Submitted:
26 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion criteria
2.2.3. Sources of Evidence
2.2.3. Search Strategies
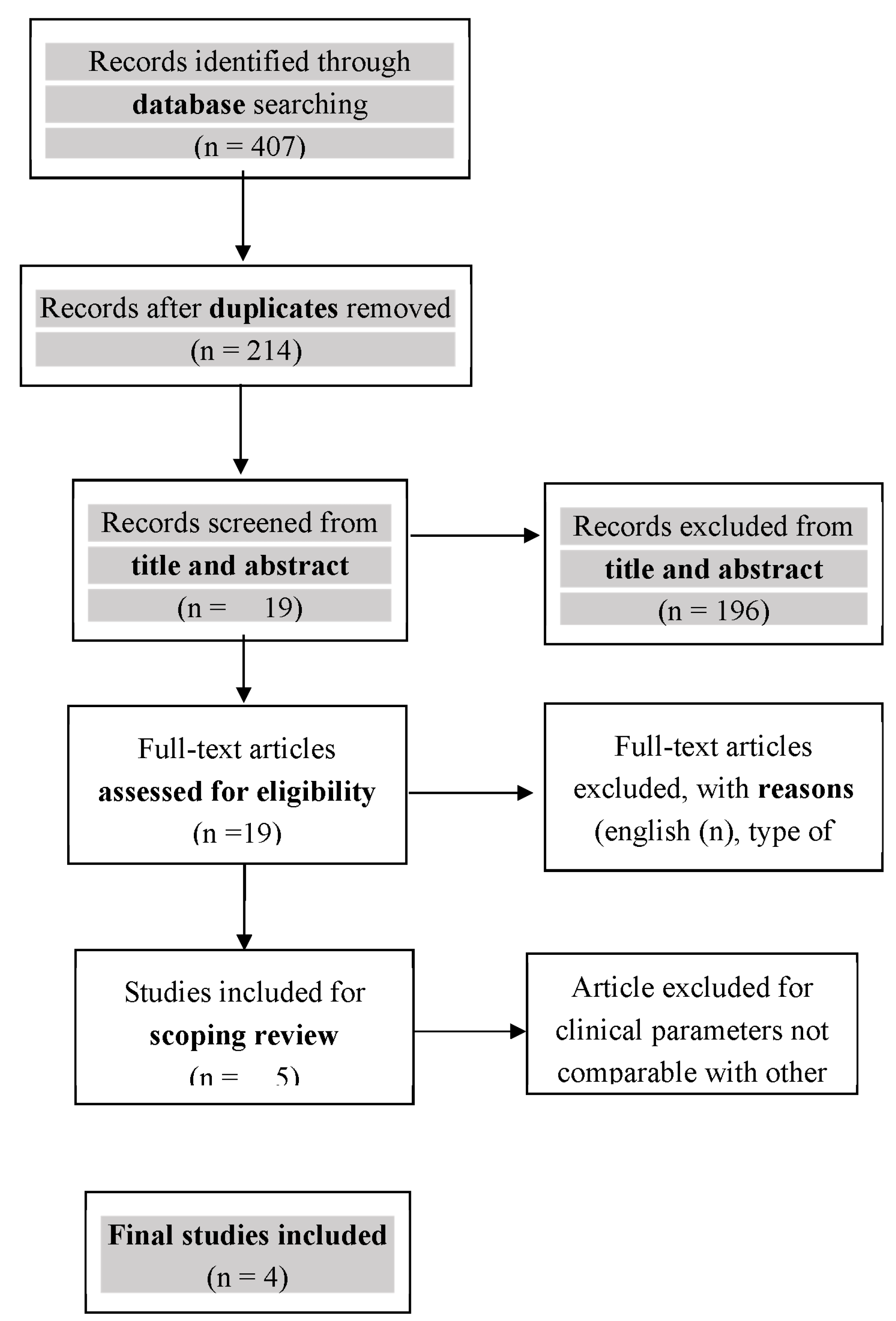
2.2.4. Selection of Sources of Evidence
3. Results
3.1. Kumar (2014) – RCT [22]
3.1.1. Sample Analysis
3.1.2. Hyaluronic acid used
3.1.3. Data
3.2. Pilloni (2017) – RCT [21]
3.2.1. Sample Analysis
3.2.2. Hyaluronic acid used
3.2.3. Data
3.2.4. Additional data
3.3. Guldener (2020) - Case series [30]
3.3.1. Sample Analysis
3.3.2. Hyaluronic acid used
3.3.3. Data
3.4. Lanzrein (2020) - Case series [29]
3.4.1. Sample Analysis
3.4.2. Hyaluronic acid used
3.4.3. Data
3.4.4. Additional data
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018, 89 Suppl 1, S313–s318. [Google Scholar] [CrossRef]
- Cortellini, P.; Bissada, N.F. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J Periodontol 2018, 89 Suppl 1, S204–s213. [Google Scholar] [CrossRef]
- Patel, R.R.; Richards, P.S.; Inglehart, M.R. Periodontal health, quality of life, and smiling patterns--an exploration. J Periodontol 2008, 79, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Mounssif, I. Periodontal plastic surgery. Periodontol 2000 2015, 68, 333–368. [Google Scholar] [CrossRef] [PubMed]
- Meza Mauricio, J.; Furquim, C.P.; Bustillos-Torrez, W.; Soto-Peñaloza, D.; Peñarrocha-Oltra, D.; Retamal-Valdes, B.; Faveri, M. Does enamel matrix derivative application provide additional clinical benefits in the treatment of maxillary Miller class I and II gingival recession? A systematic review and meta-analysis. Clin Oral Investig 2021, 25, 1613–1626. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D., Jr. A classification of marginal tissue recession. Int J Periodontics Restorative Dent 1985, 5, 8–13. [Google Scholar]
- Cairo, F.; Nieri, M.; Cincinelli, S.; Mervelt, J.; Pagliaro, U. The interproximal clinical attachment level to classify gingival recessions and predict root coverage outcomes: an explorative and reliability study. J Clin Periodontol 2011, 38, 661–666. [Google Scholar] [CrossRef]
- McGuire, M.K.; Scheyer, E.T.; Schupbach, P. A Prospective, Case-Controlled Study Evaluating the Use of Enamel Matrix Derivative on Human Buccal Recession Defects: A Human Histologic Examination. J Periodontol 2016, 87, 645–653. [Google Scholar] [CrossRef]
- McGuire, M.K.; Cochran, D.L. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 2: Histological evaluation. J Periodontol 2003, 74, 1126–1135. [Google Scholar] [CrossRef]
- França-Grohmann, I.L.; Sangiorgio, J.P.M.; Bueno, M.R.; Casarin, R.C.V.; Silvério Ruiz, K.G.; Nociti, F.H., Jr.; Casati, M.Z.; Sallum, E.A. Treatment of dehiscence-type defects with collagen matrix and/or enamel matrix derivative: Histomorphometric study in minipigs. J Periodontol 2020, 91, 967–974. [Google Scholar] [CrossRef]
