Submitted:
30 May 2023
Posted:
30 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction

2. Characteristics and functionalities of edible films and coatings
- a)
- interactions with the food texture and surfaces,
- b)
- ageing and prolonged performance with the shelf life of the food in contact,
- c)
- changes in flavor, color, and texture of food due to the interactions with edible films and coatings,
- d)
- response and sensitivity under storage/environmental conditions, and
- e)
- processing conditions, including temperature, color and thickness.
2.1. Key functionalities of edible films and coatings
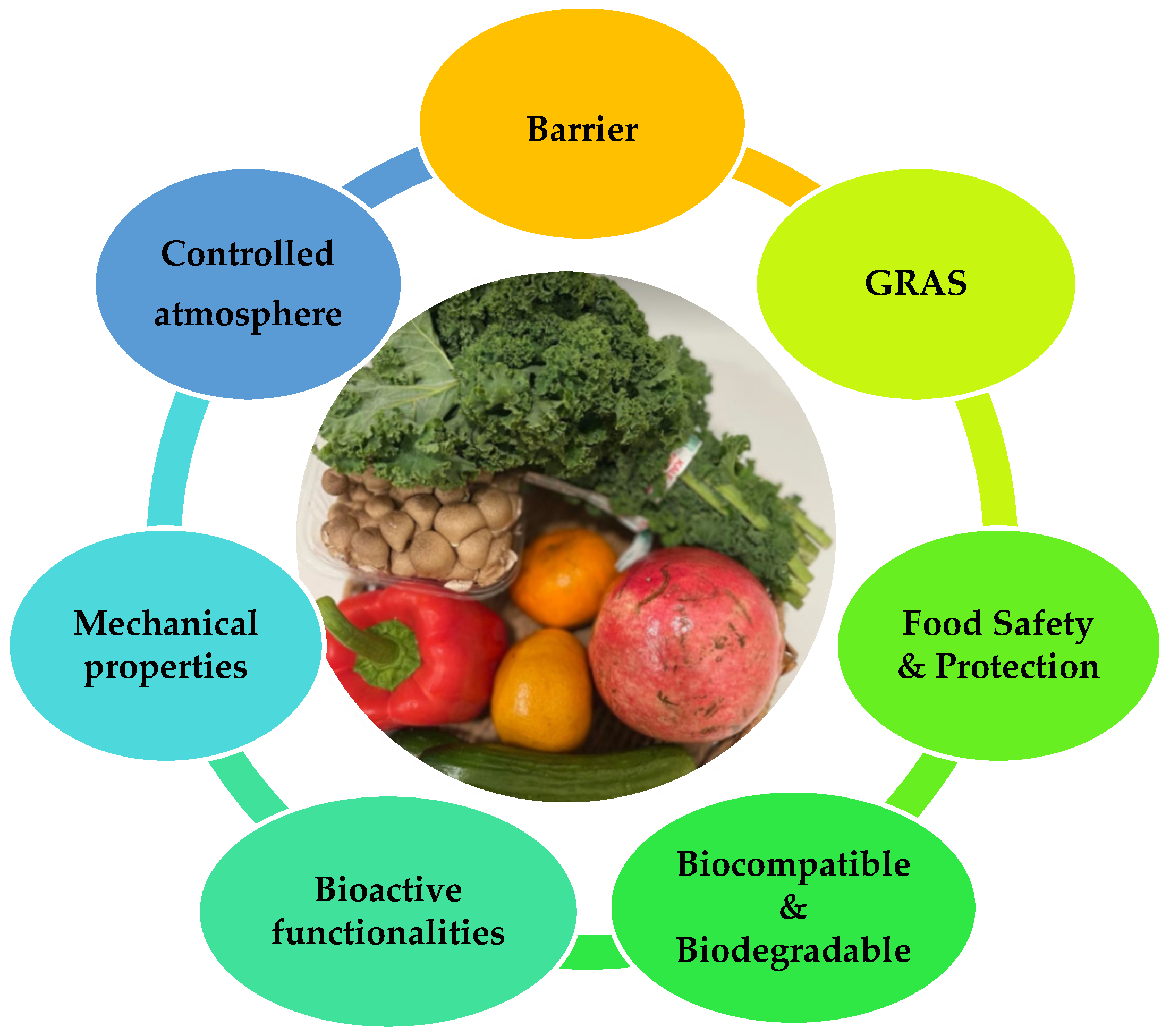
- Protection from transport, handling, mechanical damages, and UV radiation
-
Barrier properties:
- a)
- Moisture barrier: minimize water vapor transmission to prevent dehydration.
- b)
- Gas barrier: control oxygen and carbon dioxide levels pass through the protective layer
- c)
- Volatile organic compounds (VOC) barrier: protection against organic vapors such as aromas and solvents and other additives and pigments.
- Prolong shelf-life
-
Bioactivity: show antimicrobial and antifungal properties and acts as probiotics.
- Biodegradability
- Structural integrity: melt above 40 ̊ C without decomposition, water resistant, easily emulsifiable, non-sticky, or non-tacky, and deliver efficient drying.
- Maintain food quality: minimal influence on texture, flavor, or color
- Formulated from economical, relatively abundant, consumer safety GRAS materials
2.2. Common preparation methods of edible films and coatings
2.2.1. Melt extrusion method
2.2.2. Solvent casting method
3. Types of edible films and coatings and their structural and chemical significance
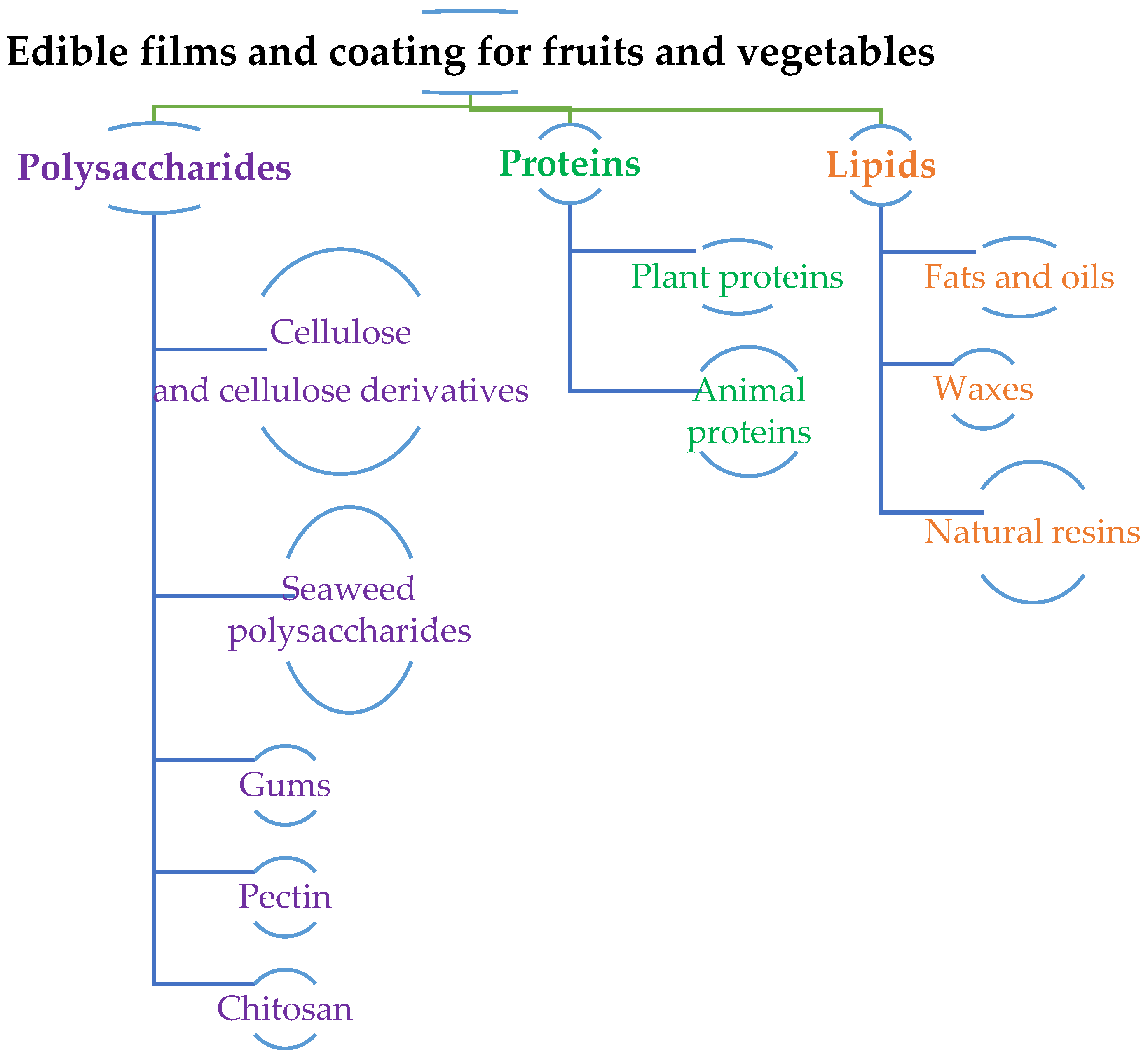
3.1. Hydrocolloids
3.2. Lipid Colloids
3.3. Composites
4. Polysaccharide based edible films and coatings
4.1. Cellulose and cellulosic derivatives
4.2. Pectin
4.3. Chitin and Chitosan
4.4. Gums
4.5. Starch
5. Protein-based edible films and coatings
5.1. Animal proteins – casein
5.2. Animal proteins – whey protein
5.3. Animal proteins – collagen
5.4. Animal proteins – Gelatin
5.5. Plant proteins- Soy and wheat proteins
6. Lipid-based edible films and coatings
| Lipids | Sources | Reference |
|---|---|---|
| Fats, oils, shortening and margarine | a) Various native fats and oil from animals, vegetables, and seeds Such as butter from dairy, lard, sunflower, mustered, olive, almond, peanut, coconut, palm, cocoa, etc b) Fractionated, concentrated, or reconstituted oils and fats ,and mono, di and tri-glycerides c) Hydrogenated or trans-esterified oils: Margarine and shortening |
[264] |
| Waxes | a) Waxes from natural animals, insects and vegetables: Beeswax, carnauba wax, candelilla wax, genuine rice bran wax, and laurel wax b) Waxes from synthetic sources; paraffin, mineral, microcrystalline, oxidized-non-oxidized polyethylene wax |
[265] |
| Natural resins | Resins from natural sources: Asafoetida, Benjoin, Chicle, Guarana, Myrrhe, oblibanum, Opponax, Sandaraque, etc | [266] |
| Essential oils and liquorices | Various essential oils from an extract from flowers, vegetables, animals and fruits; Citrus, rose, ginger, mint, etc | [267] |
6.1. Essential oil
6.2. Waxes and resins
7. Health Effects
8. Conclusion and Current Trends
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Food wastage footprint: Impacts on natural resources, FAO. 2013.
