Submitted:
27 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and reagents
2.2. Cell culture
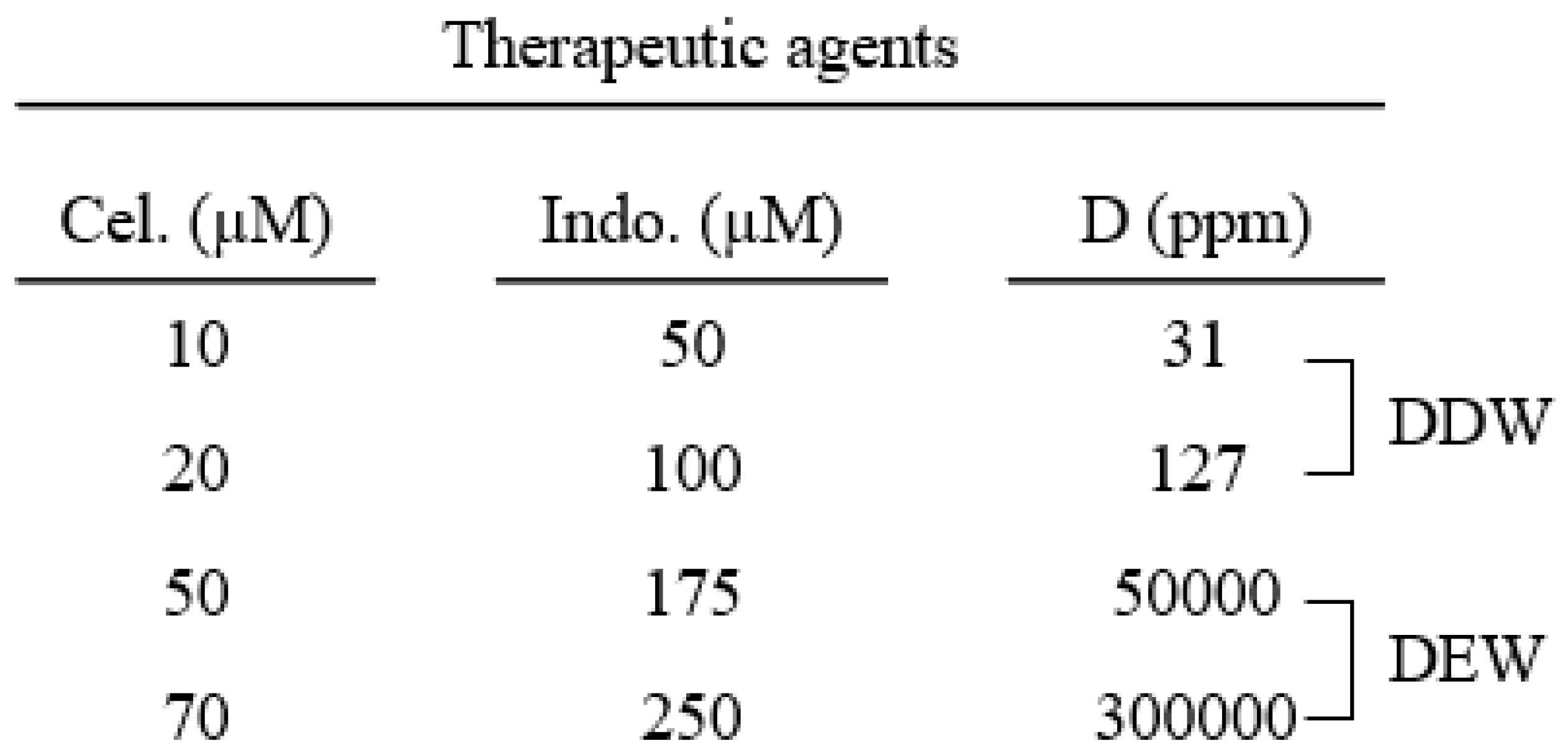
2.3. Preparation of media containing different concentrations of deuterium
2.4. MTT assay for Cellular Proliferation
2.5. Protein Isolation and Western Blotting
2.6. Statistical analysis
3. Results:
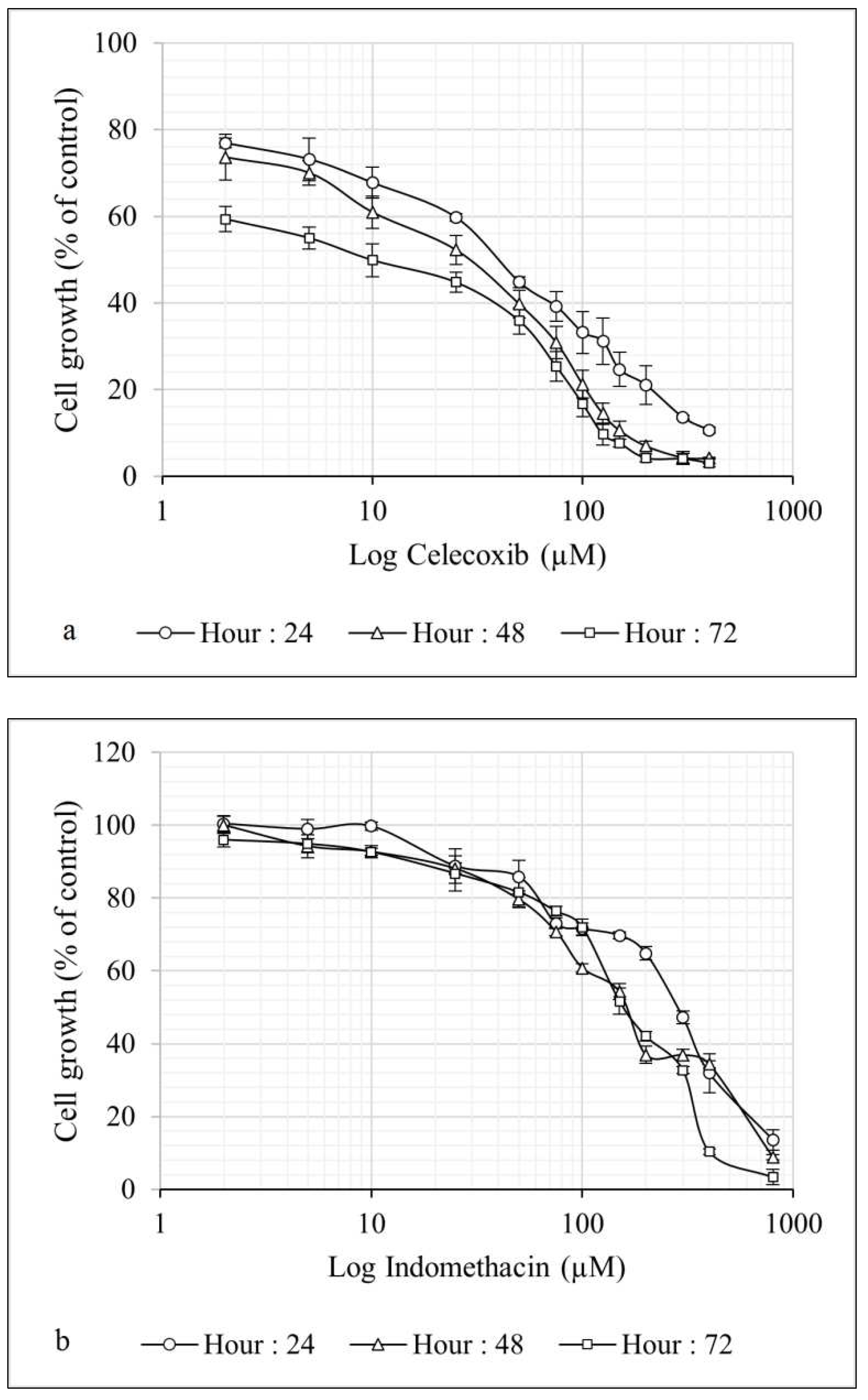
3.1. Celecoxib and indomethacin inhibited Hep G2 cell proliferation in a dose- and time-dependent manner
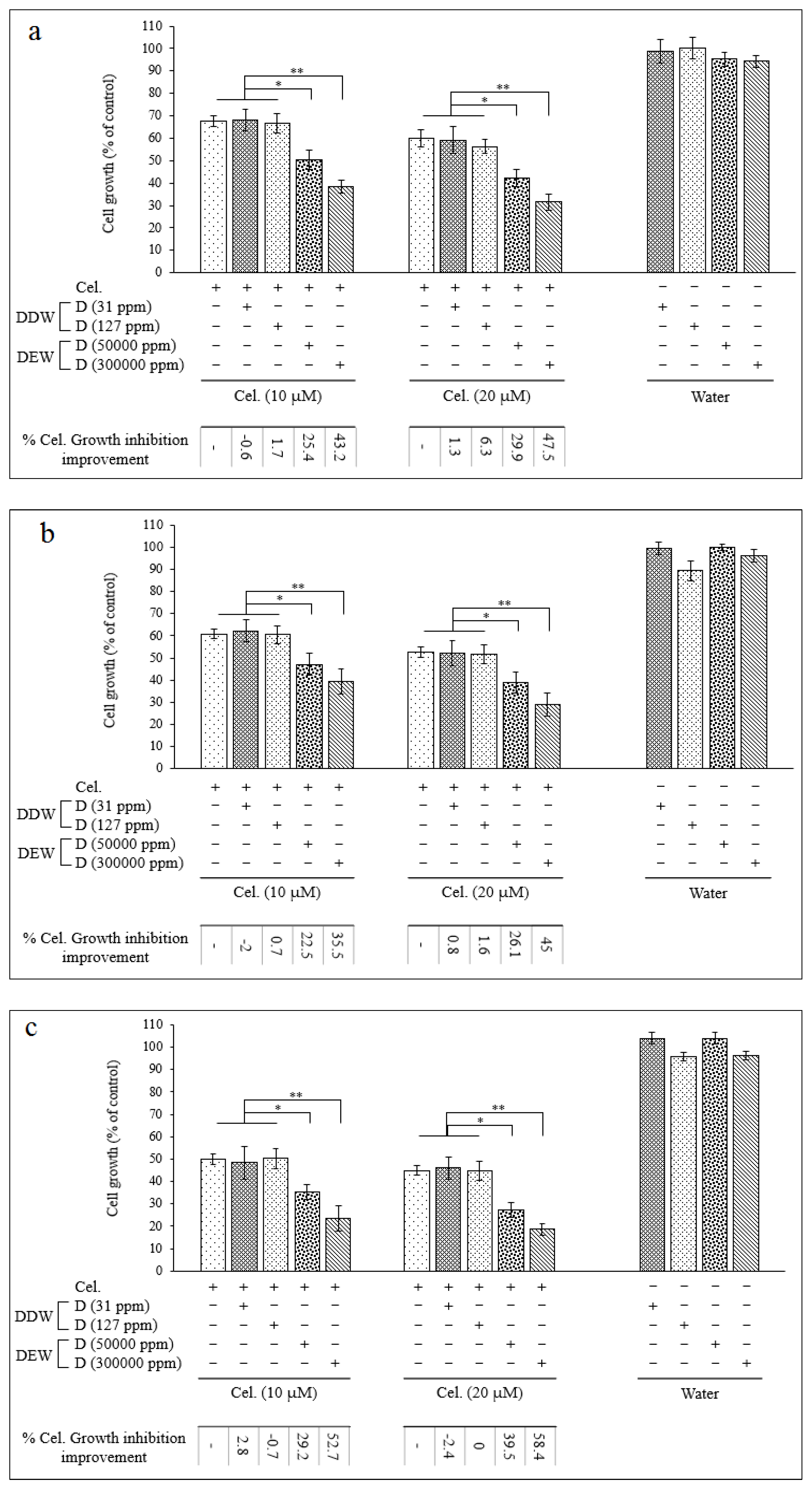
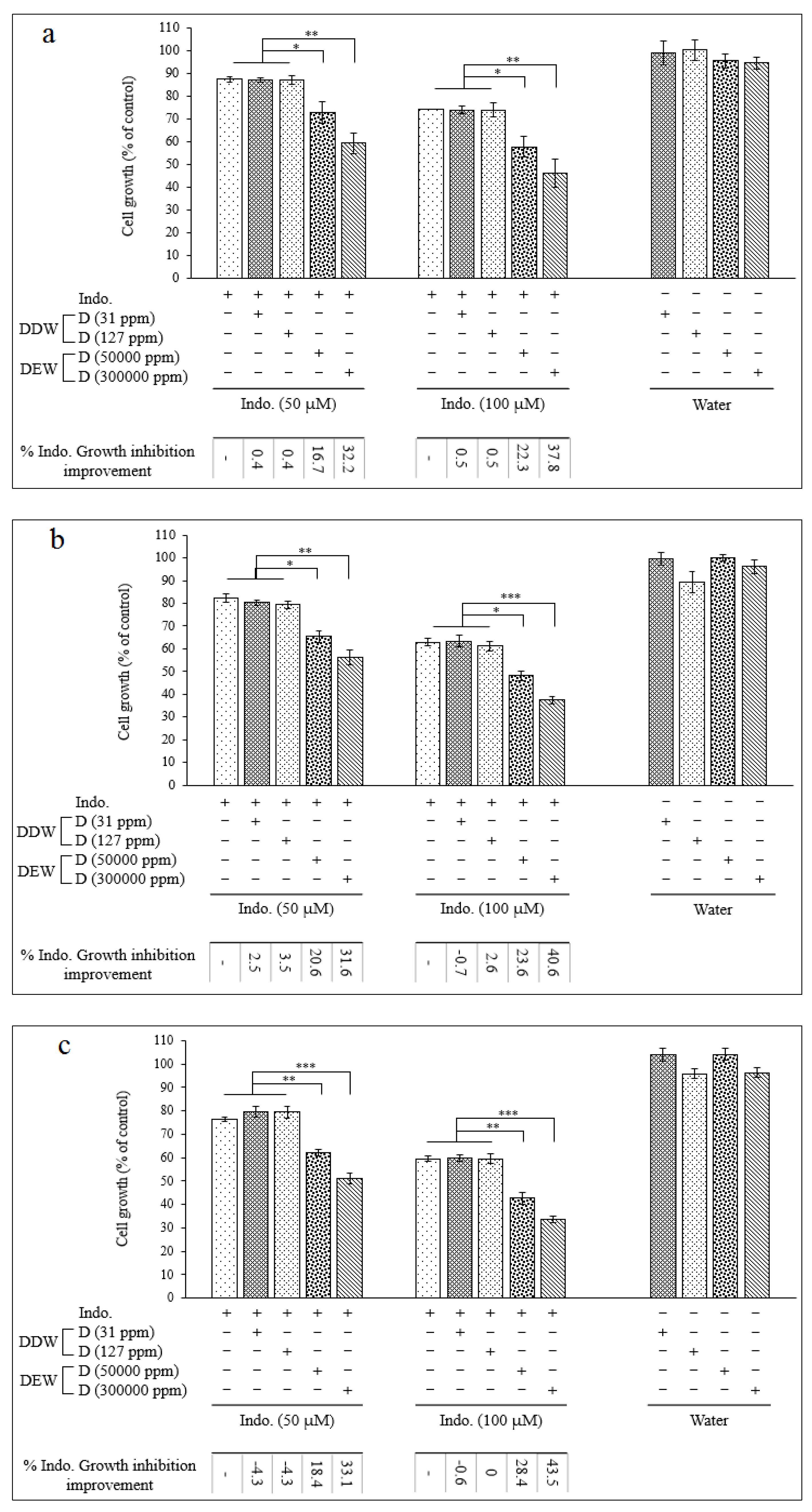
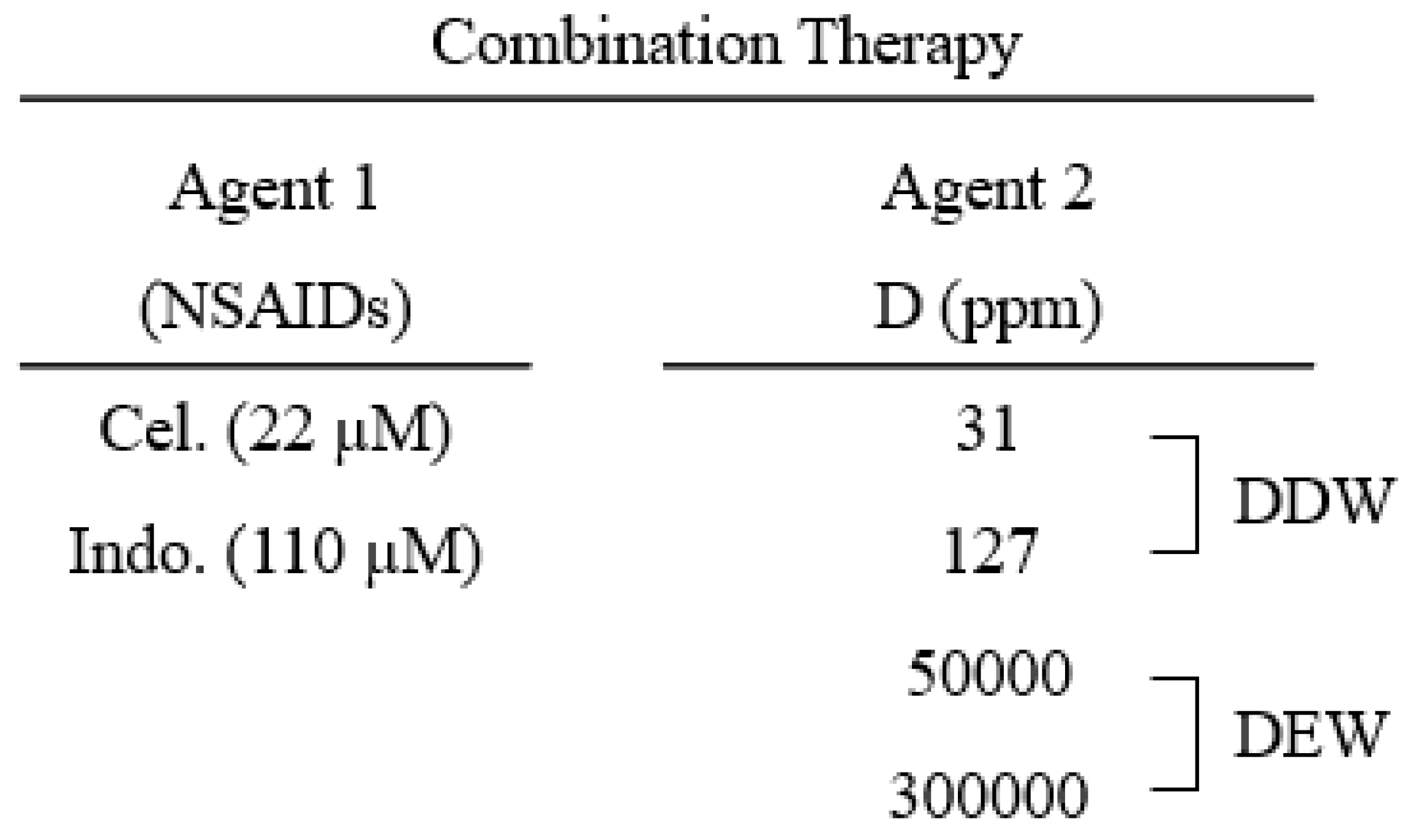
3.2. The combination of celecoxib and indomethacin with DEW but not DDW produces a more potent inhibitory effect on cell growth than either product alone
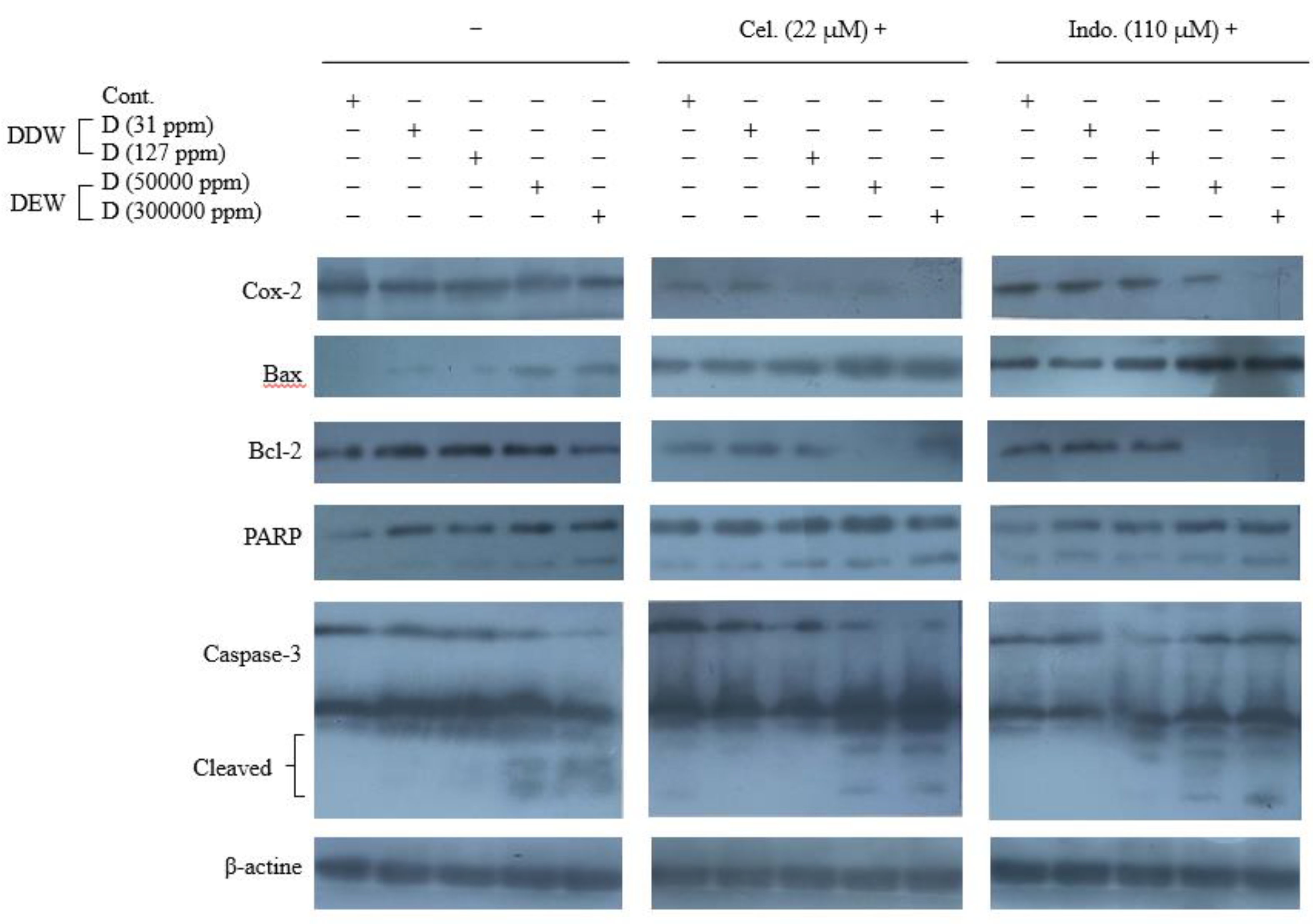
3.3. Western blotting analysis results
3.3.1. COX-2 protein expression in the cells treated with Cel, Indo, DDW, DEW and their combinations
3.3.2. Expression of Bax and Bcl-2 proteins in the cells treated with Cel, Indo, DDWs, and DEWs and their combinations
3.3.3. Caspase-3 activation and PARP cleavage assay in the cells treated with Cel, Indo, DDWs, and DEWs and their combinations
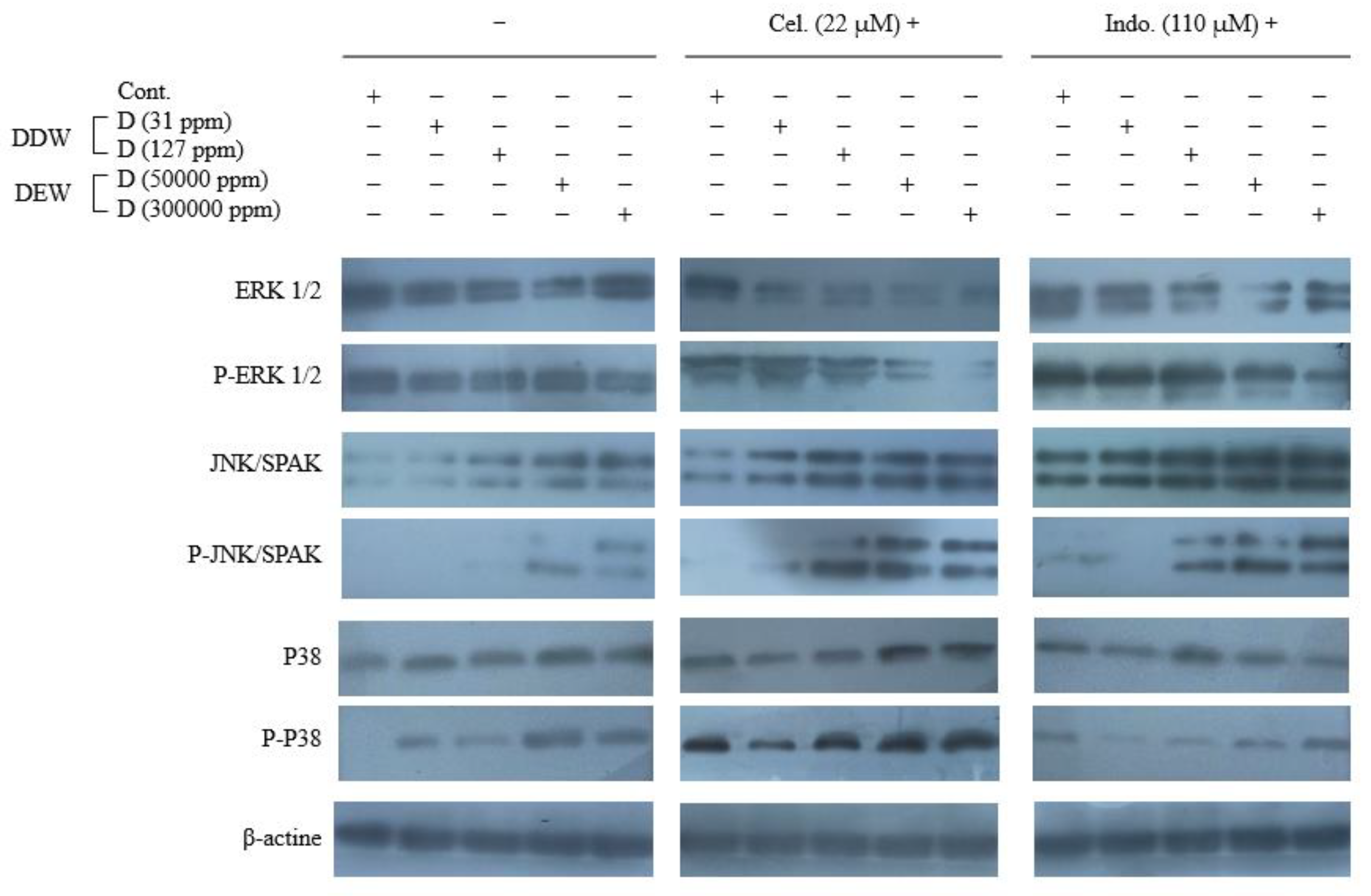
3.3.4. Expression of MAPKinase proteins in the cells treated with Cel, Indo, DDWs, and DEWs and their combinations
4. Discussion
5. Conclusion
References
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterol. 2017, 152, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Waller, L.P.; Deshpande, V.; Pyrsopoulos, N. Hepatocellular Carcinoma: A Comprehensive Review. World J. hepatol. 2015, 7, 2648–2663. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, R.; Okanoue, T.; Fujiwara, N.; Okita, K.; Kiyosawa, K.; Omata, M.; Kumada, H.; Hayashi, N.; Koike, K. Clinical Characteristics, Treatment, and Prognosis of Non-B, Non-C Hepatocellular Carcinoma: A Large Retrospective Multicenter Cohort Study. Gastroenterol. 2015, 50, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.J.H.; Wong, C.; Ng, C.H.; Poh, C.W.; Jain, S.R.; Huang, D.Q.; Muthiah, M.D. A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity. J. Clin. Med. 2021, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Mathers, C. Projections of Mortality and Causes of Deaths, 2016 to 2060. Available online: https://colinmathers.com/2022/05/10/projections-of-global-deaths-from-2016-to-2060/ (Accessed on 22 May 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin., 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in Liver Cancer and Possible Treatment Approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; Messa, C.; Guerra, V.; Carr, B.I.; D'Alessandro, R. Inflammatory Mechanisms of HCC Development. Cancers, 2020, 12, 641. [CrossRef]
