Submitted:
29 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
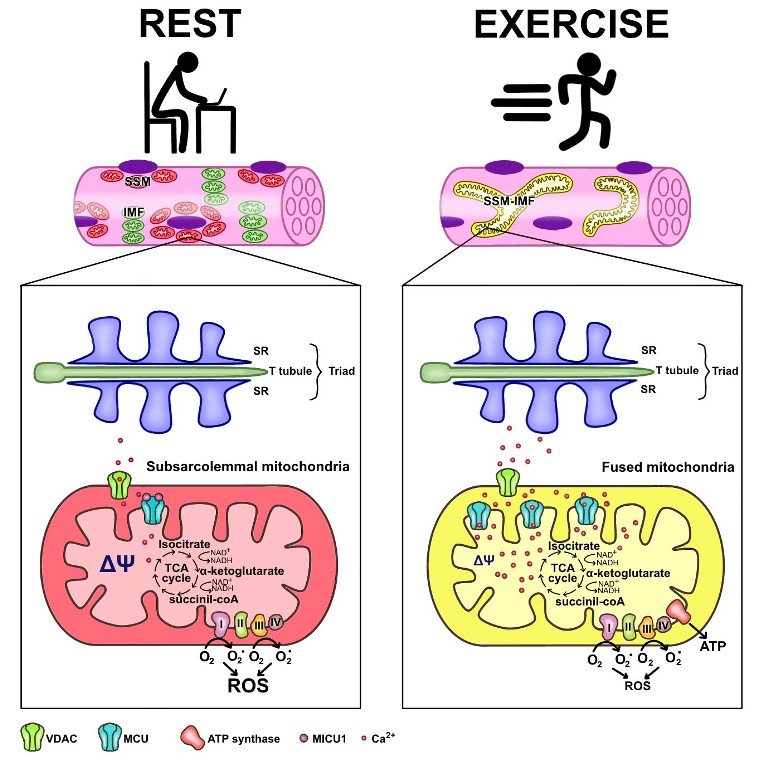
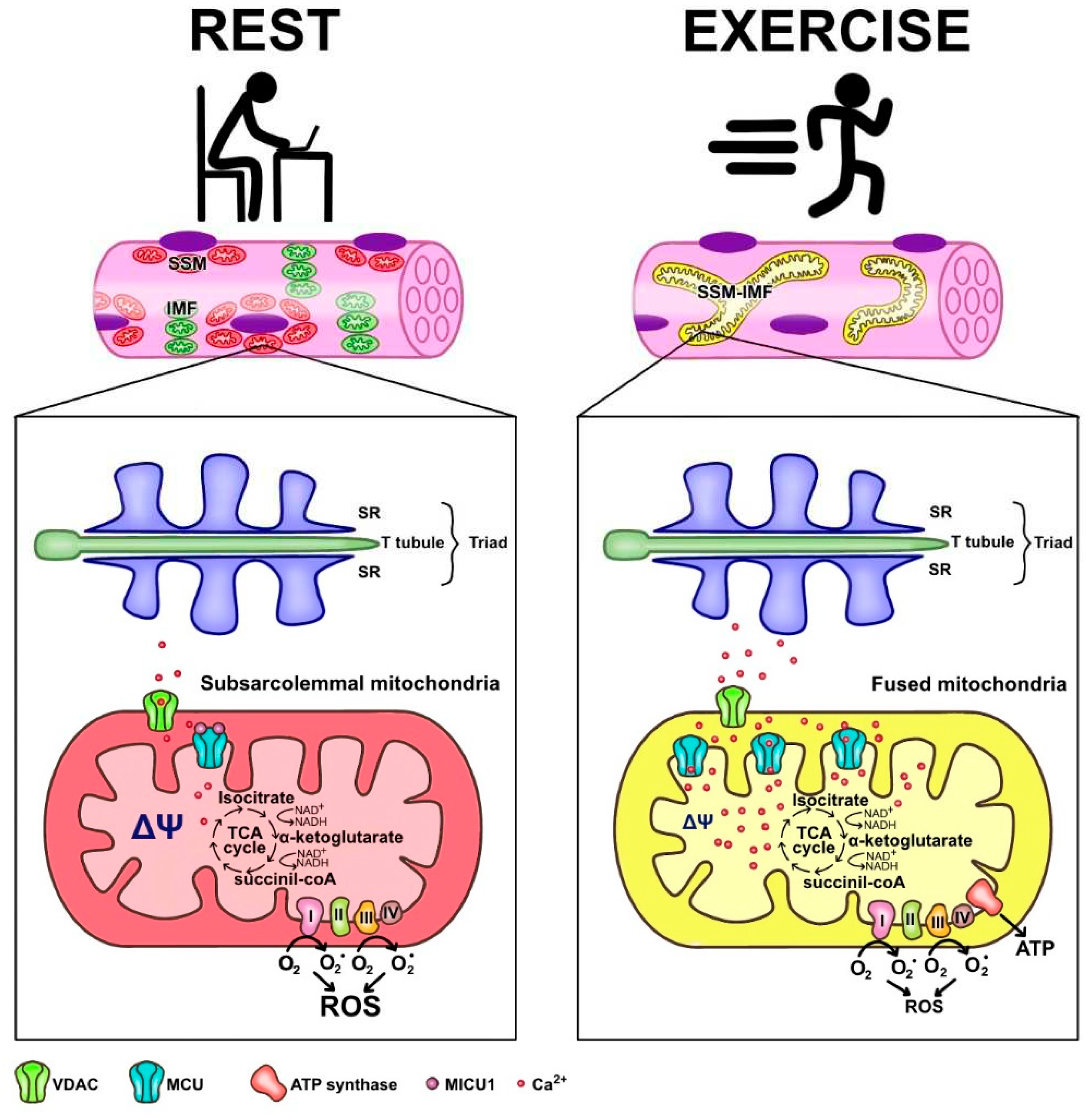
Keywords:
1. Introduction
2. H2O2-mediated physiological signals in skeletal muscle
2.1. Role of H2O2 in the excitation-contraction (E-C) coupling mechanism.
2.2. Role of H2O2 in glucose uptake in skeletal muscle
2.3. Mitochondrial ROS associated to physical activity.
2.3.1. The particular mitochondria distribution and characteristics in skeletal muscle
2.3.2. Function of the heterogeneity of mitochondria within the muscle fiber
2.3.3. Mitochondria dynamics is altered in skeletal muscle of aging subjects and in pathological conditions.
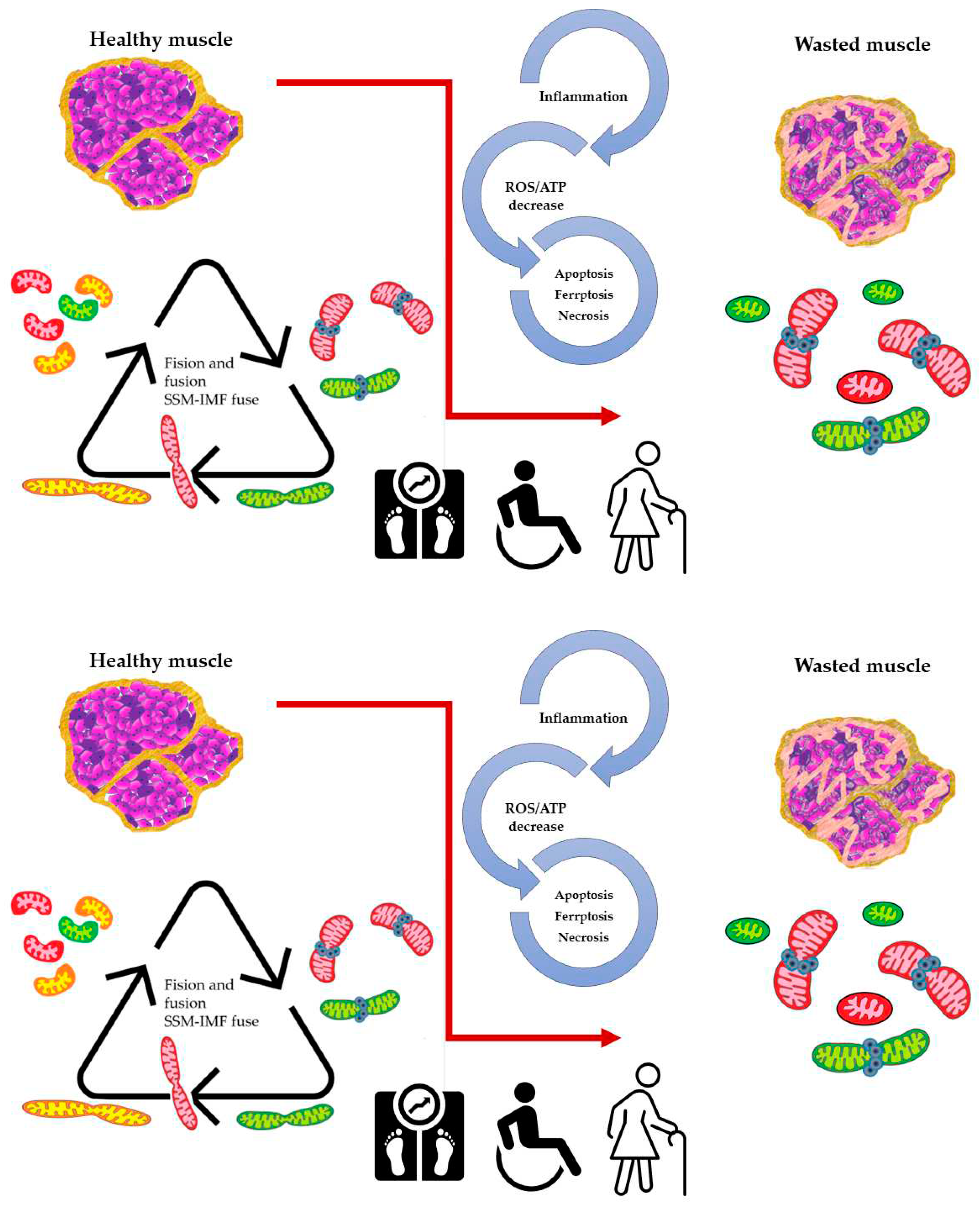
3. Conclusion
References
- Wang, P.; Li, C.G.; Qi, Z.; Cui, D.; Ding, S. Acute Exercise Induced Mitochondrial H₂O₂ Production in Mouse Skeletal Muscle: Association with p(66Shc) and FOXO3a Signaling and Antioxidant Enzymes. Oxid Med Cell Longev 2015, 2015, 536456. [Google Scholar] [CrossRef] [PubMed]
- Osório Alves, J.; Matta Pereira, L.; Cabral Coutinho do Rêgo Monteiro, I.; Pontes Dos Santos, L.H.; Soares Marreiros Ferraz, A.; Carneiro Loureiro, A.C.; Calado Lima, C.; Leal-Cardoso, J.H.; Pires Carvalho, D.; Soares Fortunato, R.; et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants (Basel) 2020, 9, 57. [Google Scholar] [CrossRef]
- Bannister, R.A.; Beam, K.G. Ca(V)1.1: The Atypical Prototypical Voltage-Gated Ca2+ Channel. Biochim Biophys Acta 2013, 1828, 1587–1597. [Google Scholar] [CrossRef]
- Ríos, E.; Gillespie, D.; Franzini-Armstrong, C. The Binding Interactions That Maintain Excitation-Contraction Coupling Junctions in Skeletal Muscle. J Gen Physiol 2019, 151, 593–605. [Google Scholar] [CrossRef]
- Rebbeck, R.T.; Karunasekara, Y.; Board, P.G.; Beard, N.A.; Casarotto, M.G.; Dulhunty, A.F. Skeletal Muscle Excitation-Contraction Coupling: Who Are the Dancing Partners? Int J Biochem Cell Biol 2014, 48, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.-H.; Pedersen, T.H.; Fraser, J.A. Reciprocal Dihydropyridine and Ryanodine Receptor Interactions in Skeletal Muscle Activation. J Muscle Res Cell Motil 2011, 32, 171–202. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xu, L.; Meissner, G. Phosphorylation of Dihydropyridine Receptor II-III Loop Peptide Regulates Skeletal Muscle Calcium Release Channel Function. Evidence for an Essential Role of the Beta-OH Group of Ser687. J Biol Chem 1995, 270, 18459–18464. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Pannell, B.K. Redox Characterization of Functioning Skeletal Muscle. Front Physiol 2015, 6, 338. [Google Scholar] [CrossRef]
- Luin, E.; Giniatullin, R.; Sciancalepore, M. Effects of H₂O₂ on Electrical Membrane Properties of Skeletal Myotubes. Free Radic Biol Med 2011, 50, 337–344. [Google Scholar] [CrossRef]
- Oba, T.; Ishikawa, T.; Takaishi, T.; Aoki, T.; Yamaguchi, M. Hydrogen Peroxide Decelerates Recovery of Action Potential after High-Frequency Fatigue in Skeletal Muscle. Muscle Nerve 2000, 23, 1515–1524. [Google Scholar] [CrossRef]
- Posterino, G.S.; Lamb, G.D. Effects of Reducing Agents and Oxidants on Excitation-Contraction Coupling in Skeletal Muscle Fibres of Rat and Toad. J Physiol 1996, 496 Pt 3, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Plant, D.R.; Lynch, G.S.; Williams, D.A. Hydrogen Peroxide Increases Depolarization-Induced Contraction of Mechanically Skinned Slow Twitch Fibres from Rat Skeletal Muscles. J Physiol 2002, 539, 883–891. [Google Scholar] [CrossRef]
- Espinosa, A.; Leiva, A.; Peña, M.; Müller, M.; Debandi, A.; Hidalgo, C.; Carrasco, M.A.; Jaimovich, E. Myotube Depolarization Generates Reactive Oxygen Species through NAD(P)H Oxidase; ROS-Elicited Ca2+ Stimulates ERK, CREB, Early Genes. J Cell Physiol 2006, 209, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Silveira, L.R.; Pereira-Da-Silva, L.; Juel, C.; Hellsten, Y. Formation of Hydrogen Peroxide and Nitric Oxide in Rat Skeletal Muscle Cells during Contractions. Free Radic Biol Med 2003, 35, 455–464. [Google Scholar] [CrossRef]
- Palomero, J.; Pye, D.; Kabayo, T.; Spiller, D.G.; Jackson, M.J. In Situ Detection and Measurement of Intracellular Reactive Oxygen Species in Single Isolated Mature Skeletal Muscle Fibers by Real Time Fluorescence Microscopy. Antioxid Redox Signal 2008, 10, 1463–1474. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Radák, Z. Hormetic Effects of Reactive Oxygen Species by Exercise: A View from Animal Studies for Successful Aging in Human. Dose Response 2009, 8, 68–72. [Google Scholar] [CrossRef]