- Liu, C.; Hotta, Y.; Santo, A.; Hengesbaugh, M.; Watabe, A.; Totoki, Y.; Allen, D.; Bengtsson, M. Food waste in Japan: Trends, current practices and key challenges. Journal of Cleaner Production 2016, 133, 557–564. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global food losses and food waste. 2011.
- FAO. Food Wastage Footprint: Impacts on Natural Resources, Summary Report 2013. 1998.
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Environmental impacts of food production. Our world in data 2020. [Google Scholar]
- Racz, A.; Vasiljev Marchesi, V.; Crnković, I. Economical, environmental and ethical impact of food wastage in hospitality and other global industries. Jahr: Europski časopis za bioetiku 2018, 9, 25–42. [Google Scholar] [CrossRef]
- FAO, F. Food wastage footprint full-cost accounting. 2014.
- Commerce, T.B.o.E.A.o.t.U.S.D.o. Gross Domestic Product, Fourth Quarter and Year 2021 (Second Estimate). 2022.
- United Nations Environment Programme. Think, Eat, Save. Reduce your Footprint. 2021.
- Assembly, U.N.G. Transforming our world: the 2030 Agenda for Sustainable Development 2015.
- Xue, L.; Liu, G. 1 - Introduction to global food losses and food waste. In Saving Food; Galanakis, C.M., Ed.; Academic Press, 2019; pp. 1–31. [Google Scholar]
- Xue, L.; Liu, G.; Parfitt, J.; Liu, X.; Van Herpen, E.; Stenmarck, Å.; O’Connor, C.; Östergren, K.; Cheng, S. Missing Food, Missing Data? A Critical Review of Global Food Losses and Food Waste Data. Environmental Science & Technology 2017, 51, 6618–6633. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Nutritional and environmental losses embedded in global food waste. Resources, Conservation and Recycling 2020, 160, 104912. [Google Scholar] [CrossRef]
- Blakeney, M. Food Loss and Food Waste: Causes and Solutions; Edward Elgar Publishing.
- Gustafsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The methodology of the FAO study: Global Food Losses and Food Waste-extent, causes and prevention”-FAO, 2011. 2013.
- Sachdeva, S.; Sachdev, T.R.; Sachdeva, R. Increasing fruit and vegetable consumption: challenges and opportunities. Indian journal of community medicine: official publication of Indian Association of Preventive & Social Medicine 2013, 38, 192. [Google Scholar]
- Southon, S.; Faulks, R. Health benefits of increased fruit and vegetable consumption. Fruit and vegetable processing: Improving quality 2002, 1, 2–20. [Google Scholar]
- FAO. International Year of Fruits and Vegetables 2021. Global Action Plan. 2021.
- Morone, P.; Koutinas, A.; Gathergood, N.; Arshadi, M.; Matharu, A. Food waste: Challenges and opportunities for enhancing the emerging bio-economy. Journal of Cleaner Production 2019, 221, 10–16. [Google Scholar] [CrossRef]
- Lintas, C. Nutritional aspects of fruits and vegetables consumption. Options Mediterraennes 1992, 19, 79–87. [Google Scholar]
- Embuscado, M.E.; Huber, K.C. Edible films and coatings for food applications; Springer, 2009; Volume 9. [Google Scholar]
- Baldwin, E.A.; Hagenmaier, R.; Bai, J.; Krochta, J.M. Edible Coatings and Films to Improve Food Quality; Taylor & Francis, 1994. [Google Scholar]
- Rossman, J.M. Commercial Manufacture of Edible Films. In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer New York: New York, NY, 2009; pp. 367–390. [Google Scholar]
- Administration, U.S.F.a.D. CFR - Code of Federal Regulations Title 21. 2022.
- Administration, U.S.F.a.D. Generally Recognized as Safe (GRAS). 2019.
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Davis, G.; Song, J. Biodegradable packaging based on raw materials from crops and their impact on waste management. Industrial crops and products 2006, 23, 147–161. [Google Scholar] [CrossRef]
- Bourtoom, T. Edible films and coatings: characteristics and properties. International food research journal 2008, 15, 237–248. [Google Scholar]
- Guimaraes, A.; Abrunhosa, L.; Pastrana, L.M.; Cerqueira, M.A. Edible films and coatings as carriers of living microorganisms: A new strategy towards biopreservation and healthier foods. Comprehensive Reviews in Food Science and Food Safety 2018, 17, 594–614. [Google Scholar] [CrossRef] [PubMed]
- Olivas, G.I.I.; Barbosa-Cánovas, G. Edible Films and Coatings for Fruits and Vegetables. In Edible Films and Coatings for Food Applications, Huber, K.C., Embuscado, M.E., Eds.; Springer New York: New York, NY, 2009; pp. 211–244. [Google Scholar]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible Films and Coatings as Food-Quality Preservers: An Overview. Foods 2021, 10. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: a review. Crit Rev Food Sci Nutr 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Hernandez-Izquierdo, V.; Krochta, J. Thermoplastic processing of proteins for film formation—a review. Journal of food science 2008, 73, R30–R39. [Google Scholar] [CrossRef]
- Rodríguez-Castellanos, W.; Martínez-Bustos, F.; Rodrigue, D.; Trujillo-Barragán, M. Extrusion blow molding of a starch–gelatin polymer matrix reinforced with cellulose. European Polymer Journal 2015, 73, 335–343. [Google Scholar] [CrossRef]
- Dahiya, M.; Saha, S.; Shahiwala, A.F. A review on mouth dissolving films. Current drug delivery 2009, 6, 469–476. [Google Scholar] [CrossRef]
- Pavlath, A.E.; Orts, W. Edible films and coatings: why, what, and how? In Edible films and coatings for food applications; Springer, 2009; pp. 1–23. [Google Scholar]
- Mellinas, C.; Valdés, A.; Ramos, M.; Burgos, N.; Garrigos, M.d.C.; Jiménez, A. Active edible films: Current state and future trends. Journal of Applied Polymer Science 2016, 133. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends in Food Science & Technology 2015, 45, 273–283. [Google Scholar]
- Bhattacharya, T. TECHNIQUES OF PREPARING EDIBLE PROTEIN FILMS. Asian Journal of Science and Technology 2013, 4, 39–41. [Google Scholar]
- Safaya, M.; Rotliwala, Y. Nanoemulsions: A review on low energy formulation methods, characterization, applications and optimization technique. Materials Today: Proceedings 2020, 27, 454–459. [Google Scholar] [CrossRef]
- Gutoff, E.B.; Cohen, E.D. Water-and solvent-based coating technology. In Multilayer flexible packaging; Elsevier, 2016; pp. 205–234. [Google Scholar]
- Wang, Q. , Chen, W., Zhu, W. et al. A review of multilayer and composite films and coatings for active biodegradable packaging. npj Sci Food 2022, 6, 18. [Google Scholar] [CrossRef]
- Gupta, V.; Biswas, D.; Roy, S. A Comprehensive Review of Biodegradable Polymer-Based Films and Coatings and Their Food Packaging Applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocolloids 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Li, J.-M.; Nie, S.-P. The functional and nutritional aspects of hydrocolloids in foods. Food Hydrocolloids 2016, 53, 46–61. [Google Scholar] [CrossRef]
