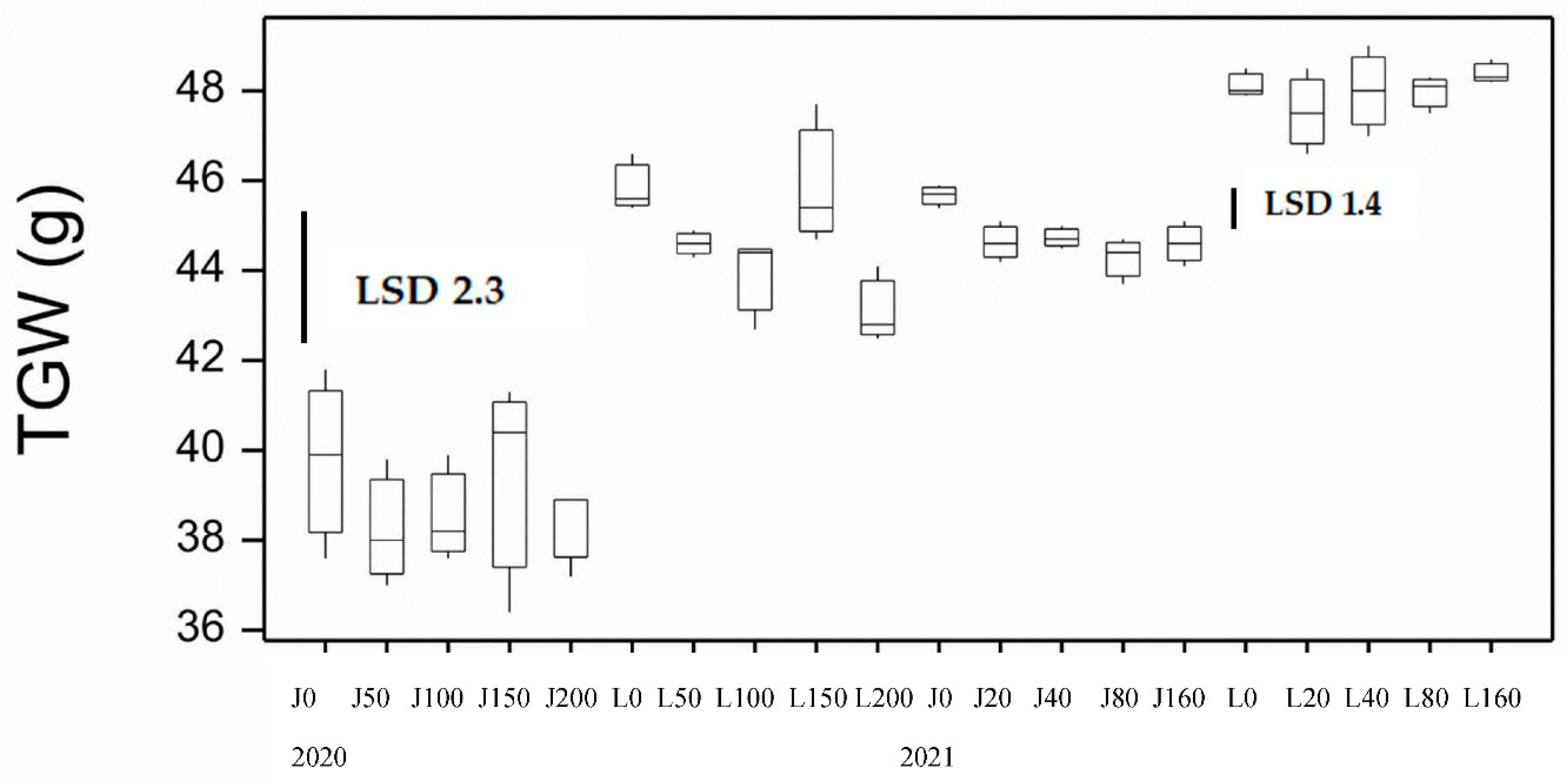
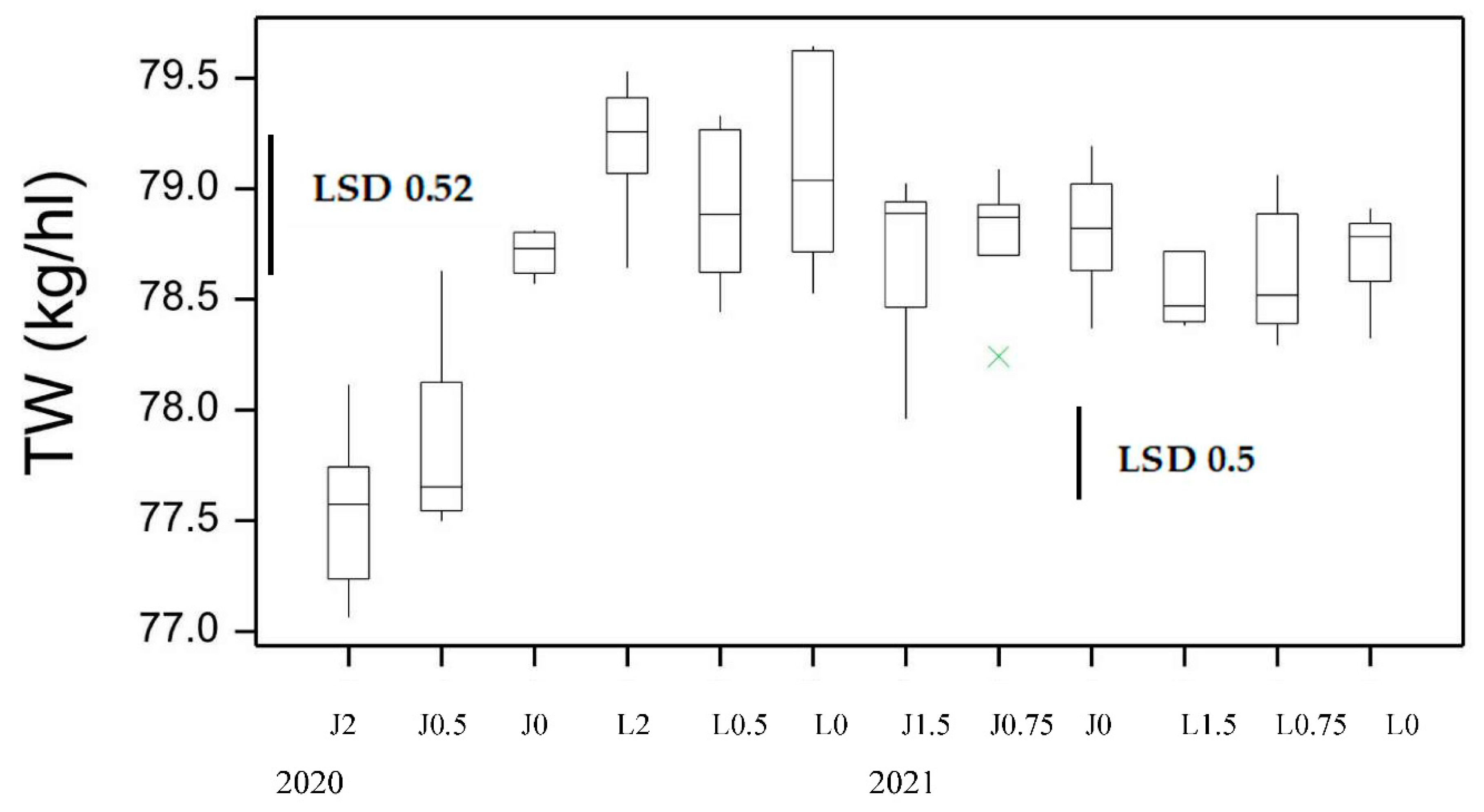
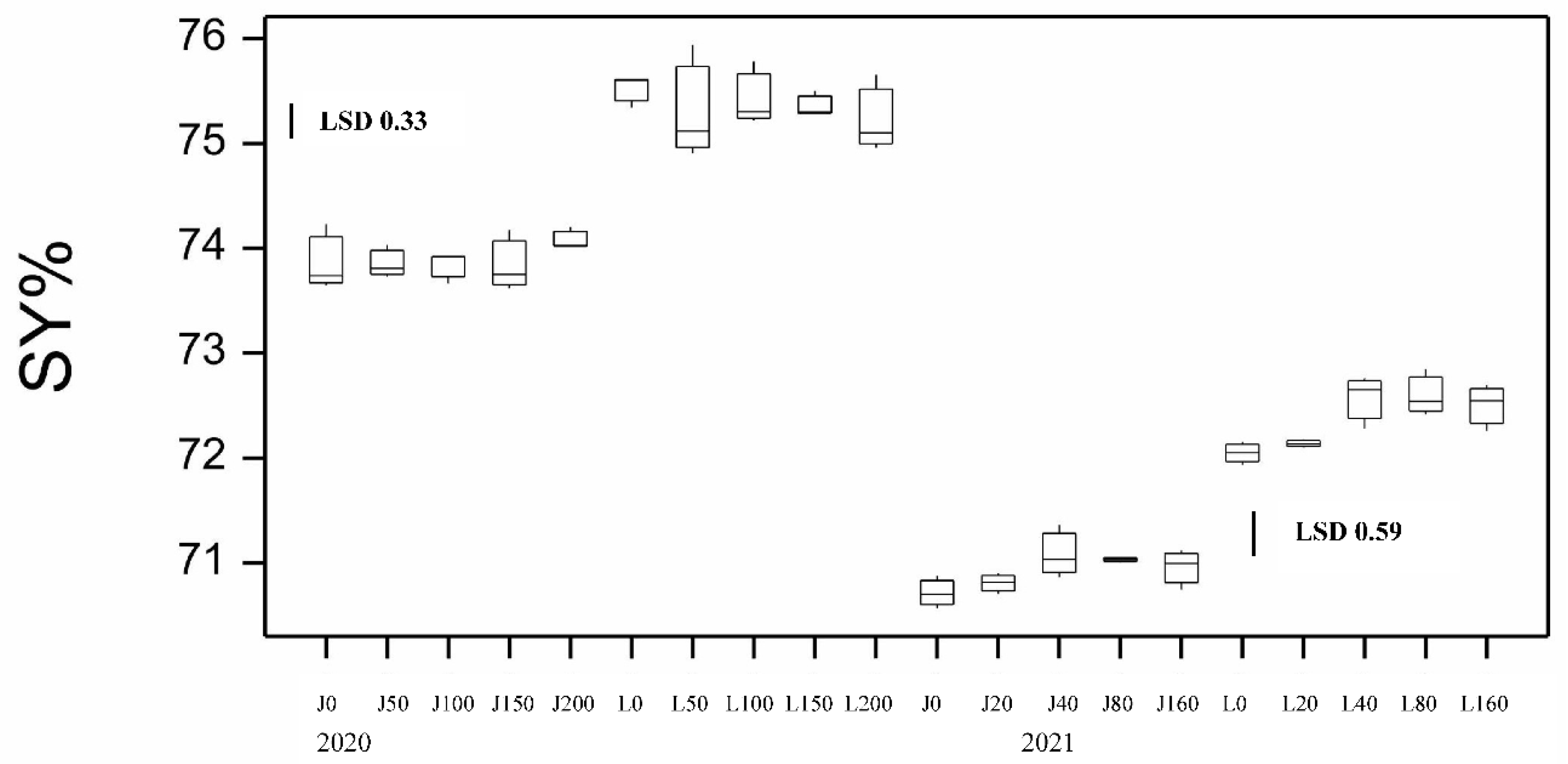
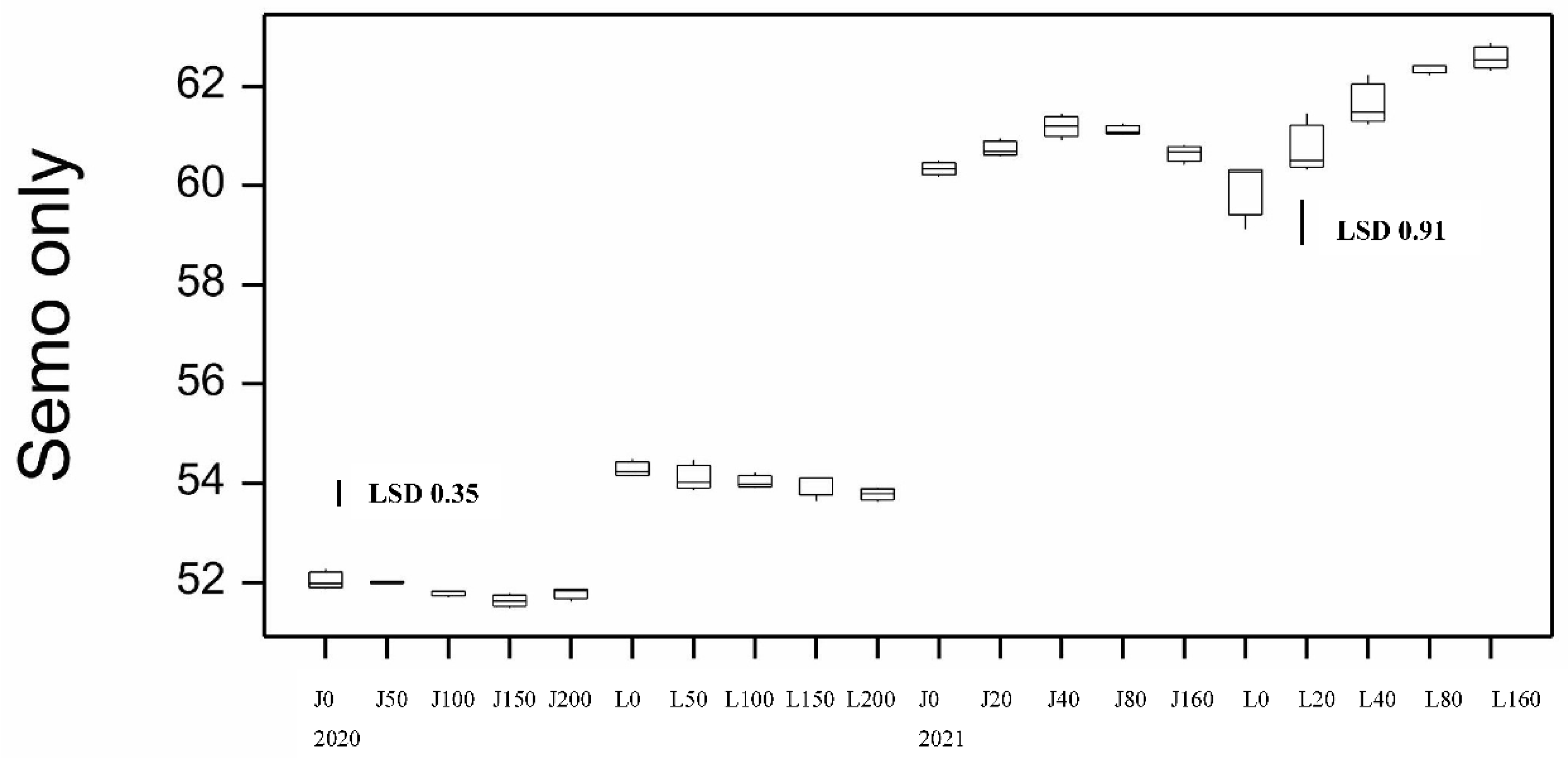
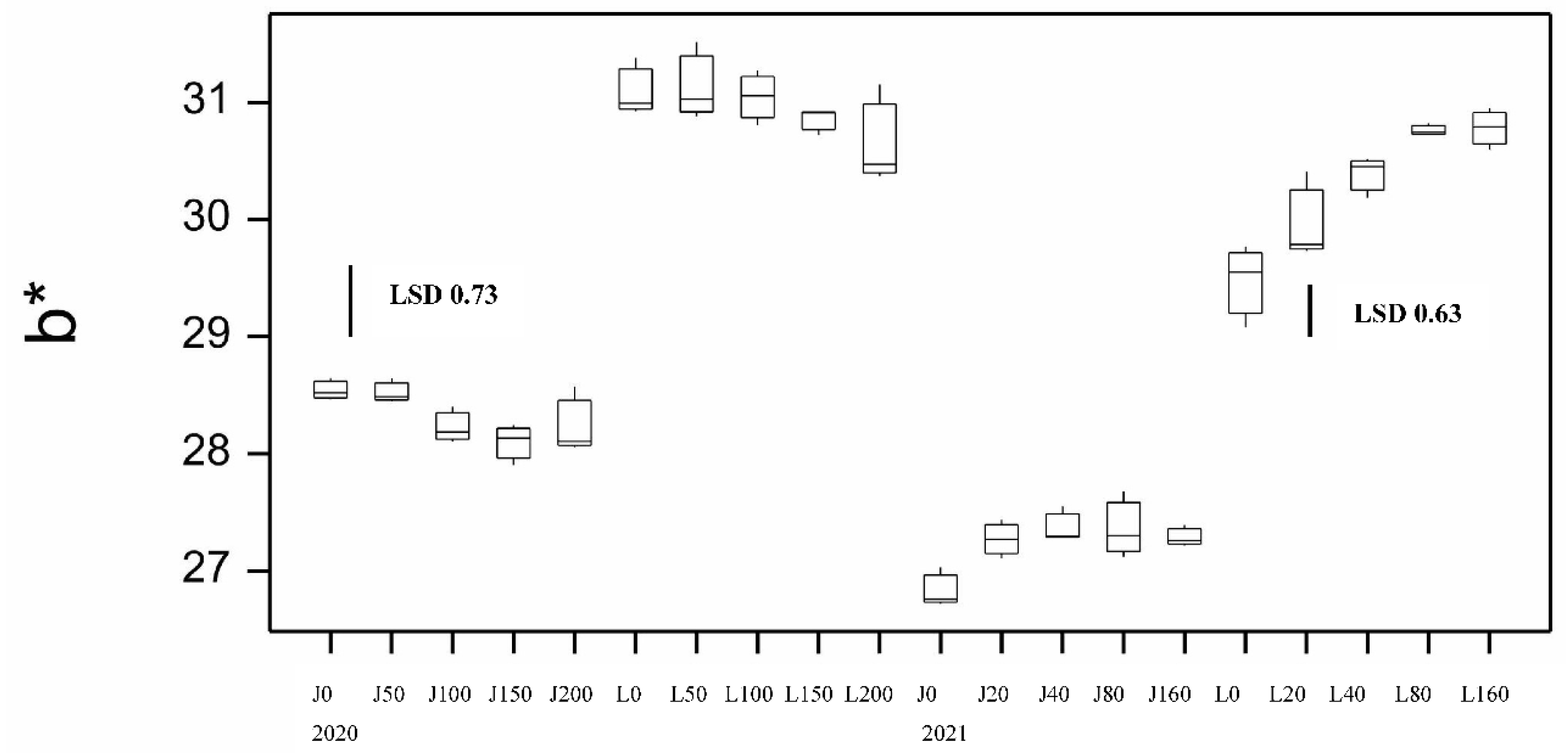
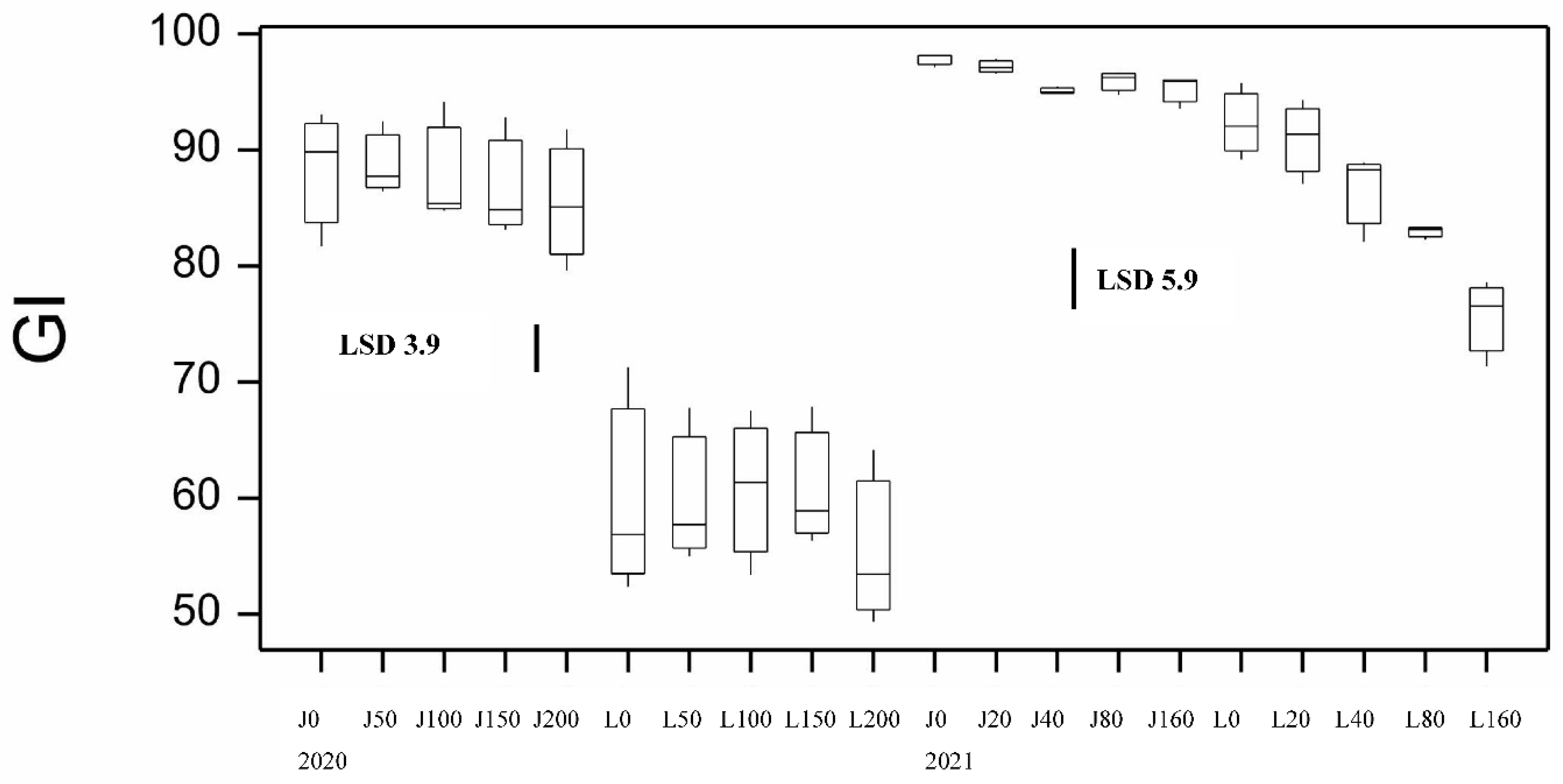