- Chambrone, L.; Pini Prato, G.P. Clinical insights about the evolution of root coverage procedures: The flap, the graft, and the surgery. J Periodontol 2019, 90, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.L.; Roberts, J.L.; Moseley, R.; Griffiths, P.C.; Thomas, D.W. Evaluation of the physical and biological properties of hyaluronan and hyaluronan fragments. Int J Pharm 2011, 420, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.; Palmer, J.W. THE POLYSACCHARIDE OF THE VITREOUS HUMOR. The Journal of biological chemistry 1934, 107, 629–634. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Laurent, U.B. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Fraser, J.R. Hyaluronan. Faseb j 1992, 6, 2397–2404. [Google Scholar] [CrossRef]
- Pirnazar, P.; Wolinsky, L.; Nachnani, S.; Haake, S.; Pilloni, A.; Bernard, G.W. Bacteriostatic effects of hyaluronic acid. J Periodontol 1999, 70, 370–374. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers (Basel) 2018, 10. [Google Scholar] [CrossRef]
- Dahiya, P.; Kamal, R. Hyaluronic Acid: a boon in periodontal therapy. N Am J Med Sci 2013, 5, 309–315. [Google Scholar] [CrossRef]
- Prato, G.P.; Rotundo, R.; Magnani, C.; Soranzo, C.; Muzzi, L.; Cairo, F. An autologous cell hyaluronic acid graft technique for gingival augmentation: a case series. J Periodontol 2003, 74, 262–267. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int J Immunopathol Pharmacol 2016, 29, 572–582. [Google Scholar] [CrossRef]
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: a randomized controlled clinical trial. Clinical oral investigations 2019, 23, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Srinivas, M.; Pai, J.; Suragimath, G.; Prasad, K.; Polepalle, T. Efficacy of hyaluronic acid (hyaluronan) in root coverage procedures as an adjunct to coronally advanced flap in Millers Class I recession: A clinical study. J Indian Soc Periodontol 2014, 18, 746–750. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O'Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Munn, Z.; Stern, C.; Aromataris, E.; Lockwood, C.; Jordan, Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC Med Res Methodol 2018, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Bateson, M. Systematic Reviews to Support Evidence-Based Medicine: How to Review and Apply Findings of Healthcare Research. Postgraduate Medical Journal 2004, 80, 123. [Google Scholar]
- Al-Ardah, A.J.; AlHelal, A.; Proussaefs, P.; AlBader, B.; Al Humaidan, A.A.; Lozada, J. Managing Titanium Mesh Exposure With Partial Removal of the Exposed Site: A Case Series Study. J Oral Implantol 2017, 43, 482–490. [Google Scholar] [CrossRef]
- Nandanwar, J.; Bhongade, M.; Puri, S.; Dhadse, P.; Datir, M.; Kasatwar, A. Comparison of effectiveness of hyaluronic acid in combination with polylactic acid/polyglycolic acid membrane and subepithelial connective tissue graft for the treatment of multiple gingival recession defects in human: A clinical study. Journal of Datta Meghe Institute of Medical Sciences University 2018, 13, 48–53. [Google Scholar] [CrossRef]
- Pilloni, A.; Schmidlin, P.R.; Sahrmann, P.; Sculean, A.; Rojas, M.A. Effectiveness of adjunctive hyaluronic acid application in coronally advanced flap in Miller class I single gingival recession sites: a randomized controlled clinical trial. Clin Oral Investig 2019, 23, 1133–1141. [Google Scholar] [CrossRef]
