1. Introduction
Frequency-specific specially tuned alternating current (AC) electric fields have demonstrated an ability to inhibit cancer cell proliferation (1). There are two current methods of delivering these fields. One method uses electrodes that are insulated and attached to the outside of the patient’s body. In this method, the electronic signal is not pulsed (1). The second method uses an electrified gas plasma enclosed in a glass tube to deliver pulsed electric fields to cancer cells up to eighteen inches away. In the first method, the frequency of treatment is modified based upon cancer cell type (1). In the second method, the pulse repetition rate (PRR) is modified based upon cancer cell type (2).
Data from experiments using both methods of delivering these two types of cancer treatment E-fields suggest that specific types of cancer cells are most vulnerable to specific frequencies. More specifically, different cancer cell types require the use of different frequencies. For TTField treatment, using attached electrodes, the rate of oscillation is specific to the cancer cell type (1). For pulsed electric fields delivered by a plasma antenna, “OPEF”, the frequency of repetition of the pulse is specific to the cancer cell type (2). We recently showed how a pulsed plasma frequency of 160kHz was able to induce an average of 38% reduction in the growth of acute lymphoblastic leukemia (3). Additional data now indicates that those same cells are most vulnerable to a narrow band of pulsed frequencies between 156kHz and 162kHz. This band of pulsed frequencies within which cancer cells are shown to be most inhibited by OPEF is here named a “Destructive Cancer Resonant Frequency Formant” (DCRFF).
2. Methods
Acute Lymphoblastic Leukemia cells (Coriell Institute Catalog #GM03638) were cultured in RPMI 1640 media (Life Technologies Catalog #61870036) with twenty percent FBS (Life Technologies catalog #A3160601) and incubated at 37C in a humidified incubator with five percent CO2. Starting cell density in flasks was 320,000 cells/mL. Cells were treated with pulsed plasma frequencies nine hours a day for three days, then incubated another forty-eight hours followed by an assay utilizing Trypan blue. Identical duplicate flasks, controls, were placed on the same heated microscope stage for nine hours a day, but without any plasma pulsed E-field treatments.
Optimal plasma treatment frequencies for the most effective reduction of leukemia cell growth were determined in vitro using heuristic methods. Various frequencies were tested nine hours per day over three consecutive days. Uniquely, all frequencies for TTFields and OPEF tested to date fall between 100kHz and 300kHz. There seems to be a correlation between the frequencies used by OPEF and TTFields for a specific cancer cell type, but this is not yet confirmed. Data from further refinement of frequency target acquisition suggest that specific types of cancers are most vulnerable to specific frequencies. For example, human glioma and ovarian cancer cells are most vulnerable to 200kHz, human breast and pancreatic cancer 150kHz, chronic leukemia 197kHz, Acute Lymphoblastic Leukemia 156kHz-162kHz, and melanoma 100kHz (1, 2, 3, 4, 5).
