1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 virus (SARS-CoV-2) had been a global health concern until very recently. Despite extensive research, the precise ramifications of this viral infection on pregnancy and its subsequent effects on offspring remain elusive [
1]. Generally, pregnant individuals are more susceptible to viral infections compared to the general population [
2]. Reports indicated that contracting SARS-CoV-2 during pregnancy can have detrimental implications for maternal well-being by increasing the risk of preeclampsia, preterm delivery, maternal mortality, neonatal intensive care unit admission, and impact neonatal outcomes with increased incidence of preterm birth and neonatal mortality [
3]. However, the intricate molecular mechanism that underlines such adverse effects during pregnancy with SARS-CoV-2 infection is not clearly understood.
According to the current reports, the SARS-CoV-2 virus relies on two key proteins, angiotensin-converting enzyme II (ACE2) and transmembrane serine protease 2 (TMPRSS2) for cell entry and membrane fusion, respectively [
4,
5]. It is reported that both, ACE2 and TMPRSS2 are enriched in many tissues in addition to the respiratory system, resulting in pathological alterations within the local tissues upon SARS-CoV-2 infection. The term placenta is one of the organs where the above-mentioned entry factors for SARS-CoV-2 along with Furin are highly expressed. Notably, the expression of ACE2 mRNA in the placenta is similar to that in the lung, the most common site for SARS-CoV-2 infection in humans [
6].
Placenta, a remarkable and intricate tissue plays a vital role in facilitating the communication channel between the mother and developing fetus by transferring nutrients, hormones, and antibodies protecting the growing fetus from immunological threats [
7]. Earlier reports suggested possible vertical transmission of SARS-CoV-2 from mother to fetus [
8,
9]. However, recent studies suggest that the placenta might act as a barrier for transplacental infection since the restriction of SARS-CoV-2 replication was found in human placental tissue [
10]. Nonetheless, controversies persist regarding the possibility of antenatal vertical transmission of SARS-CoV-2.
Hence, the primary objectives of this study were twofold. Firstly, we aimed to investigate the potential vertical transmission of the SARS-CoV-2 virus by examining its presence in both the maternal and placental compartments of the term placenta. Secondly, we aimed to explore differentially expressed genes within placental compartments that are altered in COVID-19 infection during pregnancy, with a particular focus on understanding how these genes and related pathways may contribute to pregnancy-related complications. By investigating these molecular pathways, we aimed to shed light on the underlying mechanisms that could lead to adverse outcomes in pregnant women infected with SARS-CoV-2.