- Lanzrein, C.; Guldener, K.; Imber, J.C.; Katsaros, C.; Stähli, A.; Sculean, A. Treatment of multiple adjacent recessions with the modified coronally advanced tunnel or laterally closed tunnel in conjunction with cross-linked hyaluronic acid and subepithelial connective tissue graft: a report of 15 cases. Quintessence Int 2020, 51, 710–719. [Google Scholar] [CrossRef]
- Guldener, K.; Lanzrein, C.; Eliezer, M.; Katsaros, C.; Stähli, A.; Sculean, A. Treatment of single mandibular recessions with the modified coronally advanced tunnel or laterally closed tunnel, hyaluronic acid, and subepithelial connective tissue graft: a report of 12 cases. Quintessence International 2020, 51, 456–463. [Google Scholar] [CrossRef]
- Baldi, C.; Pini-Prato, G.; Pagliaro, U.; Nieri, M.; Saletta, D.; Muzzi, L.; Cortellini, P. Coronally advanced flap procedure for root coverage. Is flap thickness a relevant predictor to achieve root coverage? A 19-case series. J Periodontol 1999, 70, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, G.; Stefanini, M.; Ganz, S.; Mazzotti, C.; Mounssif, I.; Marzadori, M. Coronally Advanced Flap with Different Designs in the Treatment of Gingival Recession: A Comparative Controlled Randomized Clinical Trial. Int J Periodontics Restorative Dent 2016, 36, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Cosgarea, R.; Stähli, A.; Katsaros, C.; Arweiler, N.B.; Brecx, M.; Deppe, H. The modified coronally advanced tunnel combined with an enamel matrix derivative and subepithelial connective tissue graft for the treatment of isolated mandibular Miller Class I and II gingival recessions: a report of 16 cases. Quintessence Int 2014, 45, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Allen, E.P. The Laterally Closed Tunnel for the Treatment of Deep Isolated Mandibular Recessions: Surgical Technique and a Report of 24 Cases. Int J Periodontics Restorative Dent 2018, 38, 479–487. [Google Scholar] [CrossRef] [PubMed]
- West, D.C.; Hampson, I.N.; Arnold, F.; Kumar, S. Angiogenesis induced by degradation products of hyaluronic acid. Science 1985, 228, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.F.; Kakkar, V.V.; Goodwin, C.A.; O'Regan, M. Inhibition of fibrinolytic activity by hyaluronan and its alcohol ester derivatives. Thromb Res 1995, 78, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Trombelli, L.; Simonelli, A.; Pramstraller, M.; Guarnelli, M.E.; Fabbri, C.; Maietti, E.; Farina, R. Clinical efficacy of a chlorhexidine-based mouthrinse containing hyaluronic acid and an antidiscoloration system in patients undergoing flap surgery: A triple-blind, parallel-arm, randomized controlled trial. Int J Dent Hyg 2018, 16, 541–552. [Google Scholar] [CrossRef]
- Vänttinen, E.; Viljanto, J. Tensile strength of new connective tissue formed in pretreated viscose cellulose implants. Ann Med Exp Biol Fenn 1965, 43, 257–259. [Google Scholar]
- Pilloni, A.; Bernard, G.W. The effect of hyaluronan on mouse intramembranous osteogenesis in vitro. Cell Tissue Res 1998, 294, 323–333. [Google Scholar] [CrossRef]
- Pilloni, A.; Rimondini, L.; De Luca, M.; Bernard, G.W. Effect of hyaluronan on calcification-nodule formation from human periodontal ligament cell culture. J Appl Biomater Biomech 2003, 1, 84–90. [Google Scholar]