- Yu, L.X.; Ling, Y.; Wang, H.Y. Role of Nonresolving Inflammation in Hepatocellular Carcinoma Development and Progression. NPJ Precis Oncol, 2018, 2, 6. [Google Scholar] [CrossRef]
- Kar, P.; Mishra, S. Management of Hepatitis B During Pregnancy. Expert Opin. Pharmacother. 2016, 17, 301–310. [Google Scholar] [CrossRef]
- Patel, S.; Nanavati, P.; Sharma, J.; Chavda, V.; Savjani, J. Functional Role of Novel Indomethacin Derivatives for the Treatment of Hepatocellular Carcinoma Through Inhibition of Platelet-Derived Growth Factor. Arch. Med. Res. 2021, 52, 483–493. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in Cancer: A Review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Stark, L.A. Aspirin Prevention of Colorectal Cancer: Focus on NF-κB Signalling and the Nucleolus. Biomedicines, 2017, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Bakır, E.; Çal, T.; Aydın Dilsiz, S.; Canpınar, H.; Eken, A.; Ündeğer Bucurgat, Ü. Assessment of the Cytotoxic, Genotoxic, and Apoptotic Potential of Flurbiprofen in HeLa and HepG2 Cell Lines. J. Biochem. Mol. Toxicol. 2021, 35, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khuder, S.; Mutgi, A. Breast Cancer and NSAID Use: A meta-Analysis. Br. J. Cancer. 2001, 84, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.; Zhao, X.; Allen, J.; Li, F.; Chang, M. A Comparison of the Effectiveness of Selected Non-Steroidal Anti-Inflammatory Drugs and their Derivatives Against Cancer Cells in Vitro. Cancer Chemother. Pharmacol. 2008, 61, 203–214. [Google Scholar] [CrossRef]
- Derry, S.; Wiffen, P.J.; Häuser, W.; Mücke, M.; Tölle, T.R.; Bell, R.F.; Moore, R.A. Oral NonsteroidalAnti-Inflammatory Drugs for Fibromyalgia in Adults. Cochrane Database Syst. Rev. 2017, 3, CD012332. [Google Scholar] [CrossRef]
- Rai, N.; Sarkar, M.; Raha, S. Piroxicam, a Traditional Non-Tteroidal Anti-Inflammatory Drug (NSAID) Causes Apoptosis by ROS Mediated Akt Activation. Pharmacol. Rep. 2015, 67, 1215–1223. [Google Scholar] [CrossRef]
- Hiľovská, L.; Jendželovský, R.; Fedoročko, P. Potency of Non-Steroidal Anti-Inflammatory Drugs in Chemotherapy. Mol. Clin. Oncol. 2015, 3, 3–12. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Sci. 2019, 3418975. [Google Scholar] [CrossRef]
