3.1. Raw bauxite charackterization
Raw bauxite was taken from the Timan deposit was pre-crushed using a rod mill and subsequently classified on vibrating sieves (NKP Mekhanobr-Tekhnika, Russia) to achieve particle size of 80 % less than 71 μm. The crushed bauxite before experiments was subjected to sieve analysis to obtain three fractions: -50 μm, +50-71 μm, and +71 μm. The average particle size of each fraction was: 48 μm, 62 μm, and 87 μm. The chemical composition of these three fractions and the raw bauxite is shown in
Table 1.
According to the data presented in
Table 1, the raw bauxite is high-iron and highly siliceous. Silica modulus of bauxite is 8.36 units, which is at the lower limit of profitability for Bayer's method.
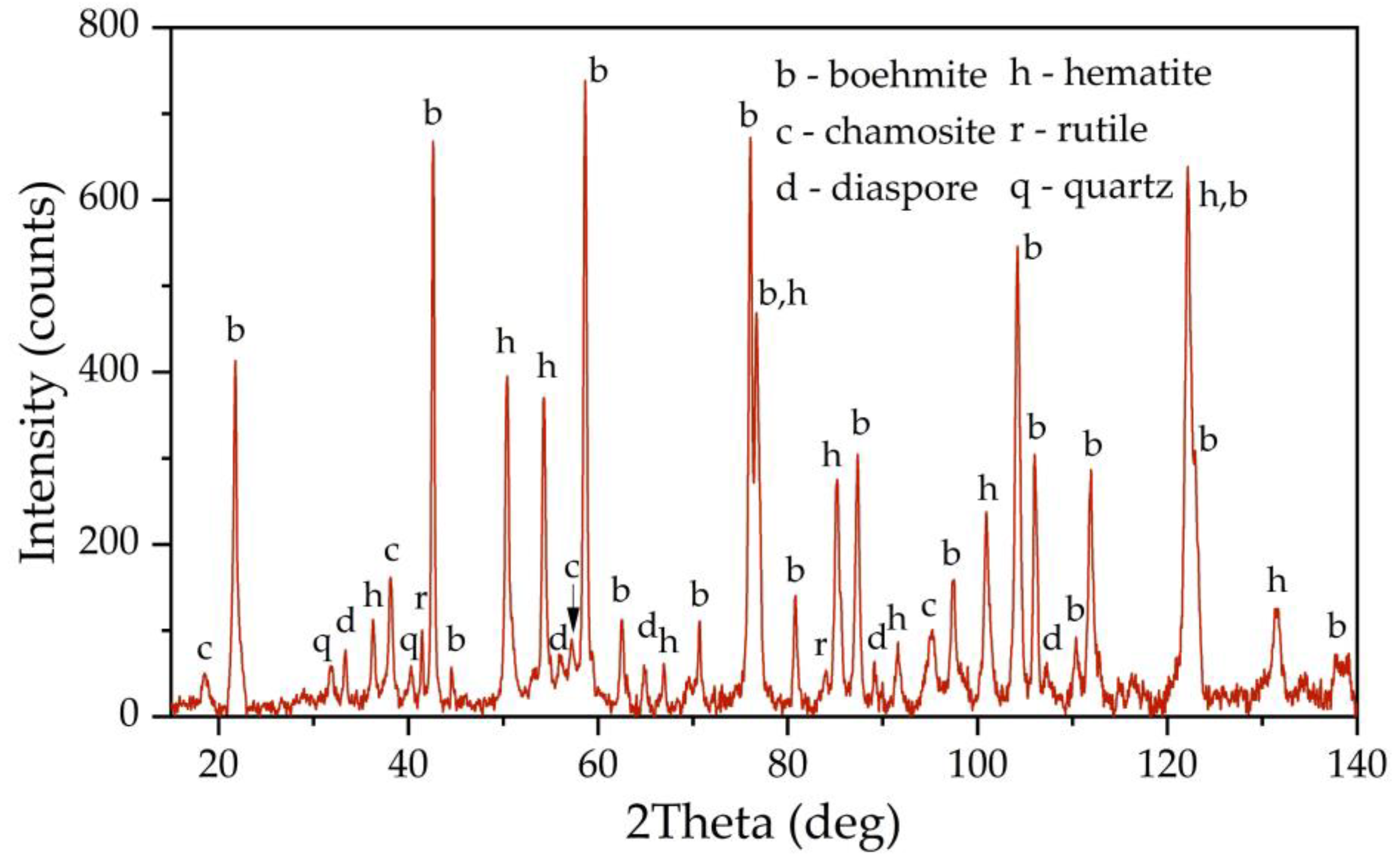
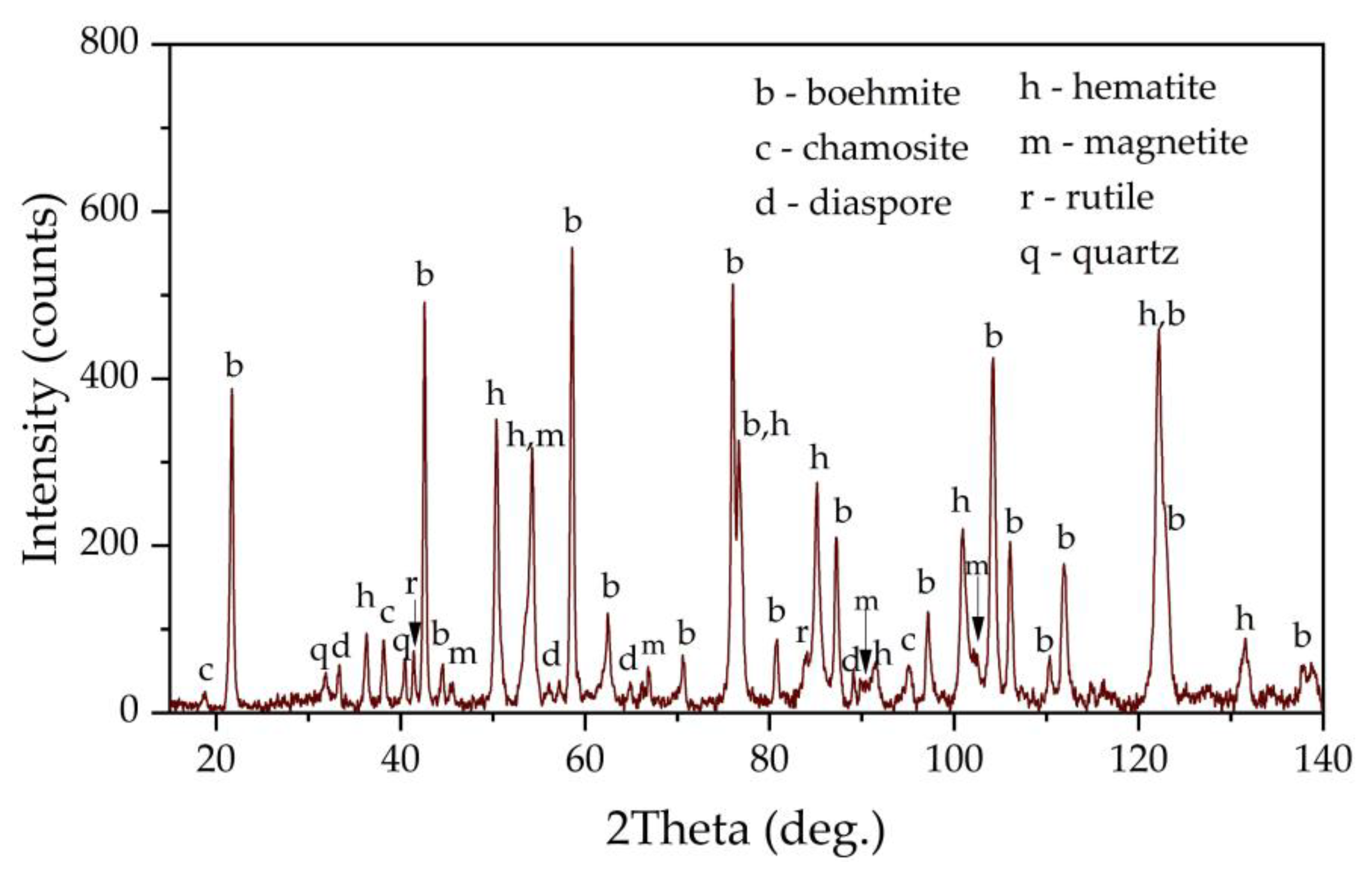
Figure 1 shows an X-ray diagram of the raw bauxite. Raw bauxite consists mainly of boehmite (AlOOH) and hematite (Fe
2O
3). Also, small amounts of rutile (TiO
2), quartz (SiO
2), diaspore (AlOOH), chamosite ((Fe²⁺,Mg,Al,Fe³⁺)₆(Si,Al)₄O₁₀(OH,O)₈) are present. A semi-quantitative analysis of the crystalline phases of the bauxite sample is shown in
Table 2. According to
Table 2, more than 62 % of the original bauxite is represented by boehmite, more than 25 % by hematite, the rest - quartz, rutile and chamosite. However, it should be noted that chamosite also has in its composition both alumina and silica, which may lead to subsequent problems during leaching (secondary aluminum losses due to the formation of DSP), also according to literature data [
6] kaolinite is often found in high-silica bauxite, but its content in this sample of Timan bauxite is insignificant or it is poorly crystalized.