- Musci, R.V.; Hamilton, K.L.; Linden, M.A. Exercise-Induced Mitohormesis for the Maintenance of Skeletal Muscle and Healthspan Extension. Sports (Basel) 2019, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, C.G.; Qi, Z.; Cui, D.; Ding, S. Acute Exercise Stress Promotes Ref1/Nrf2 Signalling and Increases Mitochondrial Antioxidant Activity in Skeletal Muscle. Exp Physiol 2016, 101, 410–420. [Google Scholar] [CrossRef]
- McArdle, F.; Spiers, S.; Aldemir, H.; Vasilaki, A.; Beaver, A.; Iwanejko, L.; McArdle, A.; Jackson, M.J. Preconditioning of Skeletal Muscle against Contraction-Induced Damage: The Role of Adaptations to Oxidants in Mice. J Physiol 2004, 561, 233–244. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Harper, M.-E. Uncoupling Proteins and the Control of Mitochondrial Reactive Oxygen Species Production. Free Radic Biol Med 2011, 51, 1106–1115. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, G.; Bo, H.; Qu, J.; Ma, G.; Cao, D.; Wen, L.; Liu, S.; Ji, L.L.; Zhang, Y. Upregulation of Uncoupling Protein-3 in Skeletal Muscle during Exercise: A Potential Antioxidant Function. Free Radic Biol Med 2009, 46, 138–145. [Google Scholar] [CrossRef]
- Cruz, C.M.; Rinna, A.; Forman, H.J.; Ventura, A.L.M.; Persechini, P.M.; Ojcius, D.M. ATP Activates a Reactive Oxygen Species-Dependent Oxidative Stress Response and Secretion of Proinflammatory Cytokines in Macrophages. J Biol Chem 2007, 282, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vegas, A.; Campos, C.A.; Contreras-Ferrat, A.; Casas, M.; Buvinic, S.; Jaimovich, E.; Espinosa, A. ROS Production via P2Y1-PKC-NOX2 Is Triggered by Extracellular ATP after Electrical Stimulation of Skeletal Muscle Cells. PLoS One 2015, 10, e0129882. [Google Scholar] [CrossRef] [PubMed]
- Jorquera, G.; Altamirano, F.; Contreras-Ferrat, A.; Almarza, G.; Buvinic, S.; Jacquemond, V.; Jaimovich, E.; Casas, M. Cav1.1 Controls Frequency-Dependent Events Regulating Adult Skeletal Muscle Plasticity. J Cell Sci 2013, 126, 1189–1198. [Google Scholar] [CrossRef]
- Casas, M.; Buvinic, S.; Jaimovich, E. ATP Signaling in Skeletal Muscle: From Fiber Plasticity to Regulation of Metabolism. Exerc Sport Sci Rev 2014, 42, 110–116. [Google Scholar] [CrossRef]