- Husain, S.S.; Szabo, I.L.; Pai, R.; Soreghan, B.; Jones, M.K.; Tarnawski, A.S. MAPK (ERK2) Kinase—A Key Target for NSAIDs-Induced Inhibition of Gastric Cancer Cell Proliferation and Growth. Life Sci. 2001, 69, 3045–3054. [Google Scholar] [CrossRef]
- Porebska, I.; Wyrodek, E.; Kosacka, M.; Adamiak, J.; Jankowska, R.; Harłozińska-Szmyrka, A. Apoptotic Markers p53, Bcl-2 and Bax in Primary Lung Cancer. In Vivo, 2006, 20, 599–604. [Google Scholar]
- Wolf, B.B.; Green, D.R. Suicidal Tendencies: Apoptotic Cell Death by Caspase Family Proteinases. J Biol Chem. 1999, 274, 20049–20052. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Kim, D.H.; Jang, J.Y.; Kang, Y.J.; Yoon, J.H.; Moon, J.O.; Chung, H.Y.; Kim, G.Y.; Choi, Y.H.; Copple, B.L.; Kim, N.D. Aspirin Induces Apoptosis in Vitro and Inhibits Tumor Growth of Human Hepatocellular Carcinoma Cells in a Nude Mouse Xenograft Model. Int. J. Oncol. 2012, 40, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.D.; Tan, W.S.; Porta, N.; Mostafid, H.; Huddart, R.; Protheroe, A.; Bogle, R.; Blazeby, J.; Palmer, A.; Cresswell, J.; Johnson, M.; Brough, R.; Madaan, S.; Andrews, S.; Cruickshank, C.; Burnett, S.; Maynard, L.; Hall, E.; BOXIT Investigators. BOXIT-A Randomised Phase III Placebo-controlled Trial Evaluating the Addition of Celecoxib to Standard Treatment of Transitional Cell Carcinoma of the Bladder (CRUK/07/004). Eur. Urol. 2019, 75, 593–601. [Google Scholar] [CrossRef]
- Li, Z.Y.; Yin, Y.F.; Guo, Y.; Li, H.; Xu, M.Q.; Liu, M.; Wang, J.R.; Feng, Z.; H. ; Duan, X.C.; Zhang, S.; Zhang, S.Q.; Wang, G.X.; Liao, A.; Wang, S.M.; Zhang, X. Enhancing Anti-Tumor Activity of Sorafenib Mesoporous Silica Nanomatrix in Metastatic Breast Tumor and Hepatocellular Carcinoma via the Co-Administration with Flufenamic Acid. Inte. J. Nanomedicine. 2020, 15, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Rayburn, E.R.; Ezell, S.J.; Zhang, R. Anti-Inflammatory Agents for Cancer Therapy. Mol. Cell Pharmacol. 2009, 1, 29–43. [Google Scholar] [CrossRef]
- Bukowska, B.; Gajek, A.; Marczak, A. Two Drugs Are Better Than One. A Short History of Combined Therapy of Ovarian Cancer. Contemp. Oncol. (Pozn). 2015, 19, 350–353. [Google Scholar] [CrossRef]
