INTRODUCTION
Since the introduction of the Fontan operation 50 years ago, the life expectancy of infants born with complex and functionally univentricular congenital heart disease (CHD) has increased significantly and most children will survive to adult life1. The Fontan palliation separates the systemic and pulmonary circulations and relieves cyanosis, with the systemic venous return being connected to the pulmonary arteries without the interposition of the right ventricle pump. At rest, a good Fontan circulation can provide a normal cardiac output (CO). However, as a consequence of its unique hemodynamic, the limitations of the Fontan circuit are exposed during exercise. In these Fontan patients, there is no pump to increase and accelerate pulmonary blood flow. Furthermore, pulmonary vascular reactivity and recruitment of vessels are limited or even absent. A patient with Fontan circulation has, therefore, a restricted ability to augment cardiac output during exercise2.
Cardiopulmonary exercise testing (CPET) is a valuable tool for assessing the exercise capacity and fitness of individuals with Fontan circulation. It involves measurements of oxygen uptake (VO2), carbon dioxide production (VCO2), and ventilatory measures during a symptom-limited exercise test. CPET provides clinicians and researchers with an integrative and comprehensive assessment of physiologic responses to exercise and cardiorespiratory fitness in this patient population3.
Many factors contribute to impaired exercise capacity in patients with Fontan circulation, such as, among others, the intrinsic haemodynamic limitations, lack of regular physical activity since childhood, muscle weakness or impaired lung function. PeakVO2 measured by CPET is broadly used to classify functional capacity. In different studies this parameter is strongly related to outcome and prognosis4,5,6. However, it requires a maximal effort for its interpretation, not always achieved by the adult with Fontan circulation. The oxygen uptake efficiency slope (OUES) is a submaximal parameter which objectively predicts the maximal exercise capacity in both healthy population and patients with acquired heart disease. However, OUES has only been used in a small number of studies with young Fontan patients7,8.
In this study we sought to provide a comprehensive assessment of exercise physiology impairments of adults with Fontan circulation using CPET; to evaluate the impact of their level of physical activity on exercise capacity and to investigate the contribution of inspiratory muscle weakness to exercise limitation. Furthermore, we study the accuracy of OUES as a submaximal parameter of functional capacity in this cohort of Fontan patients.
METHODS
Data Source and Study population:
All patients post-Fontan palliation aged>16 years at follow-up at the Adult Congenital Heart Disease Unit in the University Hospital Virgen del Rocio in Seville were identified. Demographic data, cardiac anatomy, prior therapeutic interventions, complications, diagnostic techniques and medical treatments were collected from electronic health records and are the primary data source for this study. Both patients with atriopulmonary and total cavopulmonary connection Fontan circulations, either fenestrated and non-fenestrated, were included. Exclusion criteria were: a) Clinical instability (NYHA functional class IV, protein-losing enteropathy, severe hypoxemia with O2 saturation <80%), b) Arrhythmias in the preceding 6 months prior to inclusion, c) Unstable angina, d) Recent surgery (<12 months) or changes in medication (<6 months), neurological sequelae, cognitive disability, or musculoskeletal problems that prevent performing the exercise testing.
In this prospective and cross-sectional study conducted in a single referral centre, lung function test, inspiratory muscle strength and cardiopulmonary exercise test were performed to 37 patients with Fontan circulation who were clinically stable and 19 healthy controls (HC). Healthy control participants were non-smokers, were not under drug treatment and did not have history of cardiovascular and/or pulmonary disease. All participants were informed about the details of the procedures, including the potential risks, before signing the written informed consent. The local Ethics Committee on human research approved the study.
International Physical Activity Questionnaire
All patients and controls completed the "International Physical Activity Questionnaire-Short Form”. The IPAQ-SF is a well-developed 7-question instrument that addresses the number of days and time spent on physical activity in moderate intensity, vigorous intensity and walking of at least 10-min duration the last 7 days, and also includes time spent sitting on weekdays the last 7 days9.
Lung function
Forced vital capacity (FVC), forced expiratory volume in one second (FEV1), and FEV1/FVC ratio were assessed according to the recommendations of the American Thoracic Society and European Respiratory Society10 and the predicted values calculated from the equations reported by Global Lung Function Initiative (GLI)11. At least three acceptable and reproducible maneuvers were achieved, using encouragement and positive reinforcement in order to obtain maximum values. Patients with restrictive pattern were classified into 3 groups based on predicted FVC values: mild restriction (predicted FVC 80% to 65%); moderate restriction (FVC 64% to 50%); and severe restriction (FVC <49%), based on published recommendations.
Inspiratory Muscle Strength
Maximal static inspiratory pressure (MIP): During testing, participants were sitting upright. Both patients and controls were instructed to exhale slowly and completely, seal lips firmly around the mouthpiece, and then inhale through the mouth as hard and fast as possible, with the nostrils occluded with a clamp. Ideally, the inspiratory pressure was held for 1.5 seconds so that the maximum pressure sustained for 1 second is recorded. The maximum value of three inspiratory manoeuvres that varied by less than 10% were recorded12. Reference values were calculated based on the equations developed by Evans et al.13: men = 120 - (0.41 x age) and women = 108 - (0.61 x age).
Maximal sniff nasal inspiratory pressure (SNIP): Inspiratory pressure is recorded by a pressure transducer connected to a catheter inserted into the nostril. This manoeuvre consists of sniffing quickly and deeply, generally from functional residual capacity (FRC), measuring the pressure generated. The duration of the sniff should be < 500 ms. The manoeuvre was repeated 10 times, taking the highest value reached12. Reference values in adults between 20 and 80 years of age were calculated from the equations developed by Uldry and Fitting14: men = -0.42 x age +126.8 and women = -0.22 x age + 94.9. In patients aged 16 and 17 years they have been calculated with the equations of Stefanutti et al.15: men = 3.3 x age + 70.