- Phillips, G.O.; Williams, P.A. Handbook of hydrocolloids; Elsevier, 2009. [Google Scholar]
- Nishinari, K.; Zhang, H.; Ikeda, S. Hydrocolloid gels of polysaccharides and proteins. Current opinion in colloid & interface science 2000, 5, 195–201. [Google Scholar]
- Goff, H.D.; Guo, Q. Chapter 1 The Role of Hydrocolloids in the Development of Food Structure. In Handbook of Food Structure Development; The Royal Society of Chemistry, 2020; pp. 1–28. [Google Scholar]
- Godoi, F.C.; Ningtyas, D.W.; Geoffroy, Z.; Prakash, S. Protein-based hydrocolloids: Effect on the particle size distribution, tribo-rheological behaviour and mouthfeel characteristics of low-fat chocolate flavoured milk. Food Hydrocolloids 2021, 115, 106628. [Google Scholar] [CrossRef]
- Yemenicioğlu, A.; Farris, S.; Turkyilmaz, M.; Gulec, S. A review of current and future food applications of natural hydrocolloids. International Journal of Food Science & Technology 2020, 55, 1389–1406. [Google Scholar]
- Kristl, J.; Šmid-Korbar, J.; Štruc, E.; Schara, M.; Rupprecht, H. Hydrocolloids and gels of chitosan as drug carriers. International Journal of Pharmaceutics 1993, 99, 13–19. [Google Scholar] [CrossRef]
- Wüstenberg, T. Cellulose and cellulose derivatives in the food industry: fundamentals and applications; John Wiley & Sons, 2014. [Google Scholar]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, characterisation, and application of pectin from tropical and sub-tropical fruits: a review. Food Reviews International 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Chen, J.; Liu, W.; Liu, C.-M.; Li, T.; Liang, R.-H.; Luo, S.-J. Pectin modifications: a review. Critical reviews in food science and nutrition 2015, 55, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.B. Structure and function of polysaccharide gum-based edible films and coatings. In Edible films and coatings for food applications; Springer, 2009; pp. 57–112. [Google Scholar]
- Alsabagh, A.; Abdou, M.; Khalil, A.; Ahmed, H.; Aboulrous, A. Investigation of some locally water-soluble natural polymers as circulation loss control agents during oil fields drilling. Egyptian Journal of Petroleum 2014, 23, 27–34. [Google Scholar] [CrossRef]
- Marcotte, M.; Hoshahili, A.R.T.; Ramaswamy, H. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Research International 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Kester, J.J.; Fennema, O. Edible films and coatings: a review. Food technology (USA) 1986. [Google Scholar]
- Bunjes, H. Structural properties of solid lipid based colloidal drug delivery systems. Current Opinion in Colloid & Interface Science 2011, 16, 405–411. [Google Scholar]
- Gordillo-Galeano, A.; Ponce, A.; Mora-Huertas, C.E. Surface structural characteristics of some colloidal lipid systems used in pharmaceutics. Journal of Drug Delivery Science and Technology 2021, 62, 102345. [Google Scholar] [CrossRef]
- Heurtault, B.; Saulnier, P.; Pech, B.; Proust, J.-E.; Benoit, J.-P. Physico-chemical stability of colloidal lipid particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef]
- Patel, A.R.; Velikov, K.P. Colloidal delivery systems in foods: A general comparison with oral drug delivery. LWT - Food Science and Technology 2011, 44, 1958–1964. [Google Scholar] [CrossRef]
- Wilde, P.J.; Chu, B.S. Interfacial & colloidal aspects of lipid digestion. Advances in Colloid and Interface Science 2011, 165, 14–22. [Google Scholar] [PubMed]
- Bourtoom, T.; Chinnan, M. Improvement of water barrier property of rice starch-chitosan composite film incorporated with lipids. Food Science and Technology International 2009, 15, 149–158. [Google Scholar] [CrossRef]
- Ajesh K.V; Hasan, M.; Shukadev, M.; Pravitha, M, Deepak Kumar Verma, Prem Prakash Srivastav,Trends in Edible Packaging Films and its Prospective Future in Food: A Review, Applied Food Research 2022,2(1),100118.
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, T.J. Surface and nutraceutical properties of edible films made from starchy sources with and without added blackberry pulp. Carbohydrate Polymers 2017, 165, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, C.V.; Sarkar, P. Composite edible films and coatings from food-grade biopolymers. Journal of Food Science and Technology 2018, 55, 4369–4383. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Chang, X.; Ding, Z.; Xu, H.; Kong, H.; Chen, F.; Wang, R.; Shan, Y.; Ding, S. Fabrication and Characterization of Eco-Friendly Polyelectrolyte Bilayer Films Based on Chitosan and Different Types of Edible Citrus Pectin. Foods 2022, 11, 3536. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, W.; Teng, A.; Zhang, K.; Ma, Y.; Duan, S.; Li, S.; Guo, Y. Using cellulose nanofibers to reinforce polysaccharide films: Blending vs layer-by-layer casting. Carbohydr. Polym. 2020, 227, 115264. [Google Scholar] [CrossRef]
- Gamage, A.; Thiviya, P.; Mani, S.; Ponnusamy, P.G.; Manamperi, A.; Evon, P.; Merah, O.; Madhujith, T. Environmental Properties and Applications of Biodegradable Starch-Based Nanocomposites. Polymers 2022, 14, 4578. [Google Scholar] [CrossRef]
- Wang, B.; Yan, L.; Guo, S.; Wen, L.; Yu, M.; Feng, L.; Jia, X. Structural Elucidation, Modification, and Structure-Activity Relationship of Polysaccharides in Chinese Herbs: A Review. Front. Nutr. 2022, 9, 08175. [Google Scholar] [CrossRef]
- Guo, M.Q.; Hu, X.; Wang, C.; Ai, L. Polysaccharides: structure and solubility. Solubility of polysaccharides 2017, 2, 8–21. [Google Scholar]
- Ross-Murphy, S.; Shatwell, K. Polysaccharide strong and weak gels. Biorheology 1993, 30, 217–227. [Google Scholar] [CrossRef]
- Burchard, W. Structure formation by polysaccharides in concentrated solution. Biomacromolecules 2001, 2, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Wettstein, S.G.; Alonso, D.M.; Gürbüz, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Current Opinion in Chemical Engineering 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Knauf, M.; Moniruzzaman, M. Lignocellulosic biomass processing: A perspective. International sugar journal 2004, 106, 147–150. [Google Scholar]
- Tang, X.; Zuo, M.; Li, Z.; Liu, H.; Xiong, C.; Zeng, X.; Sun, Y.; Hu, L.; Liu, S.; Lei, T. Green processing of lignocellulosic biomass and its derivatives in deep eutectic solvents. Chem. Sus. Chem. 2017, 10, 2696–2706. [Google Scholar] [CrossRef]
- Nishinari, K.; Zhang, H.; Ikeda, S. Hydrocolloid gels of polysaccharides and proteins. Current Opinion in Colloid & Interface Science 2000, 5, 195–201. [Google Scholar]
- Kester, J.J.; Fennema, O. Edible films and coatings: a review. Food technology (USA) 1986.