3. Electronics Setup
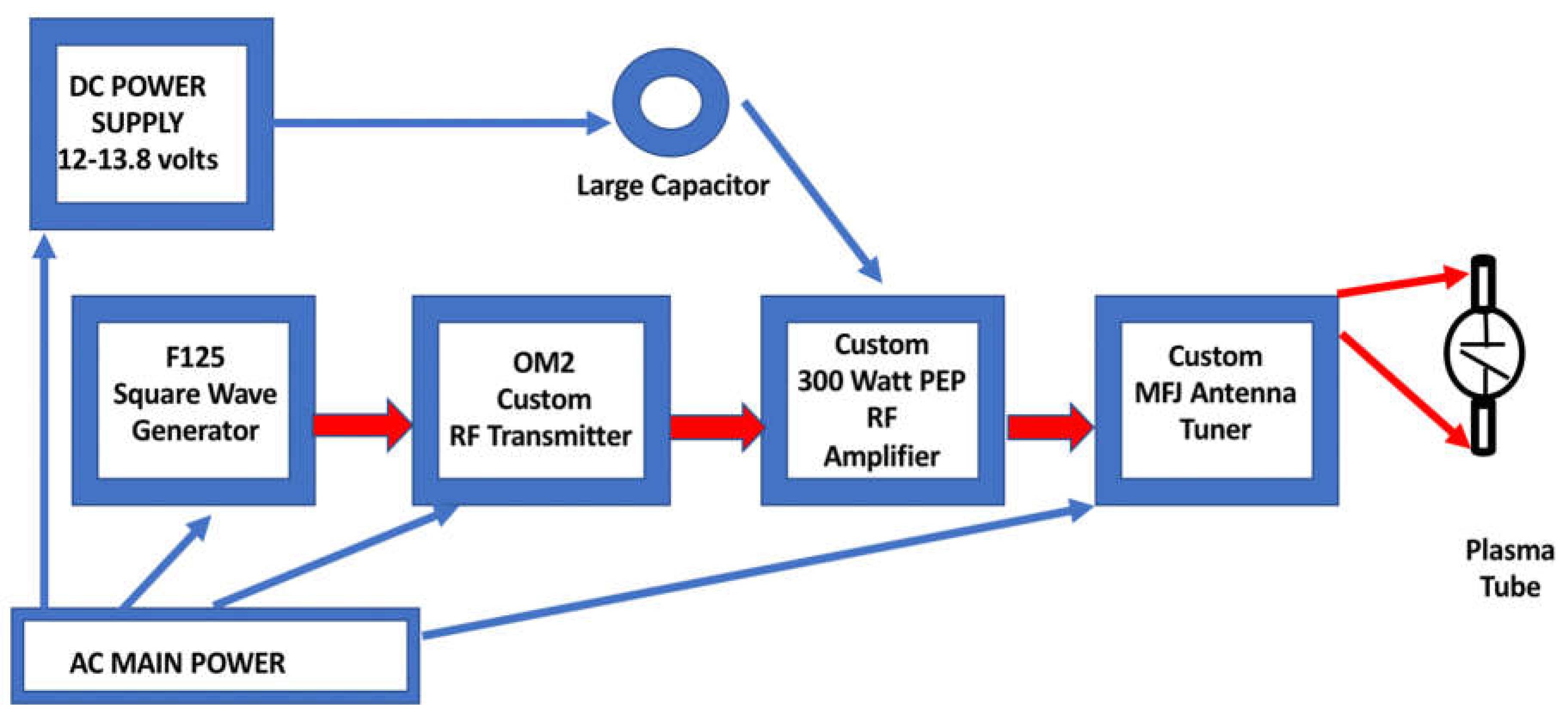
Pulsed plasma tube E-field emissions were broadcast by a specialized glass ‘Phanotron’ plasma tube antenna (billplasmatubes.com) located eighteen inches from the microscope where the cancer cells were placed on a heated stage for treatment. The plasma tube was driven by a three hundred-Watt custom radio frequency amplifier (Plasmasonics) (6). The originating signal source was a square wave generator with a fifty-percent duty cycle (F125, Atelier Robin), driving an ‘OM2’ custom radio frequency transmitter (Plasmasonics) that over-modulates the amplitude of the incoming square wave causing a pulsing of the final radio frequency output at a pulse rate that matches the fundamental frequency of the incoming square wave. Impedance tuning between the Phanotron tube and the custom RF amplifier was accomplished by a customized MFJ antenna tuner (Plasmasonics). Pulsed ‘OPEF’ E-fields are considered to cause nonthermal biological effects (7). Calculation of our E-field strength has been presented in another of our publications (8).