- Palmer, A.C.; Chidley, C.; Sorger, P.K. A Curative Combination Cancer Therapy Achieves High Fractional Cell Killing Through Low Cross-Resistance and Drug Additivity. Elife. 2019, 8, e50036. [Google Scholar] [CrossRef]
- Narayan, R.S.; et al. A Cancer Drug Atlas Enables Synergistic Targeting of Independent Drug Vulnerabilities. Nat. Commun. 2020, 11, 2935. [Google Scholar] [CrossRef]
- Chu, T.H.; et al. Celecoxib Enhances the Therapeutic Efficacy of Epirubicin for Novikoff Hepatoma in Rats. Cancer Med. 2018, 7, 2567–2580. [Google Scholar] [CrossRef]
- Abdallah, F.M.; Helmy, M.W.; Katary, M.A.; Ghoneim, A.I. Synergistic Antiproliferative Effects of Curcumin and Celecoxib in Hepatocellular Carcinoma HepG2 Cells. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; et al. Meloxicam Combined with Sorafenib Synergistically Inhibits Tumor Growth of Human Hepatocellular Carcinoma Cells via ER Stress-Related Apoptosis. Oncol. Rep. 2015, 34, 2142–2150. [Google Scholar] [CrossRef]
- Syroeshkin, A.; Levitskaya, O.; Uspenskaya, E.; Pleteneva, T.; Romaykina, D.; Ermakova, D. Deuterium Depleted Water as an Adjuvant in Treatment of Cancer. Sys. Rev. Pharm. 2019, 10, 112–117. [Google Scholar] [CrossRef]
- Cong, F.S.; Zhang, Y.R.; Sheng, H.C.; Ao, Z.H.; Zhang, S.Y.; Wang, J.Y. Deuterium-Depleted Water Inhibits Human Lung Carcinoma Cell Growth by Apoptosis. Exp. Ther. Med. 2010, 1, 277–283. [Google Scholar] [CrossRef]
- Boros, L.G.; Somlyai, I.; Kovács, B.Z.; Puskás, L.G.; Nagy, L.I.; Dux, L.; Farkas, G.; Somlyai, G. Deuterium Depletion Inhibits Cell Proliferation, RNA and Nuclear Membrane Turnover to Enhance Survival in Pancreatic Cancer. Cancer Control. 2021, 28, 1073274821999655. [Google Scholar] [CrossRef] [PubMed]
- Yavari, K.; Kooshesh, L. Deuterium Depleted Water Inhibits the Proliferation of Human MCF7 Breast Cancer Cell Lines by Inducing Cell Cycle Arrest. Nutr. Cancer. 2019, 71, 1019–1029. [Google Scholar] [CrossRef]
- Kovács, B.Z.; Somlyai, I.; Papp, A.; Somlyai, G. Deuterium-Depleted Water Delayed Hormone Therapy of Prostate Cancer. J. Clin. Rev. Case Rep. 2021, 6, 747–749. [Google Scholar]
- Zhang, X.; Gaetani, M.; Chernobrovkin, A.; Zubarev, R.A. Anticancer Effect of Deuterium Depleted Water - Redox Disbalance Leads to Oxidative Stress. Mol. Cell Proteomics. 2019, 18, 2373–2387. [Google Scholar] [CrossRef]
- Bowen, G.J.; Winter, D.A.; Spero, H.J.; Zierenberg, R.A.; Reeder, M.D.; Cerling, T.E.; Ehleringer, J.R. Stable Hydrogen andOxygen Isotope Ratios of Bottled Waters of the World. Rapid Commun. Mass Spectrom. 2005, 19, 3442–3450. [Google Scholar] [CrossRef]