- Pinheiro, C.H. da J.; Silveira, L.R.; Nachbar, R.T.; Vitzel, K.F.; Curi, R. Regulation of Glycolysis and Expression of Glucose Metabolism-Related Genes by Reactive Oxygen Species in Contracting Skeletal Muscle Cells. Free Radical Biology and Medicine 2010, 48, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Higaki, Y.; Mikami, T.; Fujii, N.; Hirshman, M.F.; Koyama, K.; Seino, T.; Tanaka, K.; Goodyear, L.J. Oxidative Stress Stimulates Skeletal Muscle Glucose Uptake through a Phosphatidylinositol 3-Kinase-Dependent Pathway. Am J Physiol Endocrinol Metab 2008, 294, E889–897. [Google Scholar] [CrossRef]
- Irrcher, I.; Ljubicic, V.; Hood, D.A. Interactions between ROS and AMP Kinase Activity in the Regulation of PGC-1alpha Transcription in Skeletal Muscle Cells. Am J Physiol Cell Physiol 2009, 296, C116–123. [Google Scholar] [CrossRef]
- Jensen, T.E.; Schjerling, P.; Viollet, B.; Wojtaszewski, J.F.P.; Richter, E.A. AMPK Alpha1 Activation Is Required for Stimulation of Glucose Uptake by Twitch Contraction, but Not by H2O2, in Mouse Skeletal Muscle. PLoS One 2008, 3, e2102. [Google Scholar] [CrossRef]
- Specht, K.S.; Kant, S.; Addington, A.K.; McMillan, R.P.; Hulver, M.W.; Learnard, H.; Campbell, M.; Donnelly, S.R.; Caliz, A.D.; Pei, Y.; et al. Nox4 Mediates Skeletal Muscle Metabolic Responses to Exercise. Mol Metab 2021, 45, 101160. [Google Scholar] [CrossRef]
- Espinosa, A.; Campos, C.; Díaz-Vegas, A.; Galgani, J.; Juretic, N.; Osorio-Fuentealba, C.; Bucarey, J.; Tapia, G.; Valenzuela, R.; Contreras-Ferrat, A.; et al. Insulin-Dependent H2O2 Production Is Higher in Muscle Fibers of Mice Fed with a High-Fat Diet. IJMS 2013, 14, 15740–15754. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS Production by NADPH Oxidase 2 Regulates Muscle Glucose Uptake during Exercise. Nat Commun 2019, 10, 4623. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ferrat, A.; Llanos, P.; Vásquez, C.; Espinosa, A.; Osorio-Fuentealba, C.; Arias-Calderon, M.; Lavandero, S.; Klip, A.; Hidalgo, C.; Jaimovich, E. Insulin Elicits a ROS-Activated and an IP₃-Dependent Ca2+ Release, Which Both Impinge on GLUT4 Translocation. J Cell Sci 2014, 127, 1911–1923. [Google Scholar] [CrossRef]
