1. Introduction
Colorectal cancer (CRC) is one of the most common solid malignant tumors worldwide. It is also top three malignancies of cancer-related cause of death. [1-3] Over 20% of patients diagnosed with CRC have distant metastasis. Which has poor prognosis of 5-year survival about 10-20%. [1, 4-6] First line treatment of metastatic CRC (mCRC) is chemotherapy and/or radiotherapy combine with surgical resection. [5, 7] Various options of chemotherapy regimens are available with biosynthetic agents while therapy also may be determined according to genetic profile of the tumor. However, treatment for mCRC is still difficult especially in patients who failed in first-line treatment and had higher disease severity such as tumor burden and site of metastasis. Recently, cell-based immunotherapy has emerged as a promising treatment option for metastatic colorectal cancer. Dendritic cell (DC)-cytokine-induced killer (CIK) cell therapy has gained enormous attention due to its potential to enhance anti-tumor immunity and improve patient outcomes.
Dendritic cell-cytokine-induced killer cells (DC-CIK) therapy involves the use of two types of immune cells: dendritic cells and cytokine-induced killer cells. Dendritic cells are antigen-presenting cells that play a critical role in initiating immune responses against foreign substances, including cancer cells. Cytokine-induced killer cells are T cells that have been activated by cytokines to specifically target cancer cells. In DC-CIK therapy, dendritic cells are initially isolated from the patient's blood and then exposed to cancer antigens to activate. These activated dendritic cells are then combined with cytokine-induced killer cells and infused back into the patient's bloodstream. [22, 23]
There are few studies revealed an efficacy of dendritic cell-cytokine-induced killer cells (DC-CIK) immunotherapy especially in progression-free survival (PFS), disease-free survival (DFS) and overall survival (OS). The preliminary results of a combination of DC-CIK immunotherapy with first line treatment seemed promising. [8-10] However, the benefit and clinical outcomes of DC-CIK immunotherapy combine with second- or later- line chemotherapy are still not definitely revealed or discussed.
In this retrospective study, CRC patients who received DC-CIK cell-based immunotherapy as adjuvant therapy of standard second- or later- line chemotherapy during July 2020 to September 2021 were recruited and their characteristics and clinical outcomes were analyzed.
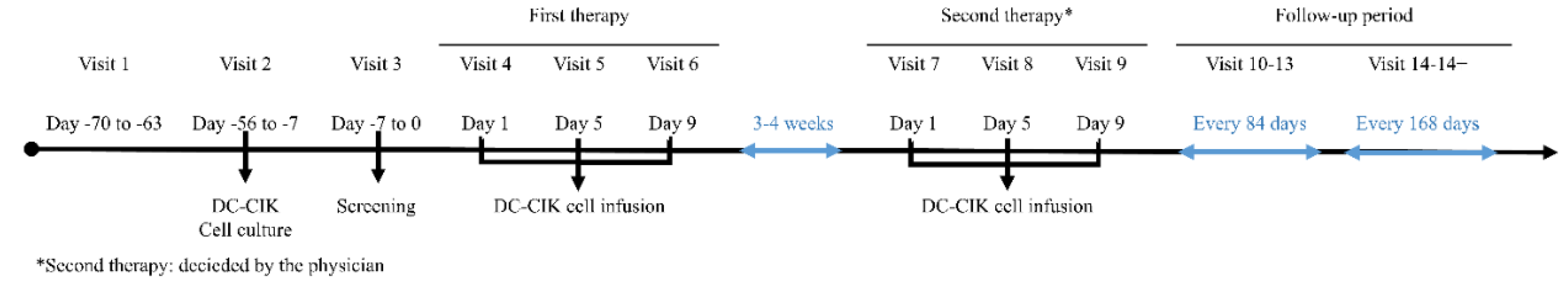
Figure 1 shows the timeline of the DC-CIK cell-based immunotherapy.