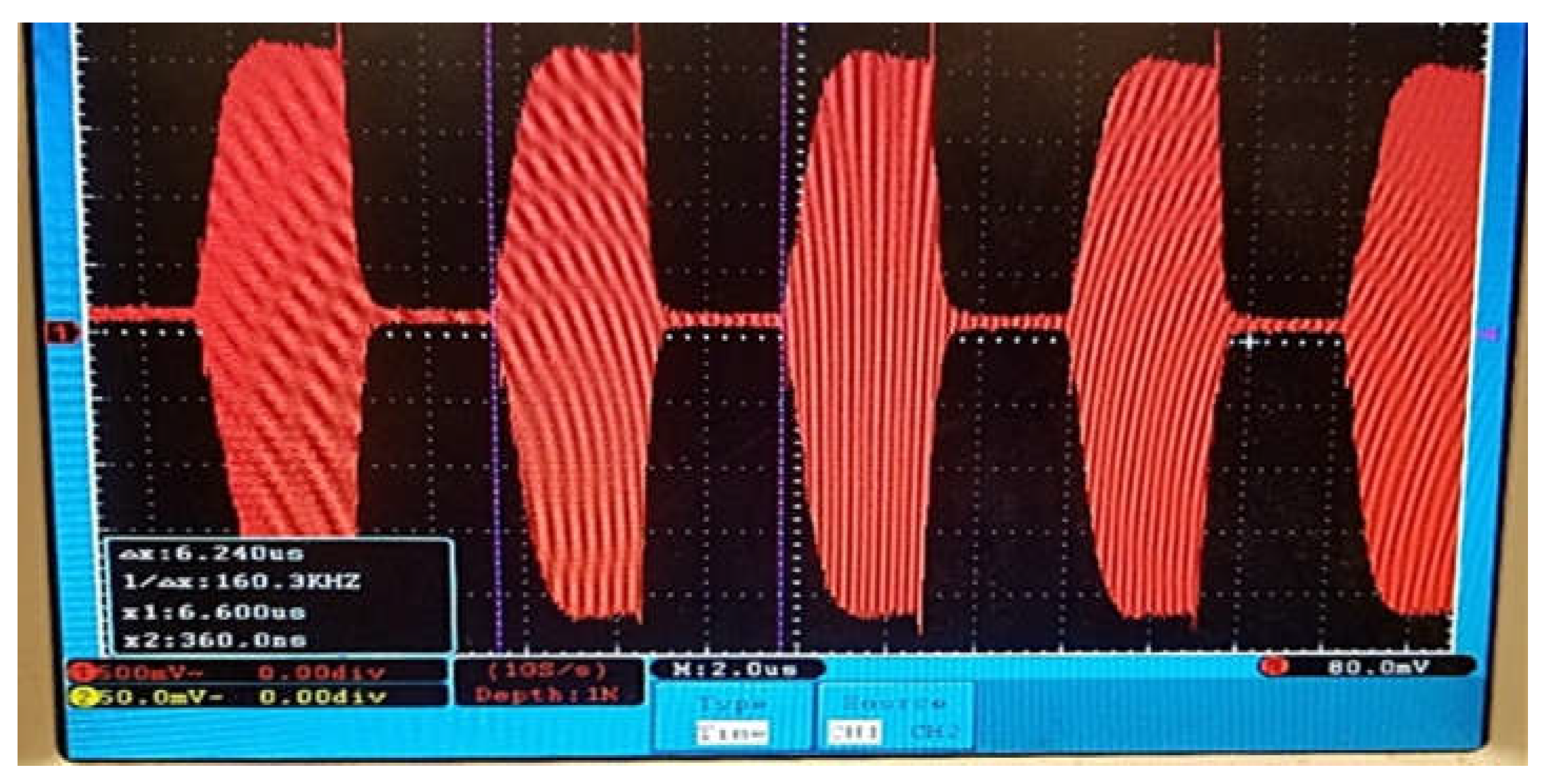
Figure 1.
160kHz OPEF Pulses.
Figure 1.
160kHz OPEF Pulses.