- Dhaka, R.; Upadhyay, A. Edible films and coatings: a brief overview. The Pharma Innovation Journal 2018, 7, 331–333. [Google Scholar]
- Lacroix, M.; Le Tien, C. 20 - Edible films and coatings from nonstarch polysaccharides. In Innovations in Food Packaging, Han, J.H., Ed.; Academic Press: London, 2005; pp. 338–361. [Google Scholar]
- Vazquez, A.; Foresti, M.L.; Moran, J.I.; Cyras, V.P. Extraction and production of cellulose nanofibers. In Handbook of polymer nanocomposites. Processing, performance and application; Springer, 2015; pp. 81–118. [Google Scholar]
- Dufresne, A. Nanocellulose: from nature to high performance tailored materials; Walter de Gruyter GmbH & Co KG, 2017. [Google Scholar]
- Kargarzadeh, H.; Ahmad, I.; Thomas, S.; Dufresne, A. Handbook of nanocellulose and cellulose nanocomposites; John Wiley & Sons, 2017. [Google Scholar]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resources Conversion 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Burchard, W.; Schulz, L. Functionality of the β (1, 4) glycosidic linkage in polysaccharides. In Proceedings of the Macromolecular Symposia; 1995; pp. 57–69. [Google Scholar]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Frontiers in Chemistry 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: chemistry, self-assembly, and applications. Chemical reviews 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhou, X.; Lyu, S.; Pan, D.; Dong, M.; Wu, S.; Ding, T.; Wei, X.; Seok, I.; Wei, S. Magnetic nanocellulose-magnetite aerogel for easy oil adsorption. Journal of colloid and interface science 2020, 560, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.M.; Cuculo, J.A. The solubility of unmodified cellulose: a critique of the literature. Journal of Macromolecular Science—Reviews in Macromolecular Chemistry 1980, 18, 1–82. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Advances and Challenges in Biopolymer-Based Films. Polymers 2022, 14, 3920. [Google Scholar] [CrossRef] [PubMed]
- Tajeddin, B. Cellulose-based polymers for packaging applications. Lignocellulosic Polymer Composites 2014, 477–498. [Google Scholar]
- Osorio, F.A.; Molina, P.; Matiacevich, S.; Enrione, J.; Skurtys, O. Characteristics of hydroxy propyl methyl cellulose (HPMC) based edible film developed for blueberry coatings. Procedia Food Science 2011, 1, 287–293. [Google Scholar] [CrossRef]
- Singh, P.; Magalhães, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-based edible films for probiotic entrapment. Food Hydrocolloids 2019, 88, 68–74. [Google Scholar] [CrossRef]
- Bastos, M.d.S.R.; Laurentino, L.d.S.; Canuto, K.M.; Mendes, L.G.; Martins, C.M.; Silva, S.M.F.; Furtado, R.F.; Kim, S.; Biswas, A.; Cheng, H.N. Physical and mechanical testing of essential oil-embedded cellulose ester films. Polymer Testing 2016, 49, 156–161. [Google Scholar] [CrossRef]
- Bastos, M.d.S.R.; da Silva Laurentino, L.; Canuto, K.M.; Mendes, L.G.; Martins, C.M.; Silva, S.M.F.; Furtado, R.F.; Kim, S.; Biswas, A.; Cheng, H. Physical and mechanical testing of essential oil-embedded cellulose ester films. Polymer Testing 2016, 49, 156–161. [Google Scholar] [CrossRef]
- Ghiasi, F.; Golmakani, M.-T.; Eskandari, M.H.; Hosseini, S.M.H. A new approach in the hydrophobic modification of polysaccharide-based edible films using structured oil nanoparticles. Industrial Crops and Products 2020, 154, 112679. [Google Scholar] [CrossRef]
- Zhuang, C.; Tao, F.; Cui, Y. Eco-friendly biorefractory films of gelatin and TEMPO-oxidized cellulose ester for food packaging application. Journal of the Science of Food and Agriculture 2017, 97, 3384–3395. [Google Scholar] [CrossRef] [PubMed]
- Bras, J.; Vaca-Garcia, C.; Borredon, M.-E.; Glasser, W. Oxygen and water vapor permeability of fully substituted long chain cellulose esters (LCCE). Cellulose 2007, 14, 367–374. [Google Scholar] [CrossRef]
- Bilbao-Sáinz, C.; Avena-Bustillos, R.J.; Wood, D.F.; Williams, T.G.; McHugh, T.H. Composite Edible Films Based on Hydroxypropyl Methylcellulose Reinforced with Microcrystalline Cellulose Nanoparticles. Journal of Agricultural and Food Chemistry 2010, 58, 3753–3760. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.; Tanada-Palmu, P.; Grosso, C. Characterization of composite biofilms of wheat gluten and cellulose acetate phthalate. Brazilian Journal of Chemical Engineering 2004, 21, 261–264. [Google Scholar] [CrossRef]
- Debeaufort, F.; Quezada-Gallo, J.-A.; Delporte, B.; Voilley, A. Lipid hydrophobicity and physical state effects on the properties of bilayer edible films. Journal of Membrane Science 2000, 180, 47–55. [Google Scholar] [CrossRef]
- KESTER, J.J.; FENNEMA, O. An Edible Film of Lipids and Cellulose Ethers: Barrier Properties to Moisture Vapor Transmission and Structural Evaluation. Journal of Food Science 1989, 54, 1383–1389. [Google Scholar] [CrossRef]
- Koh, H.-Y.; MS, C. Characteristics of corn zein and methyl cellulose bilayer edible films according to preparation protocol. Food Science and Biotechnology 2002, 11, 310–315. [Google Scholar]
- Park, J.W.; Testin, R.F.; Park, H.J.; Vergano, P.J.; Weller, C.L. Fatty Acid Concentration Effect on Tensile Strength, Elongation, and Water Vapor Permeability of Laminated Edible Films. Journal of Food Science 1994, 59, 916–919. [Google Scholar] [CrossRef]
- Shao, P.; Wu, W.; Chen, H.; Sun, P.; Gao, H. Bilayer edible films with tunable humidity regulating property for inhibiting browning of Agaricus bisporus. Food Research International 2020, 138, 109795. [Google Scholar] [CrossRef]
- 109. . Liu, Z.; Lin, D.; Lopez-Sanchez, P.; Yang, X. Characterizations of bacterial cellulose nanofibers reinforced edible films based on konjac glucomannan. International Journal of Biological Macromolecules 2020, 145, 634–645. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M. Characterization of a new biodegradable edible film based on Sago Starch loaded with Carboxymethyl Cellulose nanoparticles. Nanomedicine Research Journal 2018, 3, 25–30. [Google Scholar]
- Tabari, M. Investigation of Carboxymethyl Cellulose (CMC) on Mechanical Properties of Cold Water Fish Gelatin Biodegradable Edible Films. Foods 2017, 6, 41. [Google Scholar] [CrossRef]
- Indumathi, M.; Sarojini, K.S.; Rajarajeswari, G. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. International journal of biological macromolecules 2019, 132, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Bhopal, R. Investigation of water vapour permeation and antibacterial properties of nano silver loaded cellulose acetate film. Int. Food Res. J 2010, 17, 623–639. [Google Scholar]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Development and characterization of plant oil-incorporated carboxymethyl cellulose/bacterial cellulose/glycerol-based antimicrobial edible films for food packaging applications. Advanced Composites and Hybrid Materials 2022, 5, 973–990. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: a review. Veterinarni medicina 2013, 58. [Google Scholar] [CrossRef]
- Park, H.-J.; Rhim, J.-W.; Jung, S.-T.; Kang, S.-G.; Hwang, K.-T.; Park, Y.-K. Mechanical properties of carrageenan-based biopolymer films. Korean Journal of Packaging Science & Technology 1995, 1, 38–50. [Google Scholar]
- Balqis, A.I.; Khaizura, M.N.; Russly, A.; Hanani, Z.N. Effects of plasticizers on the physicochemical properties of kappa-carrageenan films extracted from Eucheuma cottonii. International journal of biological macromolecules 2017, 103, 721–732. [Google Scholar] [CrossRef]
- Thomas, W. Carrageenan. In Thickening and gelling agents for food; Springer, 1997; pp. 45–59. [Google Scholar]
- Baldwin, E.A.; Nisperos-Carriedo, M.O.; Baker, R.A. Use of edible coatings to preserve quality of lightly (and slightly) processed products. Critical Reviews in Food Science & Nutrition 1995, 35, 509–524. [Google Scholar]
- Lin, M.G.; Lasekan, O.; Saari, N.; Khairunniza-Bejo, S. The Effect of the Application of Edible Coatings on or before Ultraviolet Treatment on Postharvested Longan Fruits. Journal of Food Quality 2017, 2017, 5454263. [Google Scholar] [CrossRef]
- Abdou, E.; Sorour, M. Preparation and characterization of starch/carrageenan edible films. International food research journal 2014, 21, 189. [Google Scholar]
- Thakur, R.; Saberi, B.; Pristijono, P.; Golding, J.; Stathopoulos, C.; Scarlett, C.; Bowyer, M.; Vuong, Q. Characterization of rice starch-ι-carrageenan biodegradable edible film. Effect of stearic acid on the film properties. International Journal of Biological Macromolecules 2016, 93, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. International Journal of Biological Macromolecules 2020, 143, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Larotonda, F.D.S.; Torres, M.D.; Gonçalves, M.P.; Sereno, A.M.; Hilliou, L. Hybrid carrageenan-based formulations for edible film preparation: Benchmarking with kappa carrageenan. Journal of Applied Polymer Science 2016, 133. [Google Scholar] [CrossRef]
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food and Bioprocess Technology 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Paula, G.A.; Benevides, N.M.B.; Cunha, A.P.; de Oliveira, A.V.; Pinto, A.M.B.; Morais, J.P.S.; Azeredo, H.M.C. Development and characterization of edible films from mixtures of κ-carrageenan, ι-carrageenan, and alginate. Food Hydrocolloids 2015, 47, 140–145. [Google Scholar] [CrossRef]
- Hambleton, A.; Voilley, A.; Debeaufort, F. Transport parameters for aroma compounds through i-carrageenan and sodium alginate-based edible films. Food Hydrocolloids 2011, 25, 1128–1133. [Google Scholar] [CrossRef]
- Thiviya, P., Gamage, A., Liyanapathiranage,A., Makehelwala, M., Dassanayake, R.S., Manamperi, A., Merah, O., Mani, S., Koduru, J.R., Terrence Madhujith, T. Algal polysaccharides: structure, preparation and applications in food packaging, Food Chemistry, 2022, 405, Part A, 134903.