2. Materials and Methods
2.1. Patients
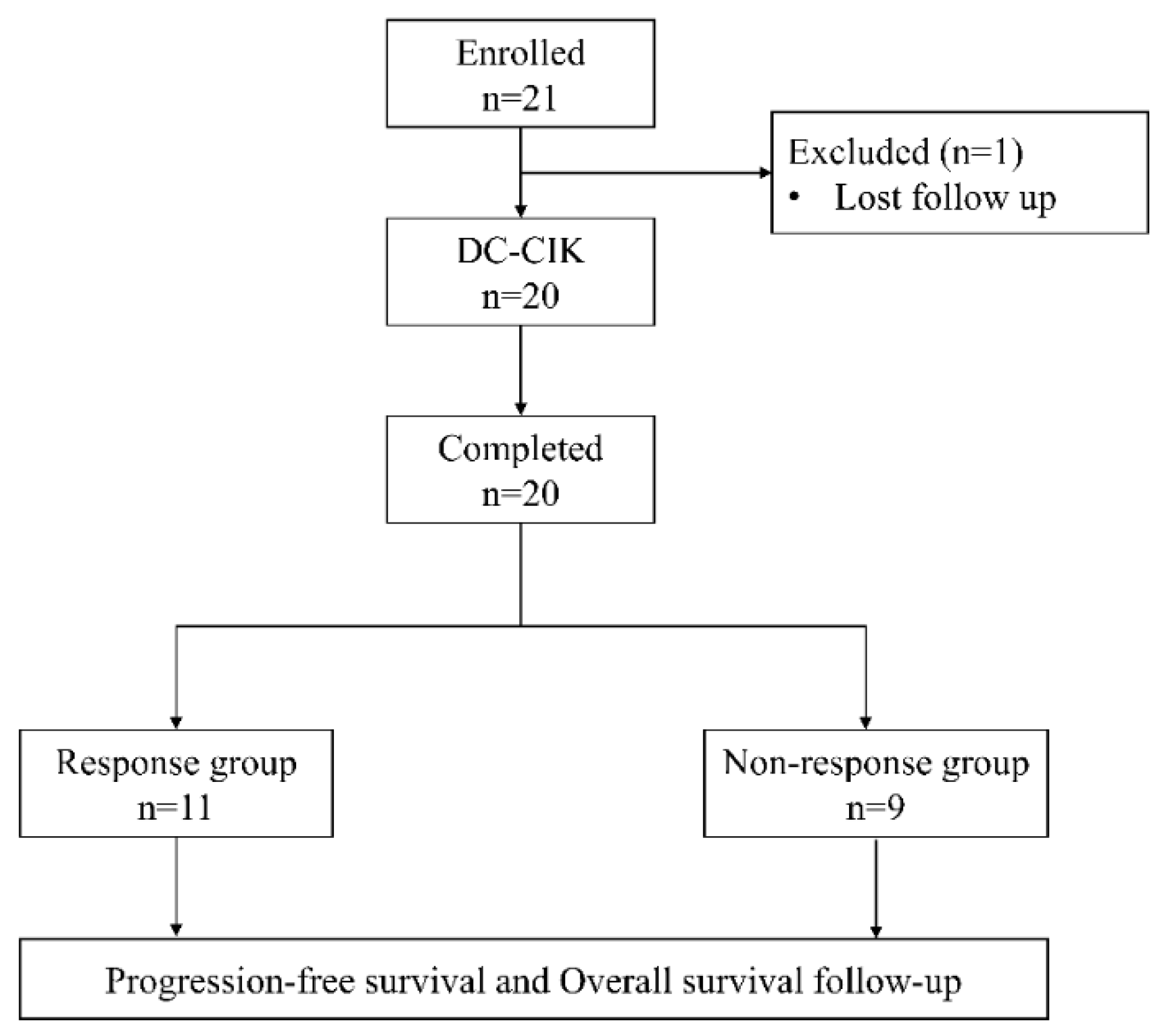
Patients with pathologically confirmed diagnoses of colorectal cancer who received DC-CIK cell-based immunotherapy as adjuvant therapy of standard chemotherapy were retrospectively enrolled from China Medical University Hospital during July 2020 to September 2021. All patient. The tumor node metastases (TNM) staging system of the American Joint Committee on Cancer/Union for International Cancer Control was used to confirm CRC stage or differentiation. The inclusion criteria were: (1) a pathological diagnosis with colorectal cancer; (2) receiving 2 full cycles of DC-CIK therapy; (3) receiving DC-CIK cell-based immunotherapy combined with second- or later-line chemotherapy. Exclusion criteria were: (1) having a follow-up period less than 12 months; (2) being lost during followed up or having incomplete data; (3) receiving DC-CIK cell-based immunotherapy combined with first-line chemotherapy. Finally, a total of 20 patients was included in this study.
Figure 2 is the flow chart of this study.