Cardiopulmonary Exercise Test
Maximal exercise testing was performed using a standardized ramp protocol on an electronically braked cycle ergometer. The participants were submitted to individualized ramp protocols with increments of 5 or 10 watts per minute in Fontan patients and 15 or 20 W/min in healthy controls.
Participants pedaled in an unloaded state for three minutes and workload was then increased continuously with a slope chosen to achieve each participant’s predicted maximal work rate after 10 to 12 min of cycling. Metabolic measurements were assessed on a breath-by-breath basis throughout exercise (Ergostik - Geratherm Respiratory GmbH, Germany). A 12-lead electrocardiogram was recorded throughout the exercise test. Blood pressure was measured at rest, every 2 min during exercise and every minute throughout recovery.
Maximal effort was defined as achieving a respiratory exchange ratio (RER) of equal to or greater than 1.10. Peak oxygen uptake (peakVO2) was determined by the mean of the last 20-30 seconds of maximal effort. Predicted maximal VO2 was calculated by Hansen-Wasserman equation16. The anaerobic threshold (AT) was determined by the V-slope method, at the point where the linear relationship between CO2 production (VCO2) and O2 consumption (VO2) disappears. VE/VCO2 slope was determined until the onset of the respiratory compensation point. The predicted maximum heart rate (HR) was obtained from the 220-age difference. The chronotropic index was calculated by maximal HR-rest HR/(220-age)-rest HR. Oxygen pulse is the VO2/HR ratio. The oxygen uptake efficiency slope (OUES) is the slope between VO2 and the logarithmic transformation of ventilation: (VO2/log10 VE)-k. Predicted OUES values were obtained by the equation of Buys et al.17 developed for Caucasian adults aged 20-60 years: OUESp Males = 1093-18.5 x age+1479 x body surface area; OUESp Females = 842-18.5 x age+1280 x body surface area. In patients aged 16-20 years the equation of Akkerman et al.18 was used.
Statistical Analysis
Continuous variables are presented as means ± SD or median and interquartile range in normally and non-normally distributed variables, respectively. Categorical variables are presented as counts and percentages. Comparisons among groups were performed using an unpaired T-test or the Mann–Whitney U test in normally and non-normally distributed variables, respectively. Intra-group comparison was performed by applying the T-test for paired samples for normally distributed variables or non-parametric Wilcoxon tests for paired samples for non-normally distributed variables. Both Pearson’s correlation coefficient or Spearman´s Rho correlation coefficient were used for measuring the correlation between continuous variables. Results were represented by coefficient of correlation (r). Statistical significance was considered for p-values <0.05. Data analyses were performed with IBM SPSS 28 statistical software.
RESULTS
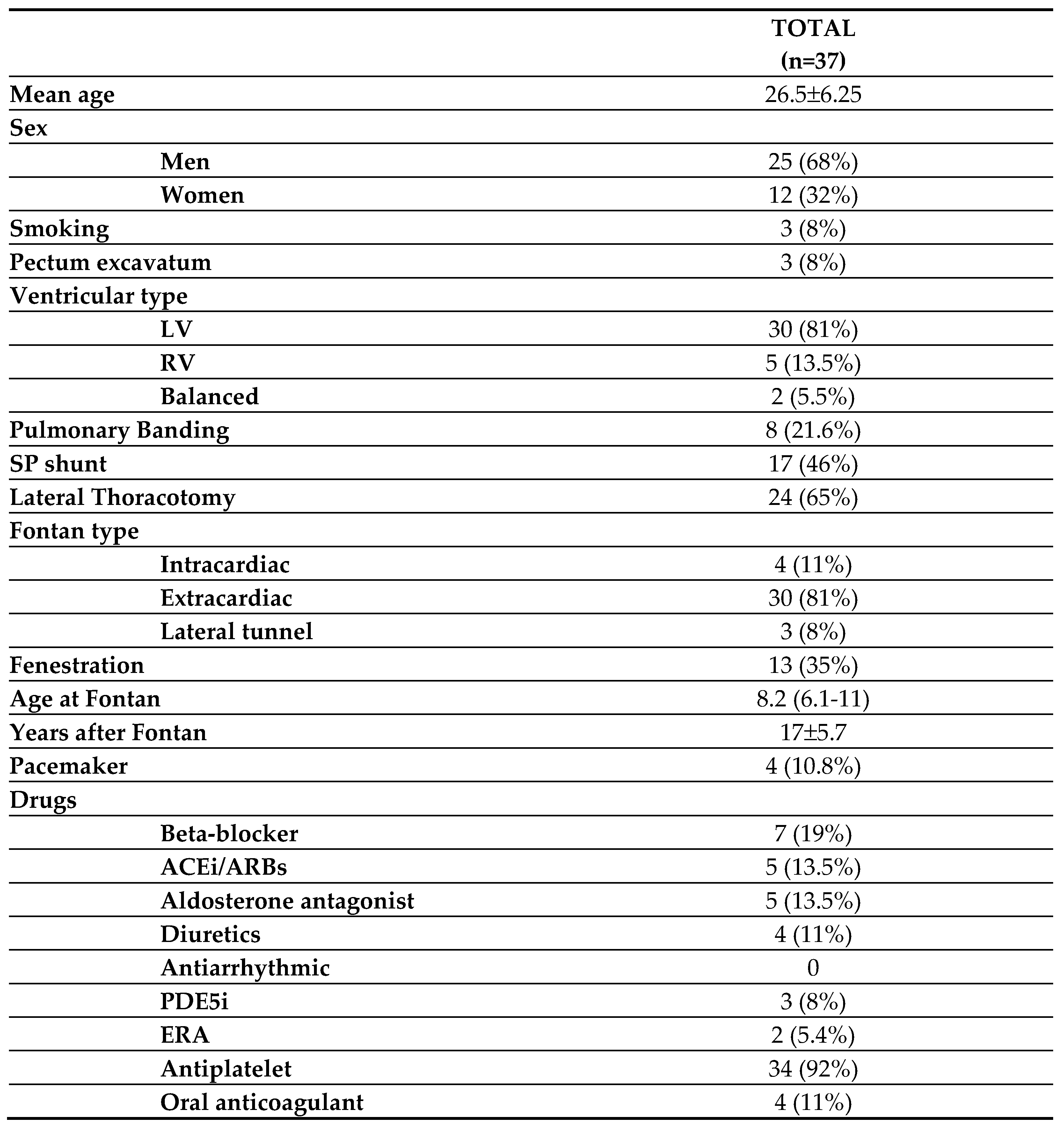
A total of 37 Fontan patients (68% male) with a mean age of 26.5±6.25 years (range 16-41 years) and 19 healthy controls (68.5% men) with a mean age of 26.1±6.8 years were recruited. There were no differences in age, gender, and body mass index (BMI) between groups.