4. Results
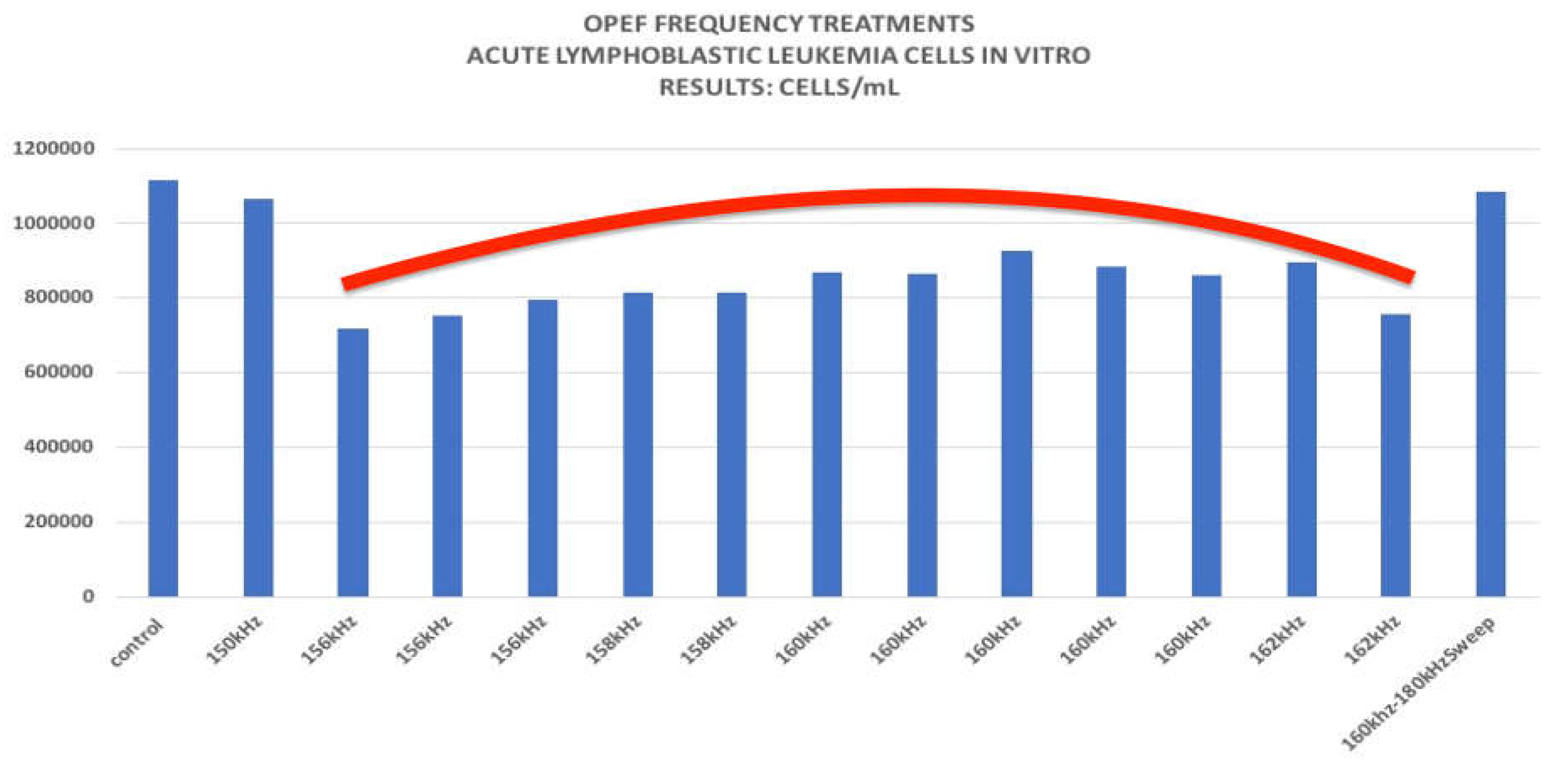
Recent experiments using OPEF demonstrated an average 38% reduction in leukemia cell growth at 160kHz (3). Acute Lymphoblastic Leukemia cell response was also tested at different frequencies and was found to be most vulnerable to electric field frequencies between 156 kHz and 162 kHz. Pulsed OPEF delivered by an enclosed gas plasma antenna within this DCRFF narrow band of frequencies demonstrated a consistent reduction in cancer cell proliferation between 24% and 43%. Our calculated P value is: P= 0.000001813
5. Discussion
Spectral formants are most typically associated with regions of the audio spectrum (20Hz-20kHz) where a narrow band of frequencies rise as a group in amplitude as compared to other spectral components. These audio-band spectral formants are often caused by resonant acoustic structures in musical instruments and create unique timbral colors associated with specific musical instruments (9,10). In the case of cancer cells, it has been posited that the inhibition of proliferation by OPEF may be due to resonant destruction of cell microtubules (8). It has also been posited that ‘cell escape’ from TTFields is due to expanding cell sizes caused by heat generated inside the cell by continuous (non-pulsed) AC electric fields delivered via specially insulated electrodes (11).
