1. Introduction
Since randomized controlled trials (RCTs) have shown its superiority or non-inferiority, respectively in high and intermediate-to-low-risk patients[
1,
2,
3,
4,
5,
6,
7], over surgical aortic valve replacement (SAVR), transcatheter aortic valve implantation (TAVI) has nowadays a well-validated role in the management of patients with severe AS.
Among others, age remains a key factor in the choice between TAVI and SAVR, and a
leitmotiv when discussing the most appropriate path of care for each patient. As aging is most often paralleled by an augmented surgical risk, a percutaneous approach is usually favored in the elderly. However, RCTs have enrolled progressively younger individuals, showing excellent procedural success and valve performance, at least through a mid-term follow-up[
2,
4,
8]. Thereby, the adagio of limiting the transcatheter approach only to the very elderly appears obsolete.
In 2021, the European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines for the management of valve diseases provided updated recommendations for the choice of intervention in patients with severe symptomatic AS[
9]. These include a class I level of evidence A (IA) recommendation in favor of transfemoral TAVI for all patients at high surgical risk or, alternatively, aging ≥75 years, irrespective of their surgical risk. On the opposite, a IB recommendation was formulated in favor of SAVR in individuals aging <75 years and deemed at low surgical risk[
9]. Hence, the aforementioned landmark (75 years) really makes the difference in intermediate and low surgical risk patients, in whom the choice of the type of intervention might be substantially influenced by age. Although the formulation of a precise age cut-off subtends practical reasons (e.g., valve durability), such a dichotomization carries the risk of overshadowing other key factors and has never shown to substantially impact on hard clinical outcomes.
Hence, we sought to compare the clinical outcomes of patients below and above the age threshold suggested by the recent ESC/EACTS guidelines and to explore the prognostic relevance of the age cut-off in intermediate-to-low-risk patients undergoing TAVI with self-expandable devices.
2. Materials and Methods
The HORSE registry is an international registry in which patients undergoing transfemoral TAVI using self-expandable valves were retrospectively enrolled across sixteen European centers between September 2014 and April 2020[
10]. All patients provided informed consent to participate in the registry and agreed with the use of their data for scientific purposes.
The research has been carried out in accordance with the Declaration of Helsinki (World Medical Association, October 2013)[
11]. Criteria for exclusion included pure aortic regurgitation, surgical prosthesis degeneration, and non-transfemoral access. Outcomes were defined according to Valve Academic Research Consortium 2 criteria[
12]. The primary outcome for the present analysis was all-cause mortality. Secondary endpoints included major and minor vascular complications, anulus rupture, new permanent pacemaker implantation, periprocedural myocardial infarction, cardiac tamponade, all-cause stroke, major bleeding, minor bleeding and acute kidney injury.
To the present analysis, patients at high surgical risk, defined as those with an STS score >8%, were excluded. Intermediate-to-low-risk patients were included irrespective of the type of prosthesis and categorized according to their age into two groups, i.e., ≥75 vs. <75 years.
Statistical Analysis
Categorical variables were reported as frequencies and percentages and compared with the Chi square test or Fisher’s test, as appropriate. Visual assessment of distribution was conducted for continuous variables, which were thereafter reported as mean (standard deviation – SD) or median [quartile 1-quartile 3 (Q1-Q3)] and compared by means of the Student´s t-test or Wilcoxon-Mann-Whitney test, as appropriate.
The cumulative unadjusted frequencies of all-cause death in patients aging ≥75 vs. <75 years were obtained with the Kaplan-Meier method and compared through the log-rank test. Univariate and multivariable Cox regression analyses were run to obtain the predictors of the same outcome, selecting candidates variables on a clinical and statistical base. Hazard ratios (HRs) along with 95% confidence intervals (Cis) were calculated. Given the time-dependent nature of the outcome, the predictive accuracy of age was assessed through a the time-dependent area under the receiver-operating characteristic curve (AUC) [
13]. The optimal cut-off point of age for the prediction of all-cause mortality was also assessed using the Youden index estimator.
Finally, to account for the possible heterogeneity across the large population of the registry, sensitivity analyses were conducted on the primary and secondary outcomes by stratifying the population according to age quartiles.
Two-tailed p-values <0.05 were considered statistically significant. The analysis was conducted using “R” software (The R foundation for statistical computing, Vienna, version 3.6.2).