Baseline characteristics of Fontan patients are displayed in
Table 1. Single ventricle morphology was left in 81% of patients. Tricuspid atresia (44%), followed by double inlet left ventricle (27.8%) were the most frequent underlying heart defect. Median age at Fontan surgery was 8.2 (6.1-11) years.
Lung function:
FVC and FEV1 were significantly lower in Fontan patients compared to their healthy peers. No differences were observed in the FEV1/FVC ratio between both groups. Twenty-one Fontan patients (56.8%) showed a mildly restrictive pattern, six (16.2%) a moderate restrictive pattern and ten patients (27%) had a normal lung function. None of the patients had a severe restrictive or obstructive pattern.
Patients with history of lateral thoracotomy showed lower FVC (FVC 72±9.5% vs 80±13%, p=0.047). We did not find statistically significant association between reduced FVC and lower peak VO2 (p=0.598) or ventilatory efficiency parameters (p=0.545).
Cardiopulmonary exercise test:
The average time between Fontan surgery and cardiopulmonary exercise test was 17±5.7 years. While all healthy controls reached maximal exercise effort (RER ≥1.10), up to 8% of Fontan patients did not achieve a maximal exercise effort (RER <1.10).
Table 3 shows CPET results of the two groups.
Table 2.
Lung function and inspiratory muscle strength.
Table 2.
Lung function and inspiratory muscle strength.
| |
Fontan patients
(n=37) |
Healthy subjects
(n=19) |
p |
| FEV1 (%) |
78.8±12.4 |
95.6±6.5 |
<0.001 |
| FVC (%) |
75±11.3 |
91±9.5 |
<0.001 |
| FEV1/FVC |
88±6.4 |
87.3±8 |
0.748 |
| MIP (cmH2O) |
79 (66-97) |
102 (84-125) |
0.005 |
| MIP (%) |
78 (65-91) |
97 (84-128) |
0.006 |
| SNIP (cmH2O) |
74 (60-88) |
89 (81-107) |
0.004 |
| SNIP (%) |
77 (59-88) |
94 (88-102) |
0.001 |
Table 3.
Cardiopulmonary exercise test parameters.
Table 3.
Cardiopulmonary exercise test parameters.
| |
Fontan patients (n=37) |
Healthy subjects (n=19) |
p |
| Age |
26.5±6.25 |
26.1±6.8 |
0.808 |
| Weight |
67±13 |
66±13 |
0.753 |
| Height |
1.69±0.06 |
1.72±0.08 |
0.226 |
| BMI (kg/m2) |
23±4 |
22±3 |
0.257 |
| pStO2 basal |
97 (95-99) |
100 (99-100) |
<0.001 |
| pStO2 max |
91 (89-93) |
98 (97-99) |
<0.001 |
| Peak load (W) |
128±29 |
209±49 |
<0.001 |
| Peak load (%) |
73±18 |
115±17 |
<0.001 |
| RER max |
1.18±0.08 |
1.22±0.07 |
0.129 |
| Peak VO2 (ml/kg/min) |
21±5.4 |
34.7±6.1 |
<0.001 |
| Peak VO2 (ml) |
1406±330 |
2363±635 |
<0.001 |
| Peak VO2 (%) |
55.5±14 |
87.6±11 |
<0.001 |
| VO2 AT (ml/kg/min) |
12.5 ±3.2 |
19.8±4.2 |
<0.001 |
| VO2 AT (%) |
32.3±8 |
50±8 |
<0.001 |
| VO2/W slope |
8.8±1.2 |
10±1.2 |
0.001 |
|
OUES (ml/min/log(L/min))
|
1608±359 |
2594±700 |
<0.001 |
| OUES (%) |
54.6±13 |
83±15 |
<0.001 |
| OUES/kg |
23 (20-28) |
41 (32-43) |
<0.001 |
| Peak HR |
157±16 |
180±16 |
<0.001 |
| MPHR (%) |
82±9.3 |
94±6.7 |
<0.001 |
| Chronotropic index |
0.65±0.15 |
0.89±0.12 |
<0.001 |
| VE max |
60.4 ± 12.6 |
91±25 |
<0.001 |
| Peak Bf |
36±8 |
41±10 |
0.088 |
| Vt max |
1.78 ± 0.44 |
2.32±0.66 |
<0.001 |
| Vt/VC (%) |
49.8 ± 10.7 |
50±7 |
0.871 |
| Breathing Reserve |
50 ± 12.7 |
33±14 |
<0.001 |
| MVV |
125 ± 27.4 |
156±28 |
<0.001 |
| VE/VCO2 AT |
30.4 ± 3.5 |
24.7±2.3 |
<0.001 |
| PETCO2 AT |
36 ± 4 |
44±5 |
<0.001 |
| VE/VCO2 slope |
28 ± 4.5 |
23.5±3.2 |
<0.001 |
| Oxygen debt |
46.2±9.3 |
36±5.4 |
<0.001 |
| VO2RD |
10 (5-15) |
5 (5-10) |
0.024 |
| HR recovery |
22±9.8 |
22±9 |
0.870 |
| Oxygen pulse (%) |
65.7±13.8 |
92±14 |
<0.001 |
| Oxygen pulse (ml/beat) |
8.7±1.8 |
13±3.4 |
<0.001 |
Mean peakVO2 was 21±5.4 ml/kg/min, an average of 55% of the predicted peakVO2 (peakVO2% predicted). The anaerobic threshold (AT) occurred at 32±8% of the predicted peak VO2 (early AT). Patients with morphologically left single ventricle showed higher peakVO2% predicted compared to those with morphologically right ventricle (57.4 ± 14.4% vs 43.4 ± 8.1%, p=0.045).