2. Results
2.1. Clinical characteristics of SARS-CoV-2 positive women
In this study, a total of 16 term placental samples from women collected between June 2020 to February 2022 were used. Out of these, seven women tested positive for SARS-CoV-2 by qPCR when admitted to the hospital during their term pregnancy for delivery and the details are given in
Table 1. Briefly, the average maternal age of women who tested positive for SARS-CoV-2 was 34.14 years (range 31 - 34 years, median 32 years). The gestational age at delivery averaged 39.1 weeks (range 34.7 – 41.3 weeks, median 39.6 weeks). Cases 4, 13, 15, and 17 were for cesarean sections due to different indications including failed induction of labor, social factor, and unexpected vaginal bleeding. All the other cases had a vaginal delivery. Except for case 15, all women had an active infection, and tested positive for SARS-CoV-2 within 5 days before their delivery. Case 15 was diagnosed with COVID-19 three months before delivery and had completely recovered at the time of delivery and thus excluded from the analysis. Cases 13, 14, 16, and 17 had mild symptoms of COVID-19, while the rest were asymptomatic when tested positive for SARS-CoV-2. The severity of COVID-19 was determined according to the published criteria released by the Society for Maternal-Fetal Medicine and the National Institutes of Health [
11]. All newborns were singletons and had normal Apgar score after birth. None of the newborns entered NICU.
2.2. Differential gene expression analysis
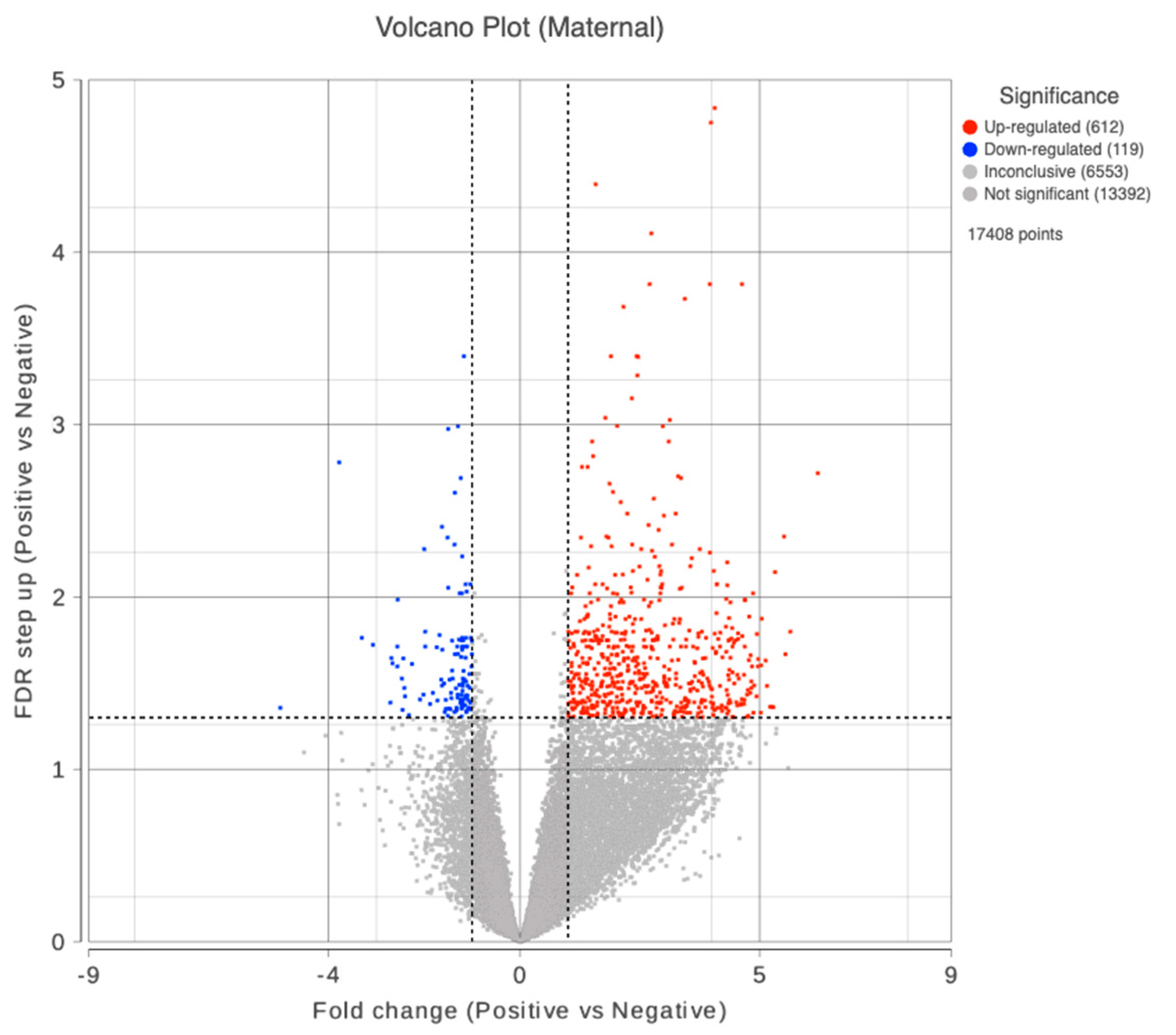
We obtained mRNA and small RNA sequencing data set from 32 placental samples (16 maternal + 16 fetal compartment) after performing quality control and batch effect correction (COVID-19 positive maternal (M) compartment n = 7; COVID-19 negative maternal compartment n = 9; COVID-19 positive fetal (F) compartment n = 7; COVID-19 negative fetal compartment n = 9). We excluded samples 10F and 10M due to potential fecal contamination. Data from 13F and 8F were filtered out due to low alignment rates to the human genome. Data from case 15 were excluded from the analysis due to its long interval from infection to delivery. We did not find any differentially expressed genes in the fetal compartment on comparing with COVID-19 positive and negative groups. However, in the maternal compartment of the placenta, a total of 727 DEGs were identified in COVID-19 positive group on comparing with the negative. In this, 608 genes were upregulated and 119 downregulated as displayed in the volcano plot (
Figure 2). The top 10 dysregulated DEGs were shown in
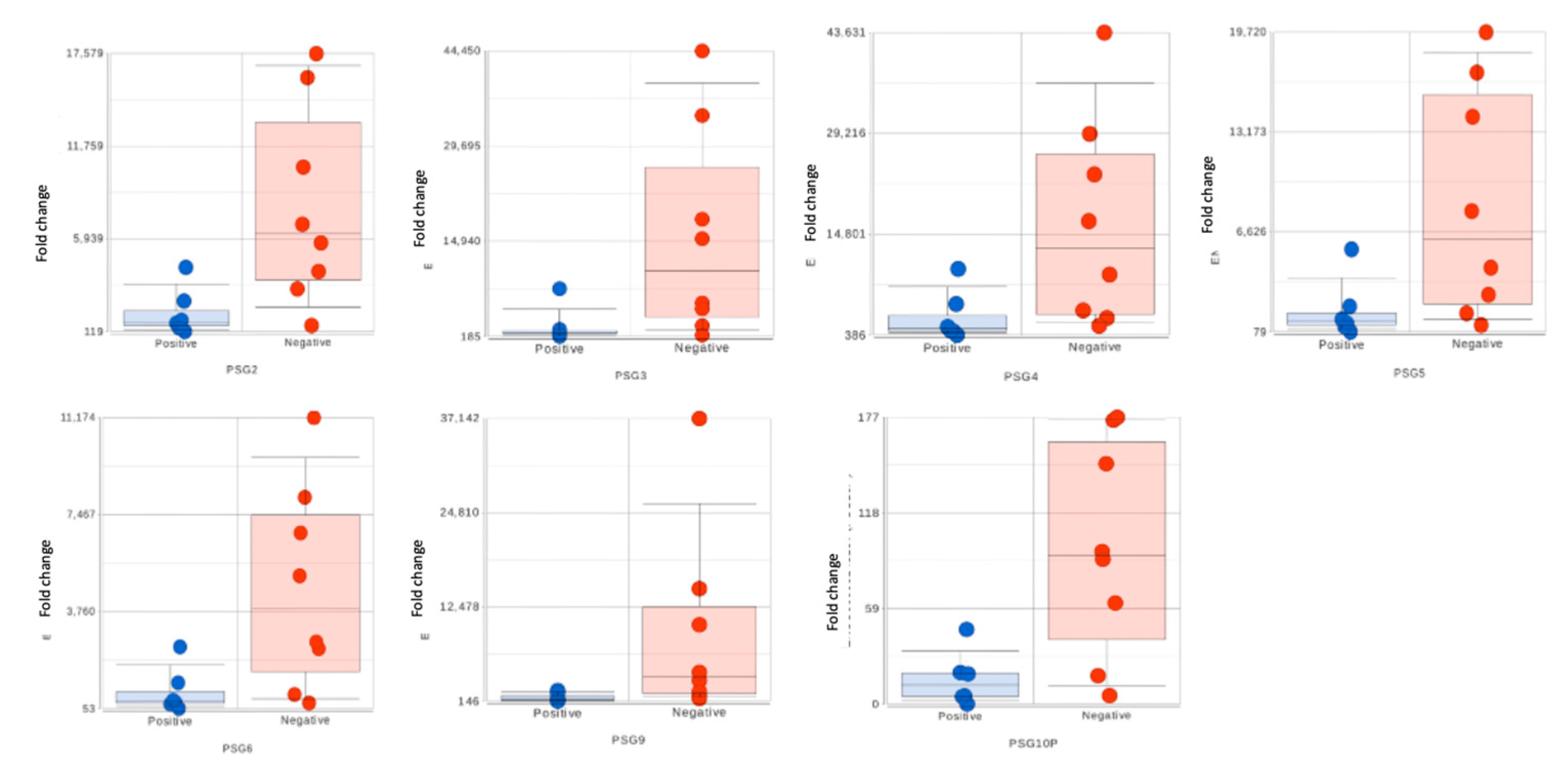
Table 2. Among the maternal DEGs, seven genes belonging to the pregnancy-specific glycoproteins (PSGs) family showed significant downregulation with active SARS-CoV-2 infection (Fold changes range -13.70 to -5.28, FDR≤0.05,
Table 3).