3. Results
3.1. Baseline and Procedural Features
Among 3389 patients initially enrolled in the registry, 411 were categorized as high surgical risk and 293 were excluded due to the absence of follow-up data. Thus, the final population encompassed 2685 individuals (age <75 years n=280; ≥75 years n=2405).
The baseline features are reported in
Table 1. The median age was 82 (IQR 79-86) years in the whole population, with patients in the two arms being divided by almost a decade of age on average. Most patients were female, with a higher proportion of males in the <75 years group (45% vs. 35%, p=0.001). Older patients had lower BMI [26.7 (24-30) vs. 28.5 (24-33), p<0.001], a higher frequency of previous pacemaker or implantable cardioverter defibrillator implantation (11% vs. 6%, p=0.019), atrial fibrillation (AF, 33% vs. 23%, p=0.010) and chronic kidney disease (CKD, 65% vs. 30%, p<0.001). Contrary, smoking (19% vs. 7%, p<0.001), diabetes (33% vs. 25%, p=0.010) and chronic obstructive pulmonary disease (25% vs. 16%, p<0.001) were more frequent in patients aging <75 years. Mean STS score was 3.60% (IQR 2.50-5.03) in the overall population, with low-risk patients being significantly more represented in the younger arm (72% vs. 55%, p<0.001) and intermediate risk in the older one (45% vs. 28%, p<0.001).
No significant differences were noted with respect to most echocardiographic data, including mean aortic valve gradient and aortic valve area. Slight, yet statistically significant, differences in terms of left ventricular ejection fraction were present [60 (55-65) in the ≥75 years arm vs. 60 (54-65) in the <75 years arm, p=0.006]. CT scan revealed a higher prevalence of porcelain aorta in younger patients (16% vs. 9%, p<0.001).