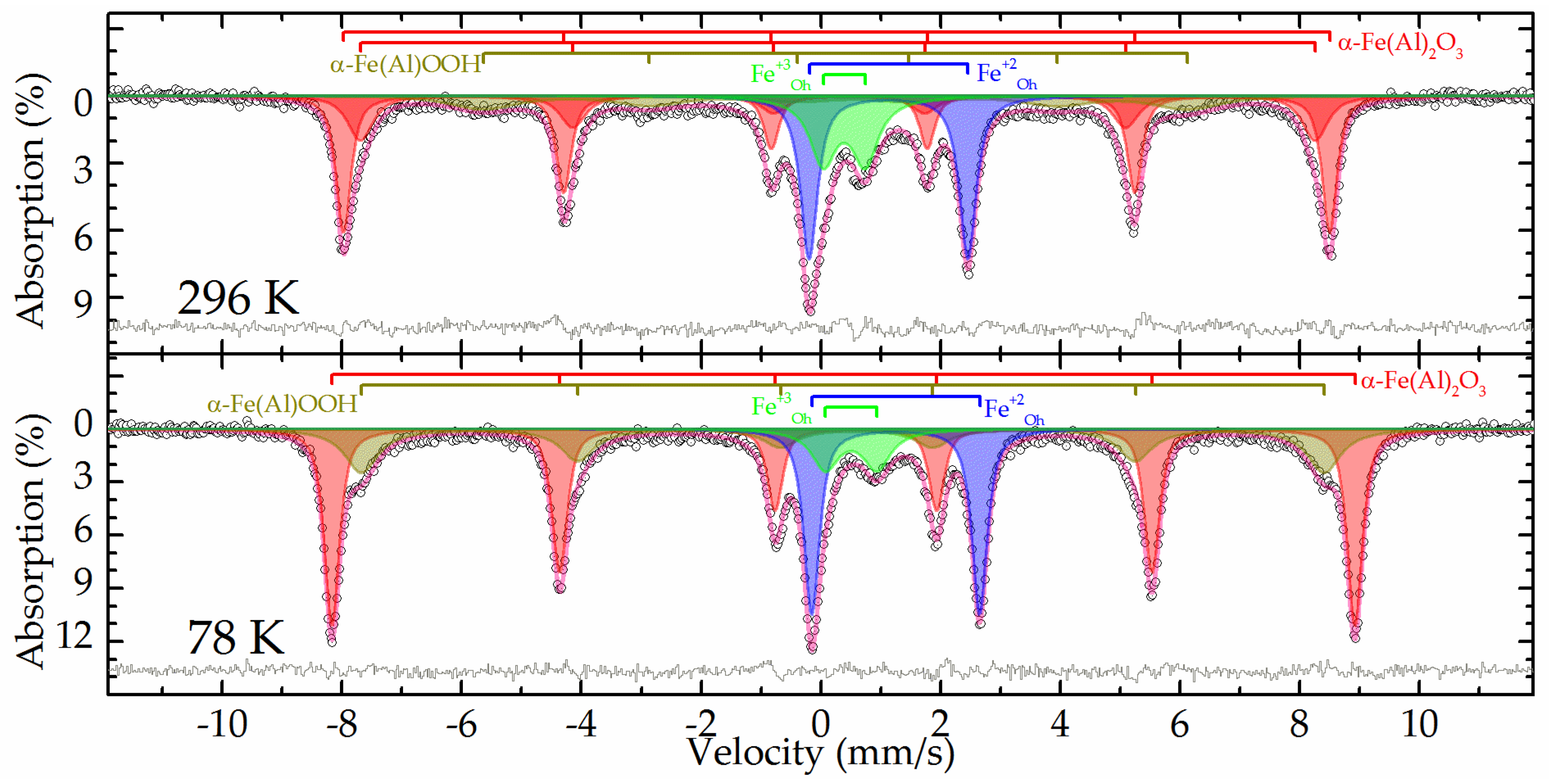
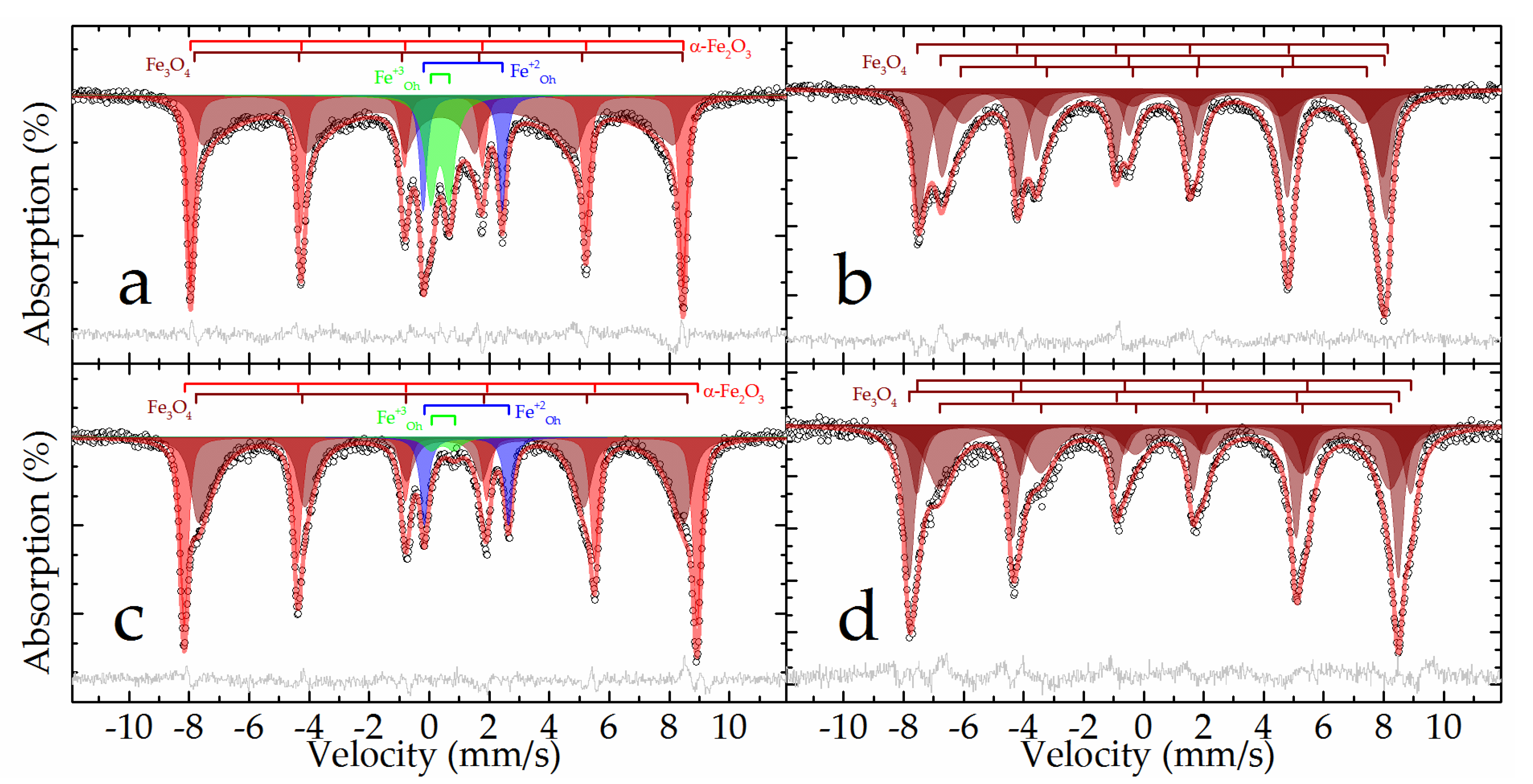
The Mössbauer spectra at both temperatures of the raw bauxite sample are a set of rather narrow resonance lines in which the presence of a sextet and a doublet with a large quadrupole splitting is clearly distinguished (
Figure 2). The experimental spectra can be satisfactorily described by a superposition of 4 or 5 subspectra, including two symmetrical doublets and two or three symmetrical sextets (
Table 3).
In the spectrum obtained at room temperature, two sextets with the maximum values of hyperfine magnetic splitting (
Table 3, subspectra 1 and 2) correspond to hematite - α-Fe
2O
3, as well as aluminum-substituted hematite [
25]. When the sample is cooled to the boiling point of nitrogen, these two sextets combine into one sextet (
Figure 2). In this case, the value of the quadrupole shift does not change sign, which indicates the absence of the Morrin transition characteristic of pure hematite, and confirms the hypothesis of alumohematite formation [
26]. The remaining sextet demonstrates a strong temperature dependence of both its profile and the hyperfine magnetic splitting (
Table 3, subspectrum 3). The hyperfine parameters of this subspectrum and the features of its temperature changes allow it to be attributed to alumogoethite, which we considered in detail in [
5]. The rest of the spectrum is described by a pair of doublets corresponding to iron atoms with charges +3 and +2 (
Table 3, subspectra 4 and 5) in the high-spin state and octahedral oxygen environment [
27]. Considering that the intensity of subspectrum 4 (
Table 3) decreases almost twofold with decreasing temperature, it can be assumed that superparamagnetic alumogoethite is partly responsible for the formation of this subspectrum. The rest of this subspectrum, as well as subspectrum 5, obviously belong to a layered aluminosilicate mineral, in particular, the hyperfine parameters make it possible to reliably assign them to chamosite [
28,
29,
30].