Medical records of the patients were reviewed for treatment response, and we focused on both followed up image finding (CT, MRI and PET) and tumor marker (CEA and CA19-9) at 6 months after the completion of DC-CIK cell-based immunotherapy. Recruited patients were then divided into two group, responder and non-responder, based on the treatment response. Definition of treatment response is a more than 20% decrease in tumor volume as shown by follow-up image, either CT, MRI or PET. Progression free survival (PFS) was defined as the time from the date of completing DC-CIK cell-based immunotherapy to the date of first progression or last contact. Overall survival (OS) was defined as the time from the date of completing DC-CIK cell-based immunotherapy to either the date of death or the date of last follow-up.
2.2. Generation of DC and CIK Cells
Peripheral blood mononuclear cells (PBMCs) were harvested from peripheral blood by apheresis (COBE BCT) and separated by Ficoll-pague (GE) gradient centrifugation. For the induction of DC-CIKs, PBMCs were firstly incubated for 7 days in the presence of IL-4 (500 U/mL; R&D Systems, Inc.), TNF-a (10 ng/mL; R&D Systems, Inc.) and GM-CSF (800 U/mL; R&D Systems, Inc.) in vitro to generate autologous DCs. Then, PBMCs were activated in vitro with recombinant cytokines IL-2 at 1,000 U/mL (R&D Systems, Inc.), IFN-γ at 1,000 U/mL (R&D Systems, Inc.) and CD3 antibody at 50 ng/mL (Takara Bio USA, Inc.) for 7 to 10 days. The phenotypes of DCs (CD3, CD86 and HLA-DR) and CIKs (CD3, CD56, CD86 and NKG2D) were characterized by flow cytometry. The proportion of CD3 cells and CD86 cells reached less than 2% and greater than 80%, respectively, among the cultured cells in the autologous DC-specific cultures. The cultured autologous DCs were then mixed with cultured CIKs at a proportion of 1:100, and then DC–CIK were harvested for intravenous administration to patients.
2.3. DC-CIK Treatment and Follow Up
Each patient received 2 cycles of DC–CIK cell infusions. One cycle of DC-CIK immunotherapy included three cellular infusions to give a total of 3 × 109 cells, spanning from 3 to 4 weeks. Patients received an infusion at Days 1, 5, and 9 during the first cycle and repeatedly the second cycle was given after the standard treatment such as chemotherapy administrated.
All patients were routinely followed at our clinic every 2 or 3 months, including blood examination, carcinoembryonic antigen (CEA) and CA19-9 levels. computed tomography (CT) scan, magnetic resonance imaging (MRI) and positron emission tomography (PET) scan to evaluate treatment response and status of the disease.
2.4. Statistical Analysis
The baseline characteristics were summarized by number with percentage for categorical variables and median with range for continuous variables. The Fisher’s exact test and Wilcoxon signed-rank test were applied to examine the difference of categorical and continuous, respectively, between two groups. The survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test. The significant level was set as 0.05. All analyses were performed by software R version 4.2.1.
3. Results
3.1. Baseline Characteristics of Patients
Twenty patients pathologically diagnosed with colorectal cancer, all at stage IV, who received DC-CIK cell-based immunotherapy combined with 2
nd- or later-line chemotherapy were retrospectively enrolled in this study. Among them, 11 were responder and 9 were non-responder.. The clinical characteristics of two groups were shown in
Table 1 to show that there was no significant difference in clinical characteristics or demographic between these two groups. Treatment strategy and medical management were also similar between these two groups.