From the procedural standpoint (
Table 2), the Evolut PRO model was predominantly implanted in the youngest arm (17% vs. 11%, p=0.001), with no other significant difference between the two groups.
3.2. Clinical Outcomes
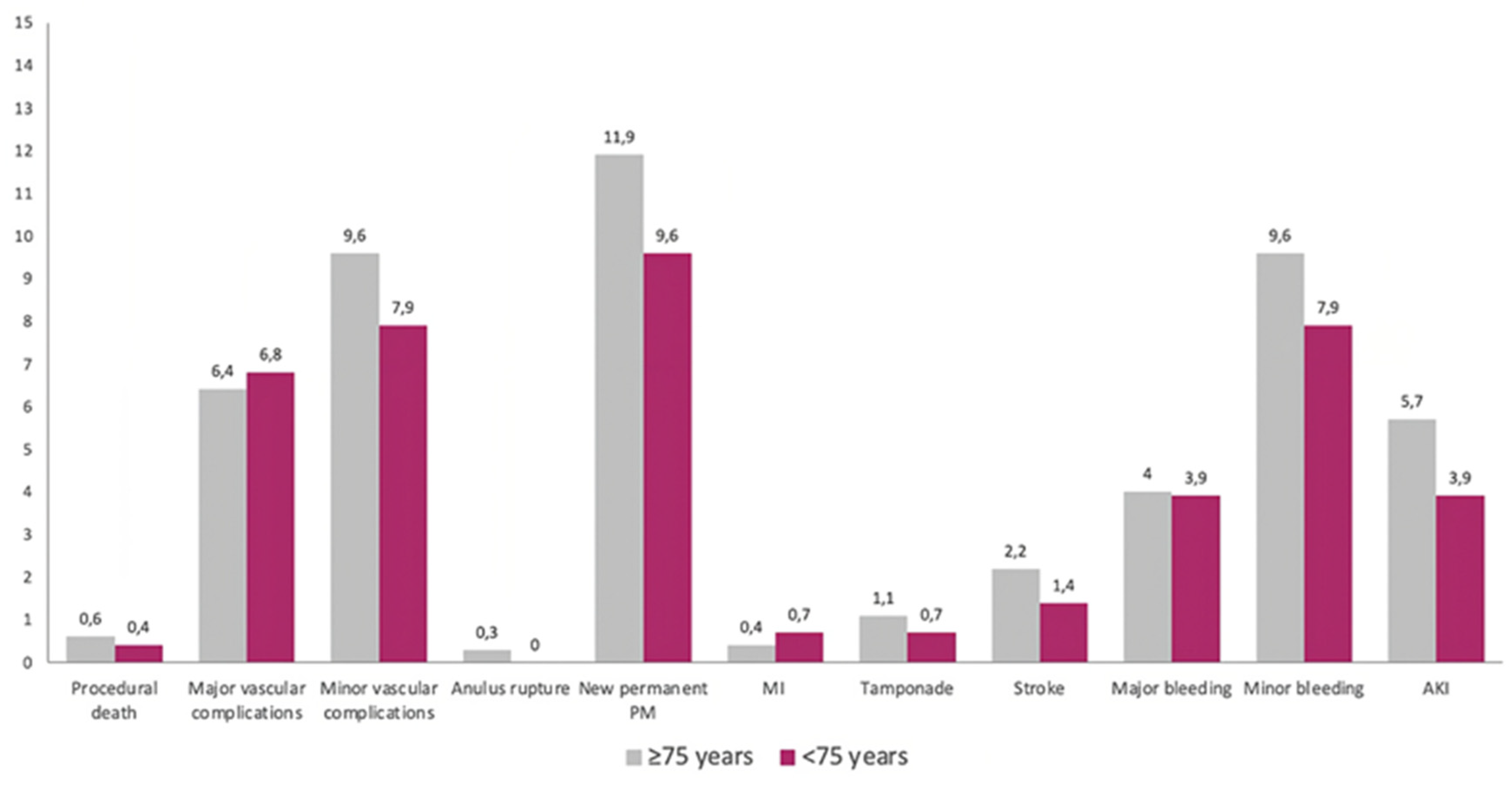
As summarized in
Figure 1, there were no significant differences across several in-hospital endpoints between the two groups.
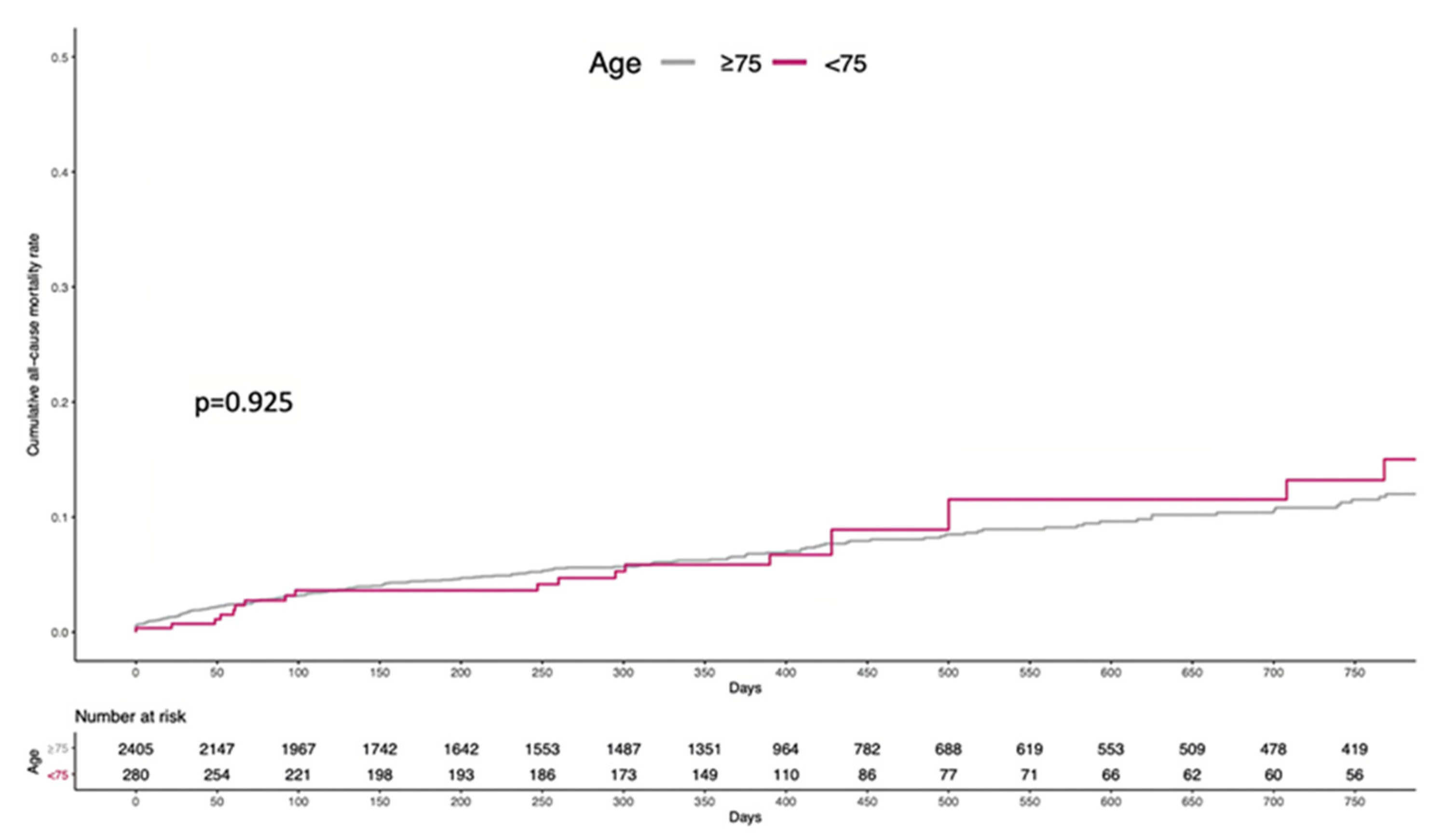
Figure 2 depicts the cumulative incidence of all-cause mortality through a mean follow-up of 437 ± 381 days (86.5% of population completed the 1-year follow-up). Overall, 198 (8.2%) and 23 (8.2%) patients died in the ≥75 and <75 years arm, respectively (log-rank p=0.925). Likewise, 1-year event-rate was comparable, with 124 (5.1%) and 13 (4.6%) deaths in the two arms, respectively, and no statistically significant difference (log-rank p=0.707).
The results of the univariate and multivariable Cox regression are reported in
Table 3. In the univariate analysis, age was not associated with the risk of all-cause mortality, either when analyzed as a continuous covariate (HR 1.01, 95% CI 0.99-1.04, p=0.294) or dichotomizing according to the prespecified cutoff of 75 years (HR 0.97, 95% CI 0.63-1.51, p=0.924). New York Heart Association class III or IV at presentation (HR 1.68, 95% CI 1.23-2.35, p=0.001), CKD (HR 1.47, 95% CI 1.05-2.08, p=0.026), atrial fibrillation (HR 1.40, 95% CI 1.06-1.84, p=0.016) and STS score (HR 1.12, 95% CI 1.03-1.21, p=0.007) significantly predicted the occurrence of the primary outcome after adjusting for covariates.