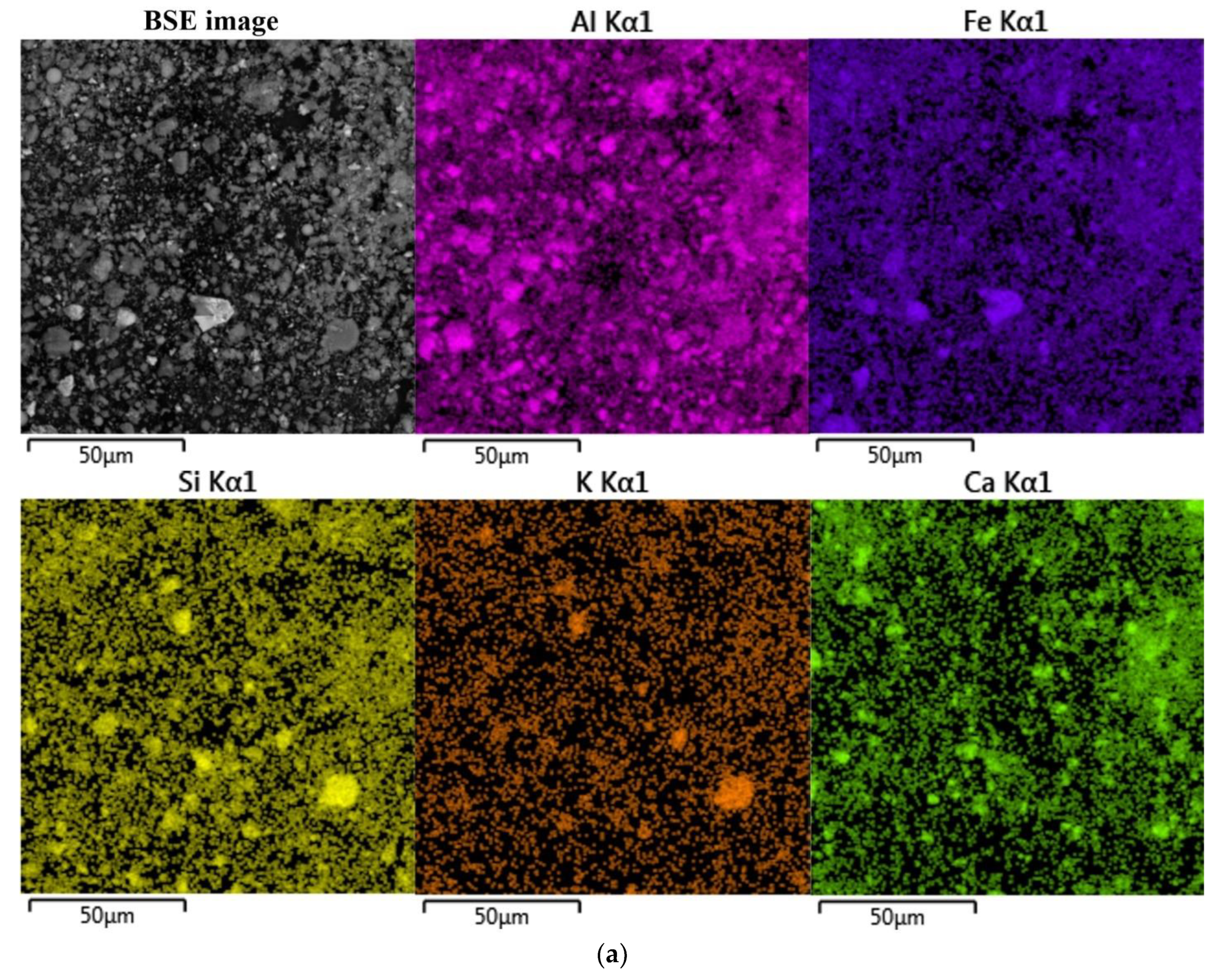
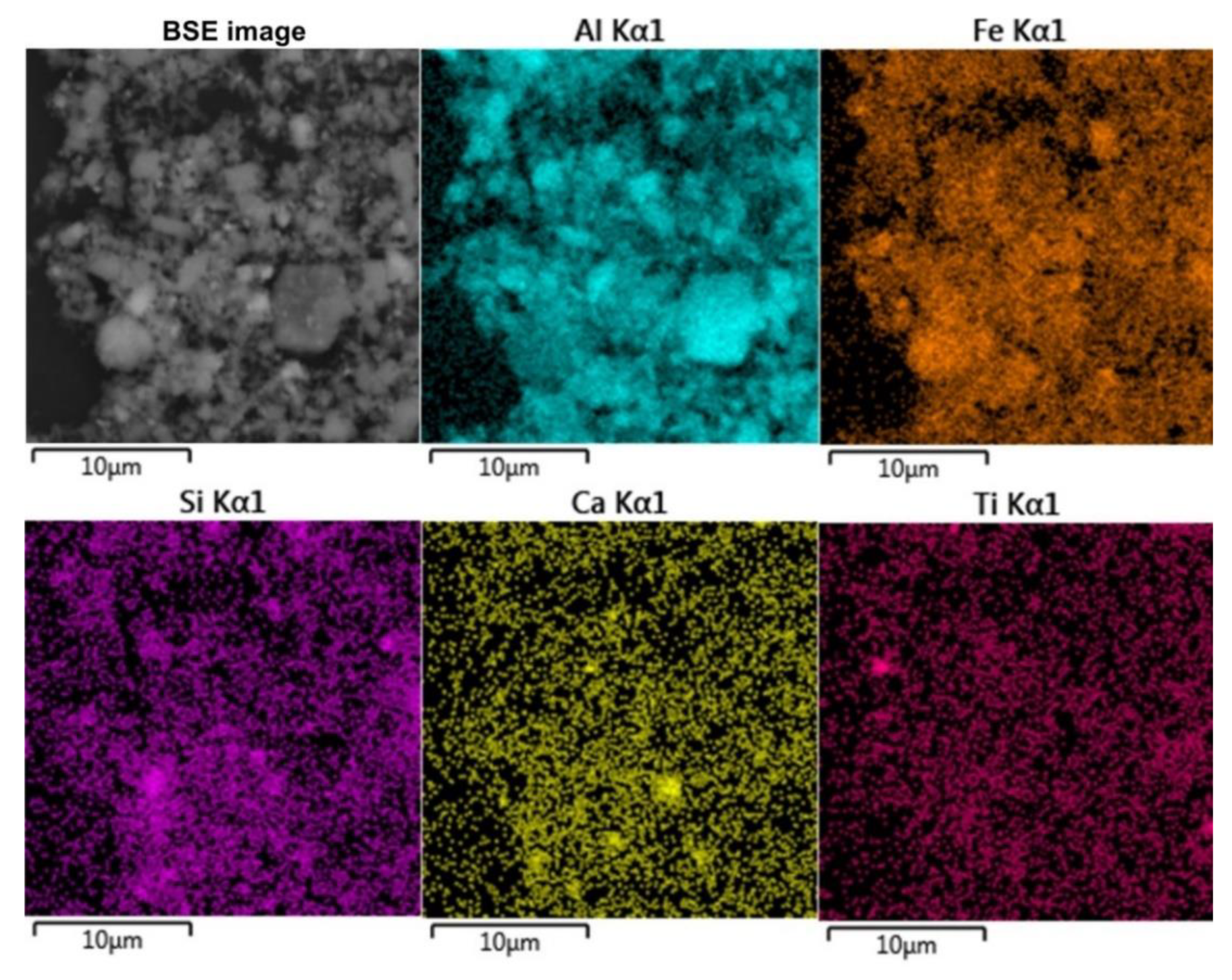
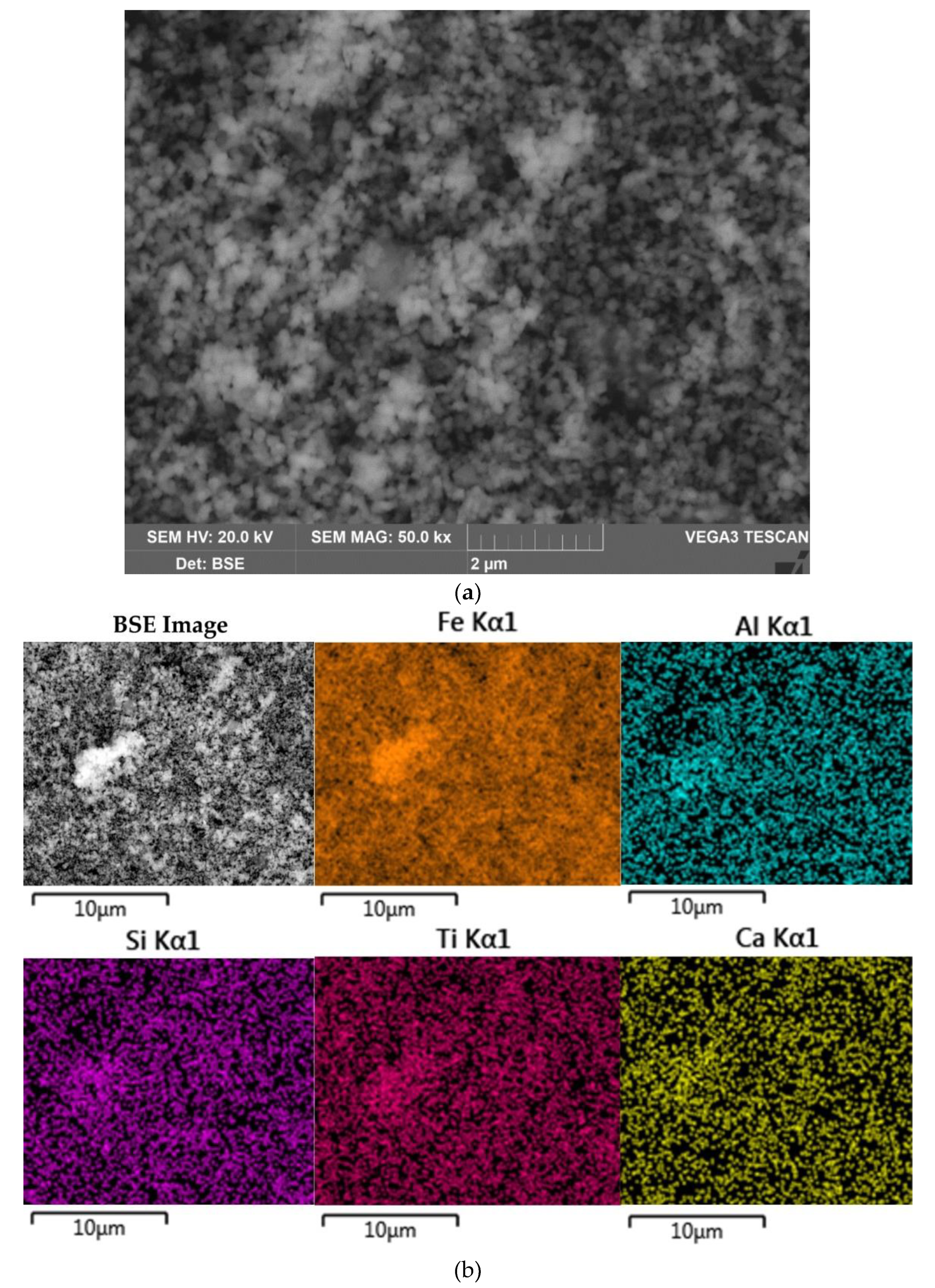
The morphology and chemical composition of the raw bauxite particles were evaluated by SEM-EDS analysis (
Figure 3,
Table 4). SEM-EDS images in
Figure 3 show that aluminum, iron, silicon, and calcium are uniformly distributed over the surface of bauxite particles, but single particles with high content of these elements can be identified. Potassium has a close association with silica, indicating its content in aluminosilicates.
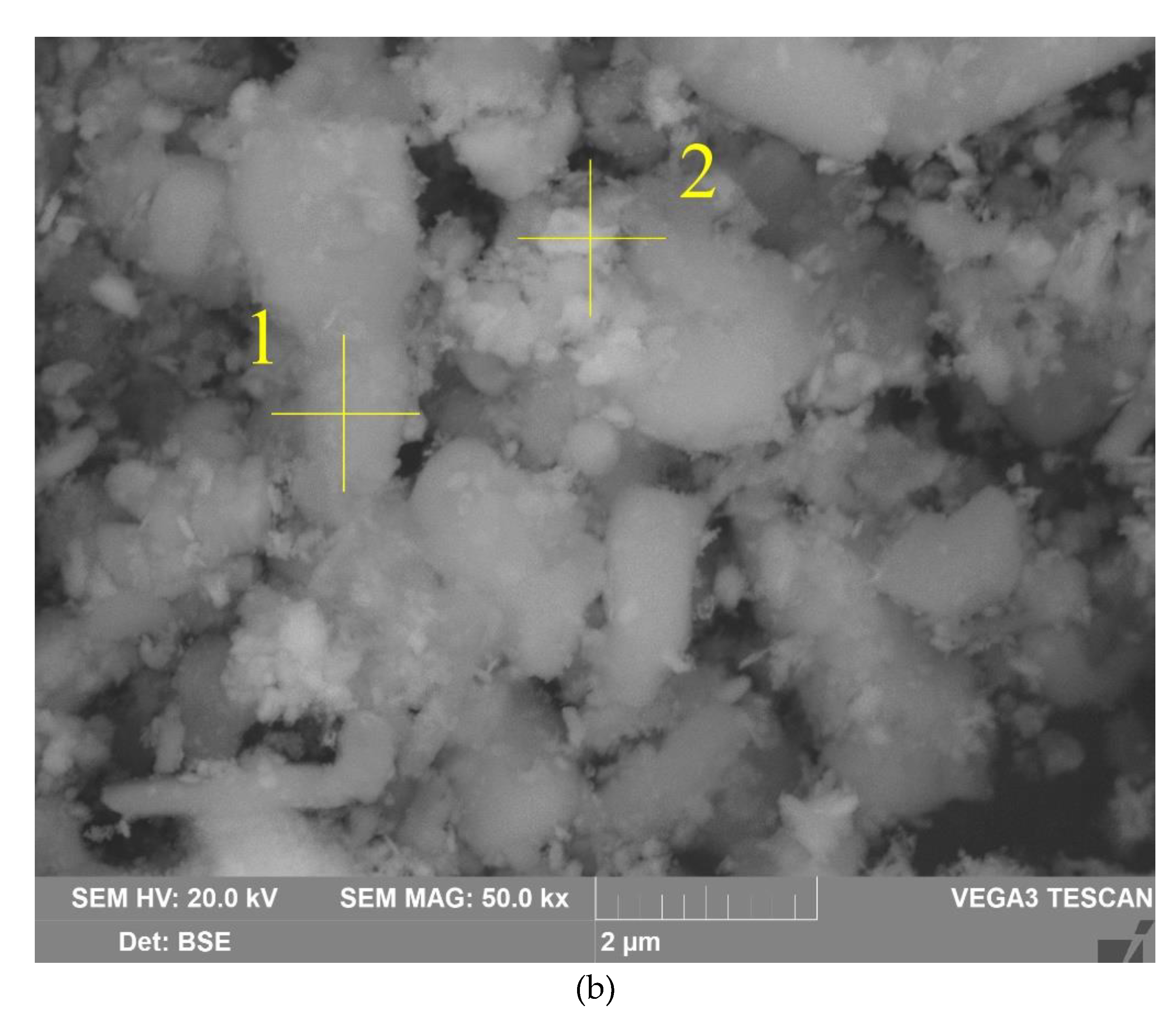
Figure 4b shows that the particles of raw bauxite have irregular shape. After grinding it is possible to observe particles of size from 100 nm to 10 μm. The results of EDS analysis of boehmite and hematite particles are shown in
Table 4 There is a close relationship between them, i.e. the boehmite particles are covered with hematite particles and vice versa.
