Submitted:
27 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Design, participants, settings and ethics
2.2. Data collection and considerations
2.3. Assessment of maternal iodine intake, status and thyroid function
2.4. Neonatal birth data and antheropometrics
2.5. Statistical analysis
3. Results
3.1. Study population
3.2. Maternal nutritional, hormonal and clinical characteristics
3.3. Maternal characteristics, pregnancy outcomes and newborn weight
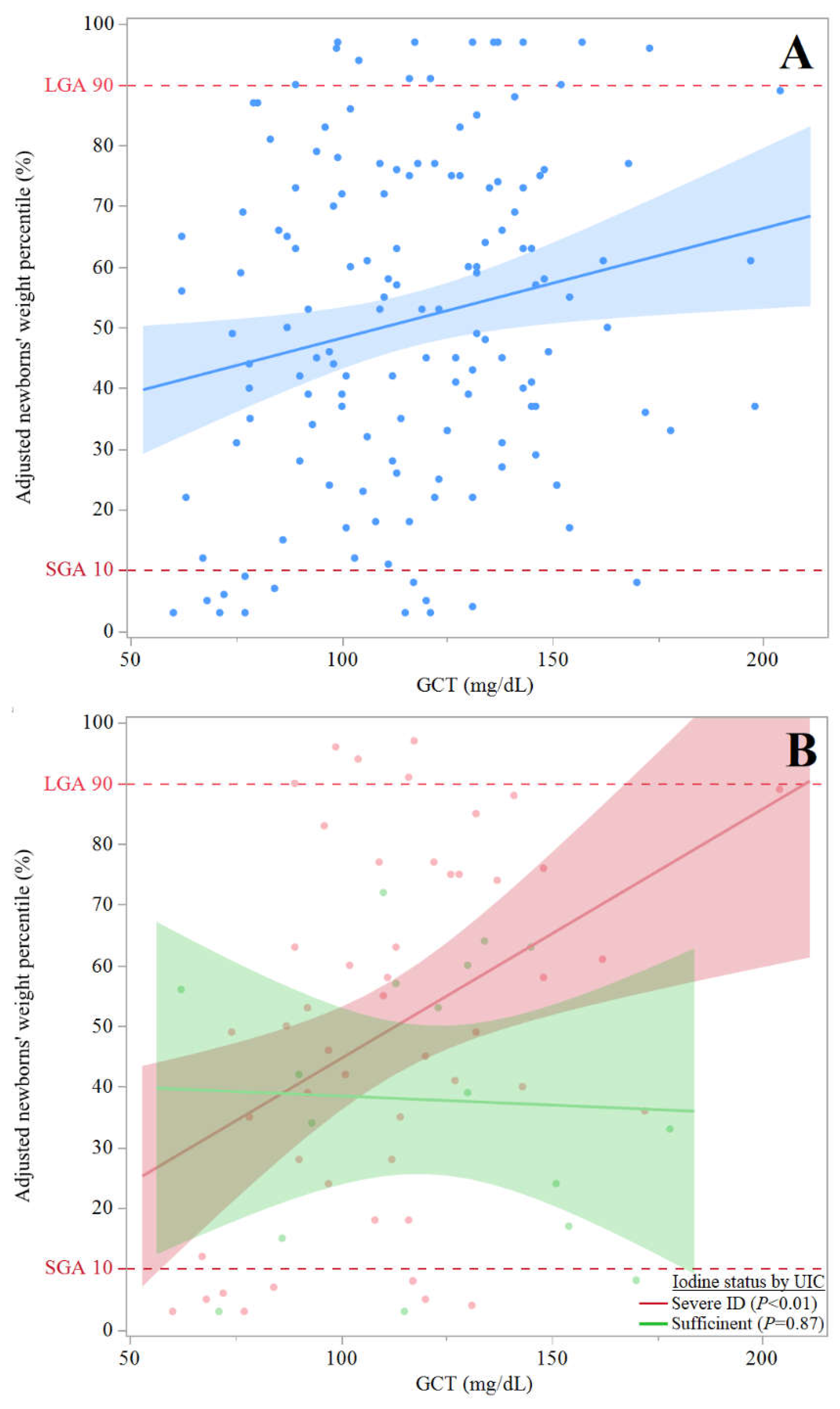
- (A)
- Scattered plot of adjusted newborns' weight percentiles (y axis) by GCT values (n=171) (x axis) of all participants with available GCT results (n=171) with dashed vertical lines to show LGA and SGA): y = 30.2 + 0.2(x), and R2 = 0.039; β (95% CI) = 0.20 (0.03, 0.33), P=0.018. The association remained significant in multivariate regression analysis adjusting for pregnant women age, smoking status, BMI at delivery, parity and gravidity: β (95 %CI) = -0.21 (0.03, 0.36), P=0.022.