- Szent-Györgyi, A. The living state and cancer. Proc. Natl. Acad. Sci. U S A. 1977, 74, 2844–2847. [Google Scholar] [CrossRef] [PubMed]
- Gat, J.R.; Gonfiantini, R. Stable Isotope Hydrology: Deuterium and Oxygen-18 in the Water Cycle. Technical Reports Series. International Atomic Energy Agency, Vienna (Austria) 1981, no. 210, 1-356.
- Altman, L.J.; Laungani, P.; Gunnarsson, G.; Wennerstorm, H.; Forsen, S. Proton, Deuterium, and Tritium Nuclear Magnetic Resonance of Intramolecular Hydrogen Bonds. Isotope Effects and the Shape of the Potential Energy Function. J. Am. Chem. Soc. 1978, 100, 8264–8266. [Google Scholar] [CrossRef]
- Westheimer, F.H. The Magnitude of thePrimary Kinetic Isotope Effect for Compounds of Hydrogen and Deuterium. Chem. Rev. 1961, 61, 265–273. [Google Scholar] [CrossRef]
- Goncharuk, V.V.; Kavitskaya, A.A.; Romanyukina, I.Y.; Loboda, O.A. Revealing Water’s Secrets: Deuterium Depleted Water. Chem. Cent. J. 2013, 7, 103. [Google Scholar] [CrossRef]
- Goncharuk; et al. Determination of Biological Activity of Water Having a Different Isotope Ratio of Protium and Deuterium. J. Water Chem. Technol. 2018, 40, 27–34. [Google Scholar] [CrossRef]
- Syroeshkin, A.V.; Antipova, N.V.; Zlatska, A.V.; Zlatskiy, I.A.; Skylska, M.D.; Grebennikova, T.V.; Goncharuk, V.V. The Effect of the Deuterium Depleted Water on the Biological Activity of the Eukaryotic Cells. J. Trace Elem. Med. Biol. 2018, 50, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Zlatska, A.; Gordiienko, I.; Vasyliev, R.; Zubov, D.; Gubar, O.; Rodnichenko, A.; Syroeshkin, A.; Zlatskiy, I. In Vitro Study of Deuterium Effect on Biological Properties of Human Cultured Adipose-Derived Stem Cells. ScientificWorldJournal. 2018, 5454367. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, B.; He, Z.; Fu, H.; Dai, Z.; Huang, G.; Li, B.; Qin, D.; Zhang, X.; Tian, L.; Fang, W.; Yang, H. Deuterium-Depleted Water (DDW) Inhibits the Proliferation and Migration of Nasopharyngeal Carcinoma Cells in Vitro. Biomed. Pharmacother. 2013, 67, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Mirică, R.E. , Deuterium-Depleted Water in Cancer Therapy. Environ. Eng. Manag. J. 2010, 9, 1543–1545. [Google Scholar] [CrossRef]
- Krempels, K.; Somlyai, I.; Somlyai, G. Molecular and Clinical Effects of Deuterium Depleted Water in Treatment and Prevention of Cancer. PositiveHealthOnline [serial on the Internet]. 2013, issue 203.