3.2. The effect of leaching parameters on bauxite desilication and transformation of iron minerals on the first stage
Modeling of the process of bauxite pretreatment with highly concentrated alkaline solutions was carried out using SANN model.
As revealed in previous study [
25], the use of high alkali concentrations and the L:S ratio eliminates the formation of DSP due to silicon retention in the solution. This allows complete extraction of alumina even from such highly silica raw materials as fly ash, regardless of how much silica was contained in the feedstock. Moreover, the boiling temperature of the highly concentrated NaOH solution (more than 330 g L
-1 Na
2O) exceeds 120 °C. This makes leaching at atmospheric pressure possible at temperatures above 100 °C. The matrix of experiments created with the Statistica 13 software package and the results of Al and Si extraction from the different bauxite fractions and the Fe and Na
2O content in the solid residue are shown in
Table 5.
As was shown [
31,
32], the use of machine learning produces more accurate models than the use of mathematical methods. The closest to the experimental data SANN model (R
2 = 0,96) obtained for alumina extraction was the multilayer perceptron (MLP) 6.10.4, where 6 is the number of input parameters, 10 is the number of hidden layers and 4 is the number of output layers.
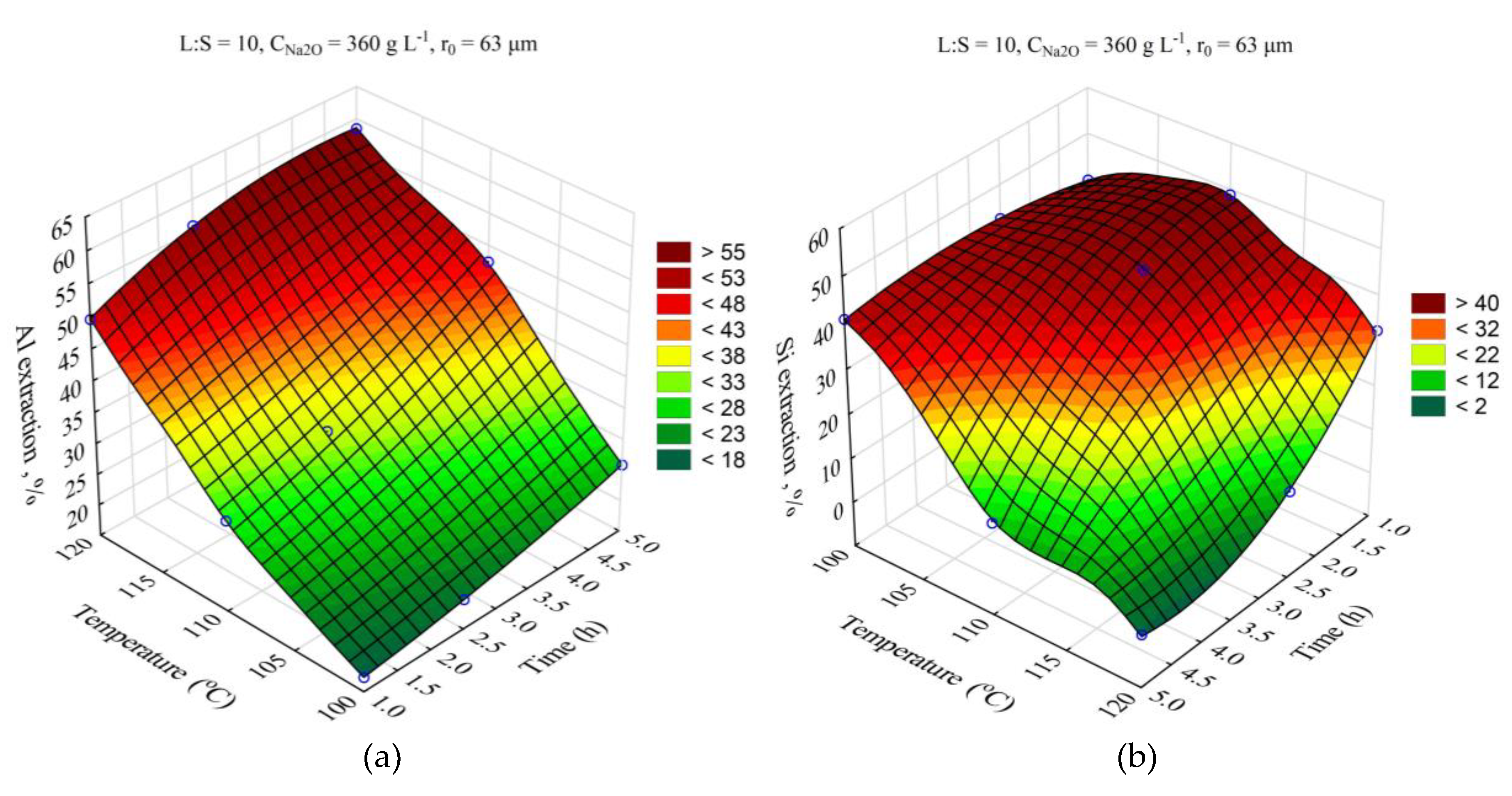
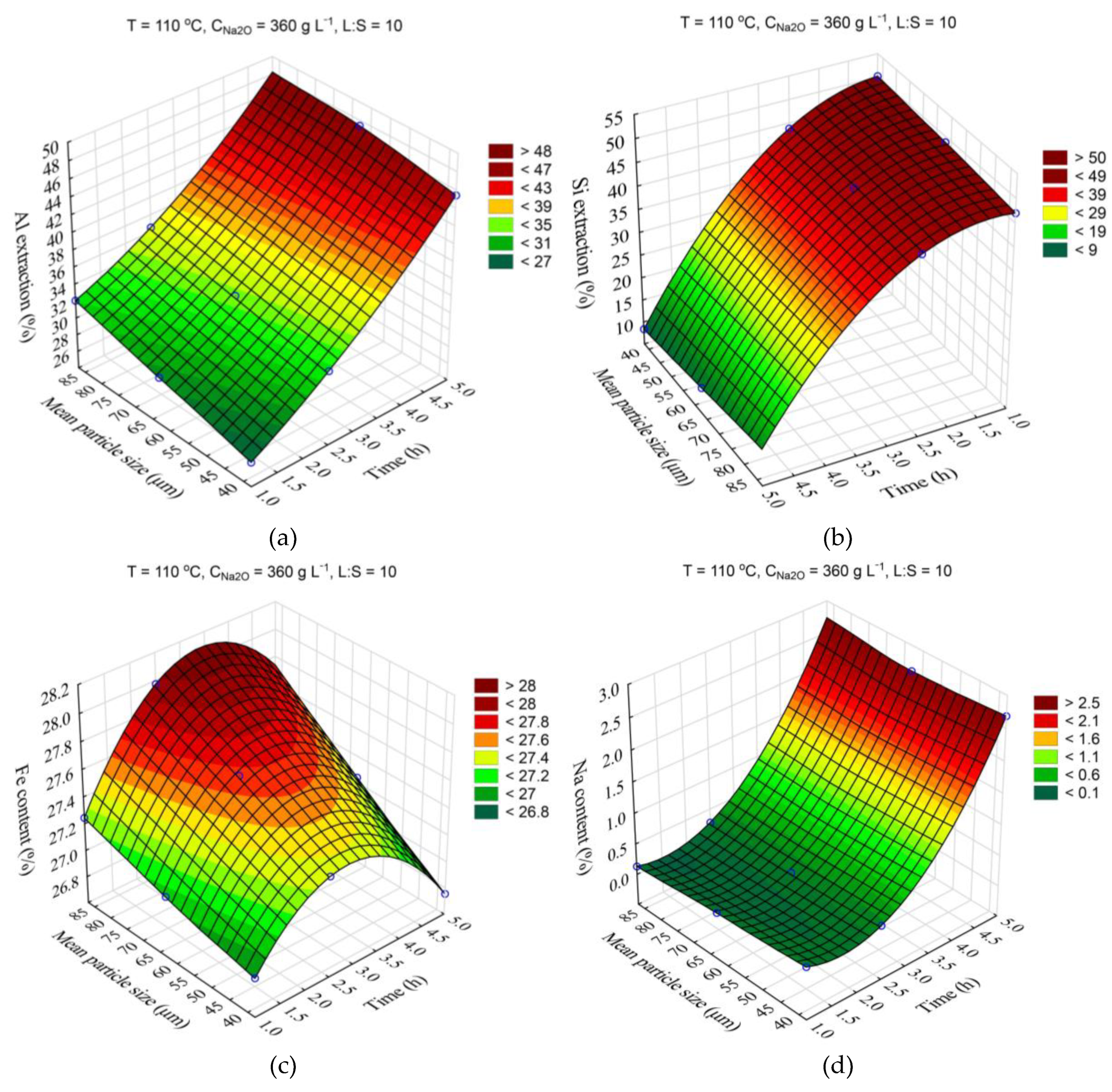
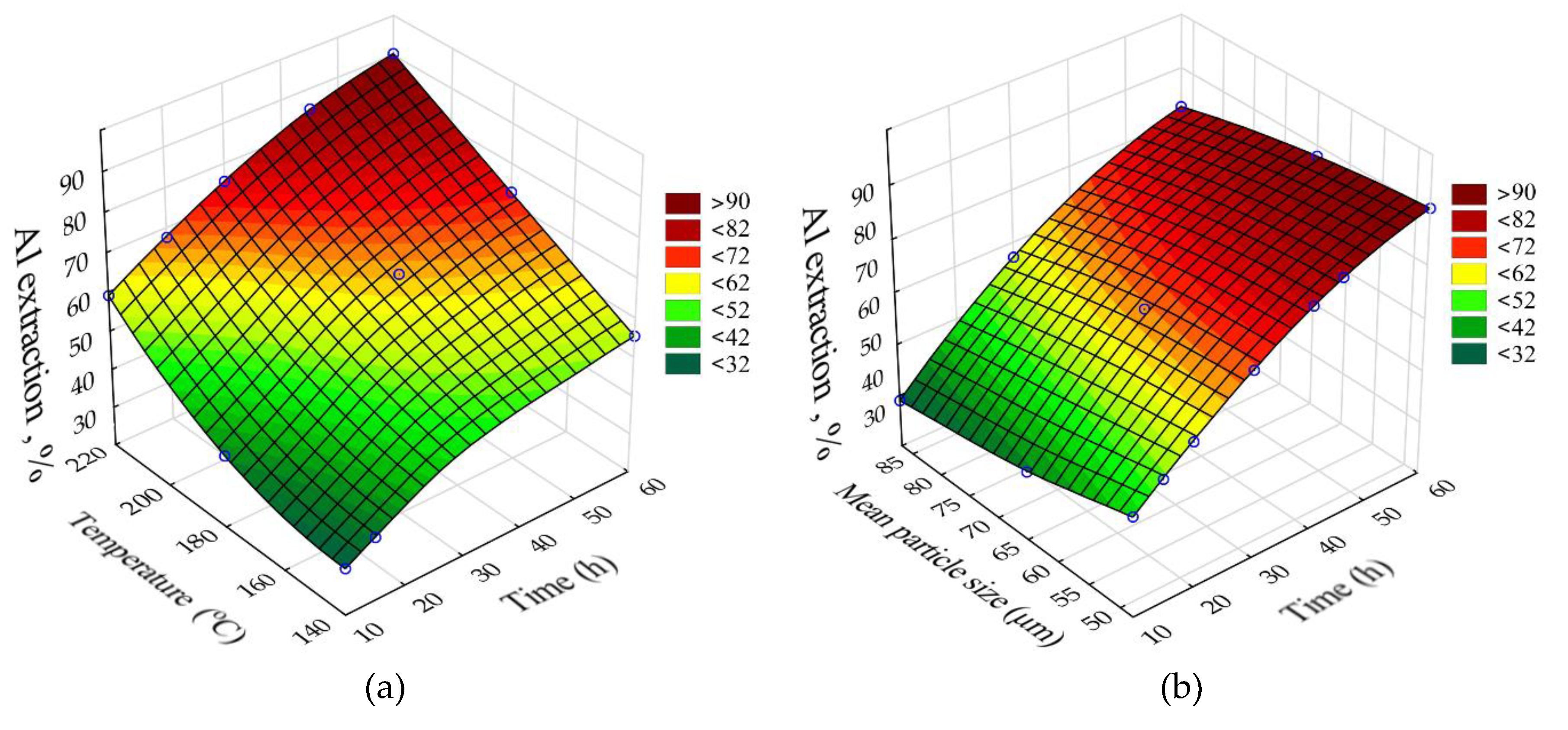
The response surfaces predicted by the SANN model for the effect of time and temperature on the degree of Al and Si extraction and the Fe and Na content in the solid residue are shown in
Figure 4. The leaching time (τ, min) was varied from 1 to 5 h, the temperature (T, °C) from 100 to 120 °C. L:S ratio, Na
2O concentration (C
