1. Introduction
Endodontic treatment is concerned with operative dental therapy which needs to be performed in conditions of irreversible inflammatory process of the dental pulp. Pulp inflammation may be caused by multiple factors, such as trauma, chronical irritation, deep caries [
1]. Globally dental caries is one of the most common problems. Factors necessary for it to occur are presence cariogenic species of oral bacteria, fermentable carbohydrates and tooth surface susceptible to acidic attack [
2]. Caries lesions may be categorized according to the depth of penetration into the tissue (e.g., enamel, dentin, pulp) [
3]. When the process of caries spreads deeply into the tooth tissues and reaches the pulp causing irreversible inflammation, endodontic treatment needs to be performed. This type of dental procedure is gaining popularity due to the desire of patients to preserve their natural teeth and increased awareness of the benefits of maintaining natural dentition. Endodontic treatment is composed of receiving an access to the pulp chamber, chemo mechanical preparation and obturation of the root canals. Such treated tooth requires functional restoration according to clinical needs. The elimination of microorganisms, necrotic tissues and accumulated hard tissue debris is crucial for the success of the treatment. Over 35% of the root canal area remains untrimmed, regardless of the instrumentation technique used. Chemical preparation using disinfectant solutions to irrigate the root canal system is essential [
4]. For this purpose, various irrigation liquids were introduced to the clinical procedures. As high properties of irrigation solutions are not sufficient to achieve the desired disinfection of the canal, some of these liquids should be activated to emphasize the positive effect which they cause in the whole endodontic treatment process.
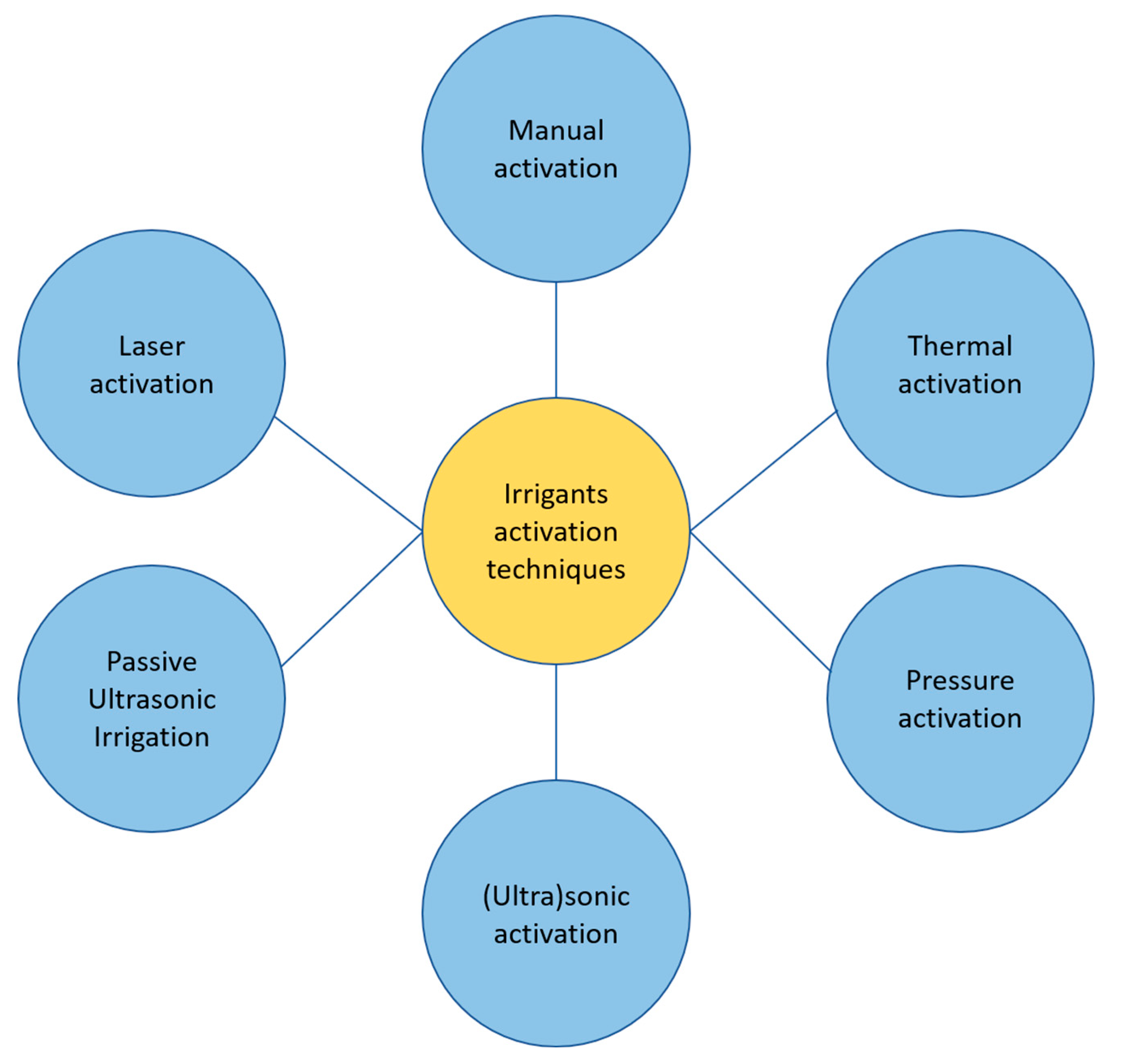
Among methods of activation of irrigants utilized during an endodontic treatment we can outline:
Manual activation
Thermal techniques (internal of external to the root)
Pressure techniques (EndoVac, Kerr Endodontics, Gilbert, USA; Rinsendo, Dürr Dental, Bietigheim, Germany)
Sonic/ultrasound techniques (EndoAcivator, Dentsply Maillefer, Ballaigues, Switzerland; EDDY, VDW, München, Germany; Ripsisonic, Medidenta International Inc, Woodside, NY; SAF, ReDent, Ra’anana, Israel).
Passive Ultrasonic Irrigation (PUI)
Laser techniques
The schematic division of irrigant activation techniques is presented on
Figure 1.
2. Materials and methods
An electronic search of the literature was conducted within PubMed, Scopus, Web of Science and Google Scholar databased. A combination of free-text words was used: irrigation, activation, sodium hypochlorite, irrigation protocols. From the search results a group of articles was selected with respect to their significance in the topic. An emphasis was put on clinical application and/or scientific-based research in the scope of activation of irrigants in endodontic treatment.
3. Irrigation protocols
Root canal irrigation during the endodontic treatment aims for removal of remaining pulp, bacteria, smear layer and other contamination. The literature describes irrigation with sodium hypochlorite (NaOCl) in concentration of 1-6% as a golden standard in endodontics [
5]. Smear layer is produced during instrumentation and is composed of organic and inorganic material. It can be removed by various techniques such as chemical, ultrasonic, laser and combination of them. As compounds which remove smear layer, we may list sodium hypochlorite in concentrations of 1-6%, chelating agents (Ethylenediaminetetraacetic acid - EDTA in concentrations of 10-19%, tetracyclines) and citric acid in concentrations of 10-50%. [
6,
7]. Many clinicians recognize chlorhexidine (CHX) as an antibacterial compound. Because of great versatility of chemicals utilized during an endodontic treatment, the procedure must be performed with help of rubber-dam.