Results of the sensitivity analysis according to age quartiles are reported in the Supplementary material (Appendix A). There was no significant difference regarding in-hospital outcomes across different quartiles except for the rate of new pacemaker implantation, which was slightly higher in patients whose age was above the median (
Supplementary Table S1).
Supplementary Figure S1 shows the cumulative incidence of all-cause mortality in the four groups, with patients in the highest quartile experiencing a numerically higher event-rate, without any statistically significant trend.
3.3. Accuracy of Age to Predict All-Cause Mortality
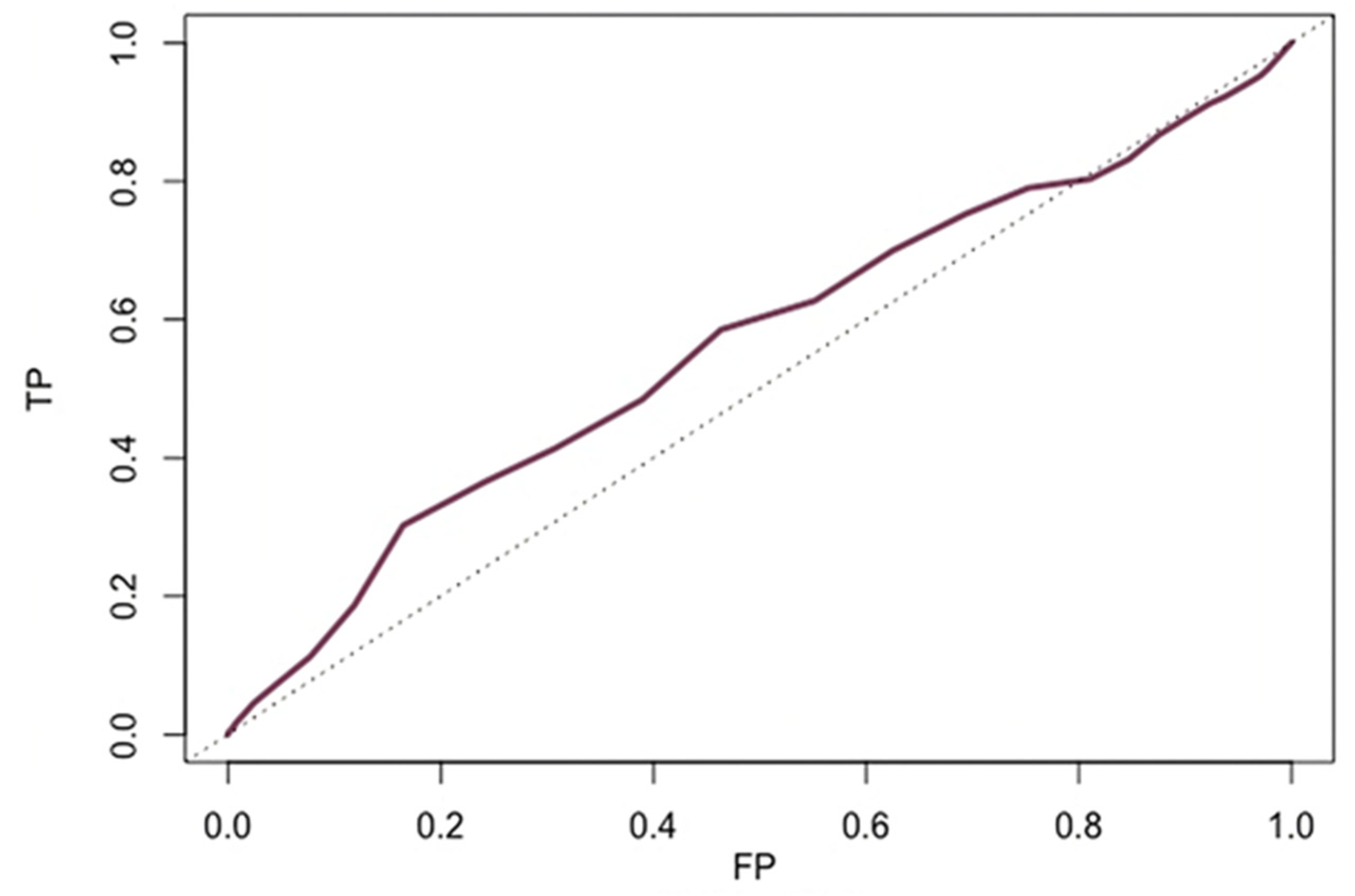
Time-dependent ROC curve showed low accuracy of age to predict either 1-year and 2-year all-cause death (both areas under the curve=0.54) (
Figure 3). The optimal age threshold to predict both 1-year (sensitivity 31%, specificity of 79%) and 2-year (sensitivity 32%, specificity of 78%) mortality was 85 years.
4. Discussion
In this large cohort of intermediate-to-low-risk individuals treated with self-expandable devices, clinical outcomes were similar in patients in and out the age cut-off recommended by current ESC/EACTS guidelines, and age alone displayed a low accuracy in predicting all-cause mortality up to two years after transfemoral TAVI.