Na2O, g L
–1), initial Al
2O
3 concentration (C
Al2O3, g L
–1), and initial mean particle size (r
0, μm) were fixed at L:S = 10, r
0 = 63 μm, C
Na2O = 360 g L
–1, C
Al2O3 = 0 g L
–1.
Obviously, increasing the temperature and time allows to increase the Al and Si extraction up to 60 and 40-50 %, respectively. However, the effect of time on the Fe content in the solid residue was low, especially at high temperature, this may be due to the fact that the desilication was completed in the first hour. Then, according to
Figure 4d, DSP begins to precipitate, which leads to an increase in the Na
2O content in the precipitate up to 3.2 % and, accordingly, it leads to decrease in the Fe content.
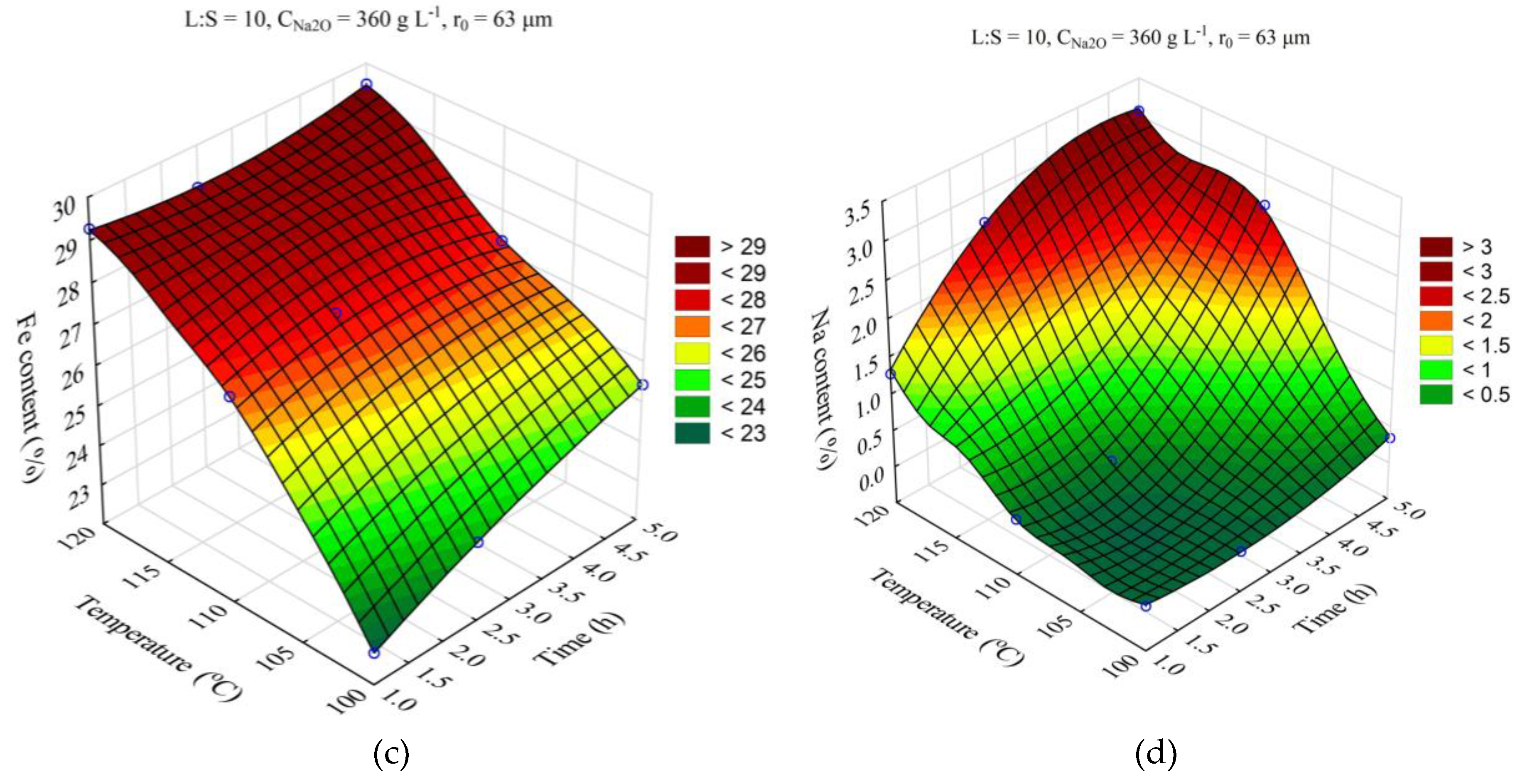
The response surfaces predicted by the SANN model for the effect of the time and the L:S ratio on the Al and Si extraction, as well as the Fe and Na content in the solid residue, are shown in
Figure 5. The leaching duration (τ, h) was varied from 1 to 5 h, the ratio L:S from 5 to 20. Other parameters were fixed at T = 110 °C, r
0 = 63 µm, C
Na2O = 360 g L
–1, C
Al2O3 = 0 g L
–1.
The increase of the L:S ratio from 5 to 20 allows to increase the solutional extraction from 28 to 41 % after 1 h of leaching (
Figure 5a), after 5 h of leaching the increase of the L:S ration from 5 to 20 results in the Al extraction increase only by 6 %. At the same time, the increase of L:S allows to significantly increase the Si extraction (
Figure 5b), which is associated with its retention in the solution, as evidenced by the Na content in the solid residue, which increases to 3 % after 5 h at L:S = 5. The high Al and Si extraction at L:S above 10 also leads to an increased Fe content in the solid residue since Fe is not leached out during the alkaline treatment and concentrates in the residue.