- Jayakody, M.M.; Vanniarachchy, M.P.G.; Wijesekara, I. Seaweed derived alginate, agar, and carrageenan based edible coatings and films for the food industry: a review. Journal of Food Measurement and Characterization 2022, 1–33. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Göksen, G.; Martínez-Abad, A.; López-Rubio, A.; Martínez-Sanz, M. Agar-based packaging films produced by melt mixing: Study of their retrogradation upon storage. Algal Research 2022, 66, 102802. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications - A review. International Journal of Biological Macromolecules 2020, 159, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Wongphan, P.; Harnkarnsujarit, N. Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films. International Journal of Biological Macromolecules 2020, 156, 80–93. [Google Scholar] [CrossRef]
- Phan The, D.; Debeaufort, F.; Voilley, A.; Luu, D. Biopolymer interactions affect the functional properties of edible films based on agar, cassava starch and arabinoxylan blends. Journal of Food Engineering 2009, 90, 548–558. [Google Scholar] [CrossRef]
- Arham, R.; Mulyati, M.; Metusalach, M.; Salengke, S. Physical and mechanical properties of agar based edible film with glycerol plasticizer. International Food Research Journal 2016, 23, 1669–1675. [Google Scholar]
- Sousa, A.M.; Sereno, A.M.; Hilliou, L.; Gonçalves, M.P. Biodegradable agar extracted from Gracilaria vermiculophylla: Film properties and application to edible coating. In Proceedings of the Materials science forum; 2010; pp. 739–744. [Google Scholar]
- Shahidi, F.; Hossain, A. Preservation of aquatic food using edible films and coatings containing essential oils: a review. Critical Reviews in Food Science and Nutrition 2022, 62, 66–105. [Google Scholar] [CrossRef] [PubMed]
- Perera, K.Y.; Sharma, S.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings). Foods 2021, 10, 2088. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M. Chemistry and uses of pectin—a review. Critical Reviews in Food Science and Nutrition 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S.; Marcotte, M. Evaluation of factors affecting barrier, mechanical and optical properties of pectin-based films using response surface methodology. Journal of food process engineering 2007, 30, 539–563. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Preparation and characterization of pectin fraction from pineapple peel as a natural plasticizer and material for biopolymer film. Food and Bioproducts Processing 2019, 118, 198–206. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Edible Films and Coatings with Pectin. In Pectin: Technological and Physiological Properties, Kontogiorgos, V., Ed.; Springer International Publishing: Cham, 2020; pp. 99–123. [Google Scholar]
- Shahrampour, D.; Khomeiri, M.; Razavi, S.M.A.; Kashiri, M. Development and characterization of alginate/pectin edible films containing Lactobacillus plantarum KMC 45. LWT 2020, 118, 108758. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Gutierrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Rodriguez-Garcia, I.; Gonzalez-Aguilar, G.A.; Ponce, A.; Moreira, M.d.R.; Roura, S.I.; Ayala-Zavala, J.F. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Frontiers in Microbiology 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Girón, Y.; Cabrera-Díaz, E.; Esparza-Merino, R.M.; Martín-del-Campo, A.; Valencia-Botín, A.J. Innovative edible films and coatings based on red color pectin obtained from the byproducts of Hibiscus sabdariffa L. for strawberry preservation. Journal of Food Measurement and Characterization 2020, 14, 3371–3380. [Google Scholar] [CrossRef]
- Rossi Marquez, G.; Di Pierro, P.; Mariniello, L.; Esposito, M.; Giosafatto, C.V.L.; Porta, R. Fresh-cut fruit and vegetable coatings by transglutaminase-crosslinked whey protein/pectin edible films. LWT 2017, 75, 124–130. [Google Scholar] [CrossRef]
- Alvarez-Pérez, O.B.; Montañez, J.; Aguilar, C.N.; Rojas, R. Pectin–Candelilla wax: An alternative mixture for edible films. Journal of Microbiology, Biotechnology and Food Sciences 2021, 2021, 167–171. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jó ´zwik-Pruska, J.; Wi´sniewska-Wrona, M. Chitin and Chitosan as Polymers of the Future—Obtaining, Modification, Life Cycle Assessment and Main Directions of Application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Roberts, G.A.; Roberts, G.A. Chitin chemistry; Springer, 1992. [Google Scholar]
- Hudson, S.; Smith, C. Polysaccharides: chitin and chitosan: chemistry and technology of their use as structural materials. In Biopolymers from renewable resources; Springer, 1998; pp. 96–118. [Google Scholar]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and antimicrobial applications of biopolymers: A review. Food Research International 2020, 136, 109327. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Téllez, C.N.; Luque-Alcaraz, A.G.; Núñez-Mexía, S.A.; Cortez-Rocha, M.O.; Lizardi-Mendoza, J.; Rosas-Burgos, E.C.; Rosas-Durazo, A.d.J.; Parra-Vergara, N.V.; Plascencia-Jatomea, M. Relationship between the Antifungal Activity of Chitosan–Capsaicin Nanoparticles and the Oxidative Stress Response on Aspergillus parasiticus. Polymers 2022, 14, 2774. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Neme¸s, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Pavinatto, A.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with chitosan-based edible films for mechanical/biological protection of strawberries. International Journal of Biological Macromolecules 2020, 151, 1004–1011. [Google Scholar] [CrossRef]
- da Mata Cunha, O.; Lima, A.M.F.; Assis, O.B.G.; Tiera, M.J.; de Oliveira Tiera, V.A. Amphiphilic diethylaminoethyl chitosan of high molecular weight as an edible film. International Journal of Biological Macromolecules 2020, 164, 3411–3420. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S.; Sabaa, M.W. Novel biodegradable and antibacterial edible films based on alginate and chitosan biguanidine hydrochloride. International Journal of Biological Macromolecules 2018, 116, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Moldão-Martins, M.; Alves, V.D. Characterization of multilayered and composite edible films from chitosan and beeswax. Food Science and Technology International 2015, 21, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Tokatlı, K.; Demirdöven, A. Effects of chitosan edible film coatings on the physicochemical and microbiological qualities of sweet cherry (Prunus avium L.). Scientia Horticulturae 2020, 259, 108656. [Google Scholar] [CrossRef]
- Doublier, J.-L.; Garnier, C.; Cuvelier, G. Gums and hydrocolloids: functional aspects. In Carbohydrates in food; CRC Press, 2017; pp. 307–354. [Google Scholar]
- Lim, W.; Shin, S.Y.; Cha, J.M.; Bae, H. Optimization of Polysaccharide Hydrocolloid for the Development of Bioink with High Printability/Biocompatibility for Coextrusion 3D Bioprinting. Polymers 2021, 13, 1773. [Google Scholar] [CrossRef] [PubMed]
- Khezerlou, A.; Zolfaghari, H.; Banihashemi, S.A.; Forghani, S.; Ehsani, A. Plant gums as the functional compounds for edible films and coatings in the food industry: A review. Polymers for Advanced Technologies 2021, 32, 2306–2326. [Google Scholar] [CrossRef]
- Islam, A.; Phillips, G.; Sljivo, A.; Snowden, M.; Williams, P. A review of recent developments on the regulatory, structural and functional aspects of gum arabic. Food Hydrocolloids 1997, 11, 493–505. [Google Scholar] [CrossRef]
- Musa, H.H.; Ahmed, A.A.; Musa, T.H. Chemistry, biological, and pharmacological properties of gum Arabic. Bioactive Molecules in Food; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 1–18. [Google Scholar]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. International Journal of Biological Macromolecules 2019, 134, 230–236. [Google Scholar] [CrossRef]
- Razak, A.S.; Lazim, A.M. Starch-based edible film with gum arabic for fruits coating. AIP Conference Proceedings 2015, 1678. [Google Scholar]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocolloids 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Li, C.; Pei, J.; Xiong, X.; Xue, F. Encapsulation of Grapefruit Essential Oil in Emulsion-Based Edible Film Prepared by Plum (Pruni Domesticae Semen) Seed Protein Isolate and Gum Acacia Conjugates. Coatings 2020, 10, 784. [Google Scholar] [CrossRef]