- Maeda, A.; Shirao, T.; Shirasaya, D.; Yoshioka, Y.; Yamashita, Y.; Akagawa, M.; Ashida, H. Piperine Promotes Glucose Uptake through ROS-Dependent Activation of the CAMKK/AMPK Signaling Pathway in Skeletal Muscle. Mol Nutr Food Res 2018, 62, e1800086. [Google Scholar] [CrossRef] [PubMed]
- Favaro, G.; Romanello, V.; Varanita, T.; Andrea Desbats, M.; Morbidoni, V.; Tezze, C.; Albiero, M.; Canato, M.; Gherardi, G.; De Stefani, D.; et al. DRP1-Mediated Mitochondrial Shape Controls Calcium Homeostasis and Muscle Mass. Nat Commun 2019, 10, 2576. [Google Scholar] [CrossRef]
- Willingham, T.B.; Ajayi, P.T.; Glancy, B. Subcellular Specialization of Mitochondrial Form and Function in Skeletal Muscle Cells. Front. Cell Dev. Biol. 2021, 9, 757305. [Google Scholar] [CrossRef]
- Takahashi, M.; Hood, D.A. Protein Import into Subsarcolemmal and Intermyofibrillar Skeletal Muscle Mitochondria. Differential Import Regulation in Distinct Subcellular Regions. J Biol Chem 1996, 271, 27285–27291. [Google Scholar] [CrossRef]
- Ferreira, R.; Vitorino, R.; Alves, R.M.P.; Appell, H.J.; Powers, S.K.; Duarte, J.A.; Amado, F. Subsarcolemmal and Intermyofibrillar Mitochondria Proteome Differences Disclose Functional Specializations in Skeletal Muscle. Proteomics 2010, 10, 3142–3154. [Google Scholar] [CrossRef]
- Csordás, G.; Weaver, D.; Hajnóczky, G. Endoplasmic Reticulum-Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol 2018, 28, 523–540. [Google Scholar] [CrossRef]
- Booth, D.M.; Enyedi, B.; Geiszt, M.; Várnai, P.; Hajnóczky, G. Redox Nanodomains Are Induced by and Control Calcium Signaling at the ER-Mitochondrial Interface. Molecular Cell 2016, 63, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Elander, A.; Sjöström, M.; Lundgren, F.; Scherstén, T.; Bylund-Fellenius, A.C. Biochemical and Morphometric Properties of Mitochondrial Populations in Human Muscle Fibres. Clin Sci (Lond) 1985, 69, 153–164. [Google Scholar] [CrossRef]
- Díaz-Vegas, A.R.; Cordova, A.; Valladares, D.; Llanos, P.; Hidalgo, C.; Gherardi, G.; De Stefani, D.; Mammucari, C.; Rizzuto, R.; Contreras-Ferrat, A.; et al. Mitochondrial Calcium Increase Induced by RyR1 and IP3R Channel Activation After Membrane Depolarization Regulates Skeletal Muscle Metabolism. Front. Physiol. 2018, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.Q.; Huo, J.; Bround, M.J.; Boyer, J.G.; Schwanekamp, J.A.; Ghazal, N.; Maxwell, J.T.; Jang, Y.C.; Khuchua, Z.; Shi, K.; et al. The Mitochondrial Calcium Uniporter Underlies Metabolic Fuel Preference in Skeletal Muscle. JCI Insight 2018, 3, e121689–121689. [Google Scholar] [CrossRef] [PubMed]