Mechanical processing should not be happening inside a dry root canal [
8]. Working in a dry environment may lead to breaking the instrument or blockage of physiological apex with shavings of machined dentin. While using endodontic instruments it is advised to use aqueous solution of NaOCl or pure distilled water or designated lubricants which may possess antibacterial properties [
9]. According to the literature, in order to create the lubrication for the tool, one might use aqueous solutions rather than readymade lubricants. They are lowering the torsional loading of NiTi tools as well as their force generated during operation [
8,
10]. Between usage different tools, it is advised to irrigate the root canal with 2 mL of NaOCl [
11]. It is not recommended to irrigate with chelating compounds during root canal processing. It might lead to erosion of the root dentin [
12]. Chelates are utilized in the final irrigation protocol in order to remove of the inorganic fraction of the smear layer [
13].
After removal of the smear layer, irrigation with antiseptic solution such as NaOCl or CHX is repeated in order to obtain induce bactericidal action on exposed root canal wall [
14]. Researchers are not in agreement what should be the most suitable concentration of NaOCl solution’s during irrigation. Some studies suggest that concentration of 5.25% is superior with eradication of
E.faecalis from infected root canals in comparison to 2.5 and 0.5% concentrations (without activation) [
15]. On the other hand, other study does not indicate any major differences between concentrations but rather highlight frequent introduction of new liquid and using relatively large volume of solution by operator as an antibacterial factors [
16].
However, using 17% of EDTA solution for 1 min in the final irrigation protocol (with subsequent irrigation with NaOCl) causes removal of smear layer and opening of the dentin tubules. Irrigation with EDTA for 10 min caused excessive peritubular and intratubular dentinal erosion [
17].
If for irrigation with EDTA an ultrasonic activation (UA) was used, then removal of smear layer and contaminations in the apical area of straight root canal is achievable in 1 min (lack of difference in comparison with 3 min of activated irrigation) [
18]. For final irrigation protocol some clinicians add CHX solution. Some experiments shown that 2% solution of CHX exceeds 5.25% solution of NaOCl in eliminating E.faecalis [
19]. On the contrary, there are results showcasing sodium hypochlorite as superior in terms of bactericidal properties [
20,
21].
4. Irrigants activation techniques
Utilizing conventional syringe for irrigation is the most common technique used in general practice. It implies a variety of problems including vapor lock effect [
22] as well as lower disinfection level of as much as 1/3 of apical part of root canal [
23]. The literature reports that activation of irrigants led to more efficient smear layer cleansing in comparison with the same liquids without activation [
24]. Moreover, it was reported that activation of sodium hypochlorite improves its capability of dissolving organic tissue [
25]. Irrigants need to have direct contact with whole root canal wall in order to act effectively. During conventional irrigation with needle, the exchange of fluids occurs only in the very short distance around the needles’ tip (approximately 1.00-1.15 mm according to the studies) [
26]. That is a reason why syringe irrigation shall be utilized only when it can be inserted within 1 mm of working length. It is not always possible considering complex anatomy on root canals and risk of pushing the solution through the tissues [
26,
27]. The velocity of administration of the liquid is a factor directly influencing flow outside of the needle. A wide distribution of aforementioned parameter was observed between operators. It makes the syringe irrigation challenging to standardize and to control [
28].
4.1. Manual activation
Manual dynamic activation (MDA) is an activation using gutta percha cone adjusted to wedge 1 mm before its working length. A gentle pumping movement is introduced to the root canal filled with irrigant. It consists of short vertical strokes (2 mm amplitude in 100 times/min pace). Activation is advised (either NaOCl or EDTA solutions) after full processing of the root canal [
29]. Using MDA for activation of EDTA or citric acid solution enhances its affinity to remove the smear layer and debris [
30]. Especially in 1/3 of the root canal apex in comparison to irrigating with EDTA without activation [
31,
32]. An improved cleansing of apical area from organic tissues was showcased by using MDS in comparison to conventional irrigation. However, with none of these techniques did one achieve a 100% of decontamination [
33].
The study implied that manual activation causes risk of post-treatment pain, occurring 24h after the procedure. MDA was compared to irrigation with needle without any activation and passive ultrasonic irrigation. The pain was evaluated after endodontic treatment of teeth with irreversible pulp inflammation [
34]. Also penetration depth of irrigants into root canal dentin is lower with MDA compared to sonic, ultrasonic and laser-induced activation techniques [
35].
4.2. Thermal techniques
Sodium hypochlorite has boiling point in the range between 96 and 120°C [
36]. Heated solution of NaOCl has greater potential for dissolving pulp and cleansing of a root canal than solution at the room temperature [
37]. Additional heating of sodium hypochlorite boosts its ability to dissolve necrotic pulp tissue as well as improves its efficiency against E. faecalis. On the other hand, heated solutions remove the organic part of dentine shavings more effectively than non-heated equivalents. High temperature raises the rate of the NaOCl reaction positively influencing its antibacterial properties as well as capability of dissolving organic residues. It is indicated that heating up to 50-60°C is an optimal range [
38].
We can outline two methods of increasing the temperature of irrigating solution: extracanal (heating of the solution before injection to the root canal) and intracanal (heating directly inside the root canal with heat carriers). Research indicates that intracanal heating of NaOCl is a more effective method than extracanal heating [
39]. The former technique caused much better cleansing of walls of the root canal from debris in comparison to the latter [
40]. It occurs most probably due to the rapid heat exchange between the tissue and environment in in vivo conditions. It results in lesser effectiveness of heating the solution extracanal than shown in the studies [
41].