Previous studies have focused on the prognostic relevance of age in patients undergoing TAVI. An analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, as well as a more recent report from the SwissTAVI registry found higher mortality rates in nonagenarians compared to younger patients, either during the first month and throughout the first year of follow-up[
14,
15]. Similar results were also reported by the Cerebrovascular EveNts in Patients Undergoing TranscathetER Aortic Valve Implantation (CENTER) collaboration[
16]. Although these observations emphasize the importance of patient selection among the very elderly, the prognostic impact of age in most patients undergoing TAVI has been proven to be limited. Indeed, RCTs on TAVI vs. SAVR in intermediate and low risk. Patients showed no significant interaction according to age subgroups for the rate of their primary endpoints. Most patients enrolled in these RCTs were substantially younger compared to earlier studies conducted on high-risk and inoperable populations, up to an average of less than 75 years in the Placement of Aortic Transcatheter Valves (PARTNER) 3 and Evolut Surgical Replacement and Transcatheter Aortic Valve Implantation in Low-Risk Patients (Evolut low risk) trials[
2,
4]. These studies provided the backbone for either the European guidelines, which substantially encourage TAVI in all patients of 75 years or beyond, and the US guidelines, which further pushed the recommendation by advocating TAVI as a possible alternative to SAVR in all patients between 65 and 80 years of age.