- Krempels, K.; Abonyi, O.; Balog, K.; Somlyai, I. Deuterium Depletion in Cancer Treatment and Prevention. PositiveHealthOnline [serial on the Internet]. 2013, issue 209.
- Goncharuk, V.V.; Syroeshkin, A.V.; Zlatskiy, I.A.; Uspenskaya, E.V.; Orekhova, A.V.; Levitskaya, O.V.; Dobrovolskiy, V.I.; Pleteneva, T.V. Quasi-Chemical Description of the Kinetics of Cell Death Spirostomum ambiguum Biosensor for Biological Activity of Aqueous Solutions. J. Water Chem. Technol. 2017, 39, 97–102. [Google Scholar] [CrossRef]
- Syroeshkin, A.; Pleteneva, T.; Uspenskaya, E.; Zlatskiy, I.; Antipova, N.; Grebennikova, T.; Levitskaya, O. D/H Control of Chemical Kinetics in Water Solutions under low Deuterium Concentrations. Chem. Eng. J. 2019, 377, 119827. [Google Scholar] [CrossRef]
- Boros, L.G.; D'Agostino, D.P.; Katz, H.E.; Roth, J.P.; Meuillet, E.J.; Somlyai, G. Submolecular Regulation of Cell Transformation by Deuterium Depleting Water Exchange Reactions in the Tricarboxylic Acid Substrate Cycle. Med. hypotheses. 2016, 87, 69–74. [Google Scholar] [CrossRef]
- Boros, L.G.; et al. Fumarate Hydratase and Deuterium Depletion Control Oncogenesis via NADPH-Dependent Reductive Synthesis: Mitochondrial Matrix Water, DNA Deuteration and Epigenetic Events. Cancer Res. 2014, 74(19_Supplement), 1426. [CrossRef]
- Kleemann, J.; Reichenbach, G.; Zöller, N.; Jäger, M.; Kaufmann, R.; Meissner, M.; Kippenberger, S. Heavy Water Affects Vital Parameters of Human Melanoma Cells in Vitro. Cancer. Manag. Res. 2020, 12, 1199–1209. [Google Scholar] [CrossRef]
- Altermatt, H.J.; Gebbers, J.O.; Laissue, J.A. Heavy Water Enhances the Antineoplastic Effect of 5-Fluoro-uracil and Bleomycin in Nude Bice Bearing Human Carcinoma. Int. J. Cancer. 1990, 45, 475–480. [Google Scholar] [CrossRef]
- Bahk, J.Y.; Lee, J.H.; Chung, H.S.; Lee, H.Y.; Chung, B.C.; Park. M.S.; Min, S.K.; Kim, M.O. Anticancer Effect of Deuterium Oxide on a Bladder Cancer Cell Related to Bcl-2 and Bax. J. Ind. Eng. Chem. 2007, 13, 501–507. [Google Scholar]
- Jandova, J.; Hua, A.B.; Fimbres, J.; Wondrak, G.T. Deuterium Oxide (D2O) Induces Early Stress Response Gene Expression and Impairs Growth and Metastasis of Experimental Malignant Melanoma. Cancers (Basel). 2021, 13, 605. [Google Scholar] [CrossRef] [PubMed]
- Capes-Davis, A.; Freshney, R.I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications. 7th ed.; Wiley-Blackwell, 2016.