While cell heating may play a role in changes in cell size and thus ‘cell escape’ from TTFields, it is also known that cell sizes vary somewhat even within the exact same cell line. Further, cancer cells, like all cells, increase their sizes during mitosis (prophase and prometaphase). The effects of TTFields and OPEF fields both, disrupt the mitotic process. Variations in cell size, even within the same cell line, require the use of several different frequencies for maximum impact in slowing the growth of cancer (12,13,14). These frequencies are often close to one another for cells within the same cell line and thus form a “band of effective frequencies” in the spectrum, i.e. a DCRFF.
‘Cell escape’ caused by cell heating is avoided with OPEF because the effects of the pulsed signal are non-thermal (7). In OPEF, field strength, PRR (frequency), pulse shape, spectral envelope shape and spectral content play an important role in the effectiveness of OPEF against cancer cells in vitro. “Cell escape” from TTFields as well as variations of cell size within the same cell line both support the concept of a “Destructive Cancer Resonant Frequency Formant” (DCRFF), with each formant range specific to cancer type. 156kHz-162kHz was found to be such a Destructive Cancer Resonant Frequency Formant range for acute lymphoblastic leukemia.
Figure 2.
Cell Density/mL for Control and OPEF Treated Cancer Cells. The Red Line Suggests a Region Termed. A “Destructive Cancer Resonant Frequency Formant”.
Figure 2.
Cell Density/mL for Control and OPEF Treated Cancer Cells. The Red Line Suggests a Region Termed. A “Destructive Cancer Resonant Frequency Formant”.
Figure 3.
Electronics Setup Schematic Diagram.
Figure 3.
Electronics Setup Schematic Diagram.
Figure 4.
Leukemia Cells Treated with Pulsed E-Fields From a Plasma Antenna.
Figure 4.
Leukemia Cells Treated with Pulsed E-Fields From a Plasma Antenna.
6. Conclusion
Specific ranges of frequencies are necessary for the treatment of cancer as a consequence of several factors, which include: natural variations in cell size, changes in cell size during mitosis, and during extended exposure to static E fields which can cause the cell to swell in size. The optimal narrow band of frequencies to which a particular cancer cell line is most vulnerable is termed the "Destructive Cancer Resonant Frequency Formant" (DCRFF). The DCRFF for Acute Lymphoblastic Leukemia cells lies between 156kHz and 162kHz.
The authors wish to express their appreciation to Tom O’Connell, of Skidmore College, for assistance in analyzing the data from these experiments.
References
- Kirson, E.D.; Dbalý, V.; Tovarys, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; Wasserman, Y.; Salzberg, M.; Ryffel, B.; Goldsher, D.; Dekel, E.; Palti, Y. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. Epub 2007 Jun 5. See SI Fig 7 (supplemental information): https://www.pnas.org/doi/10.1073/pnas.0702916104. https://www.pnas.org/doi/10.1073/pnas.0702916104#supplementary-materials. 2007, 104, 10152–7. [Google Scholar] [CrossRef] [PubMed]
- Holland, A. Cell fragmentation and inhibition of proliferation of human leukemia cells in vitro by frequency-specific amplitude modulated RF pulsed plasmas. BIOEM 2015, Monterey, California [invited poster presentation and abstract] https://tholland48258.app.box.com/s/ts9e30jl4f9qvctfma2q0grcadesluyj https://tholland48258.app.box.com/file/35267188661?s=cynzvx98ywqlmcyaj3pdub1mea7mum0k.