No significant DEG was found in the small RNA sequencing analysis between positive and negative groups on the maternal and fetal sides respectively.
2.3. Differentially expressed genes in symptomatic women
We further compared maternal placental gene expression between women symptomatic for COVID-19 at the time of delivery (n=3) with healthy women (n=9). We identified a total of 14,223 differentially regulated gene transcripts, and the complete list of these genes can be found in supplementary
Table 1. Among the DEG, PSG1 was significantly downregulated by more than 300 folds in the symptomatic cases, while IGF2 showed a substantial upregulation. Additionally, other PSG genes, including PSG3, PSG4, PSG6, PSG8, PSG9, and PSG11, were highly downregulated in the symptomatic cases. Furthermore, the expression of the inflammatory cytokine IL6 was 179 folds higher in the symptomatic patients compared to the healthy women.
2.4. Pathway enrichment analysis
2.4.1. Comparision between women with and without SARS-CoV-2 infection
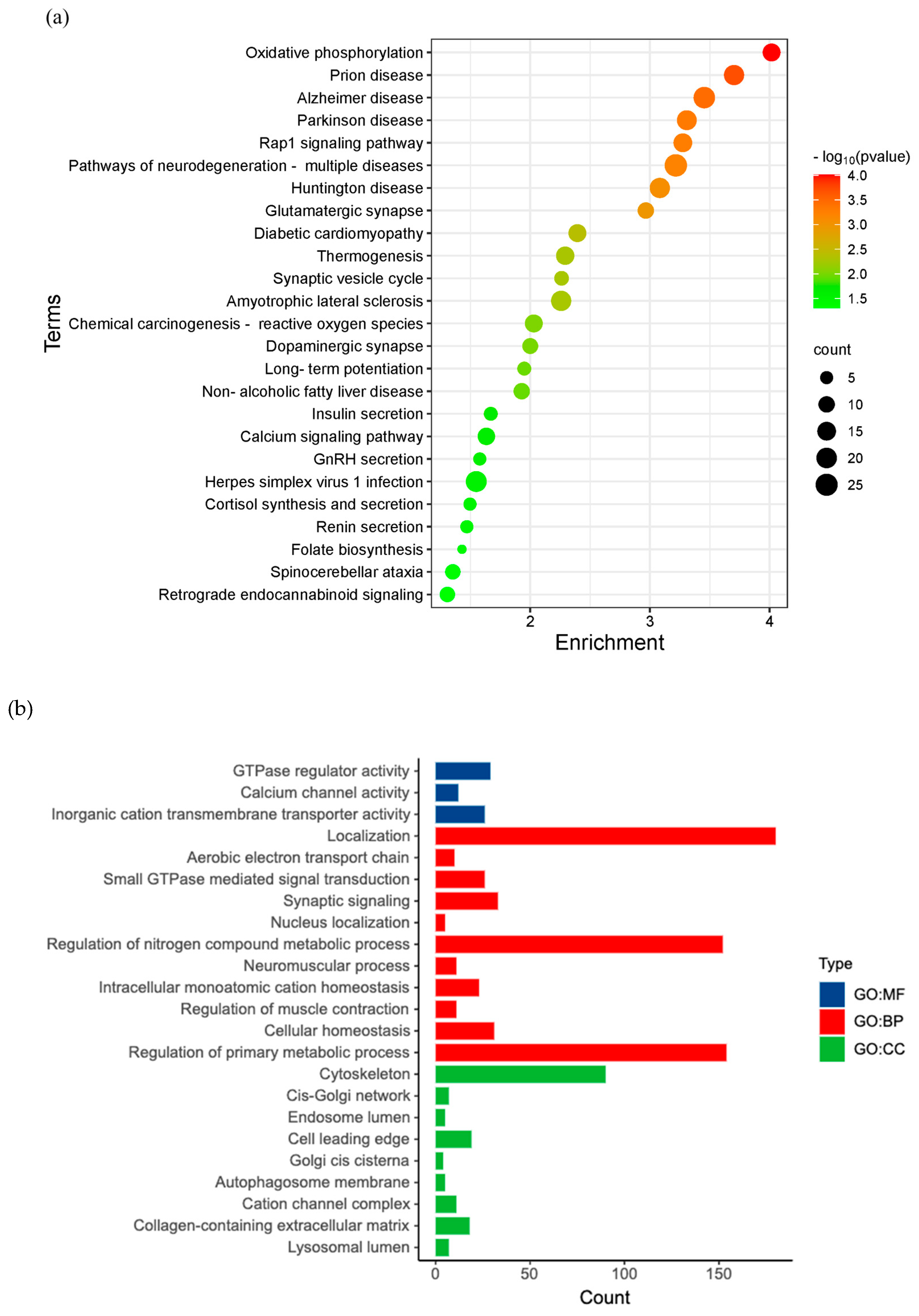
To predict the biological functions of maternal DEGs, KEGG functional enrichment analysis was performed for maternal DEGs. We observed 25 biological signaling pathways enriched with the DEG (
Figure 4a). Herpes simplex virus 1 infection signaling pathway is one of them that is relevant to viral infection in the placenta. Furthermore, the above enriched KEGG pathways were further classified into four major groups: Metabolism, environmental information processing, organismal systems, and human diseases (
Figure 4b).
2.4.2. Comparision between symptomatic women with and without SARS-CoV-2 infection
On further deep analysis, we identified several notable results with pathways enrichment analysis performed between maternal placenta of symptomatic for COVID-19 with healthy women. Please refer to
Table 3 for more detailed information on the top 10 results from the pathway enrichment analysis.