- (B)
- Scattered plot of adjusted newborns' weight percentiles (y axis) by GCT values (n=86), excluding participants with mild-to-moderate ID (x axis) with lines to show linear fit (dashed vertical lines to show LGA and SGA); For participants with sufficient iodine status (by UIC): y= 41.4 + 0.03(x), R2=0, P=NS; For participants with severe ID (by UIC): y = 3.5 + 0.4(x), and R2 = 0.16, β (95% CI) = 0.33 (0.09, 0.55), P<0.01. The association remained significant in multivariate regression analysis adjusting for pregnant women age, smoking status, BMI at delivery, parity and gravidity: β (95 %CI) = 0.4 (0.37, 1.62), P<0.01.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson C, Syddall H, Rodin I, Osmond C, Barker DJ. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry. 2001;179:450-5. https://doi.org/10.1192/bjp.179.5.450. [CrossRef]
- Koller-Smith LI, Shah PS, Ye XY, Sjörs G, Wang YA, Chow SSW, Darlow BA, Lee SK, Håkanson S, Lui K; Australian and New Zealand Neonatal Network; Canadian Neonatal Network; Swedish Neonatal Quality Register. Comparing very low birth weight versus very low gestation cohort methods for outcome analysis of high risk preterm infants. BMC Pediatr. 2017;17(1):166. https://doi.org/10.1186/s12887-017-0921-x. [CrossRef]
- Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004 Dec;93(446):26-33. https://doi.org/10.1111/j.1651-2227.2004.tb00236.x. PMID: 15702667. [CrossRef]
- Li N, An H, Jin M, Li Z, Zhang Y, Zhang L, Liu J, Ye R. Association of Infants Small for Gestational Age with Anemia under Five Years Old in Two Large Longitudinal Chinese Birth Cohorts. Nutrients. 2022;14(5):1006. https://doi.org/10.3390/nu14051006. [CrossRef]
- Scifres CM. Short- and Long-Term Outcomes Associated with Large for Gestational Age Birth Weight. Obstet Gynecol Clin North Am. 2021 Jun;48(2):325-337. https://doi.org/10.1016/j.ogc.2021.02.005. PMID: 33972069. [CrossRef]
- Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes. 2015;10(2):77-83. https://doi.org/10.1111/ijpo.230. [CrossRef]
- HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991-2002. https://doi.org/10.1056/NEJMoa0707943. [CrossRef]
- Beksac MS, Tanacan A, Hakli DA, Ozyuncu O. Use of the 50-g glucose challenge test to predict excess delivery weight. Int J Gynaecol Obstet. 2018 Jul;142(1):61-65. https://doi.org/10.1002/ijgo.12504. [CrossRef]
- Eastman CJ, Ma G, Li M. Optimal Assessment and Quantification of Iodine Nutrition in Pregnancy and Lactation: Laboratory and Clinical Methods, Controversies and Future Directions. Nutrients. 2019;11(10):2378. https://doi.org/10.3390/nu11102378. [CrossRef]
- Shields BM, Knight BA, Hill A, Hattersley AT, Vaidya B. Fetal thyroid hormone level at birth is associated with fetal growth. J Clin Endocrinol Metab. 2011 Jun;96(6):E934-8. https://doi.org/10.1210/jc.2010-2814. [CrossRef]
- Korevaar TI, Chaker L, Jaddoe VW, Visser TJ, Medici M, Peeters RP. Maternal and Birth Characteristics Are Determinants of Offspring Thyroid Function. J Clin Endocrinol Metab. 2016 Jan;101(1):206-13. https://doi.org/10.1210/jc.2015-3559. [CrossRef]
- Vulsma T, Gons MH, de Vijlder JJ. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med. 1989;321(1):13-6. https://doi.org/10.1056/NEJM198907063210103. [CrossRef]
- Mortimer RH, Galligan JP, Cannell GR, Addison RS, Roberts MS. Maternal to fetal thyroxine transmission in the human term placenta is limited by inner ring deiodination. J Clin Endocrinol Metab. 1996;81(6):2247-9. https://doi.org/10.1210/jcem.81.6.8964859. [CrossRef]