The O2 pulse was low, with a mean of 65.7±13.8% of predicted O2 pulse. As for the oxygen pulse kinetics, 51.4% showed an ascending slope and 48.6% an early flattening after reaching the AT. Furthermore, in 24.3% of the total cohort (9 patients) we observed a descending O2 pulse slope at maximal exertion. Patients with an early flattened O2 pulse and/or a descending pulse at maximal exertion had a lower peakVO2% predicted (52±14% vs 62±12.5, p=0.04 and 47.6±9% vs 60±14, p=0.018 respectively).
Chronotropic response was lower compared to healthy controls, as shown in
Table 3. Patients with chronotropic insufficiency had lower peakVO2% predicted (53±12% vs 69.8±20%, p=0.008). Chronotropic index (r=0.546, p<0.001), % maximum predicted HR (r=0.572, p<0.001) and HR reserve (r=0.452, p=0.005) showed statistically significant correlation with peakVO2% predicted. Finally, Fontan patients had higher values of ventilatory efficiency parameters compared to their healthy peers (
Table 3).
Oxygen Uptake Efficiency Slope (OUES):
Mean OUES was 1600±351, a mean of 54% of predicted OUES (%OUES). A moderate positive correlation was observed between absolute OUES and absolute peakVO2 (r=0.63, p=0.000). Strong positive correlation was observed between the %OUES and peakVO2% predicted (r=0.726, p>0.001) and between indexed OUES/kg and indexed peakVO2 (ml/kg/min) (r=0.846, p<0.001). OUES also correlated with submaximal parameters: OUES-VO2 at anaerobic threshold (AT) r=0.608, p=0.000. A negative correlation was seen between OUES and VE/VCO2 slope (r=-0.40, p=0.013).
Level of physical activity:
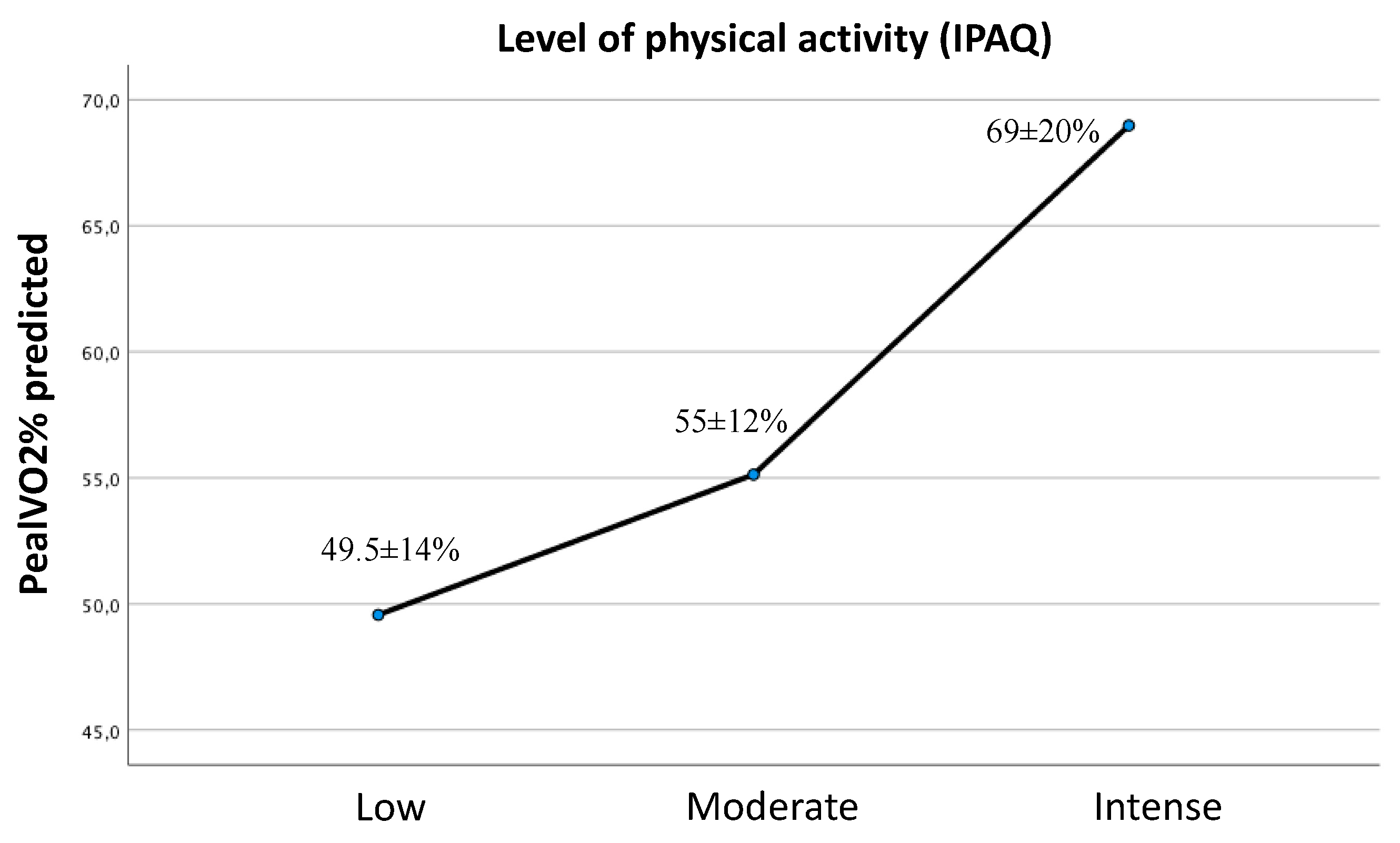
In our Fontan cohort 59.5% of patients had a moderate level of physical activity determined by the IPAQ questionnaire (n=22). 27% of the cohort (n=10) had a sedentary life and only 13.5% (5 patients) had a high level of physical activity.
