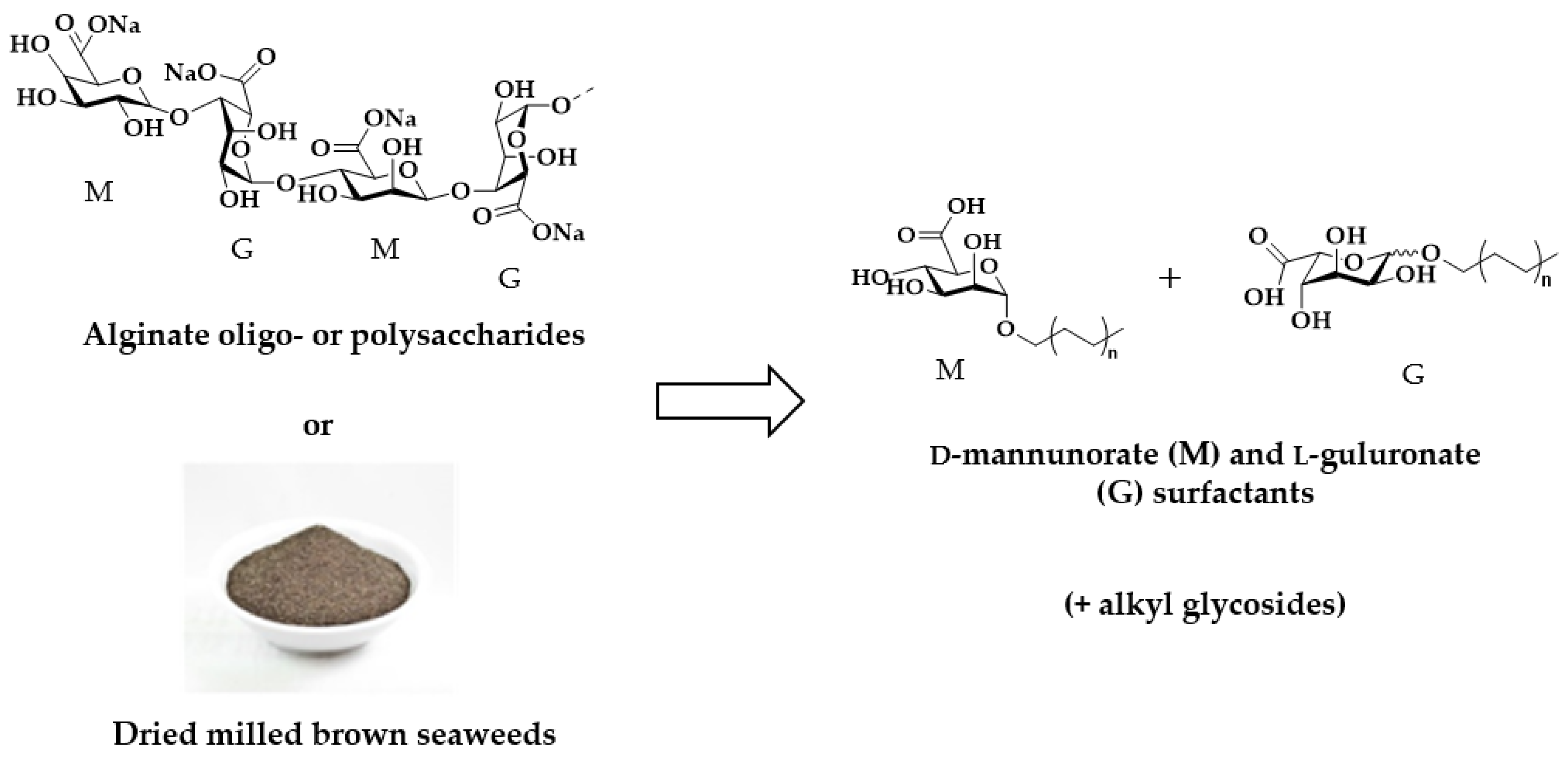
Oligomannnuronate, oligoguluronate, oligoalginate and semi-refined alginate were produced from fresh or dry seaweeds (CEVA, 83 Rue de Pen Lan, 22610 Pleubian, France) and dried milled brown seaweeds (Asco T10) were purchased from Thorverk (Iceland); all other commercially available chemicals were used without further purification. All reactions were monitored by thin layer chromatography (Kieselgel 60F254 Merck). Compounds were visualized using a H2SO4 solution (5 % H2SO4 in EtOH) or a vanillin solution (15 g of vanillin in 250 mL of EtOH and 2.5 mL of conc. H2SO4) followed by heating. Geduran 60 (40-63 µm, Merck) was used for column chromatography. NMR spectra were recorded on a Bruker Avance III 400 spectrometer operating at 400.13 MHz for 1H, equipped with a BBFO probe with a Z-gradient coil and a GREAT 1/10 gradient unit. The standard temperature was adjusted to 298 K. The zg30 Bruker pulse program was used for 1D 1H NMR, with a TD of 64k, a relaxation delay d1 = 2 s and 8 scans. The spectrum width was set to 18 ppm. Fourier transform of the acquired FID was performed with an apodization of 0.3 Hz in most of the cases. Chemical shifts are mentioned in parts per million (ppm) with tetramethylsilane as an internal standard. Coupling constants were expressed in Hertz (Hz) and the following abbreviations were used to indicate the multiplicity: s (singulet), d (doublet), t (triplet), q (quadruplet), m (multiplet), dd (doublet of doublets), dt (doublet of triplets) and br (broad signal).