- Ovadia YS, Zangen S, Rosen SR, Gefel D, Almashanu S, Benbassat C, Fytlovich S, Aharoni D, Anteby EY, Shenhav S. Maternal iodine deficiency: a newborns' overweight risk factor? A prospective study. Arch Gynecol Obstet. 2022;305(3):777-787. https://doi.org/10.1007/s00404-021-06261-x. [CrossRef]
- Zhang X, Yuan N, Sun J, Zhao X, Du J, Nan M, Zhang Q, Ji L. Association Between Iodine Nutritional Status and Adverse Pregnancy Outcomes in Beijing, China: a Single-Center Cohort Study. Biol Trace Elem Res. 2022;200(6):2620-2628. https://doi.org/10.1007/s12011-021-02887-9. [CrossRef]
- Shenhav S, Benbassat C, Gefel D, Zangen S, Rosen SR, Avrahami-Benyounes Y, Almashanu S, Groisman L, Rorman E, Fytlovich S, Anteby EY, Ovadia YS. Can Mild-to-Moderate Iodine Deficiency during Pregnancy Alter Thyroid Function? Lessons from a Mother-Newborn Cohort. Nutrients. 2022;14(24):5336. https://doi.org/10.3390/nu14245336. [CrossRef]
- Silva CM, Arnegard ME, Maric-Bilkan C. Dysglycemia in Pregnancy and Maternal/Fetal Outcomes. J Womens Health (Larchmt). 2021;30(2):187-193. https://doi.org/10.1089/jwh.2020.8853. [CrossRef]
- Pregnancy at Age 35 Years or Older: ACOG Obstetric Care Consensus No. 11. Obstet Gynecol. 2022;140(2):348-366. https://doi.org/10.1097/AOG.0000000000004873. [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins–Obstetrics. Obesity in Pregnancy: ACOG Practice Bulletin, Number 230. Obstet Gynecol. 2021;137(6):e128-e144. https://doi.org/10.1097/AOG.0000000000004395. [CrossRef]
- Zimmermann, M.B.; Andersson, M. Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 2012, 70, 553-570. https://doi.org/10.1111/j.1753-4887.2012.00528.x. [CrossRef]
- IOH. Standing committee on the Scientific Evaluation of Dietary Reference Intakes, Institute of Health; Press, N.A., Ed. National Academy Press: USA, 2001.
- CDC. Laboratory Procedure Manual: Iodine in Urine. NHANES 2003–2004 Inorganic Toxicology and Nutrition Branch, Division of Laboratory Sciences, National Center for Environmental Health: 2007.
- WHO. Assessment of iodine deficiency disorders and monitoring their elimination. A guide for programmed managers. Third Edition; World Health Organization: France, 2007.
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J., et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315-389. https://doi.org/10.1089/thy.2016.0457. [CrossRef]
- Ma, Z.F.; Skeaff, S.A. Thyroglobulin as a biomarker of iodine deficiency: a review. Thyroid 2014, 24, 1195-1209. https://doi.org/10.1089/thy.2014.0052. [CrossRef]
- Dollberg, S.; Haklai, Z.; Mimouni, F.B.; Gorfein, I.; Gordon, E.S. Birth weight standards in the live-born population in Israel. Isr Med Assoc J 2005, 7, 311-314.
- Davidson, S.; Sokolover, N.; Erlich, A.; Litwin, A.; Linder, N.; Sirota, L. New and improved Israeli reference of birth weight, birth length, and head circumference by gestational age: a hospital-based study. Isr Med Assoc J 2008, 10, 130-134.
- Greenwood, D.C.; Webster, J.; Keeble, C.; Taylor, E.; Hardie, L.J. Maternal Iodine Status and Birth Outcomes: A Systematic Literature Review and Meta-Analysis. Nutrients 2023, 15, 387. https://doi.org/10.3390/nu15020387. [CrossRef]
- Dosiou C, Medici M. MANAGEMENT OF ENDOCRINE DISEASE: Isolated maternal hypothyroxinemia during pregnancy: knowns and unknowns. Eur J Endocrinol. 2017;176(1):R21-R38. https://doi.org/10.1530/EJE-16-0354. [CrossRef]
- Glinoer D. Maternal and fetal impact of chronic iodine deficiency. Clin Obstet Gynecol. 1997;40(1):102-116. https://doi.org/10.1097/00003081-199703000-00011. [CrossRef]
- Rousset B, Dupuy C, Miot F, Dumont J. Chapter 2 Thyroid Hormone Synthesis And Secretion. In: Feingold KR, Anawalt B, Blackman MR, et al., eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; September 2, 2015.