- Groten, C.J.; MacVicar, B.A. Mitochondrial Ca2+ Uptake by the MCU Facilitates Pyramidal Neuron Excitability and Metabolism during Action Potential Firing. Commun Biol 2022, 5, 900. [Google Scholar] [CrossRef]
- Patron, M.; Raffaello, A.; Granatiero, V.; Tosatto, A.; Merli, G.; De Stefani, D.; Wright, L.; Pallafacchina, G.; Terrin, A.; Mammucari, C.; et al. The Mitochondrial Calcium Uniporter (MCU): Molecular Identity and Physiological Roles. Journal of Biological Chemistry 2013, 288, 10750–10758. [Google Scholar] [CrossRef]
- Mammucari, C.; Gherardi, G.; Zamparo, I.; Raffaello, A.; Boncompagni, S.; Chemello, F.; Cagnin, S.; Braga, A.; Zanin, S.; Pallafacchina, G.; et al. The Mitochondrial Calcium Uniporter Controls Skeletal Muscle Trophism in Vivo. Cell Rep 2015, 10, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Yi, J.; Li, X.; Zhou, J. Physiological Ca2+ Transients Versus Pathological Steady-State Ca2+ Elevation, Who Flips the ROS Coin in Skeletal Muscle Mitochondria. Front Physiol 2020, 11, 595800. [Google Scholar] [CrossRef]
- Alevriadou, B.R.; Patel, A.; Noble, M.; Ghosh, S.; Gohil, V.M.; Stathopulos, P.B.; Madesh, M. Molecular Nature and Physiological Role of the Mitochondrial Calcium Uniporter Channel. Am J Physiol Cell Physiol 2021, 320, C465–C482. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol Rev 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Khodabukus, A.; Baar, K. Contractile and Metabolic Properties of Engineered Skeletal Muscle Derived from Slow and Fast Phenotype Mouse Muscle. J Cell Physiol 2015, 230, 1750–1757. [Google Scholar] [CrossRef]
- Loucif, H.; Dagenais-Lussier, X.; Beji, C.; Telittchenko, R.; Routy, J.-P.; van Grevenynghe, J. Plasticity in T-Cell Mitochondrial Metabolism: A Necessary Peacekeeper during the Troubled Times of Persistent HIV-1 Infection. Cytokine Growth Factor Rev 2020, 55, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Shirihai, O.S.; Gentil, B.J.; Burelle, Y. Mitochondrial Morphology Transitions and Functions: Implications for Retrograde Signaling? Am J Physiol Regul Integr Comp Physiol 2013, 304, R393–406. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Hartnell, L.M.; Malide, D.; Yu, Z.-X.; Combs, C.A.; Connelly, P.S.; Subramaniam, S.; Balaban, R.S. Mitochondrial Reticulum for Cellular Energy Distribution in Muscle. Nature 2015, 523, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Quezada, E.R.; Díaz-Vegas, A.; Jaimovich, E.; Casas, M. Changes in Gene Expression of the MCU Complex Are Induced by Electrical Stimulation in Adult Skeletal Muscle. Front Physiol 2020, 11, 601313. [Google Scholar] [CrossRef]
- Horn, A.; Van der Meulen, J.H.; Defour, A.; Hogarth, M.; Sreetama, S.C.; Reed, A.; Scheffer, L.; Chandel, N.S.; Jaiswal, J.K. Mitochondrial Redox Signaling Enables Repair of Injured Skeletal Muscle Cells. Sci Signal 2017, 10, eaaj1978. [Google Scholar] [CrossRef] [PubMed]
- Crochemore, C.; Mekki, M.; Corbière, C.; Karoui, A.; Noël, R.; Vendeville, C.; Vaugeois, J.-M.; Monteil, C. Subsarcolemmal and Interfibrillar Mitochondria Display Distinct Superoxide Production Profiles. Free Radic Res 2015, 49, 331–337. [Google Scholar] [CrossRef]
- Debattisti, V.; Horn, A.; Singh, R.; Seifert, E.L.; Hogarth, M.W.; Mazala, D.A.; Huang, K.T.; Horvath, R.; Jaiswal, J.K.; Hajnóczky, G. Dysregulation of Mitochondrial Ca2+ Uptake and Sarcolemma Repair Underlie Muscle Weakness and Wasting in Patients and Mice Lacking MICU1. Cell Rep 2019, 29, 1274–1286. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative Genomics Identifies MCU as an Essential Component of the Mitochondrial Calcium Uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef]
- Eisner, V.; Lenaers, G.; Hajnóczky, G. Mitochondrial Fusion Is Frequent in Skeletal Muscle and Supports Excitation-Contraction Coupling. J Cell Biol 2014, 205, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A.; Contreras-Hernández, I.; Castro-Sepúlveda, M.; Campos, C.A.; Figueroa, R.; Tevy, M.F.; Eisner, V.; Casas, M.; Jaimovich, E. Muscle Function Decline and Mitochondria Changes in Middle Age Precede Sarcopenia in Mice. Aging (Albany NY) 2018, 10, 34–55. [Google Scholar] [CrossRef] [PubMed]