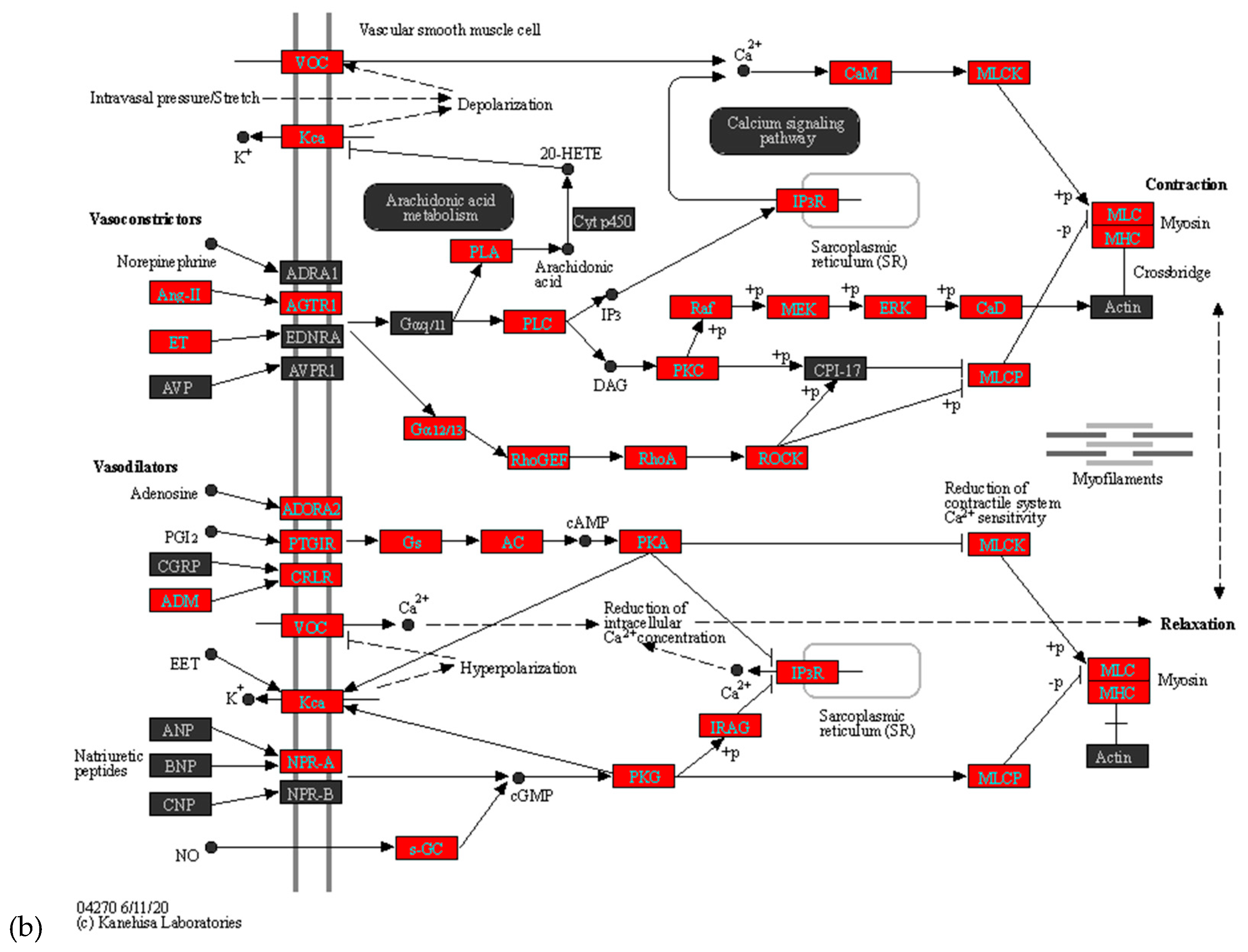
The top altered pathways in the maternal placenta of symptomatic women included Herpes simplex virus 1 infection (Supplementary Figure 1), Vascular smooth muscle contraction (
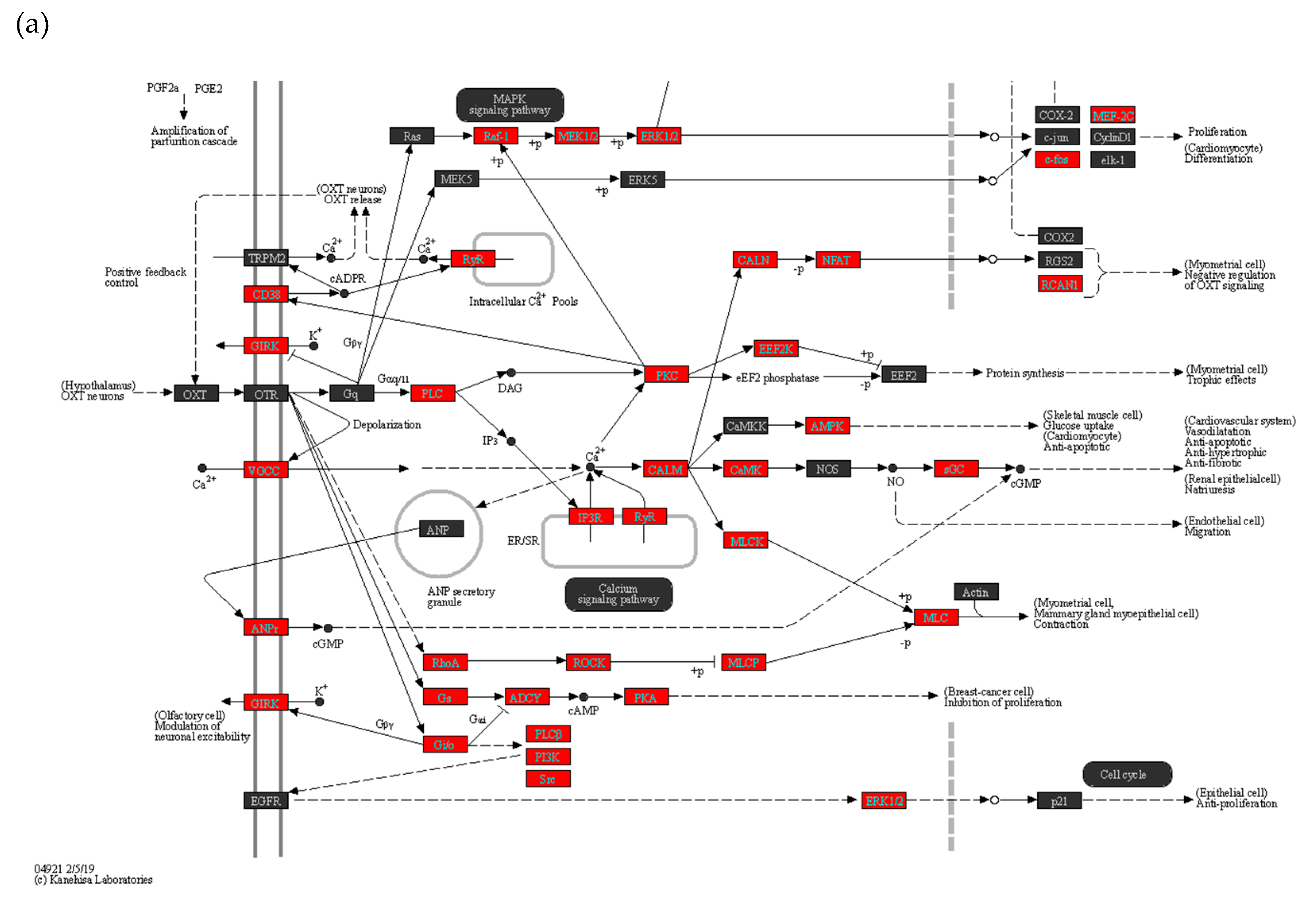
Figure 5a), and Oxytocin signaling pathways (
Figure 5b). These findings suggest potential molecular mechanisms and pathways that are influenced by COVID-19 infection during pregnancy and may contribute to pregnancy-related complications.