Contrarily to older patients, however, those in their 70s or 60s have been substantially underrepresented in RCTs published so far, with most evidence nowadays available deriving from observational studies. In the German Quality Assurance Registry on Aortic Valve Replacement (AQUA) registry[
17], for instance, SAVR and transfemoral TAVI were compared among patients <75 years. After propensity matching, in-hospital outcomes did not differ between the two cohorts except for a higher rate of permanent pacemaker implantation in TAVI patients and a higher rate of delirium in SAVR patients. Later, Witberg and colleagues[
18] compared patients <70 and ≥70 years who had undergone TAVI, showing similar incidence of in-hospital adverse events and a similar rate of all-cause mortality up to 5 years. Taken together, these observations further support the substantial equipoise of the surgical and transcatheter approaches in the youngest candidates as well, with consistent benefit of TAVI in terms of hard clinical outcomes across different ages. The present study further expands this literature, confirming a comparable safety-efficacy profile of TAVI in patients at the two extremes of the prespecified age threshold. Differently from the previously published data, however, we focused on a guideline-recommended cut-off, which makes our study of immediate practical relevance by providing clinicians and interventionalists with a tool to enhance guidelines understanding and to critically appraise their content.
Although our study did not find any relevant impact of age on clinical outcomes following transfemoral TAVI, a high number of factors tightly intertwined with patients’ age, unfortunately not fully captured by our data, should be taken into account. First and foremost, as shown by previous studies performed in patients treated with SAVR, young age is among the drivers of structural valve deterioration[
19]. Notwithstanding the similarities between the prostheses adopted for TAVI and SAVR, it remains currently uncertain whether the long-term performance of the first might reproduce that of surgical valves[
20]. Moreover, even in the era of alignment techniques, current devices for TAVI carry the risk of making coronary re-access harder compared to surgical prostheses[
21]. Therefore, they potentially hamper future percutaneous coronary revascularizations, which is of particular interest in young patients[
22].
Hence, the choice of the type of intervention should be weighted on several factors, among which age remains, without doubts, essential. However, substantial heterogeneity in terms of treatment outcomes is unlikely to be predictable exclusively by age itself. Harmonizing technical and clinical aspects as well as patient’s preferences, as encouraged by current guidelines (8,16,21), will remain a must in the next future.
Limitations
The retrospective and non-randomized design is an inherent and key limitation of the present study, preventing us to exclude the influence of confounders and limiting the capability to explore all the potential effect modifiers. Missing data (13.5%) might have influenced the reliability of the analysis. We would have aimed at performing further analyses to explore the age cut-offs supported by US guidelines: unfortunately, this was substantially impossible due to the very low number of patients aging less than 65 years. Furthermore, patients included in the dataset may not be representative of those implanted with balloon-expandable valves, which were not enrolled in the HORSE registry. Finally, due to the lack of a long-term clinical and echocardiographic follow-up, we cannot estimate the impact of structural valve deterioration.
5. Conclusions
Careful, case-by-case evaluation of patient features and preferences remains a must in severe AS. Age-related issues exist and should be considered when choosing the best treatment strategy. However, the age cut-off advocated by contemporary guidelines does not stratify the occurrence of hard adverse events, either during the hospital stay and through a mid-length follow-up.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1. In-hospital outcomes according to age quartiles; Supplementary Figure S1. All-cause mortality according to age quartiles).
Author Contributions
Conceptualization, A.S. and S.B.; methodology, A.S., S.B., and F.G.; software, A.S., S.B.; validation, A.S., W.K., A.K., T.B., S.T., R.G., F.D.M., M.A., R.D., B.R., L.N., M.B., A.R., T.P., J.R.C., I.P., A.C. and F.G; formal analysis, A.S., S.B., F.G., A.C and F.G.; investigation, A.S., W.K., A.K., T.B., S.T., R.G., F.D.M., M.A., R.D., B.R., L.N., M.B., A.R., T.P., J.R.C., I.P., A.C. and F.G.; resources, A.S., W.K., A.K., T.B., S.T., R.G., F.D.M., M.A., R.D., B.R., L.N., M.B., A.R., T.P., J.R.C., I.P., A.C. and F.G.; data curation, A.S., S.B., F.G.; writing—original draft preparation, A.S., S.B., F.G; writing—review and editing, A.S., W.K., A.K., T.B., S.T., R.G., F.D.M., M.A., R.D., B.R., L.N., M.B., A.R., T.P., J.R.C., I.P., A.C. and F.G.; visualization, A.S., S.B. and F.G.; supervision, A.S., I.P., A.C. and F.G.; project administration, A.S. and F.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and submitted as study communication to the Institutional Review Board of every participating center without a dedicated approval due to their retrospective character.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
Won-keun Kim received personal fees from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, Meril Life Sciences, Shockwave Med. Stefan Toggweiler serves as a consultant and/or proctor for Boston Scientific, Edwards Lifesciences, Medtronic, Abbott Vascular, Biosensors, Shockwave, Teleflex, Medira, AtHeart Medical, VeoSource, Polares Medical, has received institutional research grants from Boston Scientific, Biosensors, Fumedica and Novartis and holds equity in Hi-D Imaging. The other authors have nothing to disclose.