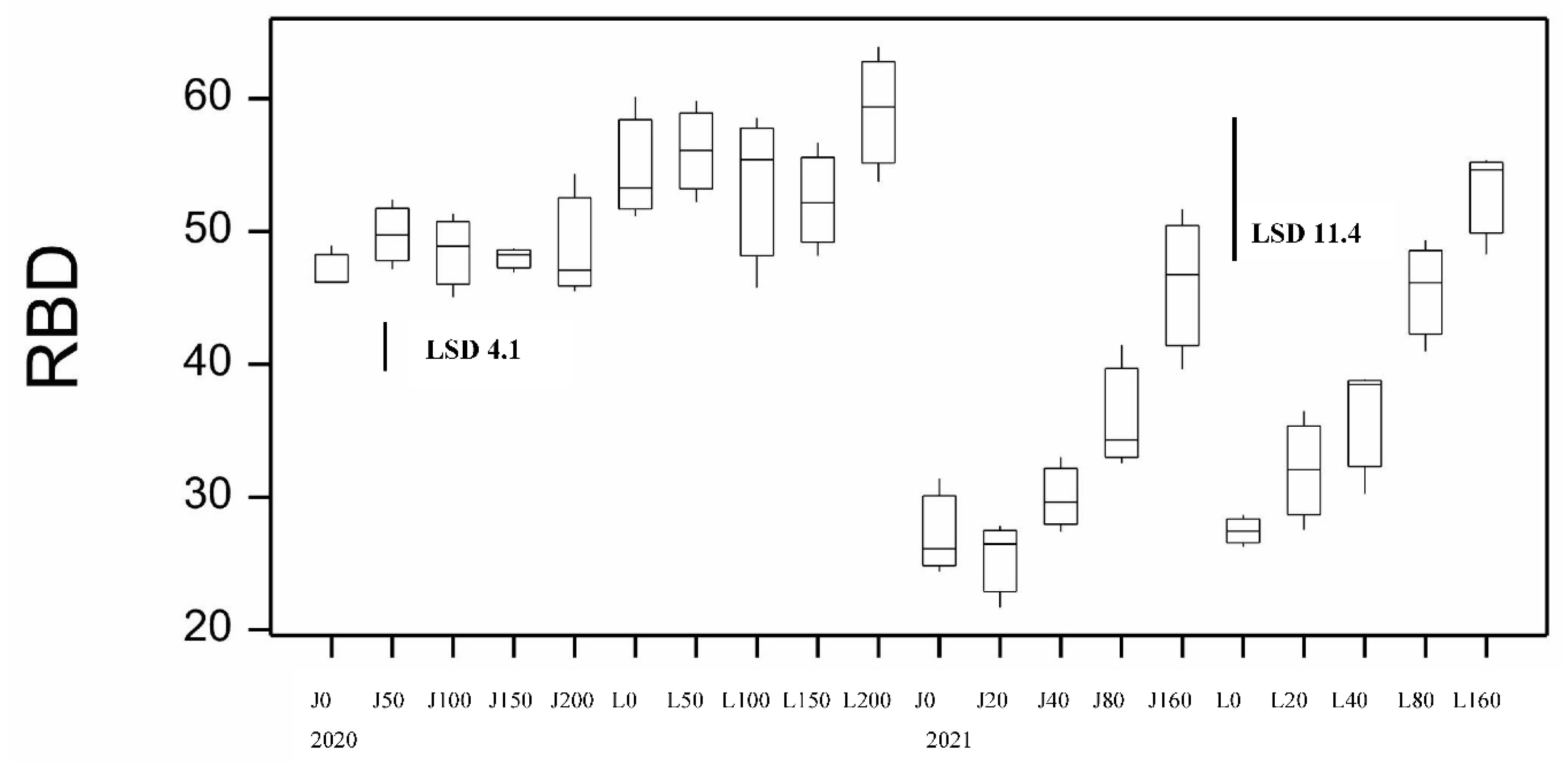
3.1. Results from the 2020 season
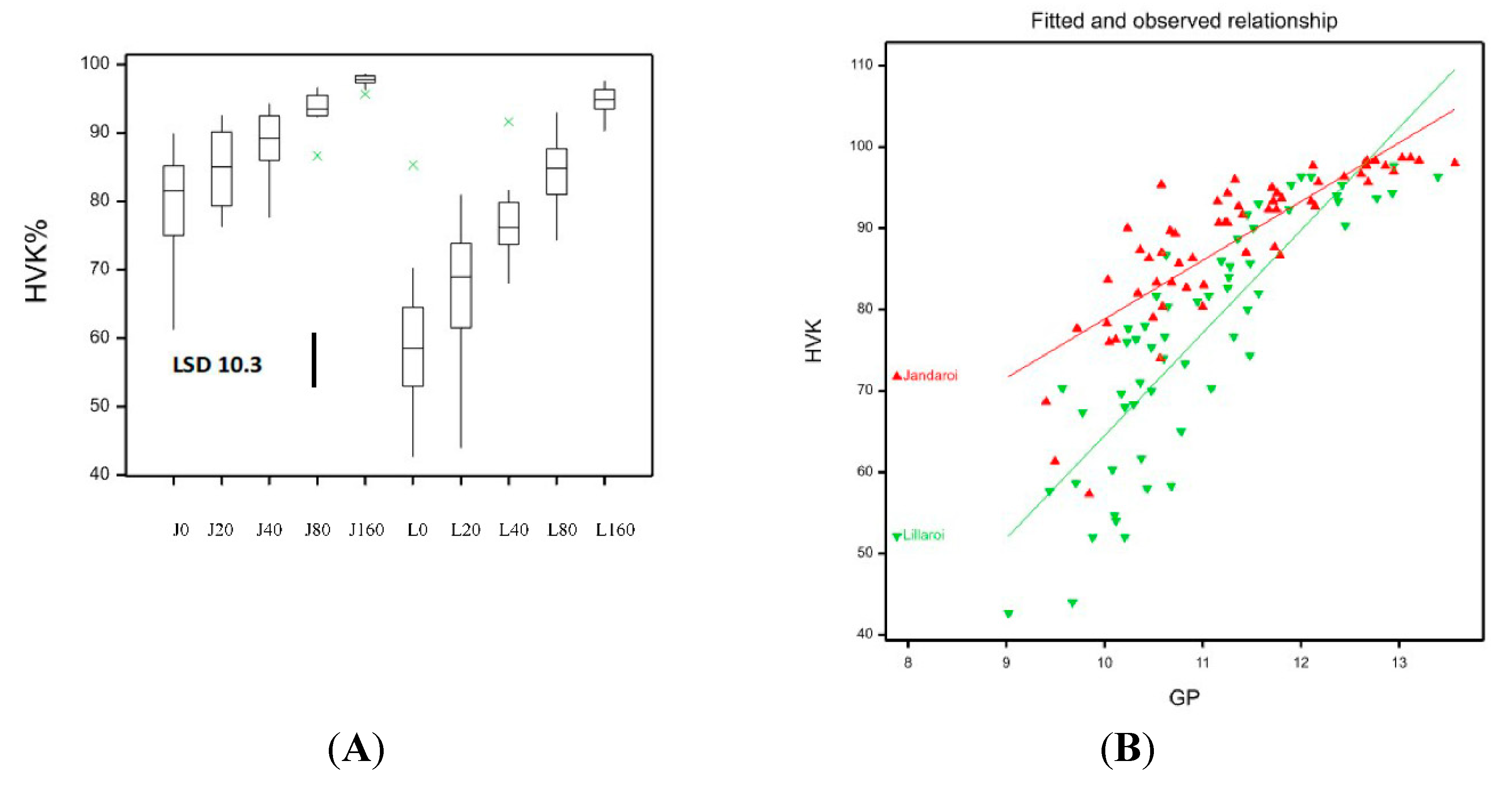
3.2. Results from the 2021 season and comparison to 2020 season
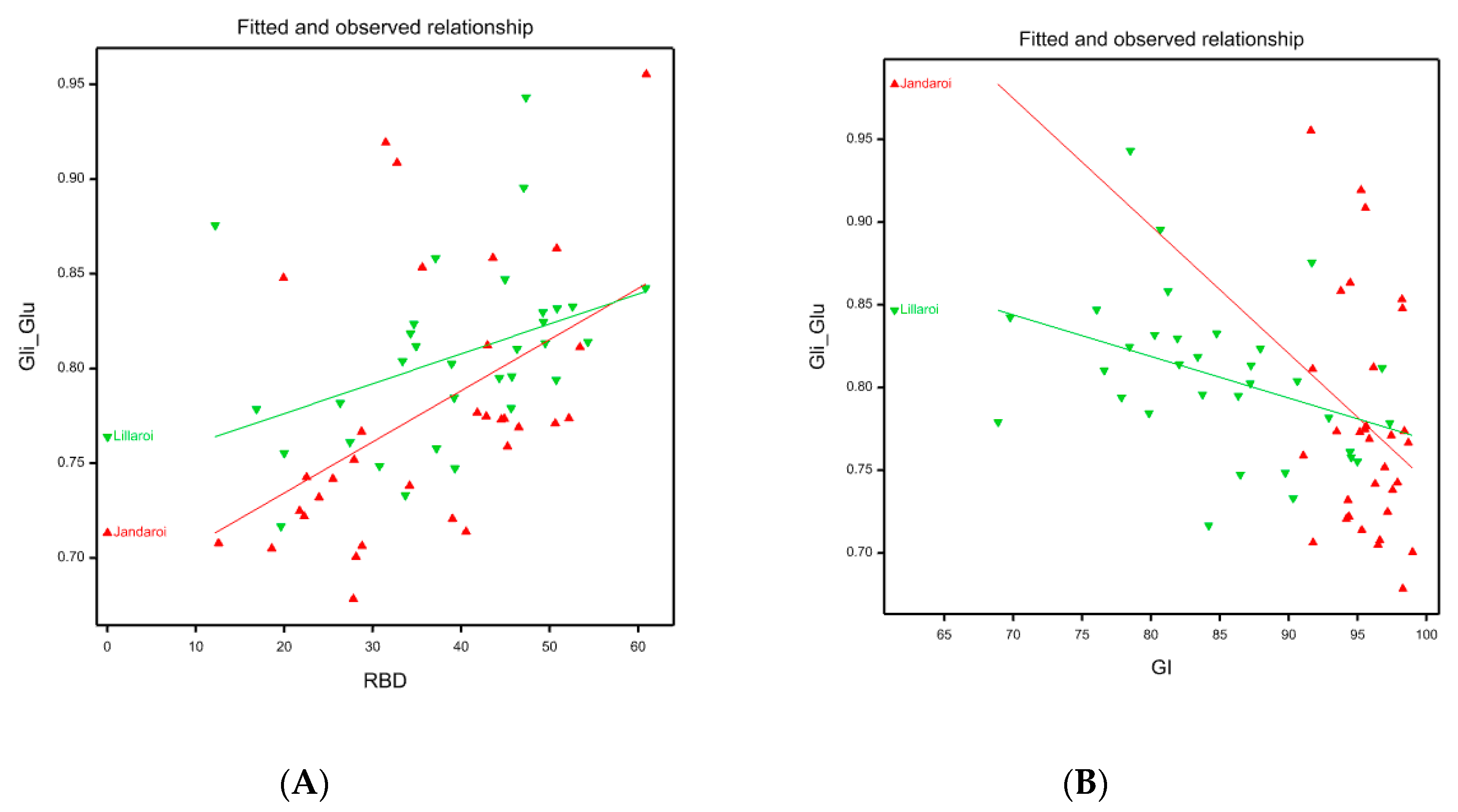