3.2. Clinical Outcomes
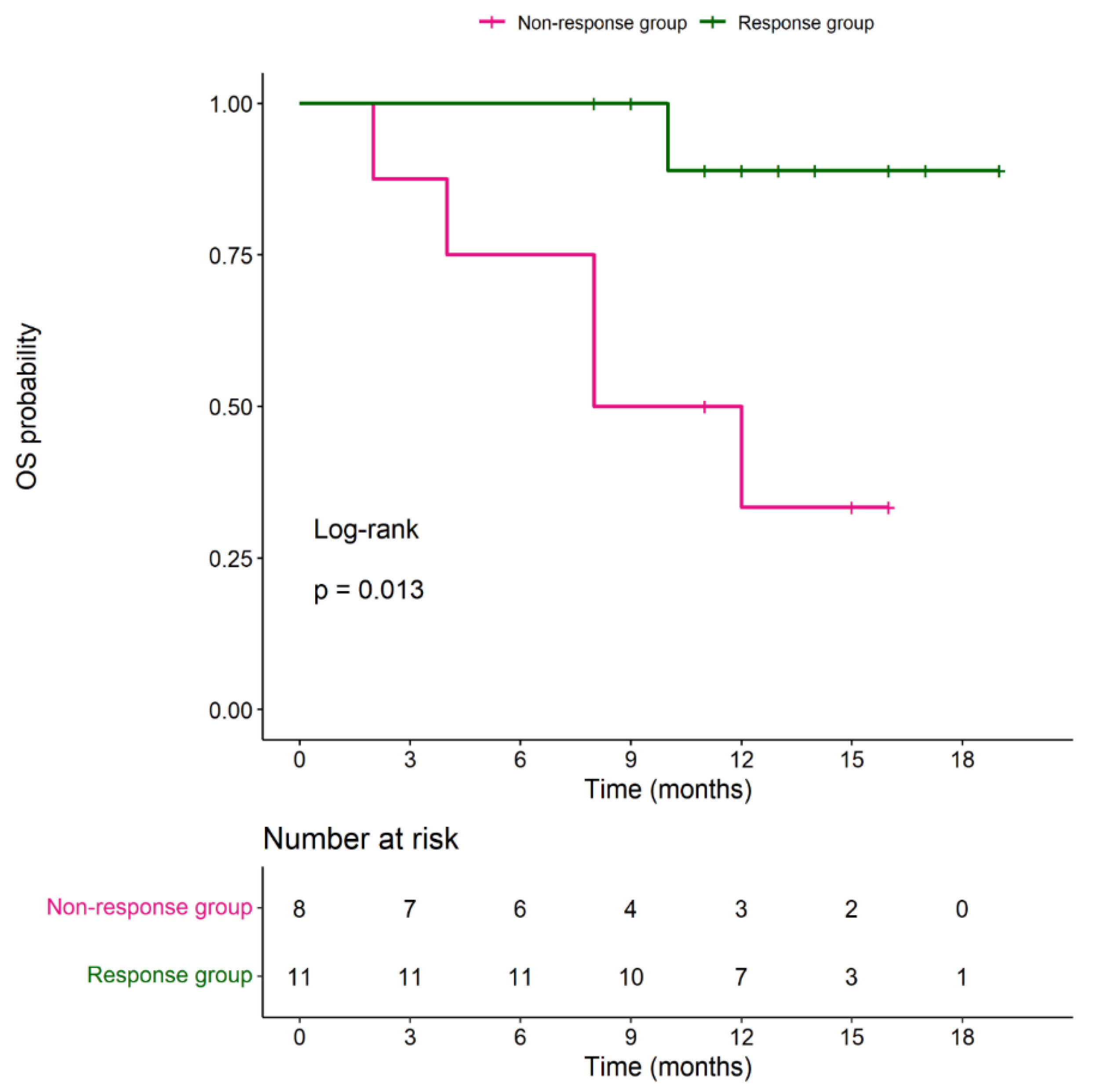
During the follow-up period, median PFS in the responder group was 7 months (with a range of 3-14 months) and median OS was 12 months (with a range of 8-19 months). Median OS in the non-responder group was 9.5 months (with a range of 2-16 months). No significant difference in OS between 2 groups (P= 0.147). Four (36.6%) patients in the responder group received surgery with curative intended 6 months after DC-CIK cell-based immunotherapy. Among these 4 patients, 2 received metastectomy (hepatectomy and splenectomy) and the other 2 underwent CRS and HIPEC surgery. The results were shown in
Table 2 and
Figure 3.
3.3. Side Effects of DC-CIK Cell Infusion
There were 3 (15%) patients had mild fever, chillness and fatigue during DC-CIK infusion. One (5%) patient developed chest tightness and tachycardia. No major event such as anaphylaxis, leukopenia, abnormal liver function tests (LFTs) or impair kidney function was noted.
