Submitted:
26 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Culture maintenance
2.2. Chemical Solutions and Analyses
2.3. Multi-generation
2.4. Acetylcholinesterase activity
2.5. Net Reproductive Rate (R0)
2.6. Hatching delay
2.7. Lifespan
2.8. Statistical analysis
3. Results
3.1. Chemical analyses
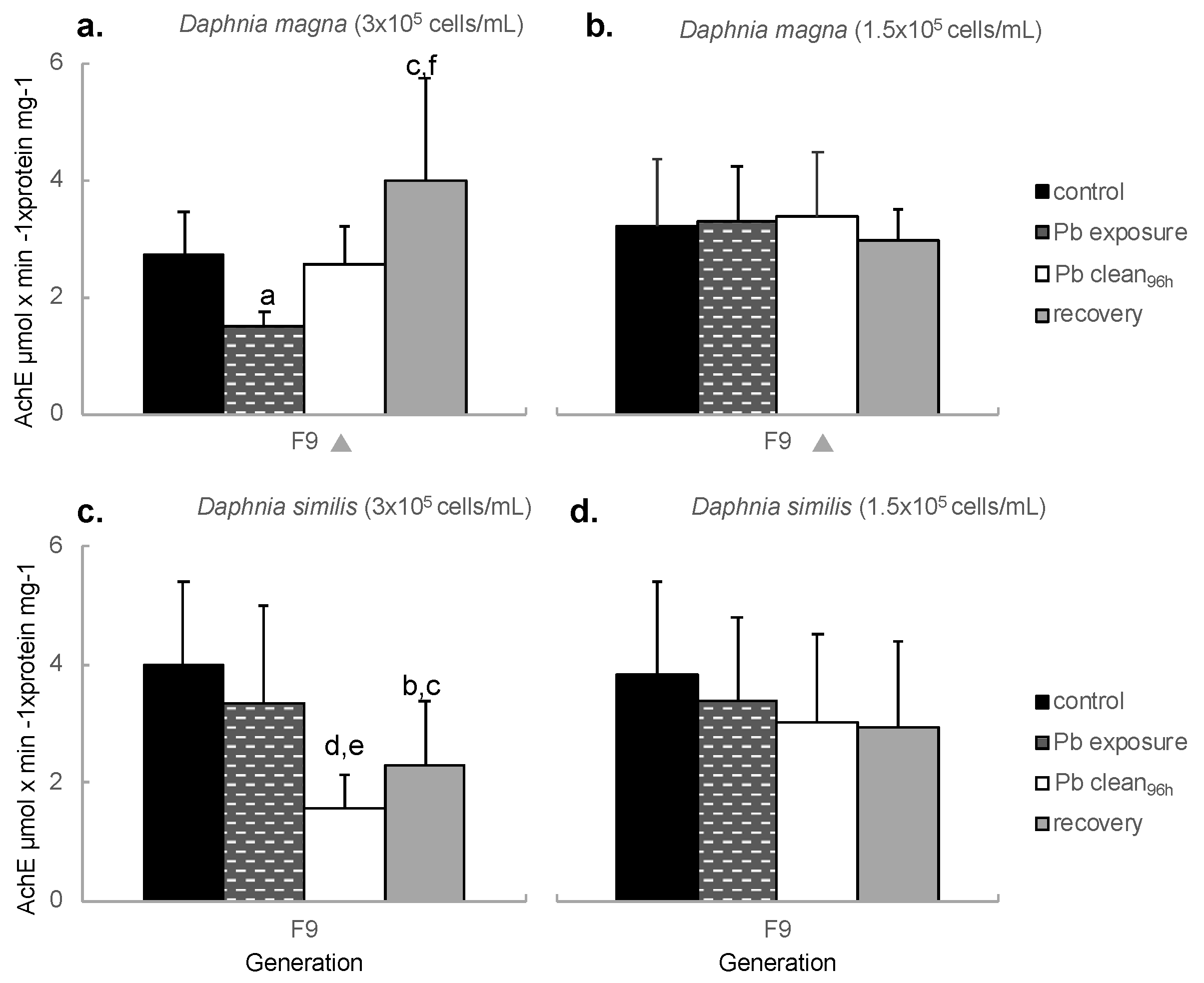
3.2. Acetylcholinesterase activity (AChE)
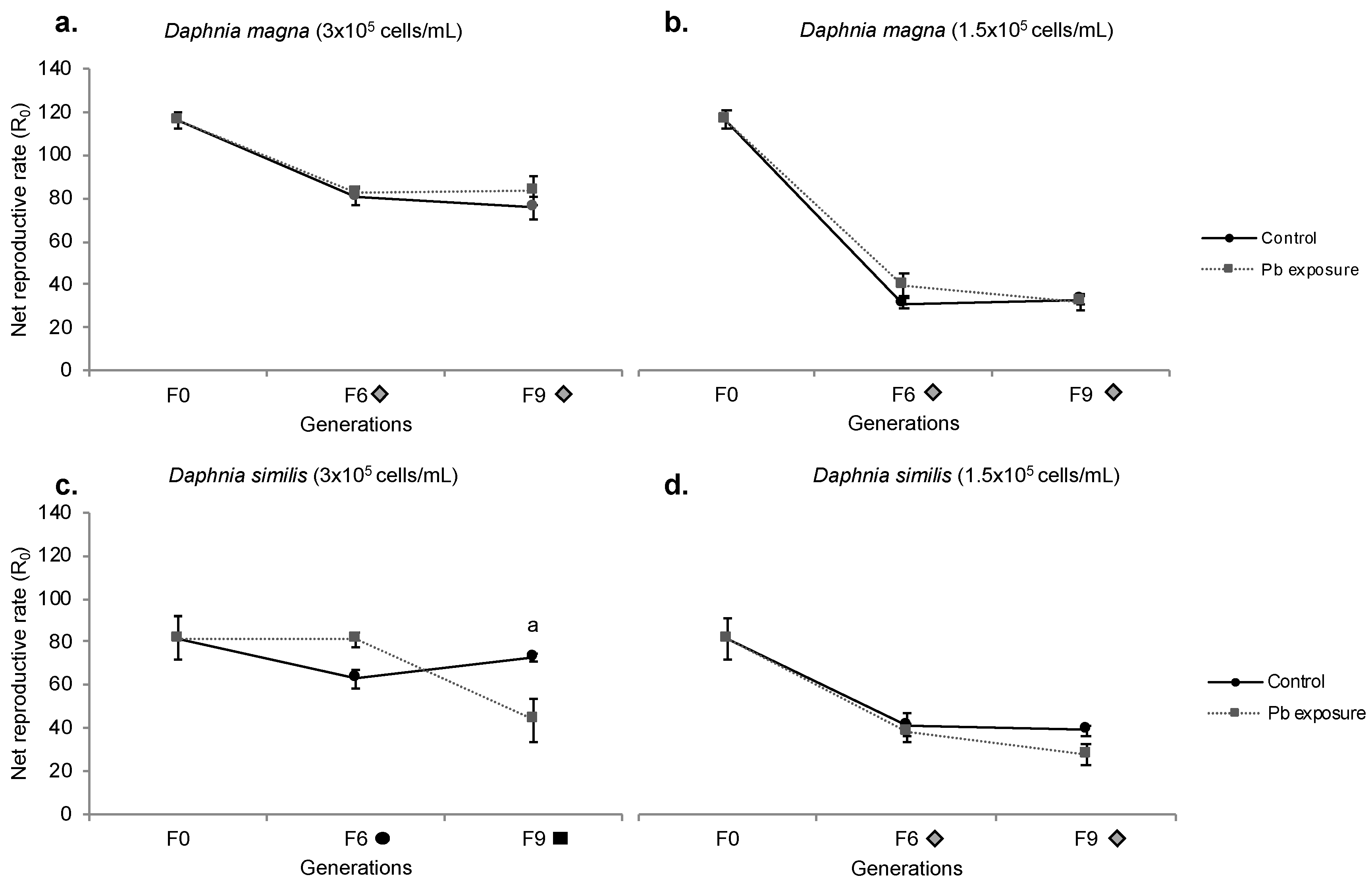
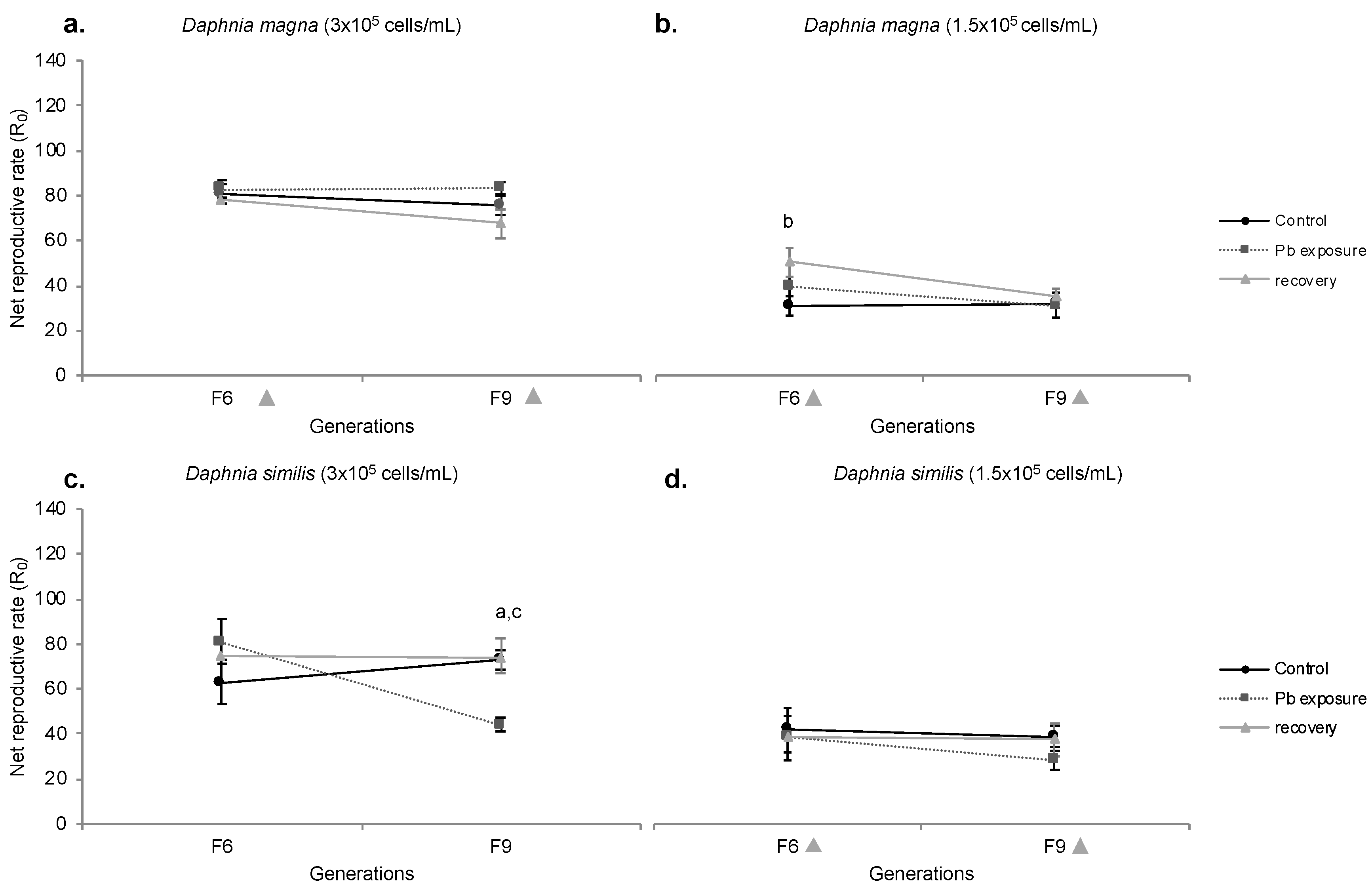
3.3. Net Reproductive Rate (R0)
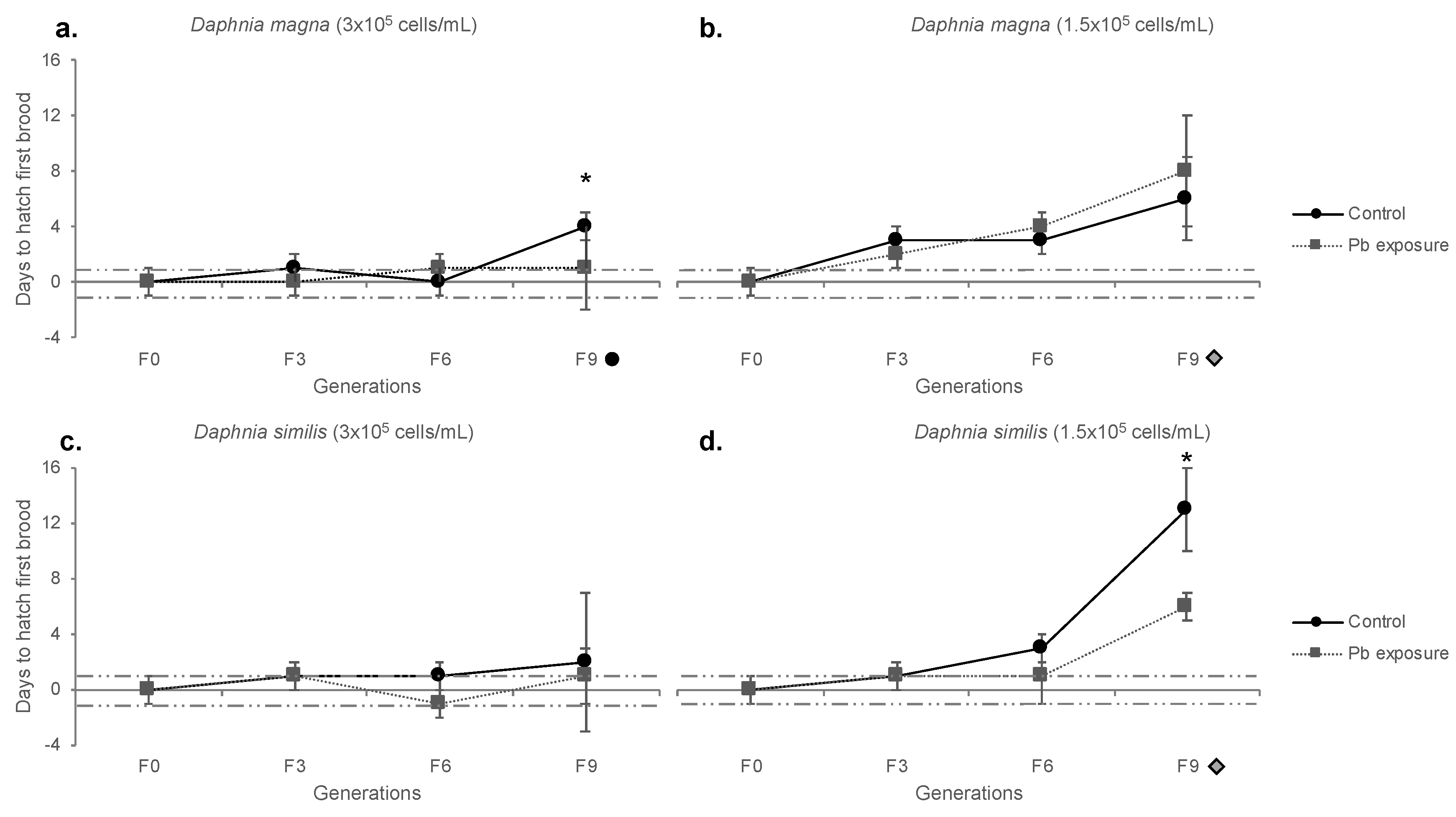
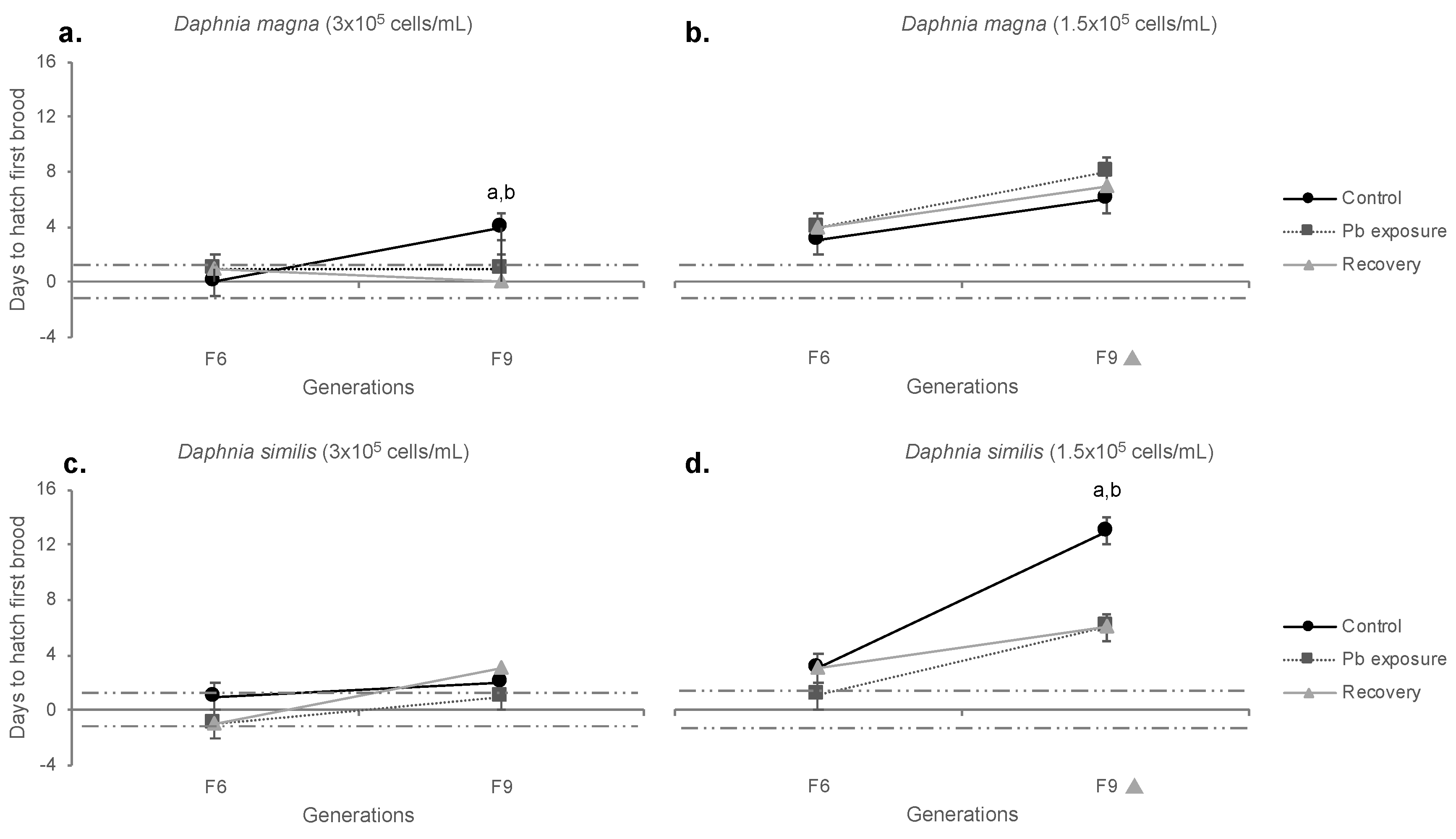
3.4. Hatching delay
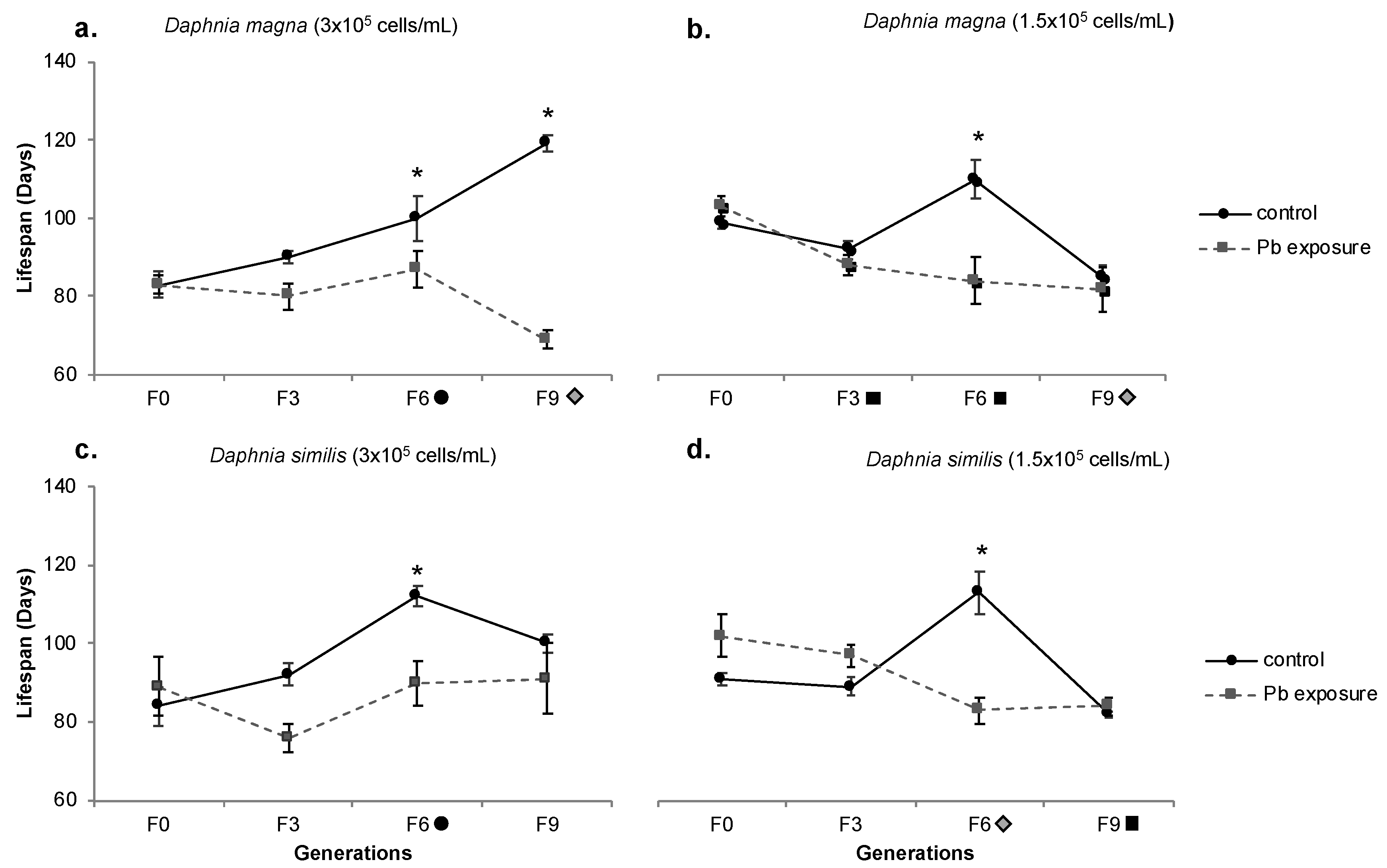
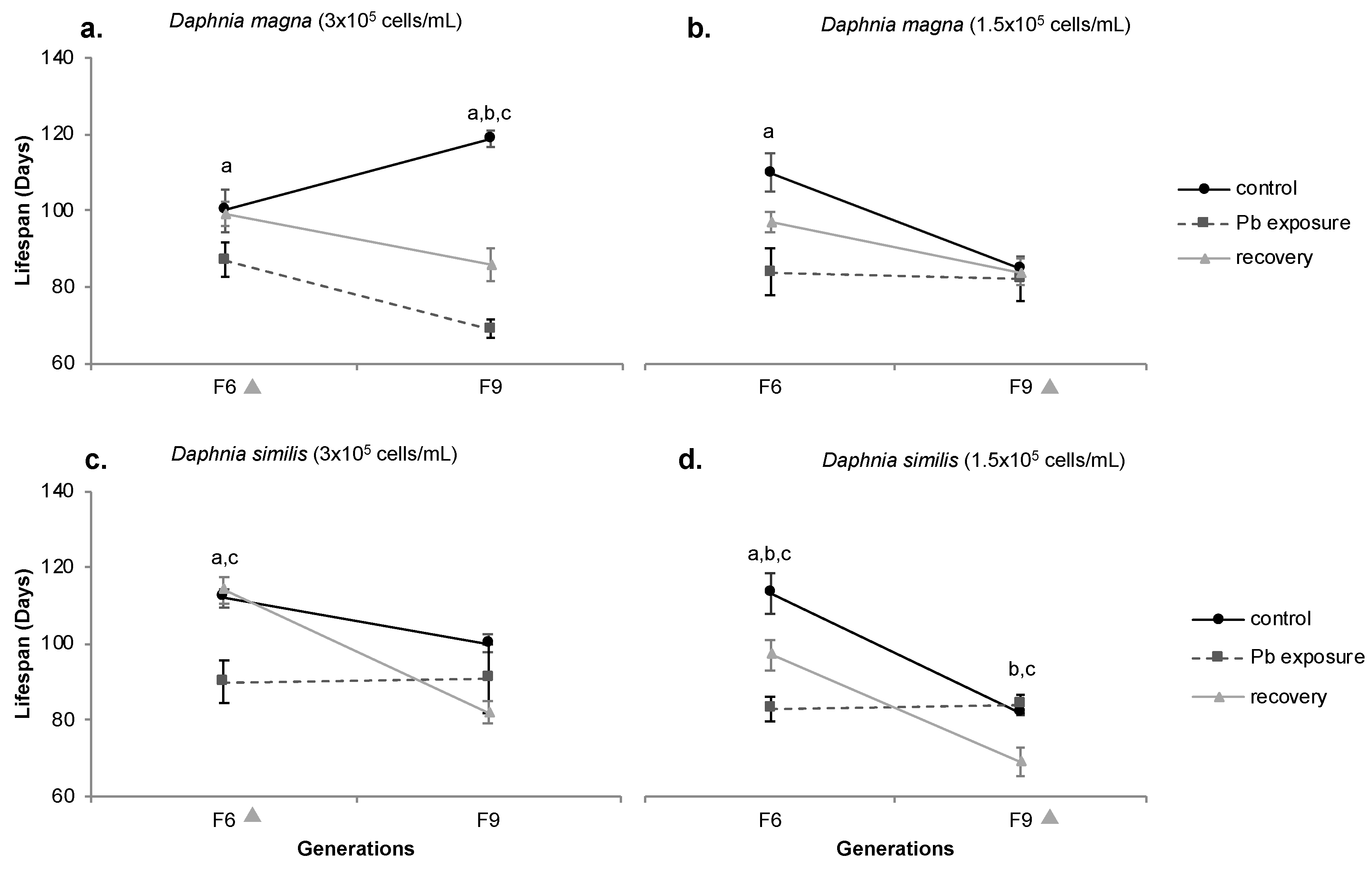