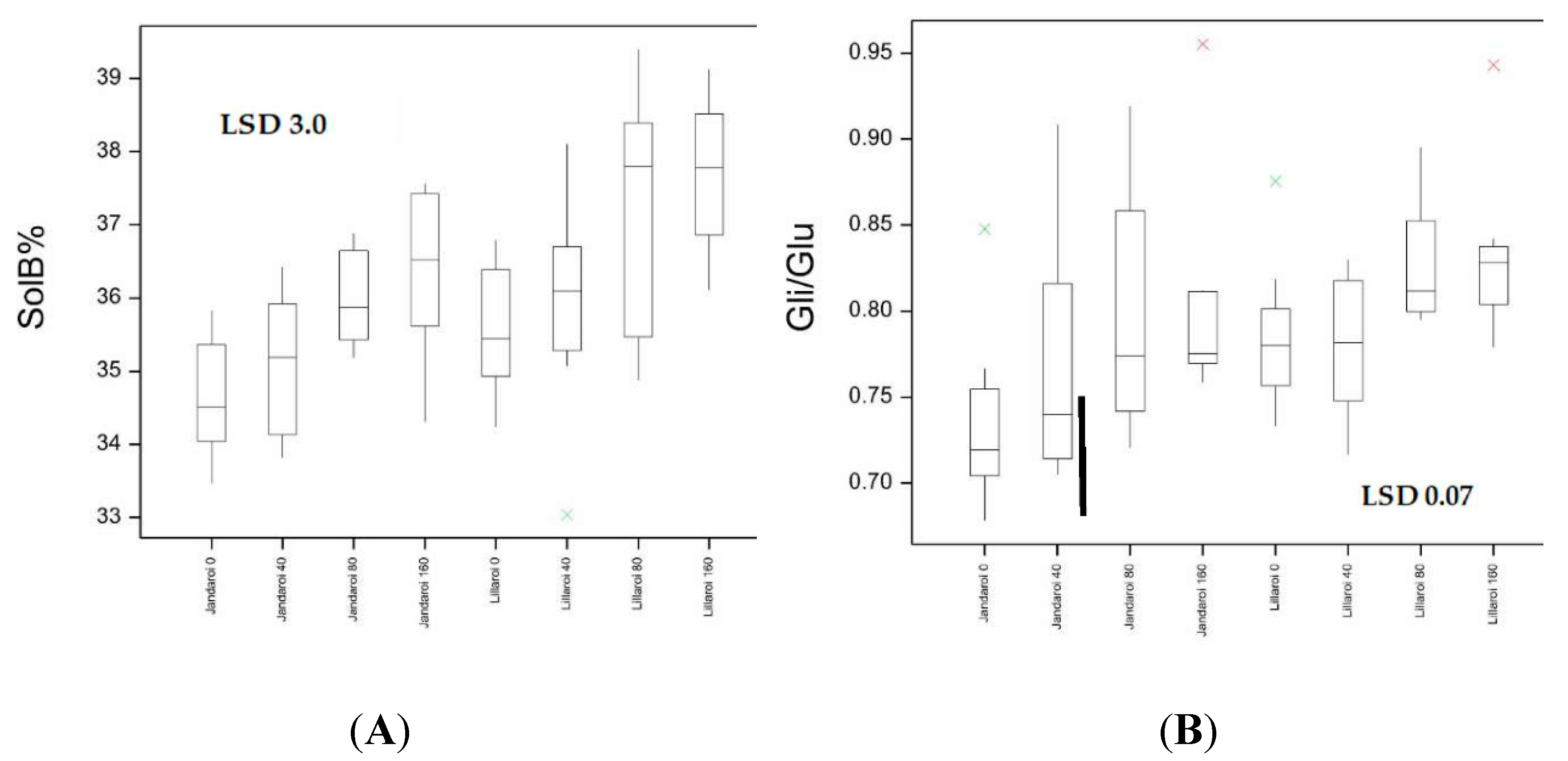
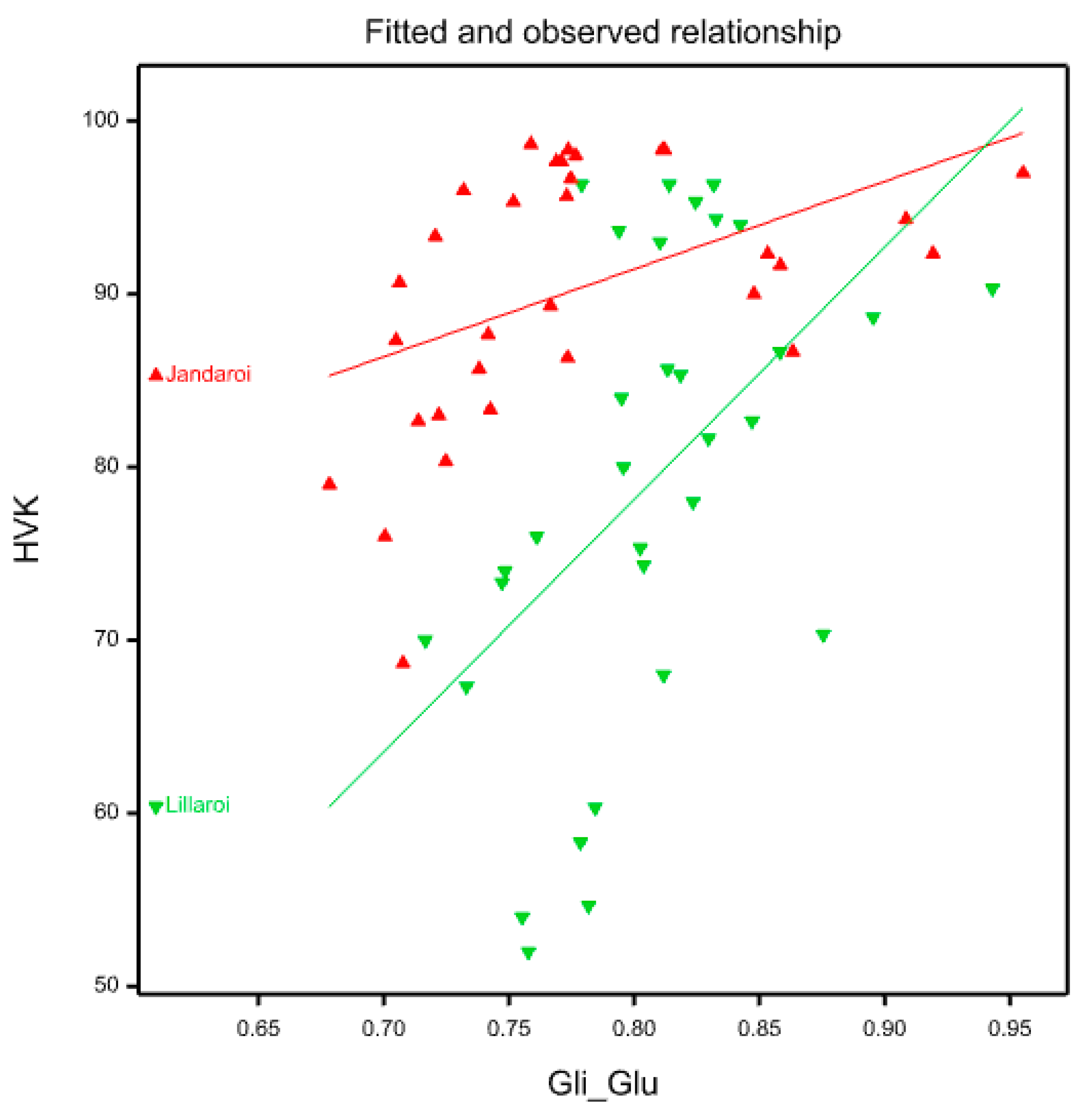
3.3. Protein compositional changes (2021 season)
4. Discussion
4.1. Comparison of seasonal impact on yield and quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richards, R.A.; Rebetzke, G.J.; Condon, A.G.; van Herwaarden, A.F. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Science 2002, 42, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.X.; Wang, K.; Dupuis, B.; Taylor, D.; Nam, S. Kernel vitreousness and protein content: Relationship, interaction and synergistic effects on durum wheat quality. Journal of Cereal Science 2018, 79, 210–217. [Google Scholar] [CrossRef]
- Anonymous. (2020). Grain Trade Australia Wheat Standards 2020/21. http://www.graintrade.org.au/sites/default/files/WHEAT%20STANDARDS%202020-2021%20All%20Grades%20updated %20061120.pdf. Accessed 22 November 2022. 22 November.