- Cree, I.A. Principles of Cancer Cell Culture. Methods Mol. Biol. 2011, 731, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Scofield, R.H. Western Blotting: Methods and Protocols. Springer: New York, USA, 2015.
- Lampiasi, N.; Azzolina, A.; Umezawa, K.; Montalto, G.; McCubrey, J.A.; Cervello, M. The Novel NF-κB Inhibitor DHMEQ Synergizes with Celecoxib to Exert Antitumor Effects on Human Liver Cancer Cells by a ROS-Dependent Mechanism. Cancer Lett. 2012, 322, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, A.; Foderà, D.; D'Alessandro, N.; Lampiasi, N.; Azzolina, A.; Montalto, G.; Cervello, M. Potentiation of the Antitumor Effects of Both Selective Cyclooxygenase-1 and Cyclooxygenase-2 Inhibitors in Human Hepatic Cancer Cells by Inhibition of the MEK/ERK Pathway. Cancer biol. ther. 2007, 6, 1457–1464. [Google Scholar] [CrossRef]
- Lampiasi, N.; et al. The Selective Cyclooxygenase-1 Inhibitor SC-560 Suppresses Cell Proliferation and Induces Apoptosis in Human Hepatocellular Carcinoma Cells. Int. J. Mol. Med. 2006, 17, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 Heterodimerizes in Vivo with a Conserved Homolog, Bax, That Accelerates Programed Cell Death. cell. 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, J.; Park, B.H.; Kinzler, K.W.; Vogelstein, B. Role of BAX in the Apoptotic Response to Anticancer Agents. Science. 2000, 290, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Chen, W.; Cobb, M. Differential Regulation and Properties of MAPKs. Oncogene. 2007, 26, 3100–3112. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP Kinase Signalling Pathways in Cancer. Oncogene. 2007, 26, 3279–3290. [Google Scholar] [CrossRef]
- Torii, S.; Yamamoto, T.; Tsuchiya, Y.; Nishida, E. ERK MAP Kinase in G1 Cell Cycle Progression and Cancer. Cancer Sci. 2006, 97, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Setia, S.; Nehru, B.; Sanyal, S.N. Upregulation of MAPK/Erk and PI3K/Akt Pathways in Ulcerative Colitis-Associated Colon Cancer. Biomed. Pharmacother. 2014, 68, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.H.; Teng, W.; Lin, C.C.; Jeng, W.J.; Chen, W.T.; Lin, C.Y.; Lin, S.M.; Sheen, I.S. Prolonged Post-Ablation Fever May Predict One-Year Tumor Recurrence in Hepatocellular Carcinoma After Radiofrequency Ablation. Int. J. Hyperthermia. 2020, 37, 1008–1015. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Venom-Derived Bioactive Compounds as Potential Anticancer Agents: A Review. Int. J. Pept. Res. Ther. 2021, 27, 129–147. [Google Scholar] [CrossRef]
- Mansour, G.H.; El-Magd, M.A.; Mahfouz, D.H.; Abdelhamid, I.A.; Mohamed, M.F.; Ibrahim, N.S.; Hady, A. Abdel Wahab, A.; Elzayat, E.M. Bee Venom and its Active Component Melittin Synergistically Potentiate the Anticancer Effect of Sorafenib Against HepG2 Cells. Bioorg. Chem. 2021, 116, 105329. [Google Scholar] [CrossRef]
- Narożna, M.; Krajka-Kuźniak, V.; Bednarczyk-Cwynar, B.; Kleszcz, R.; Kujawski, J.; Baer-Dubowska, W. The Effect of Novel Oleanolic Acid Oximes Conjugated with Indomethacin on the Nrf2-ARE And NF-κB Signaling Pathways in Normal Hepatocytes and Human Hepatocellular Cancer Cells. Pharmaceuticals (Basel). 2020, 14, 32. [Google Scholar] [CrossRef]
- Hossain, M.A.; et al. Aspirin Enhances Doxorubicin-Induced Apoptosis and Reduces Tumor Growth in Human Hepatocellular Carcinoma Cells in Vitro and in Vivo. Int. J. Oncol. 2012, 40, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE 2 Pathway: Key Roles in the Hallmarks of Cancer and Adaptation to the Tumour Microenvironment. Carcinogenesis. 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-T.; Honn, K.V. Nie, D. Cyclooxygenases, Prostanoids, and Tumor Progression. Cancer. Metastasis. Rev. 2007, 26, 525–534. [Google Scholar] [CrossRef]
- Poligone, B.; Baldwin, A.S. Positive and Negative Regulation of NF-κB by COX-2: Roles of Different Prostaglandins. J. Biol. Chem. 2001, 276, 38658–38664. [Google Scholar] [CrossRef]
- Chen, G.; Li, X.; Yang, J.; Li, J.; Wang, X.; He, J.; Huang, Z. Prognostic Significance of Cyclooxygenase-2 Expression in Patients With Hepatocellular Carcinoma: A Meta-Analysis. Arch. Med. Sci. 2016, 12, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, J.; Gou, H.; Cao, D.; Jiang, M.; Hou, M. Clinical Significance of Cox-2, Survivin and Bcl-2 Expression in Hepatocellular Carcinoma (HCC). Med. Oncol. 2011, 28, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Zhang, L.H.; Gao, J.H.; Zhao, C.; Tong, H.; Ye, C.; Huang, Z.Y.; Liu, R.; Tang, C.W. Suppressing Growth and Invasion of Human Hepatocellular Carcinoma Cells by Celecoxib Through Inhibition of Cyclooxygenase-2. Cancer Manag. Res. 2019, 11, 2831–2848. [Google Scholar] [CrossRef]
- Dong, X.; et al. Meloxicam Executes its Antitumor Effects Against Hepatocellular Carcinoma in COX-2-Dependent and -Independent Iathways. PloS One. 2014, 9, e92864. [Google Scholar] [CrossRef]
- Hu, J.W.; Chen, B.; Zhang, J.; Qi, Y.P.; Liang, J.H.; Zhong, J.H.; Xiang, B.D. Novel Combination of Celecoxib and Metformin Improves the Antitumor Effect by Inhibiting the Growth of Hepatocellular Carcinoma. J. Cancer. 2020, 11, 6437–6444. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple Functions of BCL-2 Family Proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaka, R.; Shibata, M.A.; Morimoto, J.; Tanigawa, N.; Otsuki, Y. COX-2 Inhibitor Celecoxib Suppresses Tumor Growth and Lung Metastasis of a Murine Mammary Cancer. Anticancer Res. 2006, 26, 4245–4254. [Google Scholar] [PubMed]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.C.; Yang, C.R.; Cheng, C.L.; Raung, S.L.; Hung, Y.Y.; Chen, C.J. Indomethacin Induces Apoptosis in 786-O Renal Cell Carcinoma Cells by Activating Mitogen-Activated Protein Kinases and AKT. Euro. J. Pharmacol. 2007, 563, 49–60. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, H.; Ma, C.; Li, N.; Wang, M. Celecoxib Enhances Apoptosis of the Liver Cancer Cells via Regulating ERK/JNK/P38 Pathway. J. Buon. 2021, 26, 875–881. [Google Scholar] [PubMed]