The peakVO2% predicted was progressively higher as the level of physical activity increased (mean peak VO2 at low activity level 49.5±14%, at moderate activity level 55±12%, at intense activity level 69±20%). Although there were differences between the three groups, the sample size only allowed us to obtain statistically significant differences between the extreme groups (mean difference of peakVO2% predicted: low-intense physical activity 19.4±7.5% (p=0.038), low-moderate physical activity 5.5±5.2%, (p=0.545) and moderate-intense 13.8±6.8% (p=0.121)) (
Figure 1).
Two patients of the total cohort showed a peakVO2 greater than 80% of predicted, which has been previously dubbed as "Super Fontan", with the following characteristics: VO2 at AT 49.8±3%, %OUES 77.8±7%, O2 pulse 91.5±0.7%, peak predicted load 120±4%, %FCMP 98±1.2% and chronotropic index 0.9±0.04, with statistically significant differences with the rest of the cohort. Both patients had a morphologically left single ventricle and performed regular intense physical activity.
Inspiratory muscle strength:
SNIP and MIP characteristics are displayed in
Table 2. Patients with Fontan circulation showed statistically significantly lower inspiratory muscle strength compared to healthy subjects.
We did not find statistically significant association between inspiratory muscle strength and peakVO2 or VO2 at AT. No significant correlation was observed between MIP (p=0.482) or SNIP (p=0.570) and VE/VCO2 slope.
DISCUSION
In this study we provide insights into exercise physiology response, aerobic functional capacity and factors limiting physical effort in adult patients with Fontan circulation using CPET. Furthermore, we demonstrate that OUES is a submaximal parameter that can be used in an important percentage of Fontan patients who do not achieve a maximal exercise effort. This cohort of patients may also present abnormal lung volumes and inspiratory muscle weakness. Our results support that exercise training improves functional capacity of patients with Fontan circulation.
Cardiovascular Exercise Performance
Oxygen uptake:
To maintain cardiac output (CO), the Fontan circulation relies on venous pressure to passively drive systemic venous blood through the pulmonary vascular bed to the pulmonary venous atrium, given the absence of a sub-pulmonary ventricular pump. This intrinsically limits the ability to boost systemic ventricular preload, which leads to a very high prevalence of impaired maximal aerobic exercise capacity2,19,20. Along with this, severe peripheral limitation associated with muscle mass deficit and generalized muscle weakness contribute to exercise intolerance21,22. PeakVO2 is an established and reliable measure widely used to assess exercise intolerance of patients with congenital heart disease. Consistently with previous studies4-7,23, we found a significantly worse functional capacity in our population of adults with Fontan circulation compared to healthy controls, with a mean peak VO2 of 21ml/kg/min and on average is 55% of what would be predicted for the general population according to the Hansen-Wasserman equation. As our group has previously published24, peakVO2 is higher on treadmill than on cycle ergometer in patients with Fontan circulation (up to 23.8% higher on the treadmill) and this differences in exercise modality should be taken into account in the assessment of the functional capacity of this population group. Although reduced peakVO2 is the norm, a subset of Fontan patients has normal or even supranormal peak VO2. Lastly, our study shows that Fontan patients with morphologically left single ventricle performed better at CPET25.
OUES accuracy:
Although peak VO2 is the main parameter for the assessment of an individual's functional capacity, it requires a maximal exercise effort for its interpretation, which is not always achieved in Fontan patients. Although in other studies higher percentages of submaximal effort have been described (up to 20% do not reach the maximum effort)8, in our series 8% did not reach a RER >1.10. In submaximal exercise peakVO2 should be interpreted with caution and submaximal parameters need to be used to assess functional capacity and prognosis.
When the minute ventilation (VE) over the entire exercise duration is logarithmically transformed and plotted against the VO2, the regression coefficient is the oxygen uptake efficiency slope (OUES). This makes the OUES a dimensionless and a submaximal parameter of functional capacity. In addition, OUES can also be normalised by body weight or body surface area to correct for differences in anthropometrics between patients. In our Fontan cohort, mean OUES was 1600 ± 351, about 54% of the predicted OUES. Although higher %OUES of up to 79% of predicted values have been reported in the paediatric age group8, the mean %OUES in our study is similar to that reported in older Fontan patients7. A good correlation between OUES and other parameters of maximal and submaximal functional capacity (peakVO2 and VO2 at AT) was observed. The strongest correlation was seen between %OUES and peakVO2% predicted and between OUES/kg and indexed peakVO2 (ml/kg/min), as previously demonstrated by Terol et al. in a paediatric Fontan cohort at a median age of 11 years8.
In certain patient groups, however, OUES values have to be interpreted with caution. Firstly, Giardini et al.26 observed that in cyanotic Fontan patients OUES calculated from the first and the last 50% of the entire exercise duration differs substantially. Secondly, OUES values are considerably influenced by anthropometric variables and show large interindividual variation. The interpretation of its values is dependent on comparison with adequate reference values, comparisons between subjects, or comparisons within subjects. It is as yet unclear which values, absolute or indexed to weight, height, age, BSA, or percentage of predicted, can best be used27. Finally, OUES and peakVO2 are not necessarily interchangeable parameters. However, OUES is not meant to predict maximal exercise parameters, it provides an objective and independent measure of cardiorespiratory function and it seems to be a useful submaximal alternative in Fontan patients unable to perform maximal exercise.