4. Discussion
DC-CIK therapy has shown promising results in the treatment of advanced gastrointestinal cancer. In a recent clinical trial, patients with advanced gastrointestinal cancer who received DC-CIK therapy had a significantly longer progression-free survival and improved quality of life compared to those who received standard chemotherapy alone. The therapy was well-tolerated, with few adverse effects reported. [
24]
In Taiwan, cell-based immunotherapy is under a strict regulation by the Ministry of Health and Welfare. As for solid malignant tumors, DC-CIK cell-based immunotherapy is only indicated for patients with metastatic disease. In the study of Xie, Y., et al, 142 patients with stage III/IV CRC patient were recruited to show a significant difference in 5-year overall survival rate and 5-year progression-free survival rate between patients with/without DC-CIK cell-based immunotherapy. [
10] Studies of Lin, T., et al. and Niu, J., et al. also reported similar results. [8, 9] However, these studies all aimed at patients with stage III/IV CRC receiving first-line treatment combined with DC-CIK cell-based immunotherapy. Publications or reports focusing on the use of DC-CIK cell-based immunotherapy with second- or later-line treatment for refractory mCRC patients are limited.
In our study, 20 patients with refractory mCRC who were treated at least with first-line chemotherapy were recruited. All patients showed progression or relapse afterward. However, after combining DC-CIK cell-based immunotherapy, 11 out of 20 patients (55%) responded to the treatment. Among these 11 responsive patients, 4 patients (36%) converted to surgical treatment (metastectomy or hyperthermic intraperitoneal chemotherapy) with a median PFS of 7 months. The preliminary results, although not statistically significant and without a control group, was encouraging. Afterwards, second- or later-line treatment for mCRC patients were searched with a focus on treatment response rate and PFS below.
The efficacy of current treatment options for patients with late-stage metastatic colorectal cancer (mCRC) have been limited, as shown by the median progression-free survival (PFS) reported in the literature. Epidermal growth factor receptor (EGFR) inhibitors, such as Panitumumab and Cetuximab, combined with chemotherapy have been widely used in second- or later line settings, with a median PFS of 3 to 7 months and a response rate of 18%. [11-15] Bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor, was also used to yield a median PFS of 7 months. [12, 16] In third- or later-line settings, Regorafenib or Trifluridine-Tipiracil (TAS-102) was used as single agent or combined with other medications, but the median PFS was only 1.7 to 4.6 months. [17-20] A review by Bekaii-Saab et al. focused on the clinical outcomes of mCRC patients, especially in third- and later-line treatments. [
21] The study found that the median PFS was only 1.9 to 4.5 months, indicating the limited efficacy of current treatment options for this patient population. Conversion to surgery after second- or third-line treatment in mCRC is also limited due to the nature of the disease and the decreased treatment response in these patients.
However, our study indicated that combining DC-CIK cell-based immunotherapy with conventional chemotherapy may be a potential treatment combination for mCRC. The results showed a greater response rate and median PFS than the references, with four of the patients even receiving subsequent curative surgery. DC-CIK therapy has the potential to enhance anti-tumor immunity by stimulating a broad immune response against cancer cells, leading to a more durable response and potentially fewer side effects. Overall, the combination of DC-CIK immunotherapy with conventional chemotherapy may provide a promising treatment option for patients with mCRC who have failed previous lines of therapy. Further research is needed to optimize the therapy and identify which patients will benefit the most.
The limitations of the current treatment options for patients with late-stage metastatic colorectal cancer (mCRC) highlight the need for alternative therapies that can improve patient outcomes. DC-CIK cell-based immunotherapy, a form of adoptive cell transfer (ACT) therapy that involves infusing activated immune cells into the patient's bloodstream, [
25] has emerged as a potential treatment option for mCRC. DC-CIK cells are a type of immune cell that is derived from the patient's own blood and are stimulated in the lab to enhance their anti-tumor activity. These cells are then infused back into the patient to stimulate a broad immune response against cancer cells. Preclinical studies [27, 28] have shown that DC-CIK cells have potent anti-tumor activity against various types of cancers, including colorectal cancer. In clinical trials, the combination of DC-CIK therapy with conventional chemotherapy has demonstrated promising results in patients with advanced-stage colorectal cancer. The therapy has shown good tolerability, with few adverse effects, and has produced encouraging response rates and improved survival outcomes.
One of the key advantages of DC-CIK immunotherapy is its ability to enhance the patient's immune response against cancer cells. [29, 30] Unlike traditional chemotherapy, which targets both healthy and cancerous cells, DC-CIK therapy is more targeted and specifically activates the patient's own immune system to recognize and attack cancer cells. This approach has the potential to produce more durable responses and fewer side effects compared to conventional chemotherapy. While DC-CIK immunotherapy shows promise as a treatment option for mCRC, further research is needed to optimize the therapy and identify which patients will benefit the most. This includes exploring different treatment combinations, dosing schedules, and patient selection criteria. Nonetheless, DC-CIK immunotherapy offers an exciting new avenue for the treatment of patients with advanced-stage colorectal cancer and may provide a more effective and targeted treatment option than current therapies.