- Fealy, C.E.; Grevendonk, L.; Hoeks, J.; Hesselink, M.K.C. Skeletal Muscle Mitochondrial Network Dynamics in Metabolic Disorders and Aging. Trends Mol Med 2021, 27, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab 2017, 25, 1374–1389. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nuevo, A.; Díaz-Ramos, A.; Noguera, E.; Díaz-Sáez, F.; Duran, X.; Muñoz, J.P.; Romero, M.; Plana, N.; Sebastián, D.; Tezze, C.; et al. Mitochondrial DNA and TLR9 Drive Muscle Inflammation upon Opa1 Deficiency. EMBO J 2018, 37, e96553. [Google Scholar] [CrossRef] [PubMed]
- Yeo, D.; Kang, C.; Gomez-Cabrera, M.C.; Vina, J.; Ji, L.L. Intensified Mitophagy in Skeletal Muscle with Aging Is Downregulated by PGC-1alpha Overexpression in Vivo. Free Radic Biol Med 2019, 130, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Pahlavani, H.; Laher, I.; Knechtle, B.; Zouhal, H. Exercise and Mitochondrial Mechanisms in Patients with Sarcopenia. Front Physiol 2022, 13, 1040381. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Yang, W.; Liao, Z.-Y.; Wu, Y.-X.; Fan, Z.; Guo, A.; Yu, J.; Chen, Q.-N.; Wu, J.-H.; Zhou, J.; et al. MICU3 Regulates Mitochondrial Ca2+-Dependent Antioxidant Response in Skeletal Muscle Aging. Cell Death Dis 2021, 12, 1115. [Google Scholar] [CrossRef]
- Altamirano, F.; López, J.R.; Henríquez, C.; Molinski, T.; Allen, P.D.; Jaimovich, E. Increased Resting Intracellular Calcium Modulates NF-ΚB-Dependent Inducible Nitric-Oxide Synthase Gene Expression in Dystrophic Mdx Skeletal Myotubes. J Biol Chem 2012, 287, 20876–20887. [Google Scholar] [CrossRef]
- Mechler, F.; Imre, S.; Dioszeghy, P. Lipid Peroxidation and Superoxide Dismutase Activity in Muscle and Erythrocytes in Duchenne Muscular Dystrophy. J Neurol Sci 1984, 63, 279–283. [Google Scholar] [CrossRef]
- Łoboda, A.; Dulak, J. Nuclear Factor Erythroid 2-Related Factor 2 and Its Targets in Skeletal Muscle Repair and Regeneration. Antioxid Redox Signal 2023, 38, 619–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Qu, H.; Chen, Y.; Luo, X.; Chen, C.; Xiao, B.; Ding, X.; Zhao, P.; Lu, Y.; Chen, A.F.; et al. Atorvastatin Induces Mitochondria-Dependent Ferroptosis via the Modulation of Nrf2-XCT/GPx4 Axis. Front Cell Dev Biol 2022, 10, 806081. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, B.; Shen, D.; Chen, J.; Yu, Z.; Chen, C. Ferroptosis in a Sarcopenia Model of Senescence Accelerated Mouse Prone 8 (SAMP8). Int J Biol Sci 2021, 17, 151–162. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