3.5. Lifespan
3.6. Principal Component Analysis (PCA)
4. Discussion
4.1. Population effects on control over generations (F0 vs. F9 control)
4.2. Multi-generation Pb exposure
4.3. Recovery from chemical exposure
4.4. Principal Component Analysis (PCA) evaluation
4.5. Daphnia magna vs. Daphnia similis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gandhi, S.A. The effect of organophosphorous pesticides on acetylcholinesterase activity in Daphnia carinata and Paratya australiensis. A thesis submitted in fulfilment of the requirements for the degree of Master of Science Saikrithika Arunachalam Gandhi Biotechnology. 2010.
- Komjarova, I.; Blust, R. Multi-metal interactions between Cd, Cu, Ni, Pb and Zn in water flea Daphnia magna, a stable isotope experiment. Aquat. Toxicol. 2008, 90, 138–144. [Google Scholar] [CrossRef]
- Irwin, R.J., 1997. Environmental Contaminants Encyclopedia. National Park Service. Colorado State University. Port Collins, Colorado, USA.
- Valavanidis, A.; Vlachogianni, T. Metal pollution in ecosystems. Ecotoxicology studies and risk assessment in the marine environment. Sci. Adv. Environ. Toxicol. Ecotoxicol. Issues. 2010. Available at https://www.researchgate.net/profile/Athanasios-Valavanidis/publication/236623174_Metal_Pollution_in_Ecosystems_Ecotoxicology_Studies_and_Risk_Assessment_in_the_Marine_Environment/links/00b4952fba8bb