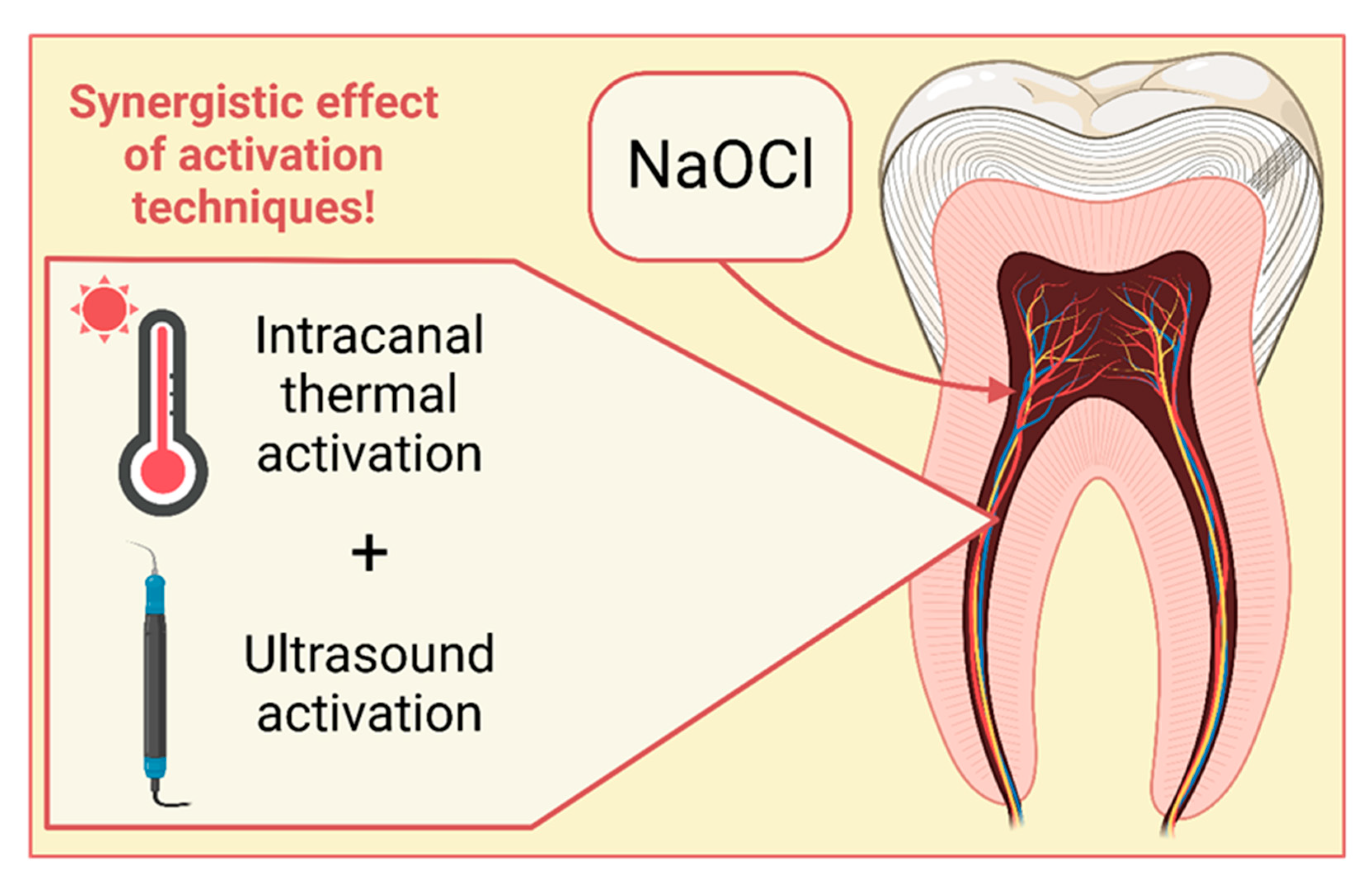
To the intracanal heating technique, heat carriers (System-B - Endodontic Heat Source, Kerr Endodontics, Gilbert, USA or alike) are used. The temperature of the device is capped at 150 to 180°C and the size of the tip is 30/04. Prior to introducing the heat carrier, a processing of the root canal is necessary. Root canal is filled with sodium hypochlorite with an endodontic needle. Heat carrier is introduced in room temperature up till 3 mm from the end of the working length and subsequently activated. Each activation cycle of the heat carrier lasts 5s with another 5s of pause. During the process, carries makes short upward and downward movement with few mm of amplitude in order to mix the NaOCl solution. After each of the aforementioned cycles, the irrigation solution is replaced with a fresh one [
36,
42]. Clinicians recommend UA of the NaOCl heated intracanal because it results with a better penetration into dentin tubules and better purity of canals in comparison to sole UA or syringe activation [
41]. The synergistic effect of those two techniques is depicted on
Figure 2. While combining thermal activation and UA, better dissolving of pulp was achieved in the side dentin tubules which remained contaminated after usage of UA or thermal activation as a sole activation techniques [
42].
4.3. Ultrasound techniques
Application of ultrasound in the course and after root canal preparation is an essential step toward improving the disinfection. The frequencies utilized in dental ultrasound equipment ranger from 25 to 40 kHz. Disinfection properties of ultrasound is based on the phenomena of cavitation and acoustic stream. Cavitation is minimized and limited to the tip of the utilized instrument while the effect of acoustic stream is more significant [
43]. It was proved that UA of NaOCl considerably enhances the efficiency of purifying the root canal area [
44], including the best lateral canal penetration [
45], antibacterial properties [
46], boosts the dissolution of necrotic tissues [
47] as well as the organic part of smear layer [
48]. UA of NaOCl in 3 cycles for 10-20 s for each root canal (with irrigant renewal after each cycle) is considered as a sufficient time to achieve cleansed canals after finished preparation. Ultrasounds seem much less efficient in enhancing the activity of EDTA. Nevertheless, they might contribute to better removal of smear layer as mentioned before [
43]. While using UA, the operator needs to insert the tip of the instrument 1 mm before the working length [
46]. Ultrasound activation is also beneficial for endodontic treatment taking place during multiple visits giving the possibility to get rid of Ca(OH)2 from the walls of root canals more efficiently in comparison to the manual syringe [
49]. Using tips for UA impose a risk of tool breakage inside the root canal or escape of irrigant outside of apical foramen [
50]. The operators must watch out not to touch the canal walls with a tip because it might lead to damping effect or unevenness on the root canal walls. [
44].
4.4. Laser activation techniques
Lasers utilized for activation of irrigants include diode laser, neodymium-doped yttrium aluminum garnet (ND:YAG) laser, erbium-doped yttrium aluminum garnet laser (Er:YAG) laser and erbium, chromium-doped yttrium, scandium, gallium and garnet (Er,Cr:YSGG) laser. We can divide laser activation techniques base on the tip placement: Laser-Activated Irrigation (LAI) where tip is placed inside the root canal and Photon-Induced Photoacoustic Streaming (PIPS) where the laser tip is placed in the pulp chamber. For the latter technique mostly Er:YAG laser is utilized. Moreover, lasers might be used for disinfection of the root canal walls without using irrigants. Yet again, paring two techniques gives a superius effect. Using laser in the canal filled with NaOCl in comparison to using the laser alone gives much better antibacterial effect [
51,
52].
Mechanism of cleansing with LAI is based on the cavitation in the solution [
53,
54]. Independent of the utilized laser, the tip placed in a root canal should be composed of glass fiber with diameter between 200 and 400 µm [
54]. The root canal shall be processed until at least ISO 30 with tool expansion of 02. Tip insertion depth should be 1 mm lower than canal working length [
55]. After placing the tip in the desired depth, one need to activate the laser and slowly remove fiber from the root canal with pace of 1 mm/s [
56]. In case of broad canals during removal of the fiber, the operator needs to make sideway, sweeping movements. It is important to activate the laser only during the removal from the canal and not while inserting the fiber toward the canal. It allows to avoid the danger of processing the canal with laser. Keeping the glass fiber in constant motion in the root canal is equally important and prevents local temperature spikes [
57].