- Holland, A.; Bare, J. Inhibition of proliferation of Acute Lymphocytic Leukemia (ALL) by frequency-specific Oscillating Pulsed Electric Fields (OPEF) broadcast by an enclosed gas plasma antenna. April 26, 2023. Currently in preprint: bioRxiv 2023.05.03.539248. https://biorxiv.org/cgi/content/short/2023.05.03.539248v1. 26 April. [CrossRef]
- Porat, Y.; Giladi, M.; Schneiderman, R.S.; Blat, R.; Shteingauz, A.; Zeevi, E.; Munster, M.; Voloshin, T.; Kaynan, N.; Tal, O.; Kirson, E.D.; Weinberg, U.; Palti, Y. Determining the optimal inhibitory frequency for cancerous cells using Tumor Treating Fields (TTFields). J Vis Exp. https://pubmed.ncbi.nlm.nih.gov/28518093/. 2017, 123, 55820. [Google Scholar] [CrossRef]
- https://novocuretrials.com/trial/panova-3/.
- Plasma Sonics Ltd. Co. Greenville, SC http://www.plasmasonics.com/.
- G.Dubost, A. Bellossi Thermal and non-thermal effects of electromagnetic fields on bio-systems 2012 ISBN: 978-1-4710-12433369. https://www.amazon.com/Thermal-thermal-effects-electromagnetic-bio-systems/dp/1471052494/ref=sr_1_1?crid=Y36RX1DYES6R&keywords=Dubost+Thermal+and+non-thermal&qid=1683036950&s=books&sprefix=dubost+thermal+and+non-thermal%2Cstripbooks%2C106&sr=1-1.
- Dubost G, Bare J, Holland A, Bellossi F. Destructive effects of pulsed electric fields on cancer cells: the microtubules mechanical resonance clue. Joint Meeting of The Bioelectromagnetics Society and the European BioElectromagnetics Association BioEM2013 Abstract Collection - Complete Collection June 10, 2013 - June 14, 2013 Thessaloniki, Greece. (abstract and poster) https://tholland48258.app.box.com/s/1r0ndnhvwawbq3t0qlw1. https://tholland48258.app.box.com/s/gp7c9x9pv1kb94tjax5f. 10 June.
- Benade, A.H. Fundamentals of musical acoustics; Oxford University Press: London, 1976. [Google Scholar]
- Standards Secretariat, Acoustical Society of America. ANSI S1.1-1994 (R2004) American National Standard Acoustical Terminology; (12.41) Acoustical Society of America: Melville, NY, 1994. [Google Scholar]
- Schneiderman R, Giladi M, Porat Y, Munster M, Weinberg U, Kirson E, Palti Y. Overcoming cell size escape from tumor treating fields using a varying frequency treatment paradigm in vitro. Journal of Clinical Oncology https://ascopubs.org/doi/10.1200/jco.2013.31.15_suppl.e22134. https://www.researchgate.net/publication/339852809_Overcoming_cell_size_escape_from_tumor_treating_fields_using_a_varying_frequency_treatment_paradigm_in_vitro. 2013, 31, No–15_suppl. [Google Scholar] [CrossRef]
- Palti, Y, Treating a tumor or the like with electric fields at different frequencies; US Patent No: US 8,706,261 B2; Date of Patent: Apr. 22, 2014; Assignee: Novocure Ltd., St. Hellier (JE). https://patentimages.storage.googleapis.com/51/87/6c/c084fb2009de00/US8706261.pdf.
- Palti Y, Dishon M, Optimizing treatment using TTFields by changing the frequency during the course of long term tumor treatment. US Patent https://patentimages.storage.googleapis.com/9c/8a/45/a7ee8ad30b3a42/US20210000528A1.pdf. US 2021/0000528 A1, 7 January 2021.
- Dubost G, Holland A, Bare J, Bellosi F. Morphological transformations of human cancer cells and microtubules caused by frequency specific pulsed electric fields broadcast by an enclosed gas plasma antenna. Proceedings-7th International Workshop on Biological Effects of EMF – October 2012 (Malta) ISBN: 978-99957- 0-361-5 https://tholland48258.app.box.com/file/11864330386?s=48dnqvpo78xw4eros9ql. 20 October.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).