However, DC-CIK therapy has its own challenges and limitations. One limitation is the difficulty in isolating and activating dendritic cells. Additionally, the therapy may not be effective in all patients, and more research is needed to determine which patients may benefit the most. [
31]
There are also several limitations in our study. First of all, this was a retrospective study without randomized comparison. Secondly, the case number of our study was relatively small. Thirdly, the follow up period was short. Despite of these limitation, conventional chemotherapy combined with DC-CIK cell-based immunotherapy seemed to have potential treatment efficacy in refractory mCRC. Studies of larger scale with randomized control and longer follow up are needed to further support our preliminary outcome.
5. Conclusions
DC-CIK cell-based immunotherapy may have some therapeutical benefit for refractory mCRC patients. Based on our data, the combination of DC-CIK cell-based immunotherapy with second- or later- line chemotherapy has a response rate over 50% with PFS of 7 months while 36% of responsive patients converted to subsequent surgical treatment, which is rare in refractory mCRC. Although without a statistical significance in this study, a larger prospective randomized control study is needed to provide more and solid support the abovementioned finding.
Author Contributions
T.-W.K., C.-W.H., W.T.-L.C., W.-C.S. and L.-B.J. conceived the study concept and design. T.-W.K., C.-W.H. and S.-C.C. performed the acquisition of data. T.-W.K., C.-W.H., W.T.-L.C., W.-C.S., and L.-B.J. contributed to the first drafting of the manuscript. C.-T.H., C.-K.T., C.-L.C., C.-L.L., and H.-T.Y. carried out the interpretation of data and statistical analysis.W.-L.H., W.-C.S., C.-H.T., D.-Y.C., and L.-B.J. supervised the study. T.-W.K., C.-W.H., W.T.-L.C., W.-C.S. and L.-B.J. contributed to the data access and responsibility and had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This retrospective study was reviewed and approved by the Research Ethics Committee of China Medical University Hospital (CMUH111-REC3-054).
Informed Consent Statement
Not applicable for the retrospective study.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
C.-H.T. was the founder of Ever Supreme Bio Technology. W.-C.S., D.-Y.C., and L.-B.J. were stockholders of the Ever Supreme Bio Technology. W.-L.H. and W.-C.S. were employed by Ever Supreme Bio Technology and China Medical University Hospital. C.-T.H., C.-K.T., and C.-L.C. were employed by Ever Supreme Bio Technology. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Siegel, R.L., K. D. Miller, and A. Jemal, Cancer statistics, 2019. CA: A Cancer Journal for Clinicians 2019, 69, 7–34. [Google Scholar] [PubMed]
- Siegel, R.L. , et al. , Colorectal cancer statistics, 2017. CA: A Cancer Journal for Clinicians 2017, 67, 177–193. [Google Scholar] [PubMed]
- Torre, L.A. , et al., Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 2015, 65, 87–108. [Google Scholar] [PubMed]
- Ansa, B. , et al., Evaluation of Colorectal Cancer Incidence Trends in the United States (2000–2014). Journal of Clinical Medicine 2018, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, N.P.M. , et al., An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. International Journal of Cancer 2018, 143, 2758–2766. [Google Scholar] [CrossRef]
- Hu, C.-Y. , et al., Time Trend Analysis of Primary Tumor Resection for Stage IV Colorectal Cancer. JAMA Surgery 2015, 150, 245. [Google Scholar] [CrossRef]
- Wang, J. , et al., Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Medicine 2020, 9, 361–373. [Google Scholar] [CrossRef]
- Lin, T. , et al., Clinical effects of autologous dendritic cells combined with cytokine-induced killer cells followed by chemotherapy in treating patients with advanced colorectal cancer: a prospective study. Tumor Biology 2016, 37, 4367–4372. [Google Scholar] [CrossRef]
- Niu, J. , et al., Retrospective Comparative Study of the Effects of Dendritic Cell Vaccine and Cytokine-Induced Killer Cell Immunotherapy with that of Chemotherapy Alone and in Combination for Colorectal Cancer. BioMed Research International 2014, 2014, 1–7. [Google Scholar]