References
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374(17):1609-1620. [CrossRef]
- Mack MJ, Leon MB, Thourani VH, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med. 2019;380(18):1695-1705. [CrossRef]
- Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376(14):1321-1331. [CrossRef]
- Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med. 2019;380(18):1706-1715. [CrossRef]
- Landes U, Webb JG, De Backer O, et al. Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J Am Coll Cardiol. 2020;75(16):1882-1893. [CrossRef]
- Søndergaard L, Steinbrüchel DA, Ihlemann N, et al. Two-Year Outcomes in Patients With Severe Aortic Valve Stenosis Randomized to Transcatheter Versus Surgical Aortic Valve Replacement: The All-Comers Nordic Aortic Valve Intervention Randomized Clinical Trial. Circ Cardiovasc Interv. 2016;9(6). [CrossRef]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. [CrossRef]
- Ruparelia N, Latib A, Buzzatti N, et al. Long-Term Outcomes After Transcatheter Aortic Valve Implantation from a Single High-Volume Center (The Milan Experience). Am J Cardiol. 2016;117(5):813-819. [CrossRef]
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561-632. [CrossRef]
- Gallo F, Gallone G, Kim WK, et al. Horizontal Aorta in Transcatheter Self-Expanding Valves: Insights From the HORSE International Multicentre Registry. Circ Cardiovasc Interv. 2021;14(9). [CrossRef]
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [CrossRef]
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J. 2012;33(19). [CrossRef]
- Arsalan M, Szerlip M, Vemulapalli S, et al. Should Transcatheter Aortic Valve Replacement Be Performed in Nonagenarians?: Insights From the STS/ACC TVT Registry. J Am Coll Cardiol. 2016;67(12):1387-1395. [CrossRef]
- Attinger-Toller A, Ferrari E, Tueller D, et al. Age-Related Outcomes After Transcatheter Aortic Valve Replacement: Insights From the SwissTAVI Registry. JACC Cardiovasc Interv. 2021;14(9):952-960. [CrossRef]
- Vlastra W, Chandrasekhar J, Vendrik J, et al. Transfemoral TAVR in Nonagenarians: From the CENTER Collaboration. JACC Cardiovasc Interv. 2019;12(10):911-920. [CrossRef]
- Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143(5):E72-E227. [CrossRef]
- Eggebrecht H, Bestehorn K, Rassaf T, et al. In-hospital outcomes after transcatheter or surgical aortic valve replacement in younger patients less than 75 years old: a propensity-matched comparison. EuroIntervention. 2018;14(1):50-57. [CrossRef]
- Witberg G, Landes U, Codner P, et al. Clinical outcomes of transcatheter aortic valve implantation in patients younger than 70 years rejected for surgery: the AMTRAC registry. EuroIntervention. 2022;17(16):1289-1297. [CrossRef]
- Johnston DR, Soltesz EG, Vakil N, et al. Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg. 2015;99(4):1239-1247. [CrossRef]
- Yerasi C, Rogers T, Forrestal BJ, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Young, Low-Risk Patients With Severe Aortic Stenosis. JACC Cardiovasc Interv. 2021;14(11):1169-1180. [CrossRef]
- 21Otto, CM. Alignment and divergence in European and North American aortic stenosis guidelines. EuroIntervention. 2022;17(14):E1123-E1125. [CrossRef]
- Khokhar AA, Ponticelli F, Zlahoda-Huzior A, et al. Coronary access following ACURATE neo implantation for transcatheter aortic valve-in-valve implantation: Ex vivo analysis in patient-specific anatomies. Front Cardiovasc Med. 2022;9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).