- Elder, D.J.; Halton, D.E.; Playle, L.C.; Paraskeva, C. The MEK/ERK Pathway Mediates COX-2-Selective NSAID-Induced Apoptosis and Induced COX-2 Protein Expression in Colorectal Carcinoma Cells. Int. J. Cancer. 2002, 99, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sinicrope, F.A. Selective Inhibitors of MEK1/ERK44/42 and p38 Mitogen-Activated Protein Kinases Potentiate Apoptosis Induction by Sulindac Sulfide in Human Colon Carcinoma Cells. Mol. Cancer Ther. 2005, 4, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, H.S.; Hah, J.W.; Jeong, W.J.; Kim, K.H.; Sung, M.W. Celecoxib Inhibits Cell Proliferation Through the Activation of ERK and p38 MAPK in Head and Neck Squamous Cell Carcinoma Cell Lines. Anticancer drugs. 2010, 21, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martindale, J.L.; Holbrook, N.J. Requirement for ERK Activation in Cisplatin-Induced Apoptosis. J. Biol. Chem. 2000, 275, 39435–39443. [Google Scholar] [CrossRef]
- Mandegary, A.; Hosseini, R.; Ghaffari, S.H.; Alimoghaddam, K.; Rostami, S.; Ghavamzadeh, A.; Ghahremani, M.H. The Expression of p38, ERK1 and Bax Proteins Has Increased During the Treatment of Newly Diagnosed Acute Promyelocytic Leukemia With Arsenic Trioxide. Ann. Oncol. 2010, 21, 1884–1890. [Google Scholar] [CrossRef]
- Somlyaia, G.; Javaherib, B.; Davaric, H.; Gyöngyid, Z.; Somlyaia, I.; Tamaddone, K.A.; Boros, L.G. Pre-Clinical and Clinical Data Confirm the Anticancer Effect of Deuterium Depletion. Biomacromol. J. 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Bayrak, B.B.; Kulak, G.Y.; Yanardag, R.; Yarat, A. Short Term Deuterium Depletion in Drinking Water Reduced Tumor Induced Oxidative Stress in Mice Liver. Pathol. Res. Prac. 2022, 240, 154186. [Google Scholar] [CrossRef] [PubMed]
- Soleyman-Jahi, S.; Zendehdel, K.; Akbarzadeh, K.; Haddadi, M.; Amanpour, S.; Muhammadnejad, S. In Vitro Assessment of Antineoplastic Effects of Deuterium Depleted Water. Asian Pac. J. Cancer Prev. 2014, 15, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, B.; Liu, C.; Fang, W.; Yang, H. [Deuterium-Depleted Water Selectively Inhibits Nasopharyngeal Carcinoma Cell Proliferation in Vitro]. Nan Fang Yi Ke Da Xue Xue Bao ( J. South. Med. Univ.). 2012, 32, 1394–1399. [Google Scholar]
- Hassanzade, A.; Mandegary, A.; Sharif, E.; Rasooli, R.; Mohammadnejad, R.; Masoumi-Ardekani, Y. Cyclooxygenase Inhibitors Combined With Deuterium-Enriched Water Augment Cytotoxicity in A549 Lung Cancer Cell Line via Activation of Apoptosis and MAPK Pathways. Iran J. Basic Med. Sci. 2018, 21, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyi, Z.; Budán, F.; Szabó, I.; Ember, I.; Kiss, I.; Krempels, K.; Somlyai, I.; Somlyai, G. Deuterium Depleted Water Effects on Survival of Lung Cancer Patients and Expression of Kras, Bcl2, and Myc Genes in Mouse Lung. Nutr. Cancer. 2013, 65, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Salomonsson, L.; Branden, G.; Brzezinski, P. Deuterium Isotope Effect of Proton Pumping in Cytochrome c Oxidase. Biochim. Biophys. Acta. 2008, 1777, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Olgun, A. , Biological Effects of Deuteronation: ATP Synthase as an Example. Theor. Biol. Med. Model. 2007, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Moritake, K.; Akiyama, Y.; Kimura, Y.; Shingu, T.; Yamasaki, T. Experimental Validation of Deuterium Oxide-Mediated Antitumoral Activity as it Relates to Apoptosis in Murine Malignant Astrocytoma Aells. J. Neurosurg. 2002, 96, 900–908. [Google Scholar] [CrossRef]
- Kalkur, R.S.; Ballast, A.C.; Triplett, A.R.; Spendier, K. Effects of Deuterium Oxide on Cell Growth and Vesicle Speed in RBL-2H3 Cells. PeerJ. 2014, 2, e553. [Google Scholar] [CrossRef]
- Mandegary, A.; Sharif, E.; Rasooli, R.; Hassanzadeh, A. The Effect of Deuterium Depleted/Enriched Water on the Growth of A549 and HepG2 Cell Lines. J. Kerman. Univ. Med. Sci. 2019, 26, 357–367. [Google Scholar] [CrossRef]
- Leonard, P.J.; Mullins, J.M. D2O Induced Alterations of Mitosis in PtK1 Cells. Exp. Cell Res. 1987, 172, 204–211. [Google Scholar] [CrossRef]
- Bader, Y.; et al. Synergistic Effects of Deuterium Oxide and Gemcitabine in Human Pancreatic Cancer Cell Lines. Cancer Lett. 2008, 259, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Jandova, J.; Hua, A.B.; Fimbres, J.; Wondrak, G.T. Deuterium Oxide (D2O) Induces Early Stress Response Gene Expression and Impairs Growth and Metastasis of Experimental Malignant Melanoma. Cancers (Basel). 2021, 13, 605. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.P.; Kadara, H.; Lotan, D.; Woo, J.K.; Lee, H.Y.; Hong, W.K.; Lotan, R. Involvement of Mitochondrial and Akt Signaling Pathways in Augmented Apoptosis Induced by a Combination of Low Doses of Celecoxib and N-(4-Hydroxyphenyl) Retinamide in Premalignant Human Bronchial Epithelial Cells. Cancer Res. 2006, 66, 9762–9770. [Google Scholar] [CrossRef]
- Liggett, J.L.; Min, K.W.; Smolensky, D.; Baek, S.J. A Novel COX-Independent Mechanism of Sulindac Sulfide Involves Cleavage of Epithelial Eell Adhesion Molecule Protein. Exp. Cell Res. 2014, 326, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