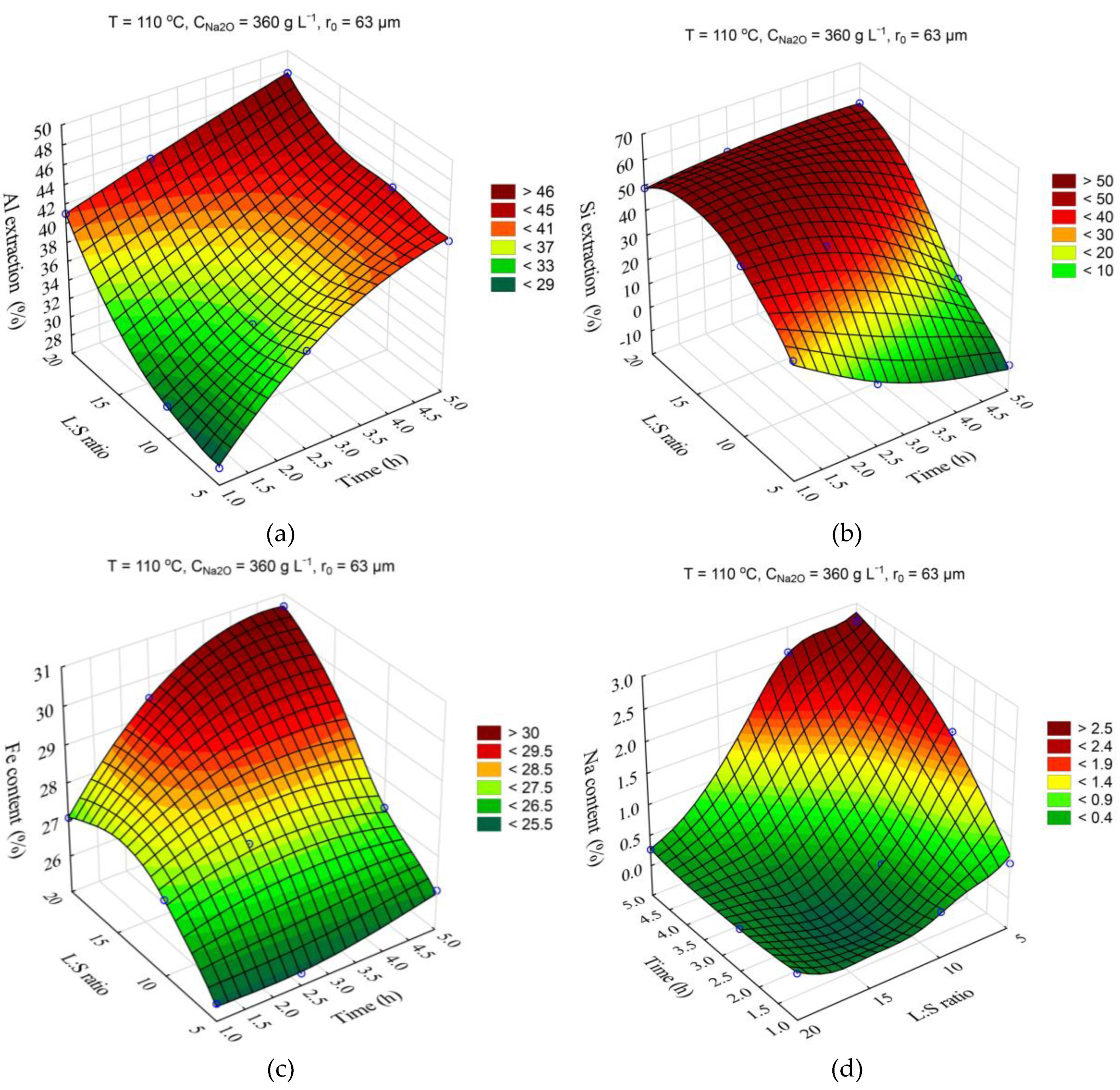
The response surfaces predicted by the SANN model for the effects of time and Na
2O concentration on the Al and Si extraction and the Fe and Na content in the solid residue are shown in
Figure 6. The leaching time (τ, min) was varied from 1 to 5 h, the Na
2O concentration – from 330 to 400 g L
–1. The other parameters were fixed at T = 110 °C, r
0 = 63 µm, L:S = 10, C
Al2O3 = 0 g L
–1.
The data shown in
Figure 6a show that solution composition has a significant impact on the Al extraction, which seems to be associated with an increase in caustic modulus and as a consequence with the incresed equilibrium concentration of Al in solution. Thus, an increase in Na
2O concentration from 330 to 400 g L
–1 after 5 h of leaching leads to an increase in Al extraction from 40 to 54%. The effect of solution concentration on the Si extraction and Na
2O content in the solid residue was insignificant (
Figure 6b,d). Increased Al extraction at high concentration reduces the yield of the solid residue and, accordingly, increases the iron content. As the leaching duration increases from 3 h to 5 h, DSP begins to form, resulting in an increased yield and higher Na
2О content in the solid residue (
Figure 6c,d).
The response surfaces predicted by the SANN model for the effects of time and initial mean particle size (r
0) on the Al and Si extraction and the Fe and Na content in the solid residue are shown in
Figure 7. The leaching time was varied from 1 to 5 h, the mean particle size from 38 to 78 μm. Other parameters were fixed at T = 110 °C, C
Na2O = 360 g L
–1, L:S = 10, C
Al2O3 = 0 g L
–1.
Decrease in the average particle size from 78 to 38 μm resulted in only a slight (2–4 %) increase of Al and Si extraction (
Figure 7a,b). The Fe content also slightly increases with the decrese of the r
0 (
Figure 8c), which is associated with a higher Al and Si extraction. After 2.5 h of leaching the Fe content begins to decrease, which is connected with the beginning of DSP formation (
Figure 7d).
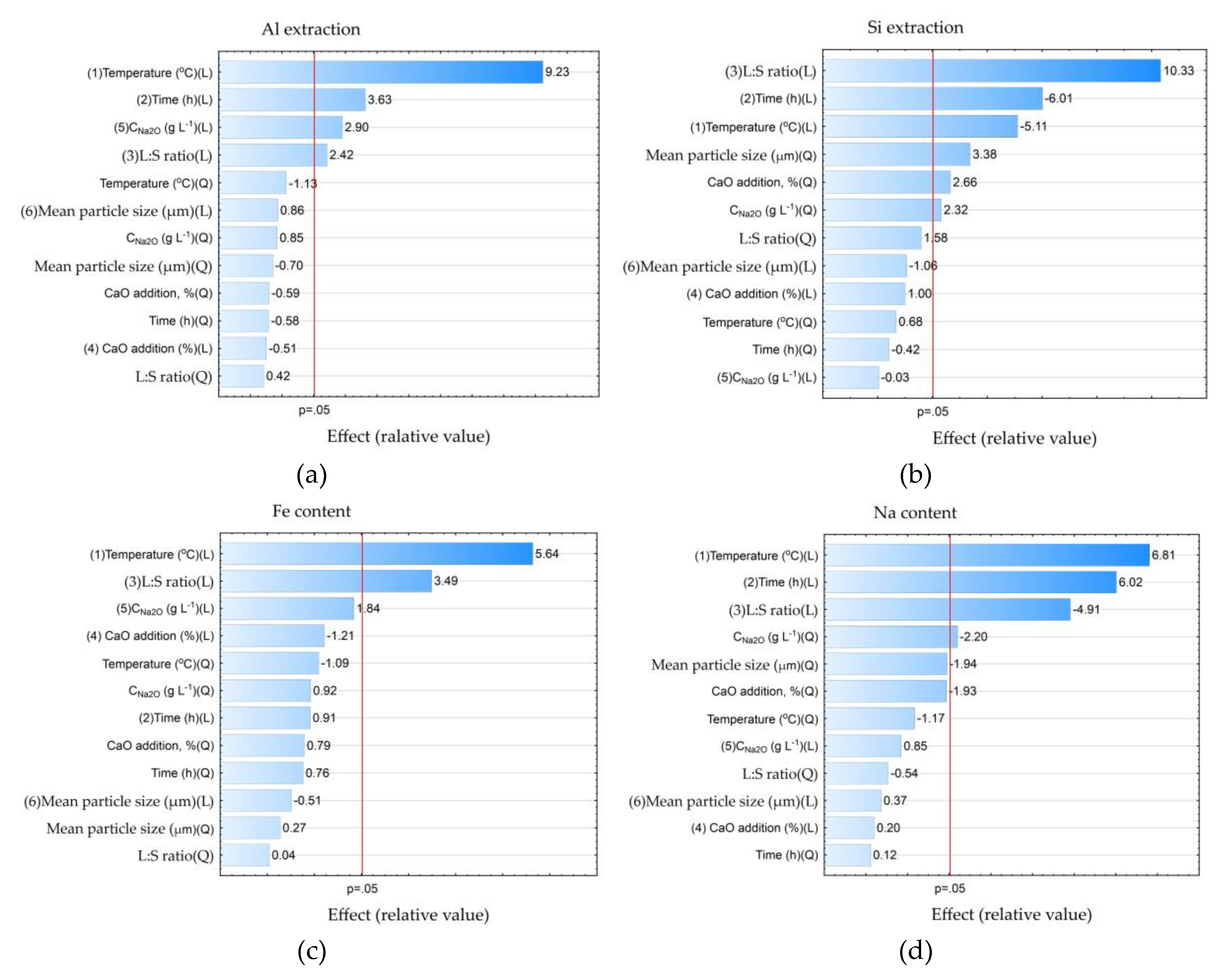
The data presented in
Table 4 were further processed in the Statistica using the ANOVA (Analysis of variance) method to study the statistical significance of certain process parameters. Pareto diagrams for each variable were constructed based on the results of ANOVA (
Figure 8).
According to the results shown in
Figure 8 with 0.95 confidence level (or significance level of 0.05), the temperature, time, concentration and L:S ratio were statistically significant for Al and Si extraction: L:S ratio, time (negative effect), temperature (negative effect), average particle size (Q – quadratic dependence); for Na in solid residue, temperature and duration were significant, while L:S ratio was significant for Na reduction in solid residue. Thus, if the task of the first stage is a selective Si extraction with minimization of Al extraction, it is necessary to take the minimum values of temperature and time and the maximum value L:S. The other parameters are of little importance for Al and Si extraction. Accordingly, the recommended parameters can be as follows: T = 100 °C, τ = 1 h and L:S = 20. At these parameters it is possible to extract up to 60 % of Si, the Al extraction can be as low as 20–24 %.