3. Discussion
This is the first study to investigate systematically, the impact of the SARS-CoV-2 virus on the human placenta at the level of global transcriptomics. This study was designed specifically to analyze the gene expression and pathways, separately in maternal and fetal placental compartments. The results were further validated in silico, corroborating with reported clinical symptoms. Here, we found a significant number of DEG with covid infection in the maternal compartment of the placenta, though we did not observe any change in the fetal compartment or in the expression of small RNA, in both compartments. Notably, we found elevated expression of IL6, one of the markers that is associated with cytokine storm [
12,
13]. Pathway enrichment analysis shows the DEGs in the maternal compartment are similar to other viral infections, namely herpes simplex virus, vascular smooth muscle contraction, and oxytocin signaling pathways. We also found no signs of SARS-CoV-2 viral load in the placental tissue both in symptomatic and non-symptomatic women who tested positive clinically. Thus, this study also supports that the placenta may shield the fetus from SARS-CoV-2 infection.
A meta-analysis shows that pregnant women with SARS-CoV-2 infection face a greater risk of maternal morbidity compared to those without the infection due to vascular dysfunction namely pre-eclampsia, thromboembolic disease, and hypertensive disorders of pregnancy [
14]. However, not many studies focused on the molecular alteration that occurs at different compartments of the placenta responsible for the above pathophysiological conditions with SARS-CoV-2. Here we have explored systematically the gene expression and pathways affected by SARS-CoV-2 around the time of delivery. On comparing the maternal compartment of placental gene expression between symptomatic women with healthy, we found pathways related to vascular smooth muscle contraction, growth hormone synthesis, and Oxytocin affected. This finding aligns well with the reported clinical symptoms, preeclampsia, and preterm birth [
14]. However, the women in our study did not present with any of the clinical symptoms for the above conditions. It can be speculated that this may be attributed to the low severity of the infection, in these individuals and less pronounced changes in the molecular expression that were not enough to cause clinical symptoms.
During vascular smooth muscle contraction, vasoconstrictors Ang-II and ET are upregulated, affecting arachidonic acid and Ca signaling pathways. This ultimately leads to an increase in Myosin light chain and myosin heavy chain expression[
15]. Hypoxia-related HIF-1 signaling pathway also impacts vasoconstriction with increased expression of IL6, IL6R, TLR4, IFNGR2, and HIF1A [
16]. This coincides with the reported risk of preeclampsia in pregnant women with COVID-19 [
16].
Pre-term delivery is another clinical challenge that is reported in some pregnant women with COVID-19. In our study, we observe increased expression of PLC, PKC, and EEF2K along with elevated molecules in the oxytocin pathway involved in the myometrial trophic effect in symptomatic women with COVID-19. This provides insight into the possible mechanism that may contribute to the increased risk for preterm delivery in women with COVID-19 infection during pregnancy.
The participants in our study either had mild symptoms or were asymptomatic on diagnosis of COVID-19. To gain a more specific understanding of placental gene expression in relation to the severity of the disease, we further compared placental gene expression between symptomatic for COVID-19 and healthy women. This comparative analysis revealed a significant number of DEGs that are associated with crucial molecular and functional pathways that may impact the health of pregnant women as well as the neonate. Interestingly, these changes in thee expression were reflected predominantly in the maternal compartment rather than the fetal side. A possible explanation for this could be the relatively short duration and lower severity of SARS-CoV-2 infection of these women.
The fact that the maternal immune system does not reject the trophoblasts, which are derived from the fetus, suggests that maternal adaptive immunity has been selectively suppressed. Placental-specific glycoproteins (PSGs) regulate placental immune function. Its expression in the placenta increases along with the gestational week and reaches its peak at late pregnancy [
17]. Maternal level of PSG is seen to be low in preeclampsia [
18]. Our study shows PSGs are significantly decreased with COVID-19 in the maternal placental, coinciding with the clinical report of increased risk for preeclampsia with COVID-19 [
19].
A recent report indicates with maternal COVID-19, that fetal demise tends to occur few days after the maternal infection with SARS-CoV-2, leading to the development of placental inflammatory lesions, related to the viral infection [
20]. Studies also report unique inflammatory responses in placenta initiated by SARS-CoV-2 infection can eventually lead to harmful consequences to the mother and the neonate [
21] consistent with the above, our results show high levels of inflammatory cytokines belonging to IL, TNF, TGF, IGF, and IGFBP families, though the SARS-CoV-2 virus was undetectable in both the maternal and fetal compartments the of placenta.