- Fernández-Real JM, López-Bermejo A, Castro A, Casamitjana R, Ricart W. Thyroid function is intrinsically linked to insulin sensitivity and endothelium-dependent vasodilation in healthy euthyroid subjects. J Clin Endocrinol Metab. 2006;91(9):3337-3343. https://doi.org/10.1210/jc.2006-0841. [CrossRef]
- Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92(2):491-496. https://doi.org/10.1210/jc.2006-1718. [CrossRef]
- Fontenelle LC, Feitosa MM, Severo JS, et al. Thyroid Function in Human Obesity: Underlying Mechanisms. Horm Metab Res. 2016;48(12):787-794. https://doi.org/10.1055/s-0042-121421. [CrossRef]
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595-1607. https://doi.org/10.2337/diab.37.12.1595. [CrossRef]
- Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes [published correction appears in Diabetes Care. 2007 Dec;30(12):3154]. Diabetes Care. 2007;30 Suppl 2:S112-S119. https://doi.org/10.2337/dc07-s202. [CrossRef]
- Schwartz R, Gruppuso PA, Petzold K, Brambilla D, Hiilesmaa V, Teramo KA. Hyperinsulinemia and macrosomia in the fetus of the diabetic mother. Diabetes Care. 1994;17(7):640-648. https://doi.org/10.2337/diacare.17.7.640. [CrossRef]
- Langer O. Fetal macrosomia: etiologic factors. Clin Obstet Gynecol. 2000;43(2):283-297. https://doi.org/10.1097/00003081-200006000-00006. [CrossRef]
- Damiano F, Rochira A, Gnoni A, Siculella L. Action of Thyroid Hormones, T3 and T2, on Hepatic Fatty Acids: Differences in Metabolic Effects and Molecular Mechanisms. Int J Mol Sci. 2017;18(4):744. https://doi.org/10.3390/ijms18040744. [CrossRef]
- Katz LS, Xu S, Ge K, Scott DK, Gershengorn MC. T3 and Glucose Coordinately Stimulate ChREBP-Mediated Ucp1 Expression in Brown Adipocytes From Male Mice. Endocrinology. 2018;159(1):557-569. https://doi.org/10.1210/en.2017-00579. [CrossRef]
- Katz LS, Argmann C, Lambertini L, Scott DK. T3 and glucose increase expression of phosphoenolpyruvate carboxykinase (PCK1) leading to increased β-cell proliferation. Mol Metab. 2022;66:101646. https://doi.org/10.1016/j.molmet.2022.101646. [CrossRef]
- Hashimoto K, Ishida E, Matsumoto S, et al. Carbohydrate response element binding protein gene expression is positively regulated by thyroid hormone. Endocrinology. 2009;150(7):3417-3424. https://doi.org/10.1210/en.2009-0059. [CrossRef]
- Verhagen NJE, Gowachirapant S, Winichagoon P, Andersson M, Melse-Boonstra A, Zimmermann MB. Iodine Supplementation in Mildly Iodine-Deficient Pregnant Women Does Not Improve Maternal Thyroid Function or Child Development: A Secondary Analysis of a Randomized Controlled Trial. Front Endocrinol (Lausanne). 2020 Oct 6;11:572984. https://doi.org/10.3389/fendo.2020.572984. [CrossRef]
- Wang F, Zhang Y, Yuan Z, et al. The association between iron status and thyroid hormone levels during pregnancy. J Trace Elem Med Biol. 2022;74:127047. https://doi.org/10.1016/j.jtemb.2022.127047. [CrossRef]
- Chu FC, Shaw SW, Lo LM, Hsieh TT, Hung TH. Association between maternal anemia at admission for delivery and adverse perinatal outcomes [published correction appears in J Chin Med Assoc. 2020 Nov;83(11):1054]. J Chin Med Assoc. 2020;83(4):402-407. https://doi.org/10.1097/JCMA.0000000000000215. [CrossRef]