Oxygen pulse kinetics:
In our series, patients with Fontan circulation have a lower oxygen pulse, with a mean of 66% of predicted, suggesting a stroke volume limitation. Furthermore, in half of the cohort the rise of oxygen pulse reached a plateau much earlier at AT, and then, flattened.
Oxygen pulse is the product of stroke volume and the arteriovenous oxygen difference during exercise. It represents oxygen consumption per heart beat and is considered a surrogate of stroke volume in the absence of anaemia or severe hypoxaemia. During the initial and intermediate phases of exercise, stroke volume has a higher relative contribution to cardiac output, but at a certain point it stops increasing and reaches a plateau. At low exercise loads, cardiac output increases due to an increase in stroke volume and HR. At higher loads, the stroke volume no longer increases and the rise in cardiac output is due to an increase in heart rate. Thus, the early flattening or decreasing O2 pulse curve in the CPET reflects a cardiac limitation to increase stroke volume during exercise or a peripheral limitation in O2 extraction in the skeletal muscles. It is noteworthy that stroke volume also represents the amount of ventricular filling during diastole and may therefore be limited by diastolic factors.
In line with the study by Bansal et al.28, early flattening of the O2 pulse slope and/or a downward displacement at peak exercise in our series is associated with lower O2 consumption. This is likely due to the Fontan circulation inability to increase pulmonary blood flow and ventricle preload during exercise and, as a consequence, a limited capacity to increase stroke volume with exertion29-33. Furthermore, we observed that those with a flat or descending oxygen pulse kinetics had a lower functional capacity. Therefore, we have seen that the morphology of oxygen pulse curve is a marker of functional capacity in these patients, reflecting a reduced stroke volume reserve.
Heart rate response:
Chronotropic insufficiency is common in patients with Fontan circulation (up to 85% in our study) and has been associated with exercise limitation34. However, the correlation between HR and functional capacity is modest and oxygen pulse was not found to be statistically different between patients with and without chronotropic insufficiency, as in other studies28,34.
It is unclear whether chronotropic insufficiency is a cause of low functional capacity or a phenomenon secondary to the limited increase in stroke volume with exercise in these patients. In healthy individuals, HR increases linearly with VO2. Factors that worsen VO2, such as ventricular dysfunction or cyanosis, cause a disproportionate increase in HR relative to VO2. In this line, Claessen G. et al.35 found that the HR for a given VO2 value is higher in Fontan patients than in healthy controls. However, although the increase in HR in relation to metabolic demand is greater than in healthy controls, the increase in HR in Fontan patients stops abruptly at a low peak value, i.e. lower peak HR. This lower peak HR at exercise could constitute a "brake" in Fontan patients, which would prevent falls in stroke volume and cardiac output due to the inability to increase preload that would be expected if higher HRs were achieved. Thus, patients with chronotropic insufficiency would have lower functional capacity as a consequence of a worse haemodynamic response to exercise.
Level of physical activity
We have seen a progressive increase in peak %VO2 as the level of physical activity (as determined by the IPAQ questionnaire) increased. Two patients in our cohort showed a peak VO2 greater than 80% of predicted, which has been dubbed as "Super Fontan". These patients showed a normal O2 pulse, normal VO2 at the AT and normal HR response with exertion. Both patients had a dominant left ventricular morphology and a high level of regular physical activity, factors previously associated with increased physical performance and this "Super Fontan" phenotype36. Regular participation in moderate and high intensity sports is important for proper development of skeletal muscle mass and prevention of sarcopenia, as the peripheral skeletal muscles act as a pump to boost venous return and increase preload and ventricular filling37. Muscle mass deficit also affects peripheral oxygen extraction. Although further studies are necessary, exercises that maintain muscle mass, especially lower extremity muscle mass, should be encouraged.
Lung Mechanics
Restrictive lung disease is common in individuals with a Fontan circulation and several studies show a reduced FEV1, FVC, and a normal or high FEV1/FVC ratio in this patient population38-41.
In Fontan patients, lung development may be adversely impacted by a variety of factors commonly seen in patients with congenital heart disease: oxygen desaturation, mechanical ventilation, lymphatic dysfunction, multiple sternotomies and thoracotomies, scoliosis or pectus deformity, and postoperative complications such as pleural adhesions and diaphragmatic palsy42.
Overall, Fontan patients in our series showed smaller lung volumes compared to healthy controls. Seventy-three percent of patients presented with a restrictive pattern on spirometry and previous lateral thoracotomy significantly associated with impaired lung function. However, in contrast to prior studies, we did not observe association between reduced FVC and lower peak VO2. Thus, Matthews et al.38 observed a correlation of 0.442 between peakVO2 and FEV1 (p=0.013) and 0.409 for FVC (p=0.022) and Callegari et al.41 found that reduced FEV1 was associated with a reduced peakVO2% predicted (r=0.43; p <0.0001). Moreover, Alonso-Gonzalez et al.42 found that moderate to severe impairment of lung function was an independent predictor of survival in a large cohort of adults with congenital heart disease.
In line with this, Turquetto et al.39 found a strong correlation between lung function and absolute peakVO2 [FVC (r= 0.86, p < 0.001); FEV1 (r= 0.83, p < 0.001)] in a cohort of Fontan patients with a prevalence of moderate restriction of up to 44%. Although an insufficient sample size to demonstrate significant differences may be the cause, since in our series the majority of patients had only a mild restrictive pattern, the low prevalence of moderate restrictive disease (only 16.2%) can also explain this discrepancy.