PIPS is an activation method in which special Er:YAG laser tip is placed inside the pulp chamber and induces cavitation within the irrigant (there is no need of placing the tool to the end of canal to obtain the disinfecting effect [
58]. High velocity irrigating streams are created in a further distance from the source of activation in comparison with UA [
59]. In the literature, a movement of created follicles in the whole internal part of the multiroot tooth is visible with the laser tip placed just in the pulp chamber [
54]. For successful PIPS activation a minimal instrumentation during canal processing is sufficient. It results in ISO 20/25 apical preparation without designation of specific taper while utilizing EndoVac system it is required for at least 35/.04 apical preparation. Despite this, the PIPS results in more significant potential of pushing NaOCl through the apical foramen than EndoVac [
60].
LAI is more efficient in cleansing the root canals, especially from intracanal bacteria than conventional or UA irrigation [
53,
61]. Using this type of activation allows for achieving more smooth surface of the root canal than while using UA. It translates into creation of stronger bonding between dentin and root canal filler [
62]. What is more, PIPS seems to be more effective than UA irrigation in removal of apically located dentin residue [
58]. Satisfactory removal of smear layer by PIPS was also reported [
63,
64] with a high quality of open tubules in the root canal dentin [
63]. PIPS appears to be more effective than conventional or sonic activation techniques in killing the bacteria deep in the dentinal tubules [
65]. No advantage in terms of disinfection was demonstrated for either LAI nor PIPS [
54].
4.5. Other activation systems
On the market, there are special devices available, designated to activation of irrigants. Among them we can enlist pressure systems (EndoVac, Kerr Endodontics, Gilbert, USA; Rinsendo, Dürr Dental, Bietigheim, Germany) or sound-wave based system (EndoAcivator, Dentsply Maillefer, Ballaigues, Switzerland; EDDY, VDW, München, Germany; Ripsisonic, Medidenta International Inc, Woodside, NY; SAF, ReDent, Ra’anana, Israel).
EndoVac uses negative pressure for irrigation. It results with cancelling of vapor lock effect and provides better cleaning at depth of 1 mm from the working length in comparison to irrigation with syringe and needle [
66]. Apical negative pressure irrigation technique was shown to be the most effective in delivering the irrigant up to the working length in mature permanent teeth [
67]. After irrigation with EndoVac system patients less frequently complained about post-treatment pain, comparing to the irrigation with needle [
68]. It might be linked with the fact that using EndoVac limits the occurrence of pushing the irrigant through the canal to the tissues surrounding the apex, in juxtaposition with Manual Dynamic Agitation (MDA) or UA [
69,
70]. Moreover, EndoVac is more effective in removal of smear layer than MDA. It is mainly because it removes vapor lock effect more efficiently and better cleansing of apical area [
61]. Efficiency of root canal processing is greater with using UA, but EndoVac is much safer in terms of potential pushing of irrigant through the apex to apical tissues [
71]. This system requires minimal apical preparation measuring 35/.04 [
60].
RinsEndo (Dürr Dental, Bietigheim, Germany) is a hydrodynamic irrigation system which utilizes pressure-sucking technology [
70]. It characterizes with greater penetration depth of the irrigants in comparison with using syringe and needle. However, it bears a greater risk of pushing the irrigant through the apical foramen [
72]. The efficiency of cleansing root canals is better with RinsEndo in comparison with using syringe and needle but worse while comparing to MDA [
50]. There are studies regarding presence of broken tool inside the root canal which is unremovable. In that scope, pressure instruments were proven to be the best choice for irrigation. They possessed better permeability of NaOCl than UD or EndoActivator. It may ensure better microbiological control despite the difficulty of tool breakage [
73].
EndoActivator system (Dentsply Maillefer, Ballaigues, Switzerland) is a soundwave-based system equipped with non-cutting tip from synthetic material for activation of the irrigant. Tip does not cause shaving of the dentin which prevents further widening of the canals. It is possible to use many types of tips depending on the size of the root canal. It must be adjusted in the way that the tip must move freely in the canal up to 2 mm before the working length [
74]. EndoActivator might be utilized to activate solution of EDTA [
75].
EDDY (VDW, München, Germany) is a tip made of elastic polyamide fiber activated with the air ultrasonic tip, designated to work in a frequency range 5000-6000 Hz. Flexible working tip is safe for root canal walls and causes no damage to dentin comparing to UA sharp tips [
76]. Sonic activation (with EDDY of EndoActivator) and PUI are better choices for removing debris than irrigation without activation with syringe in straight root canals. Moreover, EDDY and PUI removed smear layer more efficiently than manual activation [
77]. The study shows that EDDY can be also more effective than PUI at removing antibiotic pastes from the root canal [
78]. One has to bear in mind, that patients subjected to irrigation with EDDY complained about post-treatment pain more frequently than group of patients treated with MDA. It might relate to much more frequent pushing irrigant through the apical foramen caused by using EDDY [
39].
5. Efficacy of activation systems in curved canals
The curvature may affect how effectively the activation system works. Studies using root canal models with curved shapes are uncommon, the majority employ models with straight canals [
79]. Increasing the apical size above 40 may enhance disinfection when treating severely curved canals, however doing thus isn't always achievable [
80]. The adverse impacts of increased canal curvature were most obvious for the sonic techniques, but this was not noticeable for PUI. The latter, however, may have an exaggerated PUI influence due to careful ultrasonic file prebending [
79].
According to multiple research sonic agitation (Endo Activator and EDDY) performed significantly better than syringe irrigation but not significantly differently than PUI [
81]. The smear layer was substantially more responsive to all activation methods than syringe irrigation. Nevertheless, a number of studies have found a substantial difference between sonic and ultrasonic cleaning abilities. While activation with PUI produced the best results for clearing debris from uneven canal surfaces, EDDY outperformed PUI significantly in terms of antibacterial activity. EDDY and syringe irrigation eliminated the smear layer from the coronal region substantially more effectively than from the apical region. In contrast, PUI did not show any appreciable variations across levels in terms of smear layer removal. The increased streaming velocity of ultrasonic devices, which is often unaffected by the curvature of the root canal, may help to explain this finding.
6. Discussion
The state of knowledge about techniques and biomaterials used in modern medicine changes rapidly nowadays. It is one of the clinicians’ tasks to constantly educate and search for the best possible solutions for their patients. To gain the latest and reliable knowledge about clinical procedures, also in field of endodontic treatment, doctors may follow instructions provided by leading endodontic associations. They offer verified and actual knowledge presented in the formula of guidelines.
In 2006 European Society of Endodontology presented a guideline about endodontic treatment [
82]. Authors list following aims of the irrigation - elimination of microorganisms, flush out debris, lubrication of root canal instruments and dissolution of organic debris. Attention is paid to not irritating the periradicular tissues by using suitable syringe and exact amount of the irrigant. No specific data is given. The possibility of using ultrasonic or sonic systems is also outlined.