- Andersen S, Karmisholt J, Pedersen KM, Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr. 2008;99(4):813-818. https://doi.org/10.1017/S0007114507842292. [CrossRef]
- Luo J, Wang X, Yuan L, Guo L. Iron Deficiency, a Risk Factor of Thyroid Disorders in Reproductive-Age and Pregnant Women: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2021;12:629831. https://doi.org/10.3389/fendo.2021.629831. [CrossRef]

| Sub-group by maternal Tg values | >17μg/L | ≤17μg/L | P value |
|---|---|---|---|
| Pregnant women, n | 96 | 92 | |
| Age (y), mean±SD | 31±6 | 32±5 | NS |
| Gestational age (weeks) at recruitment, mean±SD | 32±7 | 31±7 | NS |
| Israeli born, n(%) | 52(54) | 42(46) | NS |
| Tertiary education, n(%) | 42(44) | 52(56) | NS |
| Secular n(%) | 28(30) | 35(38) | NS |
| IVF | 6(6) | 10(11) | NS |
| Smoking | |||
| Current smoker | 13(14) | 11(12) | NS |
| Past smoker | 18(19) | 18(20) | NS |
| Alcohol, n(%) | 0(0) | 0(0) | NS |
| Post-psychological stressful event, n(%) | 14(15) | 18(20) | NS |
| GCT (mg/dL), mean±SD | 119±28 | 114±31 | NS |
| BMI (kg/m2) | |||
| At recruitment, mean±SD | 29±5 | 28±5 | NS |
| At delivery, mean±SD | 31±6 | 29±4 | NS |
| Gravidity, mean±SD* | 4±2 | 3±2 | 0.05 |
| Parity, mean±SD* | 3±2 | 2±1 | 0.03 |
| Iodine Intake | |||
| Estimated dietary Iodine intake (μg/d), mean±SD* | 163±104 | 221± 114 | <0.01 |
| Iodine intake < RDA, n (%)F | 69(72) | 44(48) | <0.01 |
| Iodized salt use, n(%) ם | 4(4) | 5(5) | NS |
| ICS intake, n (%) F | 44(46) | 62(67) | <0.01 |
| Estimated iodine intake from ICS (μg/d), median (IQR)K ****MIQR |
1 (0-150) | 150 (0-220) | <0.01 |
| Dietary goitrogens exposure, n (%) F | 20(22) | 12(13) | NS |
| UIC | |||
| Median UIC, μg/L (IQR) | 53(39-86) | 65(41-97) | NS |
| Participants with UIC <150 μg/L, n(%) | 77(80) | 72(86) | NS |
| Participants with UIC <50 μg/L, n (%) | 34(35) | 30(33) | NS |
| TSH | |||
| Mean±SD (mIU/L), n(%)) | 1.8±1.0 | 1.8±1.0 | NS |
| Participants with SCH, n(%) | 4 (4) | 2 (2) | NS |
| FT4 | |||
| Mean±SD (μg/L), n(%) | 1.0±0.2 | 1.0±0.1 | NS |
| Participants with IHT, n(%) | 7(7) | 8(9) | NS |
| FT3 (pmol/L), mean±SD | 4.1±0.7 | 3.9±0.7 | NS |
| TPO Ab | |||
| TPO Ab (mIU/L), median(IQR) | 13(11-16) | 13(11-17) | NS |
| Positive TPO Ab, n(%) | 1(1) | 4(4) | NS |
| Tg Ab | |||
| Tg Ab (mIU/L), median(IQR) | 10(10-11) | 10(10-12) | NS |
| Positive Tg Ab, n(%) | 1(1) | 4(5) | NS |
| Newborns at birtha, n | 85 | 84 | |
| Gestational age (days), mean±SD | 266±29 | 270±13 | NS |
| Preterm birth, n (%) | 13 (15) | 11 (13) | NS |
| Gender (Female, Male) | 36, 47 | 36,48 | NS |
| Apgar score | |||
| At 1 minute after delivery, mean±SD | 8.9±0.1 | 8.9±0.1 | NS |
| At 5 minutes after delivery, mean±SD | 9.9±0.4 | 9.9±0.3 | NS |
| Birthweight | |||
| Crude weight (g), mean±SD | 3,176±652 | 3,029±580 | NS |
| LBW | 3(4) | 8(10) | NS |
| Macrosomia | 6(7) | 2(2) | NS |
| Adjusted weight percentile (%), mean±SD Do | 56±28 | 49±26 | 0.07 |
| SGA | 5(6) | 10(12) | NS |
| LGAF | 13(15) | 3(3) | 0.02 |
| Length percentile (%), mean±SDDa | 74.4±26.3 | 70.6±25.9 | NS |
| Head circumference (cm) | |||
| Mean±SD | 34.4±2.1 | 34.0±1.7 | NS |
| > 90th percentileDa, n(%) | 27(28) | 17(18) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