Preparation of C4-C4 Man from OM
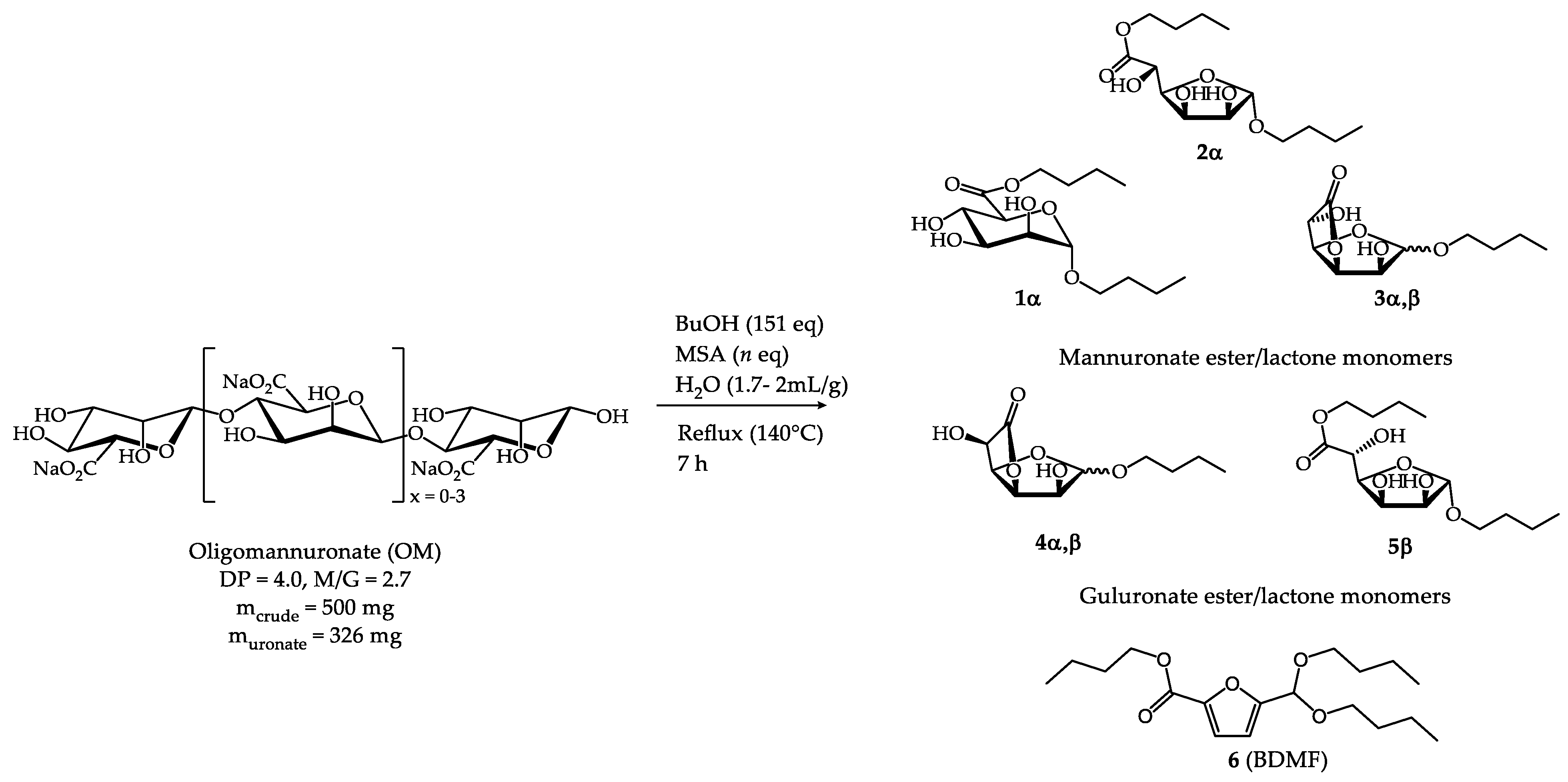
Oligomannuronate (500 mg, 1.81 mmol CO2-, 1 eq.) was dispersed in water (0.87 mL) and n-butanol (25 mL, 273 mmol, 151 eq.) in a round-bottom flask with a Dean-Stark apparatus. Methanesulfonic acid technical grade 70% wt (422 mg, 3.08 mmol, 1.7 eq.) was added and the mixture was refluxed under vigorous stirring. The water formed in the medium was gradually removed by azeotropic distillation. After 7 h, the mixture was cooled to ambient temperature. The mixture was then neutralised with 1N NaOH solution (400 µL) and concentrated. The resulting mixture was dissolved in diethyl ether (20 mL) and then filtered using celite. The celite was finally rinsed with diethyl ether (100 mL) and the filtrate was concentrated. The products obtained (495 mg) were purified by silica gel chromatography with CH2Cl2:CH3OH, (97/3–96/4, v/v) to help determine the molar composition. The molar composition of this mixture of products before silica gel chromatography is as follows: 52% (n-butyl) n-butyl-α-D-mannopyranosiduronate (1α), 7% (n-butyl) n-butyl-α-D-mannofuranosiduronate (2α), 11% n-butyl-α-D-mannofuranosidurono-6.3-lactone (3α), 13% n-butyl-β-D-mannofuranosidurono-6.3-lactone (3β), 5% BDMF (6), 5% n-butyl-α-L-gulofuranosidurono-6.3-lactone (4α), 5% n-butyl-β-L-gulofuranosidurono-6.3-lactone (4β), 3% (n-butyl) n-butyl-β-L-gulofuranosiduronate (5β). 1H NMR (400 MHz, CDCl3) δ 7.11 (d, J = 3.4 Hz, BDMF), 6.51 (dd, J = 3.5, 0.8 Hz, BDMF), 5.53 (s, BDMF), 5.14 (dd, J = 7.4, 4.7 Hz, H3-4β), 5.06 (s, H1-4β), 5.05 (d, J = 2.0 Hz, H1-3α), 5.03 (d, J = 4.6 Hz, H1-3β), 5.00 (t, J = 4.8 Hz, H3-3α), 4.95 (t, J = 4.5 Hz, H1-4α), 4.91 (d, J = 1.7 Hz, H1-1α), 4.90 (t, J = 5.0 Hz, H3-3β), 4.79 (dd, J = 6.8, 4.8 Hz, H4-3β), 4.76 (dd, J = 6.0, 4.5 Hz, H4-3α), 4.72 (dd, J = 7.6, 4.0 Hz, H4-4β), 4.69 (d, J = 5.5 Hz, H4-4α), 4.43 (d, J = 2.9 Hz, H5-2α),4.31 – 4.15 (m, OCH2), 4.08 (d, J = 9.5 Hz, H5-1α), 4.00 (t, J = 9.3 Hz, H4-1α), 3.94 (m, H2-1α), 3.87 (dd, J = 8.9, 3.5 Hz, H3-1α), 3.83 – 3.69 (m, OCH2), 3.65 (t, J = 6.6 Hz, BDMF), 3.60 – 3.38 (m, OCH2) 1.76 – 1.62 (m, CH2), 1.62 – 1.49 (m, CH2), 1.46 – 1.29 (m, CH2), 0.99 – 0.88 (m, CH3). After column chromatography, five fractions F1-F5 were isolated and characterised by 1H NMR. F1: 57 mg of BDMF (6). (Rf=0.94, CH2Cl2/MeOH (95/5, v