British Endodontic Society remarks that success of the endodontic treatment depends on accurate diagnosis and completion of each stage of treatment to a high standard [
83]. In the guided presented in 2022 it is noticed that primary objective of endodontic treatment is elimination of microorganisms from the root canal system and preventing reinfection. The aims of the irrigation are defined as: antibacterial action, tissue dissolving capabilities, reduction of the friction between the instrument and dentine, improving the cutting effectiveness of the files, cools the file or tooth, and a washing effect to flush out debris. The very important point is that irrigation is the only way to impact those areas of the root canal wall not touched by mechanical instrumentation. Irrigants recommended by British Endodontic Society are sodium hypochlorite solution (0.5% to 5.25%), EDTA (17% ) and Chlorhexidine (2%). Activation of irrigants is also recommended. They can be enhanced using dynamic agitation, ultrasonic activation, negative pressure irrigation or with heat.
Considering modern studies, we may conclude that sodium hypochlorite is the primary irrigant of choice. Usage of the chelator is also necessary in order to dissolve hard- tissue debris created during instrumentation or the inorganic components of the smear layer [
84]. Study concludes that there is no evidence that long-term clinical success of endodontic treatment can be improved by irrigants’ activation. However, it is noted that NaOCl and EDTA delivered by a syringe and needle and activated by an ultrasonic file form the most popular and efficient protocol of irrigation during root canal treatment. Such approach is promoted in the review from 2012 which stresses the role of ultrasonic irrigation as having a positive effect on chemical, biological and physical debridement of the root canal system as investigated in many in vitro studies [
85]. The study after analyses of articles available on MEDLINE and Cochrane bases, concludes that to fully compare different methods of activation, there is a need of protocols’ standardization and further tests are necessary to obtain a complete state of the art. Another review on effectiveness of ultrasonically activated irrigation from 2018 comparing 15 studies came to a similar conclusion as presented above [
86]. Authors notice that ultrasonic irrigant’s activation is more efficient in microorganism reduction compared to other irrigant activation techniques.
7. Summary
Contemporary state of dental knowledge does not indicate one optimal root canal irrigation protocol measured by criterion of the clinical success of the endodontic treatment. There are many irrigants including: NaOCl, EDTA, CHX, citric acid, distilled water, saline and many techniques of their activation. Among them, we can outline manual activation, thermal techniques (extra- and intracanal), pressure techniques, sonic/ultrasonic techniques, PUI and laser techniques. Activation methods such as EDDY, PUI or UA allow for much more efficient removal of smear layer.
Some activation techniques, like EDDY or manual activation with gutta-percha, might contribute to post-treatment pain. Literature indicates superior advantage of activation of irrigants with lack of thereof. However, one effective way of activation cannot be indicated. It is very much dependent on specific patients’ root canal anatomy and predisposition.
Author Contributions
Conceptualization, M.R. and K.S-M.; methodology, A.P.; validation, M.D., P.P. and K.S-M.; formal analysis, M.D., K.S-M, W.G. and M.M.; investigation, M.R., A.P and N.J.; resources, P.P.; writing—original draft preparation, M.R. and A.P.; writing—review and editing, P.P. and N.J.; visualization, P.P.; supervision, K.S-M., M.D., W.G. and M.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giraud, T.; Jeanneau, C.; Rombouts, C.; Bakhtiar, H.; Laurent, P.; About, I. Pulp Capping Materials Modulate the Balance between Inflammation and Regeneration. Dental Materials 2019, 35, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.P.; Dhillon, J.K. Dental Caries: A Disease Which Needs Attention. Indian J Pediatr 2018, 85, 202–206. [Google Scholar] [CrossRef] [PubMed]
- MacHiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res 2020, 54, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Cheung, G.S.P.; Lee, A.H.C.; McGrath, C.; Neelakantan, P. PROMs Following Root Canal Treatment and Surgical Endodontic Treatment. Int Dent J 2023, 73, 28–41. [Google Scholar] [CrossRef]
- Baumgartner, J.C.; Cuenin, P.R. Efficacy of Several Concentrations of Sodium Hypochlorite for Root Canal Irrigation. J Endod 1992, 18, 605–612. [Google Scholar] [CrossRef]
- Violich, D.R.; Chandler, N.P. The Smear Layer in Endodontics – a Review. 2010, 2–15. [CrossRef]
- Roberti, F.; Savaris, J.M.; Tay, F.R.; Bortoluzzi, A. Smear Layer Removal Using Passive Ultrasonic Irrigation and Different Concentrations of Sodium Hypochlorite. J Endod 2020. [Google Scholar] [CrossRef]
- Peters, O.A.; Boessler, C.; Zehnder, M. Effect of Liquid and Paste-Type Lubricants on Torque Values during Simulated Rotary Root Canal Instrumentation. Int Endod J 2005, 38, 223–229. [Google Scholar] [CrossRef]
- Wong, S.; Mundy, L.; Chandler, N.; Upritchard, J.; Purton, D.; Tompkins, G. Antibacterial Properties of Root Canal Lubricants: A Comparison with Commonly Used Irrigants. Australian Endodontic Journal 2014, 40, 111–115. [Google Scholar] [CrossRef]
- Boessler, C.; Peters, O.A.; Zehnder, M. Impact of Lubricant Parameters on Rotary Instrument Torque and Force. J Endod 2007, 33, 280–283. [Google Scholar] [CrossRef]
- Jahromi, M.Z.; Fathi, M.H.; Zamiran, S. Experimental Study of Smear Layer and Debris Remaining Following the Use of Four Root Canal Preparation Systems Using Scanning Electron Microscopy. J Islam Dent Assoc Iran 2013, 25. [Google Scholar]