82bbd000000/Metal-Pollution-in-Ecosystems-Ecotoxicology-Studies-and-Risk-Assessment-in-the-Marine-Environment.pdf (accessed May 23, 2023). /.
- Frank, J.J.; Poulakos, A.G.; Tornero-Velez, R.; Xue, J. Systematic review and meta-analyses of lead (Pb) concentrations in environmental media (soil, dust, water, food, and air) reported in the United States from 1996 to 2016. Sci. Tot. Environ. 2019, 694, 133489. [Google Scholar] [CrossRef] [PubMed]
- Hashim, R.; Han Song, T.; Zuhartini Md Muslim, N.; Peck Yen, T. Determination of Heavy Metal Levels in Fishes from the Lower Reach of the Kelantan River, Kelantan, Malaysia. Trop. Life Sci. Res., 2014, 25, 21–39. [Google Scholar] [PubMed]
- Reddy, G.R.; Basha, M.R.; Devi, C.B.; Suresh, A.; Baker, J.L.; Shafeek, A.; Heinz, J.; Chetty, C.S. Lead induced effects on acetylcholinesterase activity in cerebellum and hippocampus of developing rat. Int. J. Dev. Neurosci., 2003, 21, 347–352. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soares, A.M.V.M. Inhibition of acetylcholinesterase activity as effect criterion in acute tests with juvenile Daphnia magna. Chemosphere, 1996, 32, 727–738. [Google Scholar] [CrossRef] [PubMed]
- European Parliment. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC. Off. J. Eur. Union, 2008, 84–97. https://doi.org/http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32008L0105.