We could not detect notable viral load in the placenta by PCR. Considering the complexity of the human placenta, we speculate that another potential mechanism of placental resistance to the SARS-CoV-2 virus may be due to its intrinsic property with a high abundance of RNase activity. The high RNase activity in the placenta may offer dual protection against invading viruses including SARS-CoV-2 virus which is a single-stranded RNA genome [
21]. This specific characteristic reflects a more complex view of host-defense system and the protective effect of the human placenta. Interestingly, our analysis with pathway enrichment revealed Herpes simplex virus 1 infection as the top altered pathway, aligning with viral infection in general. Additionally other signaling pathways that are part of anti-viral process namely Toll-like receptor (TLR) and retinoic acid (RIG) signaling pathways that are part of early stage viral and
in utero infections are also differentially regulated [
22][
23].
Although this study has limitations with sample size, the consistency of results using different bioinformatic tools enhances the reliability of results obtained. The biological pathways derived from the in-silico analysis may require further validation with functional assays. A study with a larger number of samples with information on COVID-19 severity, duration of infection, viral load, pregnancy-related diseases, and other co-morbidities will be important as they affect the transcriptome signature of the placenta and alter the biological functions to a different extent. This information will be important in managing COVID-19 pregnant women with specific disease conditions. Additionally, more comprehensive research is required for a deeper understanding of the system in terms of specific operational characteristics of individual components. For this, a complex experimental setup and suitable model will be required. Often, RNA-seq studies are validated by qPCR. However, RNA-seq performed meticulously with stringent analysis is sufficient to identify the differentially expressed genes [
24]. Here we have utilized both FDR and the statistical test based on the Wald test making this data robust and well-aligned with the reported clinical observations. However, extended protein-based validation and functional study could be of much importance and may be of the scope of another study.
4. Materials and Methods
4.1. Study population
The study was approved by the national ethics authority (EPM) in Sweden (Dnr: 2020-01938). In total, 16 pregnant women during the 3rd trimester of pregnancy were recruited after informed consent. All participants were recruited before vaccination was available. None of the subjects were vaccinated for SARS-CoV-2. Before delivery, 7 women were positive for SARS-CoV-2 infection while 9 women were negative for SARS-CoV-2 during the 3rd trimester of pregnancy. The COVID-19 infection was tested with a nasal swab by RT-PCR. Clinical data of all positive women were collected. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the local Ethics Committee. No connection to the patients' identities was conceivable because all the data were anonymized.
4.2. Sample collection
Fresh placental tissues were collected within 30 minutes of delivery. All placenta weights were within normal range. Potential contamination with fecal in sample 10 was observed at the time of collection, therefore sample 10 was excluded. For each placenta sample, one piece of the maternal compartment (approximate dimensions of 1 cm x 0.5 cm x 0.5 cm) was dissected from the middle of the parenchyma. The amniotic membrane was removed from the fetal side and another piece of placenta tissue was cut from the fetal compartment. To remove blood the tissue cubes were washed with sterile phosphate-buffered saline (Gibco) and divided into three parts. The first part of the placental sample was placed into a cryovial and immediately snap-frozen with dry ice and stored in liquid nitrogen for further processing. The second part of the sample was put into a 2 ml cryotube with 1.5 ml RNAlater (Invitrogen) and stored at -80 C. To prepare a formalin-fixed paraffin-embedded tissue block, the third part was fixed with 4% paraformaldehyde solution (Sigma) in a 5ml Eppendorf tube for 24 hours, continued with serial dehydration by ethanol, clearing, paraffin infiltration, and embedding as previously described (ref) later stored at 4C for future experiments.
4.3. RNA extraction
The Quick-RNA Microprep Kit (Zymo Research, Catalogue No:R1050) was used to extract total RNA from snap-frozen placenta tissues following the manufacturer protocol with proteinase K treatment. Briefly, approximately 3 mg of placenta tissue was chopped into 0.2 mm3 pieces on ice and transferred to a tube with 600ul RNA lysis buffer added with proteinase K reaction mix. After incubating for one hour at room temperature, the tissue pieces in RNA lysis buffer were homogenized in a 2ml ZR BashingBead Lysis Tube (Zymo Research) using a Disruptor Genie® (Zymo Reserach) for 30 minutes. All the samples were DNAse treated to remove genomic DNA contamination in the downstream application. RNA quantification was measured by Qubit Flex Fluorometer (Invitrogen, Singapore) using Qubit high sensitivity RNA assay kit.
4.4. qPCR for SARS-CoV-2 detection
Real-time fluorescent RT-PCR kit for detecting SARS-CoV-2 (Beijing Genomics Institute, Shenzhen, China, MFG030015) was used to detect ORF1ab and N gene of SARS-CoV-2 using reverse transcription PCR. According to the manufacturer’s instructions, 10ul extracted RNA of each sample was added to the reaction system mixed with 18.5ul of reaction mix and 1.5ul enzyme mix. The total reaction volume is 30ul. RT-qPCR cycling was conducted on Bio-rad CFX Connect PCR system as follows: 50°C for 20 minutes, 95°C for 5 minutes, then 45 cycles of 15 seconds at 95°C and 30 seconds at 60°C. The testing result is interpreted as the specimen is positive if standard curves are in S-shape with cycle threshold (Ct) values ≤40. The specimen is negative if standard curves are not in S-shape with no Ct values or Ct > 40. The BGI assay has a limit of detection of 100 copies/ml.
4.5. mRNA library preparation and sequencing
DNA libraries for next generation sequencing were constructed from all the placental samples RNA using Smart-seq2 protocol [
25] with 30 ng of total RNA for each sample. Enzymatic fragmentation and tagmentation of DNA was performed using the Nextera XT kit (Illumina Inc, USA) along with IDT® for Illumina® DNA Unique Dual Index barcodes. The final amplified libraries were purified with AMPure XP beads in ratio of 1:1 (Sample versus beads). The final cDNA library was quantified by Qubit 1X HS DNA assay kit (Invitrogen) and quality of the libraries were analysed by the 2100 Bioanalyzer system (Agilent) using a high sensitivity DNA chip (Agilent). An equimolar concentration of 5 ng from each library was pooled. Sequencing was performed on the Illumina NovaSeq 6000 sequencing platform (Novogene, UK), with 2 x 150 bp read set up .