- Niu, W.; Yoshioka, T.; Kobayashi, C.; Suda, H. A Scanning Electron Microscopic Study of Dentinal Erosion by Final Irrigation with EDTA and NaOCl Solutions. Int Endod J 2002, 35, 934–939. [Google Scholar] [CrossRef]
- Nogo-Živanović, D.; Kanjevac, T.; Bjelović, L.; Ristić, V.; Tanasković, I. The Effect of Final Irrigation with MTAD, QMix, and EDTA on Smear Layer Removal and Mineral Content of Root Canal Dentin. Microsc Res Tech 2019, 82, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.S.; Armas, A.; Goldman, M.; Lin, P.S. A Scanning Electron Microscopic Comparison of a High Volume Final Flush with Several Irrigating Solutions: Part 3. J Endod 1983, 9, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Berber, V.B.; Gomes, B.P.F.A.; Sena, N.T.; Vianna, M.E.; Ferraz, C.C.R.; Zaia, A.A.; Souza-Filho, F.J. Efficacy of Various Concentrations of NaOCl and Instrumentation Techniques in Reducing Enterococcus Faecalis within Root Canals and Dentinal Tubules. Int Endod J 2006, 39, 10–17. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N.; Favieri, A.; Lima, K.C. Chemomechanical Reduction of the Bacterial Population in the Root Canal after Instrumentation and Irrigation with 1%, 2.5%, and 5.25% Sodium Hypochlorite. J Endod 2000, 26, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.V.; Guedes, D.F.C.; Pécora, J.D.; da Cruz-Filho, A.M. Time-Dependent Effects of Chitosan on Dentin Structures. Braz Dent J 2012, 23, 357–361. [Google Scholar] [CrossRef]
- Kuah, H.G.; Lui, J.N.; Tseng, P.S.K.; Chen, N.N. The Effect of EDTA with and without Ultrasonics on Removal of the Smear Layer. J Endod 2009, 35, 393–396. [Google Scholar] [CrossRef]
- Önçaǧ, Ö.; Hoşgör, M.; Hilmioǧlu, S.; Zekioǧlu, O.; Eronat, C.; Burhanoǧlu, D. Comparison of Antibacterial and Toxic Effects of Various Root Canal Irrigants. Int Endod J 2003, 36, 423–432. [Google Scholar] [CrossRef]
- Williamson, A.E.; Cardon, J.W.; Drake, D.R. Antimicrobial Susceptibility of Monoculture Biofilms of a Clinical Isolate of Enterococcus Faecalis. J Endod 2009, 35, 95–97. [Google Scholar] [CrossRef]
- Dunavant, T.R.; Regan, J.D.; Glickman, G.N.; Solomon, E.S.; Honeyman, A.L. Comparative Evaluation of Endodontic Irrigants against Enterococcus Faecalis Biofilms. J Endod 2006, 32, 527–531. [Google Scholar] [CrossRef]
- Dioguardi, M.; Di Gioia, G.; Illuzzi, G.; Laneve, E.; Cocco, A.; Troiano, G. Endodontic Irrigants: Different Methods to Improve Efficacy and Related Problems. Eur J Dent 2018, 12, 459–466. [Google Scholar] [CrossRef]
- Teixeira, C.S.; Felippe, M.C.S.; Felippe, W.T. The Effect of Application Time of EDTA and NaOCI on Intracanal Smear Layer Removal: An SEM Analysis. Int Endod J 2005, 38, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Pladisai, P.; Ampornaramveth, R.S.; Chivatxaranukul, P. Effectiveness of Different Disinfection Protocols on the Reduction of Bacteria in Enterococcus Faecalis Biofilm in Teeth with Large Root Canals. J Endod 2016, 42, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Stojicic, S.; Zivkovic, S.; Qian, W.; Zhang, H.; Haapasalo, M. Tissue Dissolution by Sodium Hypochlorite: Effect of Concentration, Temperature, Agitation, and Surfactant. J Endod 2010, 36, 1558–1562. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Lambrianidis, T.; Kastrinakis, E. Irrigant Flow within a Prepared Root Canal Using Various Flow Rates: A Computational Fluid Dynamics Study. Int Endod J 2009, 42, 144–155. [Google Scholar] [CrossRef]
- Abou-Rass, M.; Piccinino, M. V The Effectiveness of Four Clinical Irrigation Methods on the Removal of Root Canal Debris. Oral Surgery, Oral Medicine, Oral Pathology 1982, 54, 323–328. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Lambrianidis, T.; Kastrinakis, E.; Bekiaroglou, P. Measurement of Pressure and Flow Rates during Irrigation of a Root Canal Ex Vivo with Three Endodontic Needles. Int Endod J 2007, 40, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Barańska-Gachowska, M. Endodoncja Wieku Rozwojowego i Dojrzałego; 3rd ed.; Czelej, 2021; ISBN 9788375633061.
- Olivieri, J.G.; García Font, M.; Stöber, E.; de Ribot, J.; Mercadé, M.; Duran-Sindreu, F. Effect of Manual Dynamic Activation with Citric Acid Solutions in Smear Layer Removal: A Scanning Electron Microscopic Evaluation. J Dent Sci 2016, 11, 360–364. [Google Scholar] [CrossRef]
- Andrabi, S.M.U.N.; Kumar, A.; Mishra, S.K.; Tewari, R.K.; Alam, S.; Siddiqui, S. Effect of Manual Dynamic Activation on Smear Layer Removal Efficacy of Ethylenediaminetetraacetic Acid and SmearClear: An in Vitro Scanning Electron Microscopic Study. Australian Endodontic Journal 2013, 39, 131–136. [Google Scholar] [CrossRef]
- Khaord, P.; Amin, A.; Shah, M.; Uthappa, R.; Raj, N.; Kachalia, T.; Kharod, H. Effectiveness of Different Irrigation Techniques on Smear Layer Removal in Apical Thirds of Mesial Root Canals of Permanent Mandibular First Molar: A Scanning Electron Microscopic Study. Journal of Conservative Dentistry 2015, 18, 321–326. [Google Scholar] [CrossRef]
- Huang, T.Y.; Gulabivala, K.; Ng, Y.L. A Bio-Molecular Film Ex-Vivo Model to Evaluate the Influence of Canal Dimensions and Irrigation Variables on the Efficacy of Irrigation. Int Endod J 2008, 41, 60–71. [Google Scholar] [CrossRef]
- Topçuoğlu, H.S.; Topçuoğlu, G.; Arslan, H. The Effect of Different Irrigation Agitation Techniques on Postoperative Pain in Mandibular Molar Teeth with Symptomatic Irreversible Pulpitis: A Randomized Clinical Trial. J Endod 2018, 44, 1451–1456. [Google Scholar] [CrossRef]
- Galler, K.M.; Grubmüller, V.; Schlichting, R.; Widbiller, M.; Eidt, A.; Schuller, C.; Wölflick, M.; Hiller, K.A.; Buchalla, W. Penetration Depth of Irrigants into Root Dentine after Sonic, Ultrasonic and Photoacoustic Activation. Int Endod J 2019, 52, 1210–1217. [Google Scholar] [CrossRef]
- Iandolo, A.; Iandolo, G.; Malvano, M.; Pantaleo, G.; Simeone, M. Modern Technologies in Endodontics. G Ital Endod 2016, 30, 2–9. [Google Scholar] [CrossRef]