- Wikesjö, U.M.; Selvig, K.A. Periodontal wound healing and regeneration. Periodontol 2000 1999, 19, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, A.; Tellefsen, M.; Wikesjö, U.; Johannsen, G. Local delivery of hyaluronan as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J Periodontol 2009, 80, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, H.; Pomowski, R.; Kundt, G.; Göcke, R. Treatment of gingivitis with hyaluronan. J Clin Periodontol 2003, 30, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ficho, A.C.; de Souza Faloni, A.P.; Pennisi, P.R.C.; Borges, L.G.F.; de Macedo Bernadino, Í.; Paranhos, L.R.; Queiroz, T.P.; Santos, P.L. Is interdental papilla filling using hyaluronic acid a stable approach to treat black triangles? A systematic review. J Esthet Restor Dent 2021, 33, 458–465. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Capodiferro, S.; Grassi, F.R. Esterified hyaluronic acid and autologous bone in the surgical correction of the infra-bone defects. Int J Med Sci 2009, 6, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kediege, S.D.; Gupta, A.; Jain, K. Evaluation of gengigel® application in the management of furcation with coronally advanced flap through surgical re-entry-a split mouth clinical study. Journal of Clinical and Diagnostic Research 2017, 11, ZC27–ZC32. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, W.; Jedaiba, W.H.; Alnami, A.M.; Hussain Baiti, L.A.; Ali Manqari, S.M.; Bhati, A.; Almarghlani, A.; Assaggaf, M. Efficacy of hyaluronic acid gel and spray in healing of extraction wound: a randomized controlled study. Eur Rev Med Pharmacol Sci 2022, 26, 3444–3449. [Google Scholar] [CrossRef] [PubMed]
- Stähli, A.; Duong, H.Y.; Imber, J.C.; Roccuzzo, A.; Salvi, G.E.; Katsaros, C.; Ramseier, C.A.; Sculean, A. Recession coverage using the modified coronally advanced tunnel and connective tissue graft with or without enamel matrix derivative: 5-year results of a randomised clinical trial. Clin Oral Investig 2022, 1–9. [Google Scholar] [CrossRef]
- Górski, B.; Szerszeń, M.; Kaczyński, T. Effect of 24% EDTA root conditioning on the outcome of modified coronally advanced tunnel technique with subepithelial connective tissue graft for the treatment of multiple gingival recessions: a randomized clinical trial. Clin Oral Investig 2022, 26, 1761–1772. [Google Scholar] [CrossRef]
- Rasperini, G.; Acunzo, R.; Limiroli, E. Decision Making in Gingival Recession Treatment: Scientific Evidence and Clinical Experience. Clin Adv Periodontics 2011, 1, 41–52. [Google Scholar] [CrossRef]
- Pini Prato, G.; Pagliaro, U.; Baldi, C.; Nieri, M.; Saletta, D.; Cairo, F.; Cortellini, P. Coronally advanced flap procedure for root coverage. Flap with tension versus flap without tension: a randomized controlled clinical study. J Periodontol 2000, 71, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Pini-Prato, G.; Baldi, C.; Pagliaro, U.; Nieri, M.; Saletta, D.; Rotundo, R.; Cortellini, P. Coronally advanced flap procedure for root coverage. Treatment of root surface: root planning versus polishing. J Periodontol 1999, 70, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Lindhe, J. Clinical Periodontology and Implant Dentistry, 2 Volume Set; Wiley: 2015.