Abnormal lung mechanics impairs also the negative intrathoracic pressure required to “pull” blood through the Fontan circulation43. In this patient population, in which there is a compromised pulmonary function, mainly due to a restrictive pattern, as we confirmed, and a low functional capacity as indicated by low peakVO2, it appears that the skeletal muscle and ventilatory pumps account importantly for the increase in cardiac output during submaximal exercise in patients with Fontan circulation. It has been previously reported that patients with CHD often have abnormal body composition. Compared with healthy peers, these individuals have reduced muscle mass, increased adiposity, and shorter stature. Using maximal inspiratory pressure (MIP) and sniff nasal inspiratory pressure (SNIP), we found that inspiratory muscle strength was impaired in Fontan patients, compared to healthy controls. However, the role of inspiratory muscle training in exercise capacity of Fontan patients remains unclear. Although Greutmann et al.44 found a weak but significant correlation between MIP and peak VO2 (r=0.33, p=0.03) in a subgroup of 11 Fontan patients, we found no correlation between MIP and peakVO2, nor could it be demonstrated by Turquetto et al.39. Further research needs to be done to assess the benefits of respiratory training in exercise performance.
Limitations of the study
We must acknowledge a number of limitations. The small sample of the population study reduces the robustness of the results. However, the sample was homogeneous for age and, although the number was small, there was a significant difference between patients and healthy controls. Data on static lung volumes measured by plethysmography were not available for the scope of this study. However, forced expiratory and inspiratory lung flows in ACHD patients were recorded by trained physicians following the American Thoracic Society guidelines. Assessment of cardiac function during exercise may have shown stronger correlations with peakVO2. However, our study did not specifically address ventricular function or hemodynamic abnormalities and its potential contribution to exercise intolerance. Finally, a follow-up study may allow to identify the prognostic value of CPET parameters and inspiratory muscle function in the outcome of Fontan patients.
CONCLUSIONS
Patients with Fontan circulation have impaired exercise capacity. OUES is a useful submaximal parameter of functional capacity in those patients who fail to reach maximal exertion, showing a strong positive correlation with peak VO2. Chronotropic insufficiency and an early flattened or descending oxygen pulse slope is associated with lower peak VO2, whereas left ventricular morphology associates with better functional capacity. Those patients who perform regular intense physical activity perform better at CPET, highlighting the importance of regular exercise training. Although patients with Fontan circulation have inspiratory muscle weakness, its impact on functional capacity is unclear.
Funding
The research received no funding.
References
- Pundi KN, Johnson JN, Dearani JA, et al. 40-year follow-up after the Fontan operation: long-term outcomes of 1,052 patients. J Am Coll Cardiol 2015; 66: 1700–1710).
- Gewilling M, Brown SC; The Fontan circulation after 45 years: update in physiology. Heart. 2016 Jul 15; 102(14): 1081–1086.
- Wang-Giuffre EW, Doshi UH. Cardiopulmonary exercise test as a tool in surveillance after Fontan operation. Vessel Plus 2022;6:29.
- Egbe, Alexander C et al. “Cardiopulmonary exercise test in adults with prior Fontan operation: The prognostic value of serial testing.” International journal of cardiology vol. 235 (2017): 6-10. [CrossRef]
- Diller, Gerhard-Paul et al. “Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients.” European heart journal vol. 31,24 (2010): 3073-83. [CrossRef]
- Fernandes, Susan M et al. “Exercise testing identifies patients at increased risk for morbidity and mortality following Fontan surgery.” Congenital heart disease vol. 6,4 (2011): 294-303. [CrossRef]
- Chen, Chun-An et al. “Prognostic value of submaximal exercise data for cardiac morbidity in Fontan patients.” Medicine and science in sports and exercise vol. 46,1 (2014): 10-5. [CrossRef]
- Terol Espinosa de Los Monteros, Covadonga et al. “Prognostic Value of Maximal and Submaximal Exercise Performance in Fontan Patients < 15 Years of Age.” The American journal of cardiology vol. 154 (2021): 92-98. [CrossRef]
- Hagströmer, Maria et al. “The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity.” Public health nutrition vol. 9,6 (2006): 755-62. [CrossRef]
- Graham, Brian L et al. “Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement.” American journal of respiratory and critical care medicine vol. 200,8 (2019): e70-e88. [CrossRef]
- Quanjer, Philip H et al. “Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations.” The European respiratory journal vol. 40,6 (2012): 1324-43. [CrossRef]
- Laveneziana, Pierantonio et al. “ERS statement on respiratory muscle testing at rest and during exercise.” The European respiratory journal vol. 53,6 1801214. 13 Jun. 2019. [CrossRef]
- Evans, John A, and William A Whitelaw. “The assessment of maximal respiratory mouth pressures in adults.” Respiratory care vol. 54,10 (2009): 1348-59.
- Uldry C, Fitting JW. Maximal values of sniff nasal inspiratory pressure in healthy subjects. Thorax. 1995 Apr;50(4):371-5. doi: 10.1136/thx.50.4.371. PMID: 7785009; PMCID: PMC474280. [CrossRef] [PubMed] [PubMed Central]
- Stefanutti, D, and J W Fitting. “Sniff nasal inspiratory pressure. Reference values in Caucasian children.” American journal of respiratory and critical care medicine vol. 159,1 (1999): 107-11. [CrossRef]
- Karlman Wasserman, James E. Hansen, Darryl Y. Sue, William W. Stringer, Kathy E. Sietsema, Xing-Guo Sun, Brian J. Whipp. Clinical Exercise Testing. In: Principles of Exercise Testing and Interpretation. Including Pathophysiology and Clinical Applications. Fifth Edition. Philadelphia: Lippincott Williams & Wilkins; 2012. p. 158.