- Cunningham, W.T.; Cole, J.S.; Balekjian, A.Y. Effect of Alcohol on the Spreading Ability of Sodium Hypochlorite Endodontic Irrigant. Oral Surgery, Oral Medicine, Oral Pathology 1982, 54, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Sirtes, G.; Waltimo, T.; Schaetzle, M.; Zehnder, M. The Effects of Temperature on Sodium Hypochlorite Short-Term Stability, Pulp Dissolution Capacity, and Antimicrobial Efficacy. J Endod 2005, 31, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Gupta, S.; Nikhil, V.; Bhadoria, A.; Raj, S. Effect of Intracanal and Extracanal Heating on Pulp Dissolution Property of Continuous Chelation Irrigant. Journal of Conservative Dentistry 2021, 24, 544–548. [Google Scholar] [CrossRef]
- Iandolo, A.; Amato, M.; Dagna, A.; Poggio, C.; Abdellatif, D.; Franco, V.; Pantaleo, G. Intracanal Heating of Sodium Hypochlorite: Scanning Electron Microscope Evaluation of Root Canal Walls. Journal of Conservative Dentistry 2018, 21, 569. [Google Scholar] [CrossRef]
- Iandolo, A.; Abdellatif, D.; Amato, M.; Pantaleo, G.; Blasi, A.; Franco, V.; Neelakantan, P. Dentinal Tubule Penetration and Root Canal Cleanliness Following Ultrasonic Activation of Intracanal-Heated Sodium Hypochlorite. Australian Endodontic Journal 2020, 46, 204–209. [Google Scholar] [CrossRef]
- Amato, M.; Pantaleo, G.; Abtellatif, D.; Blasi, A.; Gagliani, M.; Iandolo, A. An in Vitro Evaluation of the Degree of Pulp Tissue Dissolution through Different Root Canal Irrigation Protocols. Journal of Conservative Dentistry 2018, 21, 175–179. [Google Scholar] [CrossRef]
- Plotino, G.; Pameijer, C.H.; Maria Grande, N.; Somma, F. Ultrasonics in Endodontics: A Review of the Literature. J Endod 2007, 33, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Sabins, R.A.; Johnson, J.D.; Hellstein, J.W. A Comparison of the Cleaning Efficacy of Short-Term Sonic and Ultrasonic Passive Irrigation after Hand Instrumentation in Molar Root Canals. J Endod 2003, 29, 674–678. [Google Scholar] [CrossRef]
- Castelo-Baz, P.; Martín-Biedma, B.; Cantatore, G.; Ruíz-Piñón, M.; Bahillo, J.; Rivas-Mundiña, B.; Varela-Patiño, P. In Vitro Comparison of Passive and Continuous Ultrasonic Irrigation in Simulated Lateral Canals of Extracted Teeth. J Endod 2012, 38, 688–691. [Google Scholar] [CrossRef]
- Caron, G.; Nham, K.; Bronnec, F.; MacHtou, P. Effectiveness of Different Final Irrigant Activation Protocols on Smear Layer Removal in Curved Canals. J Endod 2010, 36, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Al-Jadaa, A.; Paqué, F.; Attin, T.; Zehnder, M. Acoustic Hypochlorite Activation in Simulated Curved Canals. J Endod 2009, 35, 1408–1411. [Google Scholar] [CrossRef] [PubMed]
- Guerisoli, D.M.Z.; Marchesan, M.A.; Walmsley, A.D.; Lumley, P.J.; Pecora, J.D. Evaluation of Smear Layer Removal by EDTAC and Sodium Hypochlorite with Ultrasonic Agitation. Int Endod J 2002, 35, 418–421. [Google Scholar] [CrossRef]
- Van Der Sluis, L.W.M.; Wu, M.K.; Wesselink, P.R. The Evaluation of Removal of Calcium Hydroxide Paste from an Artificial Standardized Groove in the Apical Root Canal Using Different Irrigation Methodologies. Int Endod J 2007, 40, 52–57. [Google Scholar] [CrossRef]
- McGill, S.; Gulabivala, K.; Mordan, N.; Ng, Y.L. The Efficacy of Dynamic Irrigation Using a Commercially Available System (RinsEndo®) Determined by Removal of a Collagen “bio-Molecular Film” from an Ex Vivo Model. Int Endod J 2008, 41, 602–608. [Google Scholar] [CrossRef]
- Cheng, X.; Xiang, D.; He, W.; Qiu, J.; Han, B.; Yu, Q.; Tian, Y. Bactericidal Effect of Er:YAG Laser-Activated Sodium Hypochlorite Irrigation Against Biofilms of Enterococcus Faecalis Isolate from Canal of Root-Filled Teeth with Periapical Lesions. Photomed Laser Surg 2017, 35, 386–392. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Z.; Ju, Y.; Tang, X. Bactericidal Efficacy of Three Parameters of Nd:YAP Laser Irradiation against Enterococcus Faecalis Compared with NaOCl Irrigation. Lasers Med Sci 2019, 34, 359–366. [Google Scholar] [CrossRef]
- De Meyer, S.; Meire, M.A.; Coenye, T.; De Moor, R.J.G. Effect of Laser-Activated Irrigation on Biofilms in Artificial Root Canals. Int Endod J 2017, 50, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, T.; Yahata, Y.; Handa, K.; Nakano, M.; Suzuki, S.; Kakiuchi, Y.; Tanaka, T.; Kanehira, M.; Suresh Venkataiah, V.; Saito, M. Er:YAG Laser-Induced Cavitation Can Activate Irrigation for the Removal of Intraradicular Biofilm. Sci Rep 2022, 12. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, X.; Liu, B.; Liu, X.; Yu, Q.; He, W. Effect of Laser-Activated Irrigations on Smear Layer Removal from the Root Canal Wall. Photomed Laser Surg 2017, 35, 688–694. [Google Scholar] [CrossRef] [PubMed]
- George, R.; Meyers, I.A.; Walsh, L.J. Laser Activation of Endodontic Irrigants with Improved Conical Laser Fiber Tips for Removing Smear Layer in the Apical Third of the Root Canal. J Endod 2008, 34, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, M. Laseroterapia w Endodoncji. Forum Stomatologii Praktycznej 2014, 30–42. [Google Scholar]
- Arslan, H.; Capar, I.D.; Saygili, G.; Gok, T.; Akcay, M. Effect of Photon-Initiated Photoacoustic Streaming on Removal of Apically Placed Dentinal Debris. Int Endod J 2014, 47, 1072–1077. [Google Scholar] [CrossRef]
- Koch, J.D.; Jaramillo, D.E.; DiVito, E.; Peters, O.A. Irrigant Flow during Photon-Induced Photoacoustic Streaming (PIPS) Using Particle Image Velocimetry (PIV). Clin Oral Investig 2016, 20, 381–386. [Google Scholar] [CrossRef]
- Yost, R.A.; Bergeron, B.E.; Kirkpatrick, T.C.; Roberts, M.D.; Roberts, H.W.; Himel, V.T.; Sabey, K.A. Evaluation of 4 Different Irrigating Systems for Apical Extrusion of Sodium Hypochlorite. J Endod 2015, 41, 1530–1534. [Google Scholar] [CrossRef]
- De Groot, S.D.; Verhaagen, B.; Versluis, M.; Wu, M.K.; Wesselink, P.R.; Van Der Sluis, L.W.M. Laser-Activated Irrigation within Root Canals: Cleaning Efficacy and Flow Visualization. Int Endod J 2009, 42, 1077–1083. [Google Scholar] [CrossRef]