- Chambrone, L.; Tatakis, D.N. Periodontal soft tissue root coverage procedures: a systematic review from the AAP Regeneration Workshop. J Periodontol 2015, 86, S8–51. [Google Scholar] [CrossRef]
- Cairo, F.; Nieri, M.; Pagliaro, U. Efficacy of periodontal plastic surgery procedures in the treatment of localized facial gingival recessions. A systematic review. J Clin Periodontol 2014, 41 Suppl 15, S44–62. [Google Scholar] [CrossRef]
- Chambrone, L.; Lima, L.A.; Pustiglioni, F.E.; Chambrone, L.A. Systematic review of periodontal plastic surgery in the treatment of multiple recession-type defects. J Can Dent Assoc 2009, 75, 203a–203f. [Google Scholar] [PubMed]
- Chambrone, L.; Sukekava, F.; Araújo, M.G.; Pustiglioni, F.E.; Chambrone, L.A.; Lima, L.A. Root-coverage procedures for the treatment of localized recession-type defects: a Cochrane systematic review. J Periodontol 2010, 81, 452–478. [Google Scholar] [CrossRef] [PubMed]
- Pini Prato, G.P.; Baldi, C.; Nieri, M.; Franseschi, D.; Cortellini, P.; Clauser, C.; Rotundo, R.; Muzzi, L. Coronally advanced flap: the post-surgical position of the gingival margin is an important factor for achieving complete root coverage. J Periodontol 2005, 76, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Saletta, D.; Pini Prato, G.; Pagliaro, U.; Baldi, C.; Mauri, M.; Nieri, M. Coronally advanced flap procedure: is the interdental papilla a prognostic factor for root coverage? J Periodontol 2001, 72, 760–766. [Google Scholar] [CrossRef]
- Hwang, D.; Wang, H.L. Flap thickness as a predictor of root coverage: a systematic review. J Periodontol 2006, 77, 1625–1634. [Google Scholar] [CrossRef]
- Al-Khateeb, R.; Olszewska-Czyz, I. Biological molecules in dental applications: hyaluronic acid as a companion biomaterial for diverse dental applications. Heliyon 2020, 6, e03722. [Google Scholar] [CrossRef]
- Liu, H.; Yin, Y.; Yao, K.; Ma, D.; Cui, L.; Cao, Y. Influence of the concentrations of hyaluronic acid on the properties and biocompatibility of Cs-Gel-HA membranes. Biomaterials 2004, 25, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, A.; de la Rosa, M.; de la Garza, M.; Caffesse, R.G. Enamel matrix derivative and coronal flaps to cover marginal tissue recessions. J Periodontol 2006, 77, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.T.; de Menezes, C.C.; Kahn, S.; Fischer, R.G.; da Silva Figueredo, C.M.; Fernandes, G.V.d.O. Gingival recession treatment with enamel matrix derivative associated with coronally advanced flap and subepithelial connective tissue graft: a split-mouth randomized controlled clinical trial with molecular evaluation. Clinical Oral Investigations 2022, 26, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Caluseru, O.M.; Guillemette, V.; Zhang, Y.; Gemperli, A.C.; Chandad, F.; Sculean, A. Influence of enamel matrix derivative on cells at different maturation stages of differentiation. PLoS One 2013, 8, e71008. [Google Scholar] [CrossRef] [PubMed]