- Xie, Y. , et al., Effect of dendritic cell-cytokine-induced killer cells in patients with advanced colorectal cancer combined with first-line treatment. World Journal of Surgical Oncology 2017, 15, 1–6. [Google Scholar] [CrossRef]
- Amado, R.G. , et al., Wild-Type <i>KRAS</i> Is Required for Panitumumab Efficacy in Patients With Metastatic Colorectal Cancer. Journal of Clinical Oncology 2008, 26, 1626–1634. [Google Scholar] [PubMed]
- Hecht, J.R. , et al., SPIRITT: a randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clinical colorectal cancer 2015, 14, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Karapetis, C.S. , et al., <i>K-ras</i>Mutations and Benefit from Cetuximab in Advanced Colorectal Cancer. New England Journal of Medicine 2008, 359, 1757–1765. [Google Scholar] [PubMed]
- Peeters, M. , et al., Randomized Phase III Study of Panitumumab With Fluorouracil, Leucovorin, and Irinotecan (FOLFIRI) Compared With FOLFIRI Alone As Second-Line Treatment in Patients With Metastatic Colorectal Cancer. Journal of Clinical Oncology 2010, 28, 4706–4713. [Google Scholar] [CrossRef]
- Peeters, M. , et al., Final results from a randomized phase 3 study of FOLFIRI ± panitumumab for second-line treatment of metastatic colorectal cancer. Annals of Oncology 2014, 25, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Masi, G. , et al., Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Annals of Oncology 2015, 26, 724–730. [Google Scholar] [CrossRef]
- Grothey, A. , et al., Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Kuboki, Y. , et al., TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. The Lancet Oncology 2017, 18, 1172–1181. [Google Scholar] [CrossRef]
- Li, J. , et al., Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. The lancet oncology 2015, 16, 619–629. [Google Scholar] [CrossRef]
- Mayer, R.J. , et al., Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer. New England Journal of Medicine 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Bekaii-Saab, T. , et al., Third- or Later-line Therapy for Metastatic Colorectal Cancer: Reviewing Best Practice. Clinical Colorectal Cancer 2019, 18, e117–e129. [Google Scholar] [CrossRef] [PubMed]
-
XU, Huiru, et al. Analysis of the Clinical Efficacy of Dendritic Cell–cytokine Induced Killer Cell-based Adoptive Immunotherapy for Colorectal Cancer. Immunological Investigations 2021, 50, 622–633. [Google Scholar] [CrossRef] [PubMed]
- NI, Ling. Advances in human dendritic cell-based immunotherapy against gastrointestinal cancer. Frontiers in Immunology 2022, 13, 887189. [Google Scholar] [CrossRef] [PubMed]
- DU, YANG, ZHANG, et al. , Cytokine-induced killer cell/dendritic cell combined with cytokine-induced killer cell immunotherapy for treating advanced gastrointestinal cancer. BMC cancer 2020, 20, 1–11.
- Zhao, Y.; Liu, X. Clinical application and development of dendritic cell/cytokine-induced killer cell immunotherapy for cancer. Journal of Hematology & Oncology 2020, 13, 1–16. [Google Scholar]
- Liu, Zhang, Jia, et al. , Dendritic cell/cytokine-induced killer cell immunotherapy combined with S-1 in patients with advanced pancreatic cancer: a prospective study. Clinical Cancer Research 2016, 22, 1–9. [Google Scholar]
- Ren, Di, Song, et al. , Clinical study on treatment of malignant melanoma with autologous tumor-derived combined DC-CIK cells. Cellular Immunology 2015, 295, 71–77. [Google Scholar]
- Shi, Zhang, Tang, et al. , Combination of DC-CIK infusion and chemotherapy in advanced colorectal cancer. World Journal of Gastroenterology 2015, 21, 6482–6488. [Google Scholar]
- He, Li, Liu, et al. , Immunotherapy for metastatic colorectal cancer. Journal of Hematology & Oncology 2021, 14, 1–14. [Google Scholar]
- Zhou, Cui, & Qiu, Dendritic cell therapy in cancer: research progress and clinical applications. Expert Review of Anticancer Therapy 2020, 20, 1041–1051.
- XU, Huiru, et al. Analysis of the Clinical Efficacy of Dendritic Cell–cytokine Induced Killer Cell-based Adoptive Immunotherapy for Colorectal Cancer. Immunological Investigations 2021, 50, 622–633. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).