- Bogari, D.F.; Alessa, M.; Aljaber, M.; Alghamdi, F.; Alamoudi, M.; Alhamed, M.; Alghamdi, A.J.; Elsherief, S.; Almalki, M.; Alhazzazi, T.Y. The Biological and Mechanical Effect of Using Different Irrigation Methods on the Bond Strength of Bioceramic Sealer to Root Dentin Walls. Cureus 2022. [Google Scholar] [CrossRef]
- Korkut, E.; Torlak, E.; Gezgin, O.; Özer, H.; Sener, Y. Antibacterial and Smear Layer Removal Efficacy of Er:YAG Laser Irradiation by Photon-Induced Photoacoustic Streaming in Primary Molar Root Canals: A Preliminary Study. Photomed Laser Surg 2018, 36, 480–486. [Google Scholar] [CrossRef]
- Golob, B.S.; Olivi, G.; Vrabec, M.; El Feghali, R.; Parker, S.; Benedicenti, S. Efficacy of Photon-Induced Photoacoustic Streaming in the Reduction of Enterococcus Faecalis within the Root Canal: Different Settings and Different Sodium Hypochlorite Concentrations. J Endod 2017, 43, 1730–1735. [Google Scholar] [CrossRef]
- Azim AA, Aksel H, Zhuang T, Mashtare T, Babu JP, H. G. Efficacy of 4 Irrigation Protocols in Killing Bacteria Colonized in Dentinal Tubules Examined by a Novel Confocal Laser Scanning Microscope Analysis. J Endod. 2016, 42, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.A.; Craig Baumgartner, J. Comparison of the EndoVac System to Needle Irrigation of Root Canals. J Endod 2007, 33, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Ankola, A.; Peerzade, M.; Sankeshwari, R.; Hampiholi, V.; Pai Khot, A.; Shah, M.A. Comparative Efficacy of Different Irrigant Activation Techniques for Irrigant Delivery Up to the Working Length of Mature Permanent Teeth: A Systematic Review and Meta-Analysis. Eur Endod J 2023, 8, 1–19. [Google Scholar] [CrossRef]
- Gondim, E.; Setzer, F.C.; Dos Carmo, C.B.; Kim, S. Postoperative Pain after the Application of Two Different Irrigation Devices in a Prospective Randomized Clinical Trial. J Endod 2010, 36, 1295–1301. [Google Scholar] [CrossRef]
- Parente, J.M.; Loushine, R.J.; Susin, L.; Gu, L.; Looney, S.W.; Weller, R.N.; Pashley, D.H.; Tay, F.R. Root Canal Debridement Using Manual Dynamic Agitation or the EndoVac for Final Irrigation in a Closed System and an Open System. Int Endod J 2010, 43, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Himel, V. Comparative Safety of Various Intracanal Irrigation Systems. J Endod 2009, 35, 545–549. [Google Scholar] [CrossRef]
- Malentacca, A.; Uccioli, U.; Zangari, D.; Lajolo, C.; Fabiani, C. Efficacy and Safety of Various Active Irrigation Devices When Used with Either Positive or Negative Pressure: An in Vitro Study. J Endod 2012, 38, 1622–1626. [Google Scholar] [CrossRef]
- Hauser, V.; Braun, A.; Frentzen, M. Penetration Depth of a Dye Marker into Dentine Using a Novel Hydrodynamic System (RinsEndo®). Int Endod J 2007, 40, 644–652. [Google Scholar] [CrossRef]
- Uzunoglu-Özyürek, E.; Dik Güzel, C.; Dogan Buzoglu, H. Effect of Different Irrigation Methods in the Presence of a Separated Instrument: An in Vitro Study. Acta Odontol Scand 2020, 78, 409–416. [Google Scholar] [CrossRef]
- Klyn, S.L.; Kirkpatrick, T.C.; Rutledge, R.E. In Vitro Comparisons of Debris Removal of the EndoActivatorTM System, the F FileTM, Ultrasonic Irrigation, and NaOCl Irrigation Alone after Hand-Rotary Instrumentation in Human Mandibular Molars. J Endod 2010, 36, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Ruddle, C. Endodontic Disinfection: Tsunami Irrigation. Saudi Endodontic Journal 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Chu, X.; Feng, S.; Zhou, W.; Xu, S.; Zeng, X. Cleaning Efficacy of EDDY versus Ultrasonically-Activated Irrigation in Root Canals: A Systematic Review and Meta-Analysis. BMC Oral Health 2023, 23, 155. [Google Scholar] [CrossRef] [PubMed]
- Urban, K.; Donnermeyer, D.; Schäfer, E.; Bürklein, S. Canal Cleanliness Using Different Irrigation Activation Systems: A SEM Evaluation. Clin Oral Investig 2017, 21, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Güven, Y.; Uygun, A.D.; Arslan, H. Efficacy of EDDY, Ultrasonic Activation, XP-Endo Finisher and Needle Irrigation on the Removal of MTAP from Artificially Created Grooves in Root Canals. Australian Endodontic Journal 2021, 47, 639–644. [Google Scholar] [CrossRef]
- Swimberghe, R.C.D.; Buyse, R.; Meire, M.A.; De Moor, R.J.G. Efficacy of Different Irrigation Technique in Simulated Curved Root Canals. Lasers Med Sci 2021, 36, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Dewsnup, N.; Pileggi, R.; Haddix, J.; Nair, U.; Walker, C.; Varella, C.H. Comparison of Bacterial Reduction in Straight and Curved Canals Using Erbium, Chromium:Yttrium-Scandium-Gallium-Garnet Laser Treatment versus a Traditional Irrigation Technique With Sodium Hypochlorite. J Endod 2010, 36, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Haupt, F.; Meinel, M.; Gunawardana, A.; Hülsmann, M. Effectiveness of Different Activated Irrigation Techniques on Debris and Smear Layer Removal from Curved Root Canals: A SEM Evaluation. Australian Endodontic Journal 2020, 46, 40–46. [Google Scholar] [CrossRef]
- Pitt Ford, T.R.; Riccucci, D.; Saunders, E.M.; Stabholz, A.; Suter, B. Quality Guidelines for Endodontic Treatment: Consensus Report of the European Society of Endodontology. Int Endod J 2006, 39, 921–930. [Google Scholar] [CrossRef]
- British Endodontic Society British Endodontic Society: A Guide to Good Endodontic Practice. 2022.
- Boutsioukis, C.; Arias-Moliz, M.T. Present Status and Future Directions – Irrigants and Irrigation Methods. Int Endod J 2022, 55, 588–612. [Google Scholar] [CrossRef] [PubMed]
- Mozo, S.; Llena, C.; Forner, L. Review of Ultrasonic Irrigation in Endodontics: Increasing Action of Irrigating Solutions. Med Oral Patol Oral Cir Bucal 2012, 17. [Google Scholar] [CrossRef] [PubMed]
- Nagendrababu, V.; Jayaraman, J.; Suresh, A.; Kalyanasundaram, S.; Neelakantan, P. Effectiveness of Ultrasonically Activated Irrigation on Root Canal Disinfection: A Systematic Review of in Vitro Studies. Clin Oral Investig 2018, 22, 655–670. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).