4.6. Small RNA library preparation and sequencing
Small RNA transcriptome for all the placental samples was constructed as described in previously published protocol [
26] with 5 ng per sample of total RNA. The amplified libraries were purified with AMPure XP beads to samples in a 1:1 ratio. Quantification of the amplified libraries was performed using Qubit Flex Fluorometer (Invitrogen) and the Qubit 1X dsDNA high sensitivity kit (Invitrogen). The 2100 Bioanalyzer system (Agilent) and the high sensitivity DNA chip (Agilent) were used to check the small RNA library quality. 5ng of library from each sample was pooled and sequenced on the Illumina NovaSeq 6000 sequencing platform (Novogene, UK) with 150 bp read setup.
4.7. Sequencing data processing and bioinformatic analysis
4.7.1. mRNA data analysis
The raw sequencing data processing and analysis for both mRNA and small RNA transcriptome was conducted using the Partek® Flow® platform (version 10.0.22.1005). A raw data quality check was performed using FastQC (version 0.11.9) toolkit in Partek® Flow® platform. Briefly, for mRNA sequencing, the Nextera adapters were trimmed, and the trimmed reads were aligned to hg38 using STAR aligner with default parameters. 60% of alignment rate was taken as the cutoff value. After alignment, sample 8F, 13F were removed due to low alignment rate (8F 57.29%, 13F 39.44%). The aligned reads were quantified to Ensembl Transcripts release 107. Mitochondrial and ribosomal reads were filtered out afterwards and genes having a count of <1 in at least 80% of the samples were filtered out. For the downstream analysis, we split the data by placental surface (maternal and fetal). On the maternal side, 6 samples from SARS-CoV-2 positive group and 8 samples from SARS-CoV-2 negative group were included. On the fetal side, 6 samples from SARS-CoV-2 positive group and 7 samples from SARS-CoV-2 negative group were included in the analysis. Differentially expression analysis on filtered counts was performed using DESeq2 tool (version 3.5). We excluded low expressed genes in all samples that have less than 10 counts. A false discovery rate (FDR) of ≤0.05 and fold change (FC) of < -2 or >2 were considered as statistically significant. KEGG (Kyoto Encyclopedia of Genes and Genomes) database [
27]was used for DEG’s to perform pathway analysis. P<0.05 was considered significant for enriched biological signaling pathways.
4.7.2. Small RNA data analysis
The raw FASTQ files were quality checked using FastQC (Version 0.11.9) followed by trimming of standard Illumina adapters and 3’ adapters. The UMI’s were removed and appended to the read header for later analysis. The trimmed reads were aligned to human genome 38 (hg38) using Bowtie 2 tool (version 2.2.5) with default parameters and followed by UMI deduplication. The reads were quantified to Ensembl transcripts release 105 and miRbase v.22. and reads having a value <1 were filtered out. Differential analysis was performed on the filtered data using DESeq 2 tool (version 3.5) and the following criteria was used for statistically significant differentially expressed genes, FDR ≤0.05 and fold change of < -2 or >2. Differential analysis is performed independently for each placental surface comparing Covid positive to negative group.
5. Conclusions
In summary, this study provides further evidence that SARS-CoV-2 infection with mild symptoms may not result in detectable placental virus load, in both the maternal and fetal compartments. This further supports the belief that mild infection is unlikely to transmit the virus during late pregnancy. More importantly, this study sheds light on the possible molecular regulation and pathways associated with increased clinical risk of preeclampsia and preterm birth with COVID-19 during pregnancy. The findings from this study offer valuable insights that could be further explored clinically, in managing pregnant women with COVID-19.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: COVID infection mimics pathways relevant to other viral infection;
Table 1: Supplementary table 1: Differentially expressed material placental genes in symptomatic women compared with healthy women.
Author Contributions
Conceptualization, P.G.L.L. and K.G.D.; Methodology, P.G.L.L, N.R.B and Y.T; software, N.R.B., P.G.L.L and Y.T; formal analysis, P.G.L.L., N.R.B.; and Y.T.; investigation, P.G.L.L., N.R.B. and Y.T.; resources, P.G.L.L., K.G.D.; and A.A.; data curation, P.G.L.L., N.R.B. and Y.T.; writing—original draft preparation, Y.T.; writing—review and editing, P.G.L.L., N.R.B. and K.G.D; visualization, Y.T.; supervision, P.G.L.L. and K.G.D.; project administration, P.G.L.L. and K.G.D.; funding acquisition, P.G.L.L. and K.G.D.; All authors have read and agreed to the published version of the manuscript.
Funding
This research study was supported by Swedish Research Council (VR) 2021-01042 and ALF project grant, Region Stockholm/KI. Salary for doctoral education of YT was supported by MATER project - European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No.813707.
Institutional Review Board Statement
Karolinska Institutet independent ethics committee (2020-01938).
Informed Consent Statement
These placentas were anonymous samples.
Data Availability Statement
Acknowledgments
We acknowledge the skillful technical assistance of qPCR test provided by Ali Rihani, Dorina Ujvari, and Jessica Alm from National Pandemic Center (Sweden) and the clinical staff at the delivery ward at Karolinska University Hospital.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study design, sample collection, analysis and interpretation of data and manuscript writing.