- Mathur, N. Industrial galactomannan polysaccharides; CRC Press Taylor & Francis Group: Boca Raton, FL, 2012. [Google Scholar]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P.; Nagar, B.J.; Naikwadi, N.N.; Variya, B.C. Galactomannan: a versatile biodegradable seed polysaccharide. International journal of biological macromolecules 2013, 60, 83–92. [Google Scholar] [CrossRef]
- Antoniou, J.; Liu, F.; Majeed, H.; Qazi, H.J.; Zhong, F. Physicochemical and thermomechanical characterization of tara gum edible films: Effect of polyols as plasticizers. Carbohydrate Polymers 2014, 111, 359–365. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: processing, properties and food applications—a review. Journal of food science and technology 2014, 51, 409–418. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC advances 2020, 10, 27103–27136. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Safdarianghomsheh, R.; Zamanifar, P.; Salehi, S.; Niakousari, M.; Hosseini, S.M.H. Characterization of novel edible films and coatings for food preservation based on gum cordia. Journal of Food Quality 2020, 2020. [Google Scholar] [CrossRef]
- Radev, R.; Pashova, S. Application of edible films and coatings for fresh fruit and vegetables. Qual. Access Success 2020, 21, 108–112. [Google Scholar]
- Dubey, N.K.; Dubey, R. Edible films and coatings: An update on recent advances. In Biopolymer-based formulations; Elsevier, 2020; pp. 675–695. [Google Scholar]
- Dhumal, C.V.; Ahmed, J.; Bandara, N.; Sarkar, P. Improvement of antimicrobial activity of sago starch/guar gum bi-phasic edible films by incorporating carvacrol and citral. Food Packaging and Shelf Life 2019, 21, 100380. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. International Journal of Biological Macromolecules 2017, 104, 345–359. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Characterization of pea starch-guar gum biocomposite edible films enriched by natural antimicrobial agents for active food packaging. Food and Bioproducts Processing 2017, 105, 51–63. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical, Barrier, and Antioxidant Properties of Pea Starch-Guar Gum Biocomposite Edible Films by Incorporation of Natural Plant Extracts. Food and Bioprocess Technology 2017, 10, 2240–2250. [Google Scholar] [CrossRef]
- Naji-Tabasi, S.; Razavi, S.M.A. Functional properties and applications of basil seed gum: An overview. Food Hydrocolloids 2017, 73, 313–325. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A. Characterization of novel basil-seed gum active edible films and coatings containing oregano essential oil. Progress in Organic Coatings 2017, 110, 35–41. [Google Scholar] [CrossRef]
- Salehi, F. Characterization of New Biodegradable Edible Films and Coatings Based on Seeds Gum: A Review. Journal of Packaging Technology and Research 2019, 3, 193–201. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Mostaghimi, M.; Ghiasi, F.; Tavakoli, S.; Naseri, M.; Hosseini, S.M.H. The effects of fatty acids chain length on the techno-functional properties of basil seed gum-based edible films. International Journal of Biological Macromolecules 2020, 160, 245–251. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Zala, B.S.; Khutliwala, T.A. An insight into the emerging exopolysaccharide gellan gum as a novel polymer. Carbohydrate Polymers 2013, 93, 670–678. [Google Scholar] [CrossRef]
- Giavasis, I.; Harvey, L.M.; McNeil, B. Gellan gum. Critical reviews in biotechnology 2000, 20, 177–211. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends in Food Science & Technology 2011, 22, 292–303. [Google Scholar]
- Alvarado-González, J.; Chanona-Pérez, J.; Welti-Chanes, J.; Calderón-Domínguez, G.; Arzate-Vázquez, I.; Pacheco-Alcalá, S.; Garibay-Febles, V.; Gutiérrez-López, G. Optical, microstructural, functional and nanomechanical properties of Aloe vera gel/gellan gum edible films. Revista mexicana de ingeniería química 2012, 11, 193–210. [Google Scholar]
- Maan, A.A.; Reiad Ahmed, Z.F.; Iqbal Khan, M.K.; Riaz, A.; Nazir, A. Aloe vera gel, an excellent base material for edible films and coatings. Trends in Food Science & Technology 2021, 116, 329–341. [Google Scholar]
- Téllez-Rangel, E.C.; Rodríguez-Huezo, E.; Totosaus, A. Effect of gellan, xanthan or locust bean gum and/or emulsified maize oil on proteins edible films properties. Emirates Journal of Food and Agriculture 2018, 404–412. [Google Scholar]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—composition, fine structure and architecture. Journal of Cereal Science 2004, 39, 151–165. [Google Scholar] [CrossRef]
- van Soest, J.J.G.; Vliegenthart, J.F.G. Crystallinity in starch plastics: consequences for material properties. Trends in Biotechnology 1997, 15, 208–213. [Google Scholar] [CrossRef]
- Rindlav-Westling, Å.; Stading, M.; Gatenholm, P. Crystallinity and morphology in films of starch, amylose and amylopectin blends. Biomacromolecules 2002, 3, 84–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics—A Review. Critical Reviews in Food Science and Nutrition 2014, 54, 1353–1370. [Google Scholar] [CrossRef]
- Liu, Z. 19 - Edible films and coatings from starches. In Innovations in Food Packaging, Han, J.H., Ed.; Academic Press: London, 2005; pp. 318–337. [Google Scholar]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Comprehensive Reviews in Food Science and Food Safety 2016, 15, 568–580. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Hubinger, M.D. Evaluation of edible films and coatings formulated with cassava starch, glycerol, carnauba wax and stearic acid. Food Hydrocolloids 2014, 38, 20–27. [Google Scholar] [CrossRef]
- Pedreiro, S.; Figueirinha, A.; Silva, A.S.; Ramos, F. Bioactive Edible Films and Coatings Based in Gums and Starch: Phenolic Enrichment and Foods Application. Coatings 2021, 11, 1393. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. Effect of starch type on the physico-chemical properties of edible films. International Journal of Biological Macromolecules 2017, 98, 348–356. [Google Scholar] [CrossRef] [PubMed]
- García, M.A.; Martino, M.N.; Zaritzky, N.E. Lipid Addition to Improve Barrier Properties of Edible Starch-based Films and Coatings. Journal of Food Science 2000, 65, 941–944. [Google Scholar] [CrossRef]
- Rodríguez, M.; Osés, J.; Ziani, K.; Maté, J.I. Combined effect of plasticizers and surfactants on the physical properties of starch based edible films. Food Research International 2006, 39, 840–846. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of Glycerol on Physical and Mechanical Properties of Wheat Starch Edible Films. Journal of Texture Studies 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Kim, K.W.; Ko, C.J.; Park, H.J. Mechanical Properties, Water Vapor Permeabilities and Solubilities of Highly Carboxymethylated Starch-Based Edible Films. Journal of Food Science 2002, 67, 218–222. [Google Scholar] [CrossRef]
- Charles, A.L.; Motsa, N.; Abdillah, A.A. A Comprehensive Characterization of Biodegradable Edible Films Based on Potato Peel Starch Plasticized with Glycerol. Polymers 2022, 14, 3462. [Google Scholar] [CrossRef] [PubMed]
- 204 Wilpiszewska, K.; Adrian Krzysztof Antosik, A.K. ; Schmidt,B. ; Janik, J.; Rokicka, J. Hydrophilic Films Based on Carboxymethylated Derivatives of Starch and Cellulose. Polymers 2020, 12(11), 2447. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Leme, B.d.O.; Santos, G.R.S.d.; Silva, J.V.d.; Nascimento, P.B.; Soares, C.T.; Fakhouri, F.M.; de Oliveira, R.A. Edible Films and Coatings Formulated with Arrowroot Starch as a Non-Conventional Starch Source for Plums Packaging. Polysaccharides 2021, 2, 373–386. [Google Scholar] [CrossRef]
- Peressini, D.; Bravin, B.; Lapasin, R.; Rizzotti, C.; Sensidoni, A. Starch–methylcellulose based edible films: rheological properties of film-forming dispersions. Journal of Food Engineering 2003, 59, 25–32. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Guilbert, S. Proteins as Agricultural Polymers for Packaging Production. Cereal Chemistry 1998, 75, 1–9. [Google Scholar] [CrossRef]
- McHUGH, T.H.; Aujard, J.F.; Krochta, J. Plasticized whey protein edible films: water vapor permeability properties. Journal of food science 1994, 59, 416–419. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Biodegradation of three-layer laminate films based on gelatin under indoor soil conditions. Polymer Degradation and Stability 2009, 94, 1307–1313. [Google Scholar] [CrossRef]
- Jauregi, P. Bioactive peptides from food proteins: new opportunities and challenges. Food Science and Technology Bulletin: Functional Foods Volume 5 2009, 5, 11–25. [Google Scholar] [CrossRef]