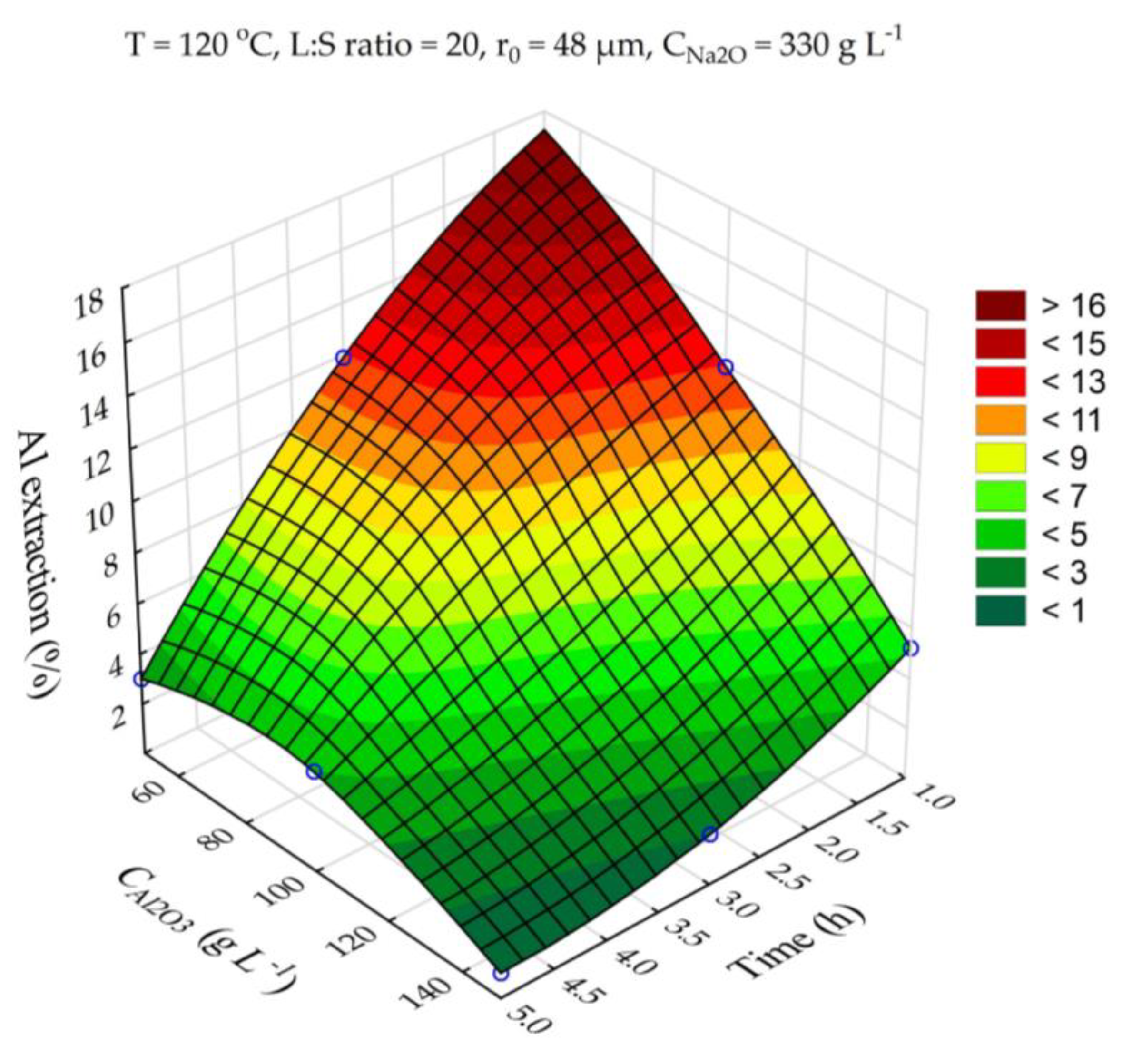
Experiments on desilication with the use of aluminate solutions of different Al concentrations were carried out to investigate the possibility of reducing Al co-extraction. It is known that the solubility of boehmite at atmospheric pressure is very low [
33], but when using highly concentrated NaOH solutions, it is sufficient to extract more than 50% of aluminum at L:S above 10. The results of experiments on the effect of Al concentration (in terms of Al
2O
3, g L
–1) on the Al extraction from bauxite during frist stage of leaching are shown in
Figure 9.
It is obvious that the use of aluminate solution can suppress the process of Al co-extraction from bauxite during its desilication even at the most severe conditions: T = 120 °C, Na
2O = 330 g L
–1 and the L:S ratio = 20. When aluminate solution with an Al
2O
3 concentration of 150 g L
–1, Na
2O = 330 g L
–1used for desilication at 100 ° C, τ = 1h and L:S ratio = 20 the Al and Si extraction were 5.1%, and 60.5%, respectively. Chemical composition of the concentrate (desilicated bauxite – DB) obtained under these conditions is shown in
Table 5. As can be seen, the silica modulus of bauxite after desilication increased to 21.34 units compared with 8.36 units for the original bauxite. The maximum theoretically Al extraction from this bauxite by the Bayer method is 95.3%.
Table 5.
Chemical composition of the desilicated bauxite (DB) obtained at optimal conditions (Al2O3 concentration of 150 g L–1, Na2O = 330 g L–1 used for desilication at 100 °C and L:S ratio = 20, τ = 1h).
Table 5.
Chemical composition of the desilicated bauxite (DB) obtained at optimal conditions (Al2O3 concentration of 150 g L–1, Na2O = 330 g L–1 used for desilication at 100 °C and L:S ratio = 20, τ = 1h).
| Main components, wt. % |
|---|
| Al2O3
|
Fe2O3 |
SiO2 |
CaO |
TiO2 |
CO2 |
Na2O |
MnO |
MgO |
K2O |
LOI |
| 47.61 |
34.79 |
2.23 |
1.45 |
2.39 |
0.67 |
0.19 |
0.58 |
0.54 |
0.12 |
9.44 |
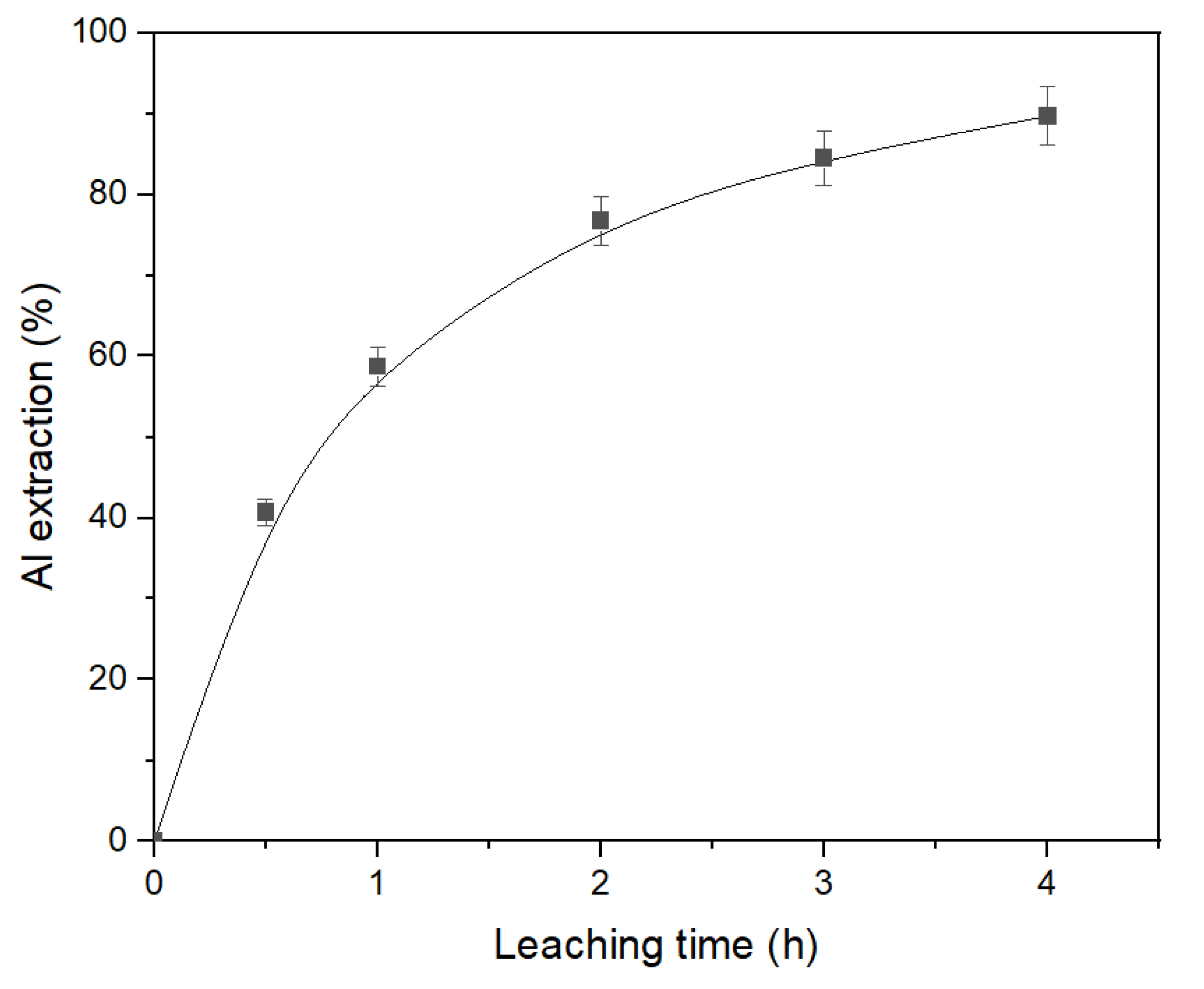
3.2. The effect of leaching parameters on Al extraction from desilicated bauxite (DB)
Desilicated bauxite obtained in section 3.1,
Table 5 was subjected to the second stage leaching under atmospheric pressure. The parameters of the leaching were: T = 120 °C, C
Na2O = 360 g L
–1, C
Al2O3 = 0 g L
-1 and the L:S ratio = 20. Th result of the effect of time on Al extraction under these condition are shown in
Figure 10. As can be seen, DB can be efficiently treated using atmospheric leaching process. After leaching the hematite transformation to magnetite was completed on 75,6 %. However, leaching time should be more than 4 h for extraction of 90 % of Al. Th resulting pregnant solution contains only 32.6 g L-1 Al
2O
3 and can not be processed using the Bayer process. Therefore, the high-pressure leaching of DB was studied.
The results of high-pressure Al leaching from DB were also processed using neural network modeling in the Statistica application package. The matrix of experiments and the results of Al extraction from desilicated bauxite are shown in
Table 6.
Table 6.
Experimental matrix and results obtained for the extraction of Al from desilicated bauxite.
Table 6.
Experimental matrix and results obtained for the extraction of Al from desilicated bauxite.
| Exp.№ |
Time (min) |
Temperature (°C) |
r0 (μm) |
Al extraction (%) |
| 1 |
10 |
220 |
38 |
58.00 |
| 2 |
30 |
220 |
38 |
76.82 |
| 3 |
40 |
220 |
38 |
86.07 |
| 4 |
60 |
220 |
38 |
94.04 |
| 5 |
22.5 |
220 |
38 |
74.20 |
| 6 |
40 |
180 |
38 |
65.80 |
| 7 |
60 |
180 |
38 |
77.00 |
| 8 |
15 |
140 |
38 |
36.70 |
| 9 |
60 |
140 |
38 |
55.79 |
| 10 |
10 |
220 |
78 |
57.30 |
| 11 |
30 |
220 |
78 |
68.20 |
| 12 |
60 |
220 |
78 |
77.80 |
| 13 |
30 |
220 |
63 |
79.62 |
| 14 |
10 |
220 |
63 |
66.50 |
| 15 |
60 |
220 |
63 |
92.52 |
The SANN model that was better fitted to the experimental data of Al extraction was the multilayer perceptron (MLP) 5.9.1 (R
2 = 0.98). The response surfaces predicted by the SANN model for Al recovery as a function of leaching time (τ, h), temperature (T, °C), and initial average bauxite particle size (r
0, μm) are shown in
Figure 11. The fixed values were C
Na2O = 330 g L
–1, C
Al2O3 = 150 g L
–1, L:S for the obtaining caustic modulus of the pregnant solution αk = 1.65.
The greatest influence (
Figure 11) on Al extraction is caused by the leaching time and temperature. Increasing the temperature from 140 °C to 220 °C increases the Al extraction after 60 min of leaching from 56 to 92 % (
Figure 11a). This may indicate that the surface chemical reaction is the limiting stage of the process. Increasing the initial particle size from 48 μm to 78 μm results in a decrease of Al extraction from 90 to 85% (
Figure 11b), which may indicate that diffusion has no influence on the kinetics of leaching process.
In order to identify the mechanism of the leaching process, the physico-chemical properties of the solid residue were studied.