- Herbert, A. An international Benchmarking Comparison of Australian Crop production and Profitability, in GRDC Updates, GRDC, Editor. 2017: Perth.
- Chakraborty, S.; Liu, C.L.; Mitter, V.; Scott, J.B.; Akinsanmi, O.A.; Ali, S.; Dill-Macky, R.; Backhouse, D.; Simpfendorfer, S. Pathogen population structure and epidemiology are keys to wheat crown rot and Fusarium head blight management. Australasian Plant Pathology 2006, 35, 643–655. [Google Scholar] [CrossRef]
- Simpfendorfer S, McKay A, Ophel-Keller K (2019). New approaches to crop disease management in conservation agriculture. In (Eds J Pratley and J Kirkegaard) “Australian Agriculture in 2020: From Conservation to Automation” pp 173-188 (Agronomy Australia and Charles Sturt University: Wagga Wagga).
- Alahmad, S.; Simpfendorfer, S.; Bentley, A.R.; Hickey, L.T. Crown rot of wheat in Australia: Fusarium pseudograminearum taxonomy, population biology and disease management. Australas. Plant Pathol. 2018, 47, 285–299. [Google Scholar] [CrossRef]
- Davis, R.A.; Huggins, D.R.; Cook, J.R.; Paulitz, T.C. Nitrogen and crop rotation effects on fusarium crown rot in no-till spring wheat. Canadian Journal of Plant Pathology, 2009, 31, 456–467. [Google Scholar] [CrossRef]
- Buster, M.; Simpfendorfer, S.; Guppy, C.; Sissons, M.; Flavel, R.J. Interactions of Fusarium Crown Rot of Wheat with Nitrogen. Plants 2023, 12, 533. [Google Scholar] [CrossRef]
- Hughes, N.; Galeano, D.; Hatfield-Dodds, S. The effects of drought and climate variability on Australian farms, ABARES insights report 2019.
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on Wheat Yield and Quality with Reduced Nitrogen Supply. Trends in Plant Science 2018, 23, 1029–1037. [Google Scholar] [CrossRef]
- Forknall, C.R.; Simpfendorfer, S.; Kelly, A.M. Using yield response curves to measure variation in the tolerance and resistance of wheat cultivars to Fusarium crown rot. Phytopathology 2019, 109, 932–941. [Google Scholar] [CrossRef]
- Sissons, M.; Ovenden, B.; Adorada, D.; Milgate, A. Durum wheat quality in high input irrigation systems in south-eastern Australia. Crop & Pasture Science 2014, 65, 411–422. [Google Scholar]
- Sissons, M.J.; Batey, I.L. Protein and Starch Properties of some Tetraploid Wheats. Cereal Chemistry 2003, 80, 468–475. [Google Scholar] [CrossRef]
- Fuertes-Mendizabal, T.A.; Aizpurua, M.B.; Gonzalez-Moro, J.M.; Estavillo, J.M. Improving wheat breadmaking quality by splitting the N fertilizer rate. Eur. J. Agron. 2010, 33, 52–61. [Google Scholar] [CrossRef]
- Johansson, V.A.; Mattern, P.J. Wheat, rye, and triticale. In Nutritional quality of cereal grains Olson, R.A.; Frey, K.J. Eds.; Agron. Monogr. 1987 28. ASA, CSSA, and SSSA, Madison, WI; pp. 133–182.
- Reznick, J.P.K.; Barth, G.; Kaschuk, G.; Pauletti, V. Nitrogen and cultivars as field strategies to improve the nutritional status of wheat grain and flour. Journal of Cereal Science 2021, 102, 103290. [Google Scholar] [CrossRef]
- Giuliani, M.M.; Giuzio, L.; De Caro, A.; Flagella, Z. Relationships between nitrogen utilization and grain technological quality in durum wheat: II. Grain yield and quality. Agronomy Journal 2011, 103, 1668–1675. [Google Scholar]
- Ehdaie, B.; Waines, J.G. Sowing date and nitrogen rate effects on dry matter and nitrogen partitioning in bread and durum wheat. Field Crops Res. 2001, 73, 47–61. [Google Scholar] [CrossRef]
- Martre, P.; Porter, J.R.; Jamieson, P.D.; Triboi, E. Modelling grain nitrogen accumulation and protein composition to understand the sink/source regulation of nitrogen remobilization for wheat. Plant Physiol. 2003, 133, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.M.; Clarke, F.R.; Pozniak, C.J. Forty-six years of genetic improvement in Canadian durum wheat cultivars. Canadian Journal of Plant Science 2010, 90, 791–801. [Google Scholar] [CrossRef]
- DePauw, R.M.; Knox, R.E.; Clarke, F.R.; Wang, H.; Fernandez, M.R.; Clarke, J.M.; McCaig, T.N. Shifting undesirable correlations. Euphytica 2007, 157, 409–415. [Google Scholar] [CrossRef]
- Motzo, R.; Fois, S.; Giunta, F. Relationship between grain yield and quality of durum wheats from different eras of breeding. Euphytica 2004, 140, 147–154. [Google Scholar] [CrossRef]
- Sissons, M.; Kadkol, G.; Taylor, J. Genotype by Environment effects on Durum Wheat Quality and Yield -Implications for Breeding. Crop Breed Genet Genom. 2020, 2, e2000XX. [Google Scholar] [CrossRef]
- Kadkol, G.; Sissons, M.; Lambert, N.; Lisle, C. Genetic improvement in grain yield and quality of Australian durum wheat over six decades of breeding. Cereal Chemistry 2022, 1–22. [Google Scholar] [CrossRef]
- Dexter, J.E.; D’Egidio, M.G. Grading factors impacting durum wheat processing quality. In Durum Wheat Chemistry and Technology, Sissons, M.S.; Abecassis, J.; Marchylo, B; Carcea, M. Eds.; 2nd edition, AACC International Press. 2012; pp. 235-250.