- Alkan, D.; Yemenicioğlu, A. Potential application of natural phenolic antimicrobials and edible film technology against bacterial plant pathogens. Food Hydrocolloids 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Banerjee, R.; Chen, H.; Wu, J. Milk protein-based edible film mechanical strength changes due to ultrasound process. Journal of Food Science 1996, 61, 824–828. [Google Scholar] [CrossRef]
- Panyam, D.; Kilara, A. Enhancing the functionality of food proteins by enzymatic modification. Trends in food science & technology 1996, 7, 120–125. [Google Scholar]
- Yada, R.Y. Proteins in food processing; Woodhead Publishing, 2017. [Google Scholar]
- Audic, J.-L.; Chaufer, B.; Daufin, G. Non-food applications of milk components and dairy co-products: A review. Le Lait 2003, 83, 417–438. [Google Scholar] [CrossRef]
- Hinz, K.; O’Connor, P.M.; Huppertz, T.; Ross, R.P.; Kelly, A.L. Comparison of the principal proteins in bovine, caprine, buffalo, equine and camel milk. Journal of Dairy Research 2012, 79, 185–191. [Google Scholar] [CrossRef]
- Petrova, S.Y.; Khlgatian, S.; Emel’yanova, O.Y.; Pishulina, L.; Berzhets, V. Current Data about Milk Caseins. Russian Journal of Bioorganic Chemistry 2022, 48, 273–280. [Google Scholar] [CrossRef]
- Swaisgood, H.E. Review and Update of Casein Chemistry1, 2. Journal of Dairy Science 1993, 76, 3054–3061. [Google Scholar] [CrossRef]
- Lacroix, M.; Cooksey, K. 18 - Edible films and coatings from animal origin proteins. In Innovations in Food Packaging, Han, J.H., Ed.; Academic Press: London, 2005; pp. 301–317. [Google Scholar]
- Shendurse, A.; Gopikrishna, G.; Patel, A.; Pandya, A. Milk protein based edible films and coatings–preparation, properties and food applications. J Nutr Health Food Eng 2018, 8, 219–226. [Google Scholar] [CrossRef]
- Chen, H. Functional Properties and Applications of Edible Films Made of Milk Proteins. Journal of Dairy Science 1995, 78, 2563–2583. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.G.; Whitehead, D.M.; Kinsella, J. Introduction to Functional Properties of Proteins. In Structure–Function Properties of Food Proteins, Phillips, L.G., Whitehead, D.M., Kinsella, J., Eds.; Academic Press: Boston, 1994; pp. 107–109. [Google Scholar]
- Avena-Bustillos, R.J.; Cisneros-Zevallos, L.A.; Krochta, J.M.; Saltveit, M.E. Application of casein-lipid edible film emulsions to reduce white blush on minimally processed carrots. Postharvest Biology and Technology 1994, 4, 319–329. [Google Scholar] [CrossRef]
- Yangılar, F.; Oğuzhan Yıldız, P. Casein/natamycin edible films efficiency for controlling mould growth and on microbiological, chemical and sensory properties during the ripening of Kashar cheese. Journal of the Science of Food and Agriculture 2016, 96, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- AVENA-BUSTILLOS, R.J.; KROCHTA, J.M. Water Vapor Permeability of Caseinate-Based Edible Films as Affected by pH, Calcium Crosslinking and Lipid Content. Journal of Food Science 1993, 58, 904–907. [Google Scholar] [CrossRef]
- Chick, J.; Hernandez, R.J. Physical, Thermal, and Barrier Characterization of Casein-Wax-Based Edible Films. Journal of Food Science 2002, 67, 1073–1079. [Google Scholar] [CrossRef]
- Chevalier, E.; Chaabani, A.; Assezat, G.; Prochazka, F.; Oulahal, N. Casein/wax blend extrusion for production of edible films as carriers of potassium sorbate—A comparative study of waxes and potassium sorbate effect. Food Packaging and Shelf Life 2018, 16, 41–50. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.Y. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnology advances 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.; Müller, K. Whey protein-based packaging films and coatings. In Whey proteins; Elsevier, 2019; pp. 407–437. [Google Scholar]
- de Castro, R.J.S.; Domingues, M.A.F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food structure 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Kandasamy, S.; Yoo, J.; Yun, J.; Kang, H.-B.; Seol, K.-H.; Kim, H.-W.; Ham, J.-S. Application of Whey Protein-Based Edible Films and Coatings in Food Industries: An Updated Overview. Coatings 2021, 11, 1056. [Google Scholar] [CrossRef]
- Di Pierro, P.; Mariniello, L.; Giosafatto, V.L.; Esposito, M.; Sabbah, M.; Porta, R. Chapter 13 - Dairy Whey Protein-Based Edible Films and Coatings for Food Preservation. In Food Packaging and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press, 2018; pp. 439–456. [Google Scholar]
- Ramos, Ó.L.; Fernandes, J.C.; Silva, S.I.; Pintado, M.E.; Malcata, F.X. Edible Films and Coatings from Whey Proteins: A Review on Formulation, and on Mechanical and Bioactive Properties. Critical Reviews in Food Science and Nutrition 2012, 52, 533–552. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Optical, mechanical, and moisture sorption properties of whey protein edible films. Journal of Food Process Engineering 2019, 42, e13245. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocolloids 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Gounga, M.E.; Xu, S.-Y.; Wang, Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. Journal of Food Engineering 2007, 83, 521–530. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Moisture Sensitivity, Optical, Mechanical and Structural Properties of Whey Protein-Based Edible Films Incorporated with Rapeseed Oil. Food Technol Biotechnol 2016, 54, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Dangaran, K.; Tomasula, P.M.; Qi, P. Structure and Function of Protein-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications, Huber, K.C., Embuscado, M.E., Eds.; Springer New York: New York, NY, 2009; pp. 25–56. [Google Scholar]
- Linsenmayer, T.F. Collagen. In Cell Biology of Extracellular Matrix: Second Edition, Hay, E.D., Ed.; Springer US: Boston, MA, 1991; pp. 7–44. [Google Scholar]
- Brodsky, B.; Ramshaw, J.A. The collagen triple-helix structure. Matrix biology 1997, 15, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Deiber, J.A.; Peirotti, M.B.; Ottone, M.L. Rheological characterization of edible films made from collagen colloidal particle suspensions. Food Hydrocolloids 2011, 25, 1382–1392. [Google Scholar] [CrossRef]
- O’Sullivan, A.; Shaw, N.B.; Murphy, S.C.; van de Vis, J.W.; van Pelt-Heerschap, H.; Kerry, J.P. Extraction of Collagen from Fish Skins and Its Use in the Manufacture of Biopolymer Films. Journal of Aquatic Food Product Technology 2006, 15, 21–32. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.; Zhou, X.; Lao, M.; Cai, L. The physiochemical and preservation properties of anthocyanidin/chitosan nanocomposite-based edible films containing cinnamon-perilla essential oil pickering nanoemulsions. LWT 2022, 153, 112506. [Google Scholar] [CrossRef]
- Song, D.-H.; Hoa, V.B.; Kim, H.W.; Khang, S.M.; Cho, S.-H.; Ham, J.-S.; Seol, K.-H. Edible Films on Meat and Meat Products. Coatings 2021, 11, 1344. [Google Scholar] [CrossRef]
- Fadini, A.L.; Rocha, F.S.; Alvim, I.D.; Sadahira, M.S.; Queiroz, M.B.; Alves, R.M.V.; Silva, L.B. Mechanical properties and water vapour permeability of hydrolysed collagen–cocoa butter edible films plasticised with sucrose. Food Hydrocolloids 2013, 30, 625–631. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Wang, H. Scale-Up Preparation and Characterization of Collagen/Sodium Alginate Blend Films. Journal of Food Quality 2017, 2017, 4954259. [Google Scholar] [CrossRef]
- Ma, D.; Jiang, Y.; Ahmed, S.; Qin, W.; Liu, Y. Antilisterial and physical properties of polysaccharide-collagen films embedded with cell-free supernatant of Lactococcus lactis. International Journal of Biological Macromolecules 2020, 145, 1031–1038. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Rodrigues, P.R.; da Silva, R.G.; Vieira, R.P.; Alves, R.M.V. Sustainable Packaging Films Composed of Sodium Alginate and Hydrolyzed Collagen: Preparation and Characterization. Food and Bioprocess Technology 2021, 14, 2336–2346. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Y.; Liu, Q.; Jiang, S.; Mu, G. Improved structure-stability and packaging characters of crosslinked collagen fiber-based film with casein, keratin and SPI. Journal of the Science of Food and Agriculture 2019, 99, 4942–4951. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. International Journal of Biological Macromolecules 2020, 160, 340–351. [Google Scholar] [CrossRef]
- Poppe, J. Gelatin. In Thickening and gelling agents for food; Springer, 1992; pp. 98–123. [Google Scholar]
- Keenan, T.R. Gelatin. In Kirk-Othmer Encyclopedia of Chemical Technology.