The greatest influence (
Figure 11) on Al extraction is caused by the leaching time and temperature. Increasing the temperature from 140 °C to 220 °C increases the Al extraction after 60 min of leaching from 56 to 92 % (
Figure 11a). This may indicate that the surface chemical reaction is the limiting stage of the process. Increasing the initial particle size from 48 μm to 78 μm results in a decrease of Al extraction from 90 to 85% (
Figure 11b), which may indicate that diffusion has no influence on the kinetics of leaching process.
In order to identify the mechanism of the leaching process, the physico-chemical properties of the solid residue were studied.
3.3. Solid resue characterization
Figure 12 shows the elemental surface distribution maps for desilicated bauxite after first stage of leaching at T = 100 °C, C
Na2O = 330 g L
–1 C
Al2O3 150 g L
–1 and L:S ratio = 20. According to the elemental distribution, Fe, Si, Ca, and Ti are evenly distributed over the particle surfaces. Particles of boehmite (particles with high Al content) may be clearly visible. Fragmented iron particles are also found, but in general the iron particles after magnetization appear to be sufficiently finely dispersed.
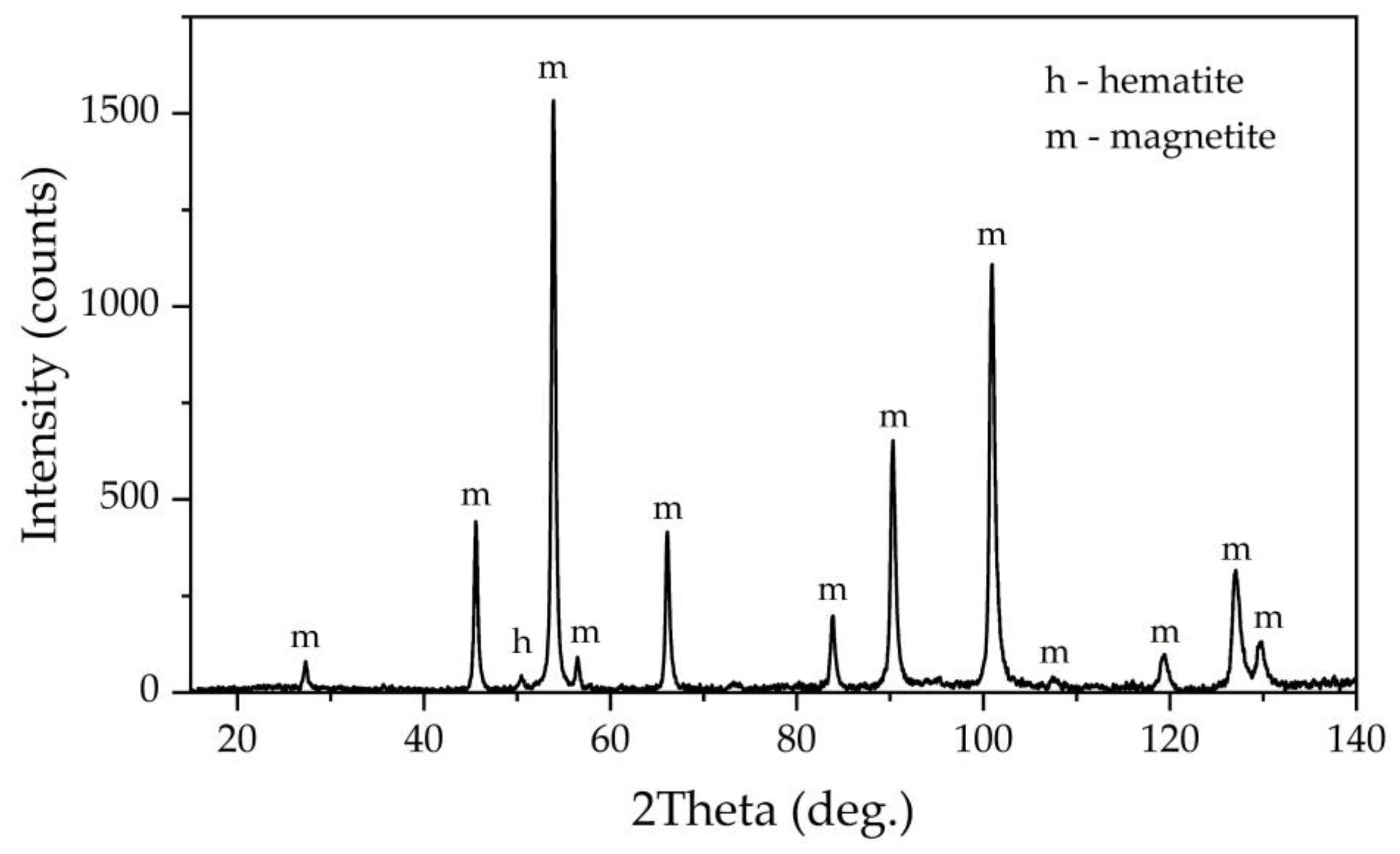
Figure 13 shows an XRD patter of bauxite desilicated under optimum conditions. After desilication in the presence of divalent iron a new phase, magnetite, appeared, although the intensity of the peaks is low. Also, after desilication and the magnetization the chamosite peaks disappeared, but quartz, which is insoluble at atmospheric pressure, is visible in the solid residue. It should be noted that under parameters of the Bayer process chamosite is almost inert until 200 °C [
34].
Chemical composition of the solid residue obtained after leaching of the DB under conditions similar to the industrial one (T = 220 °C, τ = 120 min, C
Na2O = 330 g L
–1, C
Al2O3 = 150 g L
–1 and L:S ratio that needed to obtain caustic modulus 1.65 in the pregnant solution) is presented in
Table 7. The yield of the solid residue was 41.5 % from the initial mass of the bauxite sample before desilication. It can be seen that Fe and Ti content in the residue increased significantly compared to the feedstock, and, according to XRD analysis, almost all iron is represented by magnetite. Alumina content was decreased by a factor of 20, and silica content by a factor of two. This indicates that practically all the alumina from the chamosite and all the boehmite were leached after 2 h of leaching - the total Al extraction after two stages was 97 %. At the same time, the Na
2O content remained very low even after two stages; this means that DSP was practically not formed in the leaching of DB in the simultaneous presence of ferrous iron, which was also confirmed by XRD analysis (
Figure 14).
Table 7.
Chemical composition of the solid residue (red mud) obtained by leaching of desilicated bauxite by mother aluminate solution T = 220 °C, τ = 120 min, CNa2O = 330 g L-1, CAl2O3 = 150 g L-1.
Table 7.
Chemical composition of the solid residue (red mud) obtained by leaching of desilicated bauxite by mother aluminate solution T = 220 °C, τ = 120 min, CNa2O = 330 g L-1, CAl2O3 = 150 g L-1.
| Main components, wt. % |
|---|
| Fe3O4
|
TiO2
|
Al2O3
|
SiO2
|
CaO |
MgO |
Na2O |
MnO |
CO2 |
SO3
|
P2O5
|
| 83.82 |
6.60 |
2.67 |
1.60 |
1.56 |
1.11 |
1.03 |
0.96 |
0.45 |
0.05 |
0.01 |
As can be seen from the XRD pattern in
Figure 14, the peaks of boehmite and hematite was disappeared, while the peaks of magnetite was increased significantly - magnetite remains almost the only phase in this BR. This fact suggests that the leaching of boehmite is complete. However, the absence of DSP and rutile on the XRD means that magnetization also helps to transform both silica and titania in a new phase [
23].
The results of the XRD patterns in
Figure 13 and 14 was confirmed by Mössbauer spectroscopy. The Mössbauer spectra of DB sample obtained at both temperatures (
Figure 15a,c) can be satisfactorily described by the superposition of two sextets and two doublets (
Table 3).
The outer sextet with the maximum hyperfine magnetic splitting and narrow resonance lines corresponds to hematite partially substituted by aluminum and is close in parameters to the analogous subspectrum of the raw bauxite sample (
Table 3). The intensity of this sextet noticeably increases when going from 296 to 78 K, with a simultaneous decrease in the intensity of the doublet described by subspectrum 3 (
Table 3), which suggests that this doublet mainly corresponds to superparamagnetic nanosized hematite. The hyperfine parameters of subspectrum 4, corresponding to iron(+2) atoms, are similar to the corresponding parameters for the initial bauxite, and obviously correspond to the incompletely reacted chamosite (
Table 3). There were no subspectra that could correspond to goethite in this sample. The rest of the spectrum can only be described using the many-state superparamagnetic relaxation model [
35]. The models for the spectra obtained at different temperatures were consistent with each other through the ratio of the energy of the magnetic anisotropy of particles to the thermal energy:
where K - magnetic anisotropy constant, V - volume of the magnetic domain, k
B - Boltzmann constant, T - temperature [
36]. Obviously, this subspectrum refers to the forming particles of nanomagnetite, possibly partially oxidized [
37]. From the parameters obtained using Equation (5) and making the assumption that the particles are spherical, and the magnetic anisotropy constant does not depend on temperature and is equal to 2*10
4 J m
-3 [
38,
39], one can estimate the sizes of magnetic domains for nanomagnetite as 10.32±0.06 nm.
The Mössbauer spectra of the BR sample have a form characteristic of magnetite [
40]: a sextet with a characteristic splitting of 1-3 resonance lines and an increased intensity of 4-6 lines in the spectra at room temperature; a noticeable asymmetric distortion of the sextet resonance lines in the spectra at the boiling point of nitrogen (
Figure 15b,d). The general broadening of resonance lines to the inner region of the spectrum indicates the manifestation of superparamagnetism by the material [
36]. Both spectra are satisfactorily described by the superposition of three sextets, the profile of each of which is specified within the many-state superparamagnetic relaxation model [
35] (
Table 3). In this case, within the same spectrum, the sextets were interconnected by relaxation parameters, and the spectra at different temperatures were additionally consistent with each other through the ratio of the energy of the magnetic anisotropy of particles to the thermal energy (Equation (4)). Similar to the method described above for the example of the DB sample, the sizes of the magnetic domains of nanomagnetite were estimated, which amounted to 19.2 ± 0.2 nm. No other components corresponding to those observed in the raw bauxite or DB samples or not observed in them were recorded in the described spectra, that means the complete magnetization of iron minerals after high-pressure leaching of the DB.
The morphology and elemental composition of the bauxite residue particles were also investigated using SEM EDS analysis (
Figure 16).
The data obtained above are confirmed by SEM-EDS analysis (
Figure 16).
Figure 16a,b show that the particle size of BR (mostly magnetite) is less than 200 nm. At the same time Al, Si, Ti and Ca are evenly distributed on the particle surface which may indicate their inclusion in the iron containing phases. It should be noted that, because of the complete Al extraction and no DSP formation, the obtained BR is enriched in rare-earth elements (REE). For example, the scandium content in BR reaches 130 mg kg
-1. Therefore, high iron content and concentration of REE makes this BR are valuable by-product for metals extraction.