- Buys, Roselien et al. “The oxygen uptake efficiency slope in 1411 Caucasian healthy men and women aged 20-60 years: reference values.” European journal of preventive cardiology vol. 22,3 (2015): 356-63. [CrossRef]
- Akkerman, Moniek et al. “Oxygen uptake efficiency slope in healthy children.” Pediatric exercise science vol. 22,3 (2010): 431-41. [CrossRef]
- Gewillig MH, Lundström UR, Bull C, Wyse RKH, Deanfield JE. Exercise responses in patients with congenital heart disease after fontan repair: Patterns and determinants of performance. J Am Coll Cardiol. 1990;15(6):1424-1432. [CrossRef]
- Gewillig M, Goldberg DJ. Failure of the fontan circulation. Heart Fail Clin. 2014;10(1):105-116. [CrossRef]
- Cordina R, O’Meagher S, Gould H, et al. Skeletal muscle abnormalities and exercise capacity in adults with a Fontan circulation. Heart. 2013;99(20):1530-1534. [CrossRef]
- Avitabile CM, Leonard MB, Zemel BS, et al. Lean mass deficits, vitamin D status and exercise capacity in children and young adults after Fontan palliation. Heart. 2014;100(21):1702-1707. [CrossRef]
- Kempny, Aleksander et al. “Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life--single centre experience and review of published data.” European heart journal vol. 33,11 (2012): 1386-96. [CrossRef]
- Ladrón-Abia, R, Manso García, B, Cejudo Ramos, P, Gaboli M, Rodriguez Puras, MJ, Gallego, P. Differences between cycle ergometer and treadmill in cardiopulmonary exercise testing in patients with Fontan circulation. Revista Española de Cardiología. ISSN 0300-8932. [CrossRef]
- Ohuchi, H et al. “Influence of ventricular morphology on aerobic exercise capacity in patients after the Fontan operation.” Journal of the American College of Cardiology vol. 37,7 (2001): 1967-74. [CrossRef]
- Giardini, Alessandro et al. “Accuracy of oxygen uptake efficiency slope in adults with congenital heart disease.” International journal of cardiology vol. 133,1 (2009): 74-9. [CrossRef]
- Akkerman, Moniek et al. “The oxygen uptake efficiency slope: what do we know?.” Journal of cardiopulmonary rehabilitation and prevention vol. 30,6 (2010): 357-73. [CrossRef]
- Bansal, Manish et al. “Oxygen pulse kinetics in Fontan patients during treadmill ramp protocol cardiopulmonary exercise testing.” Pediatric cardiology vol. 33,8 (2012): 1301-6. [CrossRef]
- Brili, Stella V et al. “Dobutamine stress echocardiography for the evaluation of cardiac reserve late after Fontan operation.” Hellenic journal of cardiology: HJC = Hellenike kardiologike epitheorese vol. 48,5 (2007): 252-7.
- Wong, James et al. “Pressure-volume loop-derived cardiac indices during dobutamine stress: a step towards understanding limitations in cardiac output in children with hypoplastic left heart syndrome.” International journal of cardiology vol. 230 (2017): 439-446. [CrossRef]
- Robbers-Visser, Daniëlle et al. “Usefulness of cardiac magnetic resonance imaging combined with low-dose dobutamine stress to detect an abnormal ventricular stress response in children and young adults after fontan operation at young age.” The American journal of cardiology vol. 101,11 (2008): 1657-62. [CrossRef]
- Strömvall Larsson, Eva, and Bengt O Eriksson. “Haemodynamic adaptation during exercise in fontan patients at a long-term follow-up.” Scandinavian cardiovascular journal: SCJ vol. 37,2 (2003): 107-12. [CrossRef]
- Ohuchi, Hideo. “Cardiopulmonary response to exercise in patients with the Fontan circulation.” Cardiology in the young vol. 15 Suppl 3 (2005): 39-44. [CrossRef]
- Diller, Gerhard-Paul et al. “Impaired heart rate response to exercise in adult patients with a systemic right ventricle or univentricular circulation: prevalence, relation to exercise, and potential therapeutic implications.” International journal of cardiology vol. 134,1 (2009): 59-66. [CrossRef]
- Claessen, Guido et al. “Heart Rate Reserve in Fontan Patients: Chronotropic Incompetence or Hemodynamic Limitation?.” Journal of the American Heart Association vol. 8,9 (2019): e012008. [CrossRef]
- Tran, Derek L et al. “The "Super-Fontan" Phenotype: Characterizing Factors Associated With High Physical Performance.” Frontiers in cardiovascular medicine vol. 8 764273. 7 Dec. 2021. [CrossRef]
- Cordina, Rachael L et al. “Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology.” International journal of cardiology vol. 168,2 (2013): 780-8. [CrossRef]
- Matthews, Iren Lindbak et al. “Reduced pulmonary function in children with the Fontan circulation affects their exercise capacity.” Cardiology in the young vol. 16,3 (2006): 261-7. [CrossRef]
- Turquetto, Aída L R et al. “Impaired Pulmonary Function is an Additional Potential Mechanism for the Reduction of Functional Capacity in Clinically Stable Fontan Patients.” Pediatric cardiology vol. 38,5 (2017): 981-990. [CrossRef]
- Opotowsky, Alexander R et al. “Abnormal spirometry after the Fontan procedure is common and associated with impaired aerobic capacity.” American journal of physiology. Heart and circulatory physiology vol. 307,1 (2014): H110-7. [CrossRef]
- Callegari, Alessia et al. “A restrictive ventilatory pattern is common in patients with univentricular heart after Fontan palliation and associated with a reduced exercise capacity and quality of life.” Congenital heart disease vol. 14,2 (2019): 147-155. [CrossRef]
- Alonso-Gonzalez, Rafael et al. “Abnormal lung function in adults with congenital heart disease: prevalence, relation to cardiac anatomy, and association with survival.” Circulation vol. 127,8 (2013): 882-90. [CrossRef]
- Van der Woude, Séline F S et al. “The Influence of Respiration on Blood Flow in the Fontan Circulation: Insights for Imaging-Based Clinical Evaluation of the Total Cavopulmonary Connection.” Frontiers in cardiovascular medicine vol. 8 683849. 5 Aug. 2021. [CrossRef]
- Greutmann, Matthias et al. “Generalised muscle weakness in young adults with congenital heart disease.” Heart (British Cardiac Society) vol. 97,14 (2011): 1164-8. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).