- Arvanitoyannis, I.; Psomiadou, E.; Nakayama, A.; Aiba, S.; Yamamoto, N. Edible films made from gelatin, soluble starch and polyols, Part 3. Food Chemistry 1997, 60, 593–604. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biology and Technology 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Maria Martelli, S.; Canhadas Bertan, L.; Yamashita, F.; Innocentini Mei, L.H.; Collares Queiroz, F.P. Edible films made from blends of manioc starch and gelatin – Influence of different types of plasticizer and different levels of macromolecules on their properties. LWT 2012, 49, 149–154. [Google Scholar] [CrossRef]
- Sobral, P.J.A.; Menegalli, F.C.; Hubinger, M.D.; Roques, M.A. Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocolloids 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Bioscience 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Preparation and functional properties of fish gelatin–chitosan blend edible films. Food Chemistry 2013, 136, 1490–1495. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.-H.; Yin, S.-W.; Yang, X.-Q.; Wang, Q.; Liu, F.; Wei, Z.-H. Characterization of gelatin-based edible films incorporated with olive oil. Food Research International 2012, 49, 572–579. [Google Scholar] [CrossRef]
- Chambi, H.; Grosso, C. Edible films produced with gelatin and casein cross-linked with transglutaminase. Food Research International 2006, 39, 458–466. [Google Scholar] [CrossRef]
- Buffo, R.A.; Han, J.H. 17 - Edible films and coatings from plant origin proteins. In Innovations in Food Packaging, Han, J.H., Ed.; Academic Press: London, 2005; pp. 277–300. [Google Scholar]
- Chiralt, A.; González-Martínez, C.; Vargas, M.; Atarés, L. 18 - Edible films and coatings from proteins. In Proteins in Food Processing (Second Edition); Yada, R.Y., Ed.; Woodhead Publishing, 2018; pp. 477–500. [Google Scholar]
- BRANDENBURG, A.H.; WELLER, C.L.; TESTIN, R.F. Edible Films and Coatings from Soy Protein. Journal of Food Science 1993, 58, 1086–1089. [Google Scholar] [CrossRef]
- Hill, K.; Höfer, R. Natural fats and oils. Sustainable solutions for modern economies. The Royal Society of Chemistry 2009, 167–237. [Google Scholar]
- Rhim, J.W.; Shellhammer, T.H. 21 - Lipid-based edible films and coatings. In Innovations in Food Packaging, Han, J.H., Ed.; Academic Press: London, 2005; pp. 362–383. [Google Scholar]
- Hernandez, E. Edible coatings from lipids and resins. Edible coatings and films to improve food quality 1994, 279–303. [Google Scholar]
- Debeaufort, F.; Voilley, A. Lipid-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications, Huber, K.C., Embuscado, M.E., Eds.; Springer New York: New York, NY, 2009; pp. 135–168. [Google Scholar]
- Gordon, M. Fats and Fatty Foods. In Food Industries Manual, Ranken, M.D., Kill, R.C., Eds.; Springer US: Boston, MA, 1993; pp. 288–327. [Google Scholar]
- Duarte, M.; Duarte, R.; Rodrigues, R.; Rodrigues, M. Essential oils and their characteristics. Essential Oils in Food Processing: Chemistry, Safety and Applications 2018, 1-19.
- D Antunes, M.; M Gago, C.; M Cavaco, A.; G Miguel, M. Edible coatings enriched with essential oils and their compounds for fresh and fresh-cut fruit. Recent patents on food, nutrition & agriculture 2012, 4, 114–122. [Google Scholar]
- Bassolé, I.H.N.; Juliani, H.R. Essential oils in combination and their antimicrobial properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.-C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. International journal of biological macromolecules 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Kavoosi, G.; Rahmatollahi, A.; Dadfar, S.M.M.; Purfard, A.M. Effects of essential oil on the water binding capacity, physico-mechanical properties, antioxidant and antibacterial activity of gelatin films. LWT-Food Science and Technology 2014, 57, 556–561. [Google Scholar] [CrossRef]
- Aitboulahsen, M.; El Galiou, O.; Laglaoui, A.; Bakkali, M.; Hassani Zerrouk, M. Effect of plasticizer type and essential oils on mechanical, physicochemical, and antimicrobial characteristics of gelatin, starch, and pectin-based films. Journal of Food Processing and Preservation 2020, 44, e14480. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Debeaufort, F. Bioactive edible films for food applications: Influence of the bioactive compounds on film structure and properties. Critical reviews in food science and nutrition 2019, 59, 1137–1153. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.-W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef]
- Shellhammer, T.; Rumsey, T.; Krochta, J. Viscoelastic properties of edible lipids. Journal of Food Engineering 1997, 33, 305–320. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P.M. Edible polymers: challenges and opportunities. Journal of Polymers 2014, 2014. [Google Scholar] [CrossRef]
- Baldwin, E.A. Surface treatments and edible coatings in food preservation. In Handbook of food preservation; CRC Press, 2020; pp. 507–528. [Google Scholar]
- Wang, D.; Huang, J.; Guo, Z.; Liu, W. Durable mixed edible wax coating with stretching superhydrophobicity. Journal of Materials Chemistry A 2021, 9, 1495–1499. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in Edible Coatings for Fresh Fruits and Vegetables: A Review. Critical Reviews in Food Science and Nutrition 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Hagenmaier, R.D. Wax microemulsion formulations used as fruit coatings. In Proceedings of the Proceedings of the Florida State Horticultural Society, 1998; pp. 251–254.
- Hagenmaier, R.D.; Baker, R.A. Edible Coatings from Morpholine-Free Wax Microemulsions. Journal of Agricultural and Food Chemistry 1997, 45, 349–352. [Google Scholar] [CrossRef]
- Oregel-Zamudio, E.; Angoa-Pérez, M.V.; Oyoque-Salcedo, G.; Aguilar-González, C.N.; Mena-Violante, H.G. Effect of candelilla wax edible coatings combined with biocontrol bacteria on strawberry quality during the shelf-life. Scientia Horticulturae 2017, 214, 273–279. [Google Scholar] [CrossRef]
- Baldwin, E.; Burns, J.; Kazokas, W.; Brecht, J.; Hagenmaier, R.; Bender, R.; Pesis, E. Effect of two edible coatings with different permeability characteristics on mango (Mangifera indica L.) ripening during storage. Postharvest Biology and Technology 1999, 17, 215–226. [Google Scholar] [CrossRef]
- Ruiz-Martínez, J.; Aguirre-Joya, J.A.; Rojas, R.; Vicente, A.; Aguilar-González, M.A.; Rodríguez-Herrera, R.; Alvarez-Perez, O.B.; Torres-León, C.; Aguilar, C.N. Candelilla Wax Edible Coating with Flourensia cernua Bioactives to Prolong the Quality of Tomato Fruits. Foods 2020, 9, 1303. [Google Scholar] [CrossRef]
- De León-Zapata, M.A.; Sáenz-Galindo, A.; Rojas-Molina, R.; Rodríguez-Herrera, R.; Jasso-Cantú, D.; Aguilar, C.N. Edible candelilla wax coating with fermented extract of tarbush improves the shelf life and quality of apples. Food Packaging and Shelf Life 2015, 3, 70–75. [Google Scholar] [CrossRef]
- Cruz RMS, Krauter V, Krauter S, et al. Bioplastics for Food Packaging: Environmental Impact, Trends and Regulatory Aspects. Foods 2022, 11, 3087. [CrossRef] [PubMed]
- Pham TT, Nguyen LLP, Dam MS, et al. Application of Edible Coating in Extension of Fruit Shelf Life: Review. AgriEngineering. 2023, 5, 520–536. [CrossRef]
- Gupta I, Cherwoo L, Bhatia R, et al. Biopolymers: Implications and application in the food industry. Biocatal Agric Biotechnol. Elsevier Ltd; 2022.
- Ding, D. A review about edible food coatings and films [master’s thesis]. Cornell University; 2021.
- Pellicer E, Nikolic D, Sort J, et al. Advances in applications of industrial biomaterials. Advances in Applications of Industrial Biomaterials. Springer International Publishing; 2017.
- V. M R, Edison LK. Safety Issues, Environmental Impacts, and Health Effects of Biopolymers. Handbook of Biopolymers. Springer Nature Singapore; 2022. p. 1–27.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