- Pini-Prato, G.P.; Cairo, F.; Nieri, M.; Franceschi, D.; Rotundo, R.; Cortellini, P. Coronally advanced flap versus connective tissue graft in the treatment of multiple gingival recessions: a split-mouth study with a 5-year follow-up. J Clin Periodontol 2010, 37, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, S.; Özener, H.; Doğan, B.; Kuru, B. Effect of topically applied hyaluronic acid on pain and palatal epithelial wound healing: An examiner-masked, randomized, controlled clinical trial. J Periodontol 2018, 89, 36–45. [Google Scholar] [CrossRef]
- Cairo, F.; Rotundo, R.; Miller, P.D.; Pini Prato, G.P. Root coverage esthetic score: a system to evaluate the esthetic outcome of the treatment of gingival recession through evaluation of clinical cases. J Periodontol 2009, 80, 705–710. [Google Scholar] [CrossRef]
- Rotundo, R.; Nieri, M.; Mori, M.; Clauser, C.; Prato, G.P. Aesthetic perception after root coverage procedure. J Clin Periodontol 2008, 35, 705–712. [Google Scholar] [CrossRef]
- Roccuzzo, M.; Bunino, M.; Needleman, I.; Sanz, M. Periodontal plastic surgery for treatment of localized gingival recessions: a systematic review. J Clin Periodontol 2002, 29 Suppl 3, 178-194; discussion 195-176. 3. [CrossRef]
| Article | Design | Recession type | Single/multiple | HA | Surgical technique | Test | Control | Outcome | Follow up |
|---|---|---|---|---|---|---|---|---|---|
| 2014 Kumar |
RCT split (7M – 3F) 10 20 rec |
RT1 | Single | Hyaluronic acid (Gengigel 0.2% gel which is 0.2% hyaluronan gel marketed by Ricerfarma pharmaceuticals, Milan, Italy) | CAF | CAF+HA GEL (10) |
CAF (1 0) |
RD, PD, CAL | 1, 3, 6, 12, 24 Weeks |
| 2017 Pilloni |
RCT (M – F) 30 |
RT1 | Single | 1,6% cross-linked HA, 0.2% linear HA (Hyadent BG, Regedent) | CAF | CAF+HA (15) | CAF (15) | RD, PD, CAL, KT, CRC, MRC, VAS | 18 mounths |
| 2020 Guldener | CASE SERIES 12 |
RT1 | Single | 1,6% cross-linked HA, 0.2% linear HA (Hyadent BG, Regedent) | MCAT+sCTG +HA LCT+sCTG +HA |
- | - | RD, PD, CAL, KT, CRC, MRC | range 6 to 30 months |
| 2020 Lanzrein | CASE SERIES 15 |
RT1 and RT2 | Multiple | 1,6% cross-linked HA, 0.2% linear HA (Hyadent BG, Regedent) | MCAT+sCTG+HA LCT+sCTG+HA |
- | - | RD, PD, CAL, KT, CRC, MRC, RES | range 6 to 33 months |
| FU | RD | PD | CAL | MRC | CRC | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow up | Baseline | Follow up | Baseline | Follow up | Follow up | Follow up | ||
| Kumar 2014 | 24 weeks | Test: 3.20 mm | Test: 1.10 mm | Test: 1.80 mm | Test: 1.70 mm | Test: 5 mm | Test: 2.80 mm | Test: 68,3% | Test: 40% |
| Control: 2.90 mm | Control: 1.00 mm | Control: 2.00 mm | Control: 2.00 mm | Control: 4.90 mm | Control: 3.00 mm | Control:61,6% | Control: 20% | ||
|
Pilloni 2017 |
18 months |
Test: 3.0[1.0] mm | Test: 0.0[0.0] mm | Test: 1.0[0.0] mm | Test: 1.0[1.0] mm | Test:4.0[1.0] mm | Test: 1.0[0.0] mm | Test: 93,8% | Test: 80% |
| Control:3.0[1.0] mm | Control:1.0[1.0] mm | Control:1.0[0.0] mm | Control:2.0[1.0] mm | Control:4.0[1.0] mm | Control:2.0[0.0] mm | Control: 73,1% | Control: 30% | ||
|
Guldener 2020 |
6±33 months | 4.6[0.9]mm | 0.5[0.6] mm | 1.8[0.9]mm | 1.3[0.5] | 6.4 mm | 1.8[0.5] | 96.09%. | 50% |
|
Lanzrein 2020 |
6±30 months | 3.3[0.8]mm | 0.8[1.0] mm | 1.3[0.5]mm | 1.5[0.5] mm | 4.6 mm | 2.3 mm | 85.1% | 20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