References
- Seymen, C.M. Being Pregnant in the COVID-19 Pandemic: Effects on the Placenta in All Aspects. J Med Virol 2021, 93, 2769–2773. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Read, J.S.; Jamieson, D.J. Pregnancy and Infection. N Engl J Med 2014, 370, 2211. [Google Scholar] [CrossRef] [PubMed]
- Simbar, M.; Nazarpour, S.; Sheidaei, A. Evaluation of Pregnancy Outcomes in Mothers with COVID-19 Infection: A Systematic Review and Meta-Analysis. J Obstet Gynaecol 2023, 43, 2162867. [Google Scholar] [CrossRef] [PubMed]
- Taglauer, E.; Benarroch, Y.; Rop, K.; Barnett, E.; Sabharwal, V.; Yarrington, C.; Wachman, E.M. Consistent Localization of SARS-CoV-2 Spike Glycoprotein and ACE2 over TMPRSS2 Predominance in Placental Villi of 15 COVID-19 Positive Maternal-Fetal Dyads. Placenta 2020, 100, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhou, B.; Zhang, L.; Balaji, K.S.; Wei, C.; Liu, X.; Chen, H.; Peng, J.; Fu, J. Expressions and Significances of the Angiotensin-Converting Enzyme 2 Gene, the Receptor of SARS-CoV-2 for COVID-19. Mol Biol Rep 2020, 47, 4383–4392. [Google Scholar] [CrossRef]
- Ace, F.; Ouyang, Y.; Bagalkot, T.; Fitzgerald, W.; Sadovsky, E.; Chu, T.; Martínez-Marchal, A.; Brieño-Enríquez, M.; Su, E.J.; Margolis, L.; et al. Term Human Placental Trophoblasts Express SARS-CoV-2 Entry. mSphere 2021, 6, 1–14. [Google Scholar] [CrossRef]
- Kreis, N.-N.; Ritter, A.; Louwen, F.; Yuan, J. A Message from the Human Placenta: Structural and Immunomodulatory Defense against SARS-CoV-2. Cells 2020, 9, 1–24. [Google Scholar] [CrossRef]
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.R.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 Vertical Transmission during Pregnancy. Nat Commun 2020, 11. [Google Scholar] [CrossRef]
- Sharps, M.C.; Hayes, D.J.L.; Lee, S.; Zou, Z.; Brady, C.A.; Almoghrabi, Y.; Kerby, A.; Tamber, K.K.; Jones, C.J.; Adams Waldorf, K.M.; et al. A Structured Review of Placental Morphology and Histopathological Lesions Associated with SARS-CoV-2 Infection. Placenta 2020, 101, 13–29. [Google Scholar] [CrossRef]
- Takada, K.; Shimodai-Yamada, S.; Suzuki, M.; Trinh, Q.D.; Takano, C.; Kawakami, K.; Asai-Sato, M.; Komatsu, A.; Okahashi, A.; Nagano, N.; et al. Restriction of SARS-CoV-2 Replication in the Human Placenta. Placenta 2022, 127, 73–76. [Google Scholar] [CrossRef]
- Brogden A K, Guthmiller M J, T.C.E. Society for Maternal-Fetal Medicine Special Statement: COVID-19 Research in Pregnancy: Progress and Potential. Ann Oncol 2020, 2–5.
- Santa Cruz, A.; Mendes-Frias, A.; Oliveira, A.I.; Dias, L.; Matos, A.R.; Carvalho, A.; Capela, C.; Pedrosa, J.; Castro, A.G.; Silvestre, R. Interleukin-6 Is a Biomarker for the Development of Fatal Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia. Front Immunol 2021, 12. [Google Scholar] [CrossRef]
- Chen, L.Y.C.; Hoiland, R.L.; Stukas, S.; Wellington, C.L.; Sekhon, M.S. Assessing the Importance of Interleukin-6 in COVID-19. Lancet Respir Med 2021, 9, e13. [Google Scholar] [CrossRef]
- Smith, E.R.; Oakley, E.; Grandner, G.W.; Ferguson, K.; Farooq, F.; Afshar, Y.; Ahlberg, M.; Ahmadzia, H.; Akelo, V.; Aldrovandi, G.; et al. Adverse Maternal, Fetal, and Newborn Outcomes among Pregnant Women with SARS-CoV-2 Infection: An Individual Participant Data Meta-Analysis. BMJ Glob Health 2023, 8, e009495. [Google Scholar] [CrossRef]
- Liu, Z.; Khalil, R.A. Evolving Mechanisms of Vascular Smooth Muscle Contraction Highlight Key Targets in Vascular Disease. Biochem Pharmacol 2018, 153, 91–122. [Google Scholar] [CrossRef]
- Taggart, M.J.; Wray, S. Hypoxia and Smooth Muscle Function: Key Regulatory Events during Metabolic Stress. J Physiol 1998, 509, 315–325. [Google Scholar] [CrossRef]
- Blois, S.M.; Sulkowski, G.; Tirado-González, I.; Warren, J.; Freitag, N.; Klapp, B.F.; Rifkin, D.; Fuss, I.; Strober, W.; Dveksler, G.S. Pregnancy-Specific Glycoprotein 1 (PSG1) Activates TGF-β and Prevents Dextran Sodium Sulfate (DSS)-Induced Colitis in Mice. Mucosal Immunology 2014 7:2 2013, 7, 348–358. [Google Scholar] [CrossRef]
- Temur, M.; Serpim, G.; Tuzluoğlu, S.; Taşgöz, F.N.; Şahin, E.; Üstünyurt, E. Comparison of Serum Human Pregnancy-Specific Beta-1-Glycoprotein 1 Levels in Pregnant Women with or without Preeclampsia. 2019, 40, 1074–1078. [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; Do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr 2021, 175, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Alcover, N.; Regiroli, G.; Benachi, A.; Vauloup-fellous, C.; Vivanti, A.J.; De luca, D. Systematic Review and Synthesis of Stillbirths and Late Miscarriages Following SARS-CoV-2 Infections. Am J Obstet Gynecol 2023. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.; Nicol, A.; Nuovo, G.J. The Differential Expression of Toll like Receptors and RIG-1 in the Placenta of Neonates with in Utero Infections. Ann Diagn Pathol 2023, 62, 152080. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sato, S.; Sotoyama, Y.; Orba, Y.; Sawa, H.; Yamauchi, H.; Sasaki, M.; Takaoka, A. RIG-I Triggers a Signaling-Abortive Anti-SARS-CoV-2 Defense in Human Lung Cells. Nat Immunol 2021, 22, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Cui, X. Design and Validation Issues in RNA-Seq Experiments. Brief Bioinform 2011, 12, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Picelli, S.; Faridani, O.R. ; Bj, sa K.; Winberg, sta; Sagasser, S.; Sandberg, R. Full-Length RNA-Seq from Single Cells Using Smart-Seq2. 2013. [CrossRef]
- Hagemann-Jensen, M.; Abdullayev, I.; Sandberg, R.; Faridani, O.R. Small-Seq for Single-Cell Small-RNA Sequencing. Nat Protoc 2018, 13, 2407–2424. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a Reference Resource for Gene and Protein Annotation. Nucleic Acids Res 2016, 44, D457–D462. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).