- Brasil (República Federativa). Resolução CONAMA No 357, de 17 de março de 2005. Dispõe sobre a classificação dos corpos de água e diretrizes ambientais para o seu enquadramento, bem como estabelece as condições e padrões de lançamento de efluentes, e dá outras providências. Ministério do Meio Ambiente, Brasil, DF. 2005.
- Tessier, A.J.; Leibold, M.A.; Tsao, J. A fundamental trade-off in resource exploitation by Daphnia and consequences to plankton communities. Ecology, 2000, 81, 826–841. [Google Scholar] [CrossRef]
- Wiklund, A.K.E.; Adolfsson-Erici, M.; Liewenborg, B.; Gorokhova, E. Sucralose induces biochemical responses in Daphnia magna. PLoS One. 2014, 9. [Google Scholar] [CrossRef]
- OECD. Guideline 202: Daphnia sp., Acute Immobilisation Test. OECD Guidel. Test. Chem. 2004. 1–12.
- OECD. Guideline 211: Daphnia magna reproduction test. OECD Guidel. Test. Chem. 2012, Section 2, 23. [CrossRef]
- Tanaka, Y.; Nakanishi, J. Chronic effects of p-nonylphenol on survival and reproduction of Daphnia galeata: Multigenerational life table experiment. Environ. Toxicol., 2002, 17, 487–492. [Google Scholar] [CrossRef]
- Shaw, J.R.; Colbourne, J.K.; Glaholt, S.P.; Turner, E.; Folt, C.L.; Chen, C.Y. Dynamics of Cadmium Acclimation in Daphnia pulex: Linking Fitness Costs, Cross-Tolerance, and Hyper-Induction of Metallothionein. Environ. Sci. Technol., 2019, 53, 14670–14678. [Google Scholar] [CrossRef]
- Bae, E.; Samanta, P.; Yoo, J.; Jung, J. Effects of multigenerational exposure to elevated temperature on reproduction, oxidative stress, and Cu toxicity in Daphnia magna. Ecotoxicol. Environ. Saf., 2016, 132, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Dudycha, J.L.; Tessier, A.J. Natural Genetic Variation of Life Span, Reproduction, and Juvenile Growth in Daphnia. Evolution (NY)., 1999, 53, 1744–1756. [Google Scholar] [CrossRef]
- Printes, L.B.; Fellowes, M.D.E.; Callaghan, A. Clonal variation in acetylcholinesterase biomarkers and life history traits following OP exposure in Daphnia magna. Ecotoxicol. Environ. Saf. 2008, 71, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Rellstab, C.; Spaak, P. Lake origin determines Daphnia population growth under winter conditions. J. Plankton Res., 2009, 31, 261–271. [Google Scholar] [CrossRef]
- Tessier, A.J.; Henry, L.L.; Goulden, C.E.; Henri, L.L.; Goulden, C.E.; Durand, M.W. Starvation in Daphnia: Energy reserves and reproductive allocation. Limnol. Oceanogr., 1983, 28, 667–676. [Google Scholar] [CrossRef]
- Wacker, A.; Martin-Creuzburg, D. Allocation of essential lipids in Daphnia magna during exposure to poor food quality. Funct. Ecol., 2007, 21, 738–747. [Google Scholar] [CrossRef]
- Smolders, R.; Baillieul, M.; Blust, R. Relationship between the energy status of Daphnia magna and its sensitivity to environmental stress. Aquat. Toxicol 2005, 73, 155–170. [Google Scholar] [CrossRef]
- ASTM. Methods for Acute Toxicity Tests With Fish, Macroinvertebrates and Amphibians. Ecol. Res. Ser. 2002. EPA-600/3-75-009 96, 61.
- Baird, D.J.; Barber, I.; Bradley, M.; Calow, P.; Soares, A.M.V.M. The Daphnia bioassay : a critique. Hydrobiology, 1989, 188, 403–406. [Google Scholar] [CrossRef]
- Araujo, G.S.; Pinheiro, C.; Pestana, J.L. Araujo, G.S.; Pinheiro, C.; Pestana, J.L.T; Soares; A.M.V.M; Abessa, D.M.S; Loureiro, S.L. Toxicity of Lead and Mancozeb differs in two monophyletic Daphnia species. Ecotoxicol. Environ. Saf 2019, 178, 230–238. [Google Scholar] [CrossRef]
- Araujo, G.S.; Soares, A.; Abessa, D.M.S.; Loureiro, S. Multi-generational effects under single and pulse exposure scenarios in two monophyletic Daphnia species. Sci. Tot. Environ 2019, 697, 134031. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres Jr., V.; Featherstone, R.M. A new and rapid colorimetric determination of Acetylcholinesterase activity. Biochem. Pharmacol., 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 1976, 72, 248–54. [Google Scholar] [CrossRef] [PubMed]
- Coen, W.M.; Janssen, C.R. The missing biomarker link : relationships between effects on the cellular energy allocation biomarker of toxicant-stressed daphnia magna and corresponding population characteristics. Environ. Toxicol. Chem., 2003, 22, 1632–1641. [Google Scholar] [PubMed]
- Ginjupalli, G.K.; Baldwin, W.S. The time- and age-dependent effects of the juvenile hormone analog pesticide, pyriproxyfen on Daphnia magna reproduction. Chemosphere, 2013, 92, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Garratt, M.; Mcardle, F.; Stockley, P.; Vasilaki, A.; Beynon, R.J.; Jackson, M.J.; Hurst, J.L. Tissue-dependent changes in oxidative damage with male reproductive effort in house mice. Funct. Ecol., 2012, 26, 423–433. [Google Scholar] [CrossRef]
- Speakman, J.R.; Garratt, M. Oxidative stress as a cost of reproduction: Beyond the simplistic trade-off model. BioEssays 2014, 36, 93–106. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, R.; Wang, C.; Li, S.; Zhang, T.; Ren, Z.; Yang, M.; Pan, H.; Xu, S.; Zhu, J.; Wang, X. The Role of AChE in Swimming Behavior of Daphnia magna: Correlation Analysis of Both Parameters Affected by Deltamethrin and Methomyl Exposure. J. Toxicol. 2017, 3265727. [Google Scholar] [CrossRef]
- Venkataraman, B.V.; Shetty, P.S.; Joseph, T.; Stephen, P.M. Acetylcholinesterase activity of rat brain and heart in starvation and protein restriction. Indian J. Physiol. Pharmacol., 1985, 29, 123–125. [Google Scholar]
- Toumi, H.; Boumaiza, M.; Millet, M.; Radetski, C.M.; Camara, B.I.; Felten, V.; Ferard, J.F. Investigation of differences in sensitivity between 3 strains of Daphnia magna (crustacean Cladocera) exposed to malathion (organophosphorous pesticide). J. Environ. Sci. Heal.—Part B Pestic. Food Contam. Agric. Wastes 2015, 50, 34–44. [Google Scholar] [CrossRef]
- Toumi, H.; Boumaiza, M.; Millet, M.; Radetski, C.M.; Felten, V.; Férard, J.F. Is acetylcholinesterase a biomarker of susceptibility in Daphnia magna (Crustacea, Cladocera) after deltamethrin exposure? Chemosphere 2015, 120, 351–356. [Google Scholar] [CrossRef]
- Schwartz, T.S.; Pearson, P.; Dawson, J.; Allison, D.B.; Gohlke, J.M. Effects of fluctuating temperature and food availability on reproduction and lifespan. Exp Gerontol., 2016, 86, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hood, W.R. Current versus future reproduction and longevity: a re-evaluation of predictions and mechanisms. J. Exp. Biol., 2016, 219, 3177–3189. [Google Scholar] [CrossRef] [PubMed]
- Frost, P.C.; Ebert, D.; Larson, J.H.; Marcus, M.A.; Wagner, N.D.; Zalewski, A. Transgenerational effects of poor elemental food quality on Daphnia magna. Oecologia, 2010, 162, 865–872. [Google Scholar] [CrossRef]
- Stige, L.C.; Hessen, D.O.; Vøllestad, L.A. Severe food stress has no detectable impact on developmental instability in Daphnia magna. Oikos, 2004, 107, 519–530. [Google Scholar] [CrossRef]
- Latta, C.L.; Frederick, S.; Pfrender, M.E. Diet Restriction and Life-History Trade-Offs in Short- and Long-Lived Species of Daphnia. J Exp Zool A Ecol Genet Physiol. 2011, 315A, 610–617. [Google Scholar] [CrossRef]
- Kim, E.; Ansell, C.M.; Dudycha, J.L. Resveratrol and Food Effects on Lifespan and Reproduction in the Model Crustacean Daphnia. J Exp Zool A Ecol Genet Physiol. 2014, 321, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Xuereb, B.; Lefèvre, E.; Garric, J.; Geffard, O. Acetylcholinesterase activity in Gammarus fossarum (Crustacea Amphipoda): Linking AChE inhibition and behavioural alteration. Aquat. Toxicol., 2009, 94, 114–122. [Google Scholar] [CrossRef]
- Chandini, T. Survival, growth and reproduction of Daphnia carinata (Crustacea: Cladocera) exposed to chronic cadmium stress at different food (Chlorella) levels. Environ. Pollut., 1989, 60, 29–45. [Google Scholar] [CrossRef]
- Ward, T.J.; Robinson, W.E. Evolution of cadmium resistance in Daphnia magna. Environ. Toxicol. Chem., 2005, 24, 2341–2349. [Google Scholar] [CrossRef]
- Gust, K.A.; Kennedy, A.J.; Melby, N.L.; Wilbanks, M.S.; Laird, J.; Meeks, B.; Muller, E.B.; Nisbet, R.M.; Perkins, E.J. , Daphnia magna’s sense of competition: intra-specific interactions (ISI) alter life history strategies and increase metals toxicity. Ecotoxicology, 2016, 25, 1126–1135. [Google Scholar] [CrossRef]
- Roff, D., 2001. Life History Evolution. Encycl. Biodivers. Second Ed. 3, 715–728. [CrossRef]
- Heugens, E.H.W.; Hendriks, A.J.; Dekker, T.; van Straalen, N.M.; Admiraal, W. A Review of the Effects of Multiple Stressors on Aquatic Organisms and Analysis of Uncertainty Factors for Use in Risk Assessment. Crit. Rev. Toxicol., 2001, 31, 247–284. [Google Scholar] [CrossRef] [PubMed]
- Coniglio, L.; Baudo, R. Life-tables of Daphnia obtusa (Kurz) surviving exposure to toxic concentrations of chromium. Hydrobiologia, 1989, 188–189, 407–410. [Google Scholar] [CrossRef]
- Münzinger, A. Effects of Nickel on Daphnia magna during chronic exposure and alterations in the toxicity to generations pre-exposed to Nickel. Wat. Res., 1990, 24, 845–852. [Google Scholar] [CrossRef]
- Labrot, F.; Ribera, D.; Denis, M. Saint; Narbonne, J.F. In vitro and in vivo studies of potential biomarkers of lead and uranium contamination: lipid peroxidation, acetylcholinesterase, catalase and glutathione peroxidase activities in three non-mammalian species. Biomarkers, 1996, 1, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Fancey, L.L.; Kiceniuk, J.W. Response and Recovery of Brain Acetylcholinesterase Activity in Atlantic Salmon (Salmo salar) Exposed to Fenitrothion. Can. J. Fish. Aquat. Sci., 1990, 47, 1652–1654. [Google Scholar] [CrossRef]
- Narra, M.R.; Regatte, R.R.; Kodimyala, R. Sub-acute toxicity effects of chlorpyrifos on acetylcholinesterase activity and recovery in the freshwater field crab Barytelphusa guerini. Int. J. Environ. Sci., 2012, 3, 98–107. [Google Scholar]
- Abdullah, A.R.; Kumar, A.; Chapman, J.C. Inhibition of acetylcholinesterase in the Australian freshwater shrimp (Paratya australiensis) by profenfos. Envirom.Sci.Technol., 1994, 13, 1861–1866. [Google Scholar]
- Pieters, B.J.; Paschke, A.; Reynaldi, S.; Kraak, M.H.S.; Admiraal, W.; Liess, M. Influence of food limitation on the effects of fenvalerate pulse exposure on the life history and population growth rate of Daphnia magna. Environ. Toxicol. Chem., 2005, 24, 2254–2259. [Google Scholar] [CrossRef]
- Berglind, R.; Dave, G.; Sjöbeck, M.L. The effects of lead on delta-aminolevulinic acid dehydratase activity, growth, hemoglobin content, and reproduction in Daphnia magna. Ecotoxicol. Environ. Saf., 1985, 9, 216–229. [Google Scholar] [CrossRef]
- Biesinger, K.E.; Christensen, G.M. Effects of Various Metals on Survival, Growth, R. eproductiono and Metabolism of Daphnia magna. J. Fish. Res. Bd. Canada, 1972, 29, 1691–1700. [Google Scholar] [CrossRef]
- Printes, L.B.; Callaghan, A. Atividade de Acetilcolinesterase em Daphnia: Um Bom Biomarcador de Avaliação Ambiental? J. Brazilian Soc. Ecotoxicol., 2006, 1, 89–92. [Google Scholar] [CrossRef]
- Bainy, A.; Medeiros, M.; Di Mascio, P.; Almeida, E. In vivo effects of metals on the acetylcholinesterase activity of the Perna perna mussel’s digestive gland. Biotemas, 2006, 19, 35–39. [Google Scholar]
- Pedrozo, C.; Bohrer, M. Effects of culture medium and food quantity on the growth, fecundity and longevity of the cladoceran Daphnia similis Claus. Acta Limnol. Bras., 2003, 15, 43–49. [Google Scholar]
- Guan, R.; Wang, W.X. Multigenerational cadmium acclimation and biokinetics in Daphnia magna. Environ. Pollut., 2006, 141, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Enserink, E.L.; Kerkhofs, M.J.J.; Baltus, C.A.M.; Koeman, J.H. Influence of food quantity and lead exposure on maturation in Daphnia magna; Evidence for a trade-off mechanism. Funct. Ecol., 1995, 9, 175–185. [Google Scholar] [CrossRef]
- Pieters, B.J.; Liess, M. Maternal nutritional state determines the sensitivity of Daphnia magna offspring to short-term Fenvalerate exposure. Aquat. Toxicol., 2006, 76, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Agra, A.R.; Soares, A.M.V.M.; Barata, C. Life-history consequences of adaptation to pollution. “Daphnia longispina clones historically exposed to copper.” Ecotoxicology, 2011, 20, 552–562. [Google Scholar] [CrossRef]
- Doke, D.; Hudson, S.; Dawson, J.; Gohlke, J. 2017. Effects of early life exposure to methylmercury in Daphnia pulex on standard and reduced food ration. Reprod Toxicol., 2017, 49, 219–225. [Google Scholar] [CrossRef]
- Winner, R.W. A comparison of body length, brood size and longevity as indices of chronic copper and zinc stresses in Daphnia magna. Environ. Pollution. Ser. A, Ecol. Biol., 1981, 26, 33–37. [Google Scholar] [CrossRef]
- Schultz, C.L.; Wamucho, A.; Tsyusko, O.V.; Unrine, J.M.; Crossley, A.; Svendsen, C.; Spurgeon, D.J. Multigenerational exposure to silver ions and silver nanoparticles reveals heightened sensitivity and epigenetic memory in Caenorhabditis elegans. Proc. R. Soc. B Biol. Sci., 2016, 283, 20152911. [Google Scholar] [CrossRef]
- Barata, C.; Markich, S.J.; Baird, D.J.; Taylor, G.; Soares, A.M.V.M. Genetic variability in sublethal tolerance to mixtures of cadmium and zinc in clones of Daphnia magna Straus. Aquat. Toxicol., 2002, 60, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Duquesne, S.; Reynaldi, S.; Liess, M. Effects of the organophosphate paraoxon-methyl on survival and reproduction of Daphnia magna: Importance of exposure duration and recovery. Environ. Toxicol. Chem., 2006, 25, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sheng, L.; Xu, J.; Tong, H.; Jiang, H. The induction of metallothioneins during pulsed cadmium exposure to Daphnia magna: Recovery and trans-generational effect. Ecotoxicol. Environ. Saf., 2016, 126, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wang, C.; Chen, X.; Qin, Z.; Li, X.; Wang, C. Toxicity assessments with Daphnia magna of Guadipyr, a new neonicotinoid insecticide and studies of its effect on acetylcholinesterase (AChE), glutathione S-transferase (GST), catalase (CAT) and chitobiase activities. Ecotoxicol. Environ. Saf. 2013, 98, 339–344. [Google Scholar] [CrossRef]


| Eigenvalues | |||
|---|---|---|---|
| Total | % of Variance | Cumulative % | |
| 1 | 9.116 | 56.97 | 56.97 |
| 2 | 4.305 | 26.90 | 83.88 |
| Component | |||
| PC1 | PC2 | ||
| AChE (D. similis)_restricted | 0.995 | ||
| R0 (D. magna)_restricted | −0.961 | ||
| Lifespan (D. magna)_restricted | −0.923 | ||
| Hatch. delay (D. similis)_restricted | 0.914 | ||
| AChE (D. similis) | 0.898 | ||
| Hatch. delay (D. magna)_restricted | 0.892 | ||
| Lifespan (D. similis) | 0.891 | ||
| R0 (D. similis) | −0.888 | ||
| R0 (D. magna) | −0.864 | ||
| Hatch. Delay (D. magna) | 0.683 | ||
| AChE (D. magna) | −0.959 | ||
| AChE (D. magna)_restricted | 0.956 | ||
| Lifespan (D. similis)_restricted | 0.919 | ||
| Hatch. Delay (D. similis) | 0.652 | −0.752 | |
| Lifespan (D. magna) | |||
| R0 (D. similis)_restricted | −0.515 | ||
| Scores | |||
| PC1 | PC2 | ||
| F0 | −1.428 | ||
| Control (F9) | 1.496 | ||
| Pb_exposure (F9) | 0.785 | −0.603 | |
| Recovery period (F9) | 0.582 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