- Dexter, J.E.; Williams, P.C.; Edwards, N.M.; Martin, D.G. The relationships between durum wheat vitreousness, kernel hardness and processing quality. J. Cereal Sci. 1988, 7, 169–181. [Google Scholar] [CrossRef]
- Samson, M.-F.; Mabille, F.; Cheret, R.; Abecassis, J.; Morel, M.H. Mechanical and physicochemical characterization of vitreous and mealy durum wheat endosperm. Cereal Chemistry 2005, 82, 81–87. [Google Scholar] [CrossRef]
- Boukef, S.; Karmous, C.; Trifa, Y.; Rezgui, S. Durum Wheat Grain Quality Traits as Affected by Nitrogen Fertilization Sources under Mediterranean Rainfed Conditions. Journal of Agriculture and Sustainability 2013, 4, 99–114. [Google Scholar]
- Sieber, A.N; Würschum, T.; Longin, C.G.H. Vitreosity, its stability and relationship to protein content in durum wheat. Journal of Cereal Science 61 2015, 71–77. [Google Scholar] [CrossRef]
- Wang, K.; Taylor, D.; Chen, Y.; Suchy, J.; Fu, B.X. Effect of Kernel Size and Its Potential Interaction with Genotype on Key Quality Traits of Durum Wheat. Foods 2021, 10, 2992. [Google Scholar] [CrossRef] [PubMed]
- Sissons, M.; Abecassis, J.; Cubadda, R.; Marchylo, B. Methods used to assess and predict quality of durum wheat, semolina and pasta In: Durum wheat chemistry and technology, Sissons, M.S.; Abecassis, J.; Marchylo, B; Carcea, M. Eds.; 2nd edition, AACC International Press. 2012; pp. 213-234.
- Ficco, D.B.M.; Mastrangelo, A.M.; Trono, D.; Borrelli, G.M.; Vita, P.D.; Fares, C.; Beleggia, R.; Platani, C.; Papa, R. The colours of durum wheat: a review. Crop & Pasture Science, 2014, 65, 1–15. [Google Scholar] [CrossRef]
- Feillet, P.; Autran, J.C.; Icard-Verniere, C. Pasta brownness: an assessment. J. Cereal Sci. 2000, 32, 215–233. [Google Scholar] [CrossRef]
- Dalla Marta, A.D.; Grifoni, M.; Mancini, G.; Zipoli, S.; Orlandini, S. The influence of climate on durum wheat quality in Tuscany, central Italy. Int. J. Biometeorol. 2011, 55, 87–96. [Google Scholar] [CrossRef]
- Fois, S.; Schlichting, L.; Marchylo, B.; Dexter, J.; Motzoa, R.; Giuntaa, F. Environmental conditions affect semolina quality in durum wheat (Triticum turgidum ssp. durum L.) cultivars with different gluten strength and gluten protein composition. J. Sci. Food Agric. 2011, 91, 2664–73. [Google Scholar] [CrossRef]
- Saint Pierre, C.; Peterson, C.J.; Ross, A.S.; Ohm, J.B.; Verhoeven, M.C.; Larson, M.; Hoefer, B. Winter wheat cultivars under different levels of nitrogen and water stress: Changes in grain protein composition. J. Cereal Sci. 2008, 47, 407–416. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Khatkar, B.S. Influence of gliadin and glutenin fractions on rheological, pasting, and textural properties of dough. Int. J. Food. Prop. 2014, 17, 1428–1438. [Google Scholar] [CrossRef]
- Tang, J.W.; Liu, J.J.; Zhang, P.P.; Zhang, Y.; Xiao, Y.G.; Qu, Y.Y.; Zhang, Y.; He, Z.H. Effects of gluten protein fractions on dough property and products quality in common wheat. Sci. Agric. Sin. Sci. 2008, 41, 2937–2946. [Google Scholar]
- AbuHammad, W.A.; Elias, E.M.; Manthey, F.A.; Alamri, M.S.; Mergoum, M. A comparison of methods for assessing dough and gluten strength of durum wheat and their relationship to pasta cooking quality. International Journal Food Science Technology, 2012, 47, 2561–2673. [Google Scholar] [CrossRef]
- Horvat, D.; Šimi´c, G.; Dvojkovi´c, K.; Ivi´c, M.; Plavšin, I.; Novoselovi´c, D. Gluten Protein Compositional Changes in Response to Nitrogen Application Rate. Agronomy 2021, 11, 325. [Google Scholar] [CrossRef]

| Metric | Jan | Feb | March | April | May | June | July | Aug | Sept | Oct | Nov | Dec | Annual |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainfall 2020 | 97.4 | 202.2 | 83.4 | 49.0 | 39.2 | 29.4 | 37.0 | 29.2 | 26.8 | 73.6 | 3.0 | 223.6 | 893.8 |
| Rainfall 2021 | 23.6 | 108.2 | 162.0 | 22.6 | 24.6 | 125.0 | 65.4 | 48.4 | 36.8 | 52.0 | 191.0 | 70.6 | 930.2 |
| Rainfall LTA | 61.2 | 73.9 | 57.2 | 25.3 | 30.4 | 53.4 | 40.9 | 39.5 | 46.3 | 58.3 | 82.3 | 80.4 | 638.5 |
| Min T 2020 | 21.4 | 19.0 | 14.4 | 9.8 | 5.1 | 3.7 | 3.5 | 2.9 | 6.6 | 10.3 | 13.3 | 17.0 | |
| Min T 2021 | 15.3 | 15.7 | 14.4 | 7.0 | 4.1 | 3.7 | 2.9 | 3.4 | 4.1 | 8.7 | 14.0 | 14.7 | |
| Min T LTA | 17.7 | 16.9 | 14.5 | 10.0 | 6.0 | 3.6 | 2.3 | 2.8 | 5.8 | 9.7 | 13.3 | 15.7 | |
| Max T 2020 | 36.3 | 29.7 | 27.5 | 24.1 | 18.9 | 17.5 | 16.9 | 17.6 | 23.3 | 27.1 | 31.8 | 30.4 | |
| Max T 2021 | 30.8 | 29.9 | 27.4 | 23.9 | 20.5 | 16.8 | 16.6 | 19.9 | 22.1 | 25.4 | 25.9 | 29.9 | |
| Max T LTA | 33.0 | 31.5 | 29.2 | 25.5 | 20.8 | 17.0 | 16.5 | 18.5 | 22.0 | 25.6 | 28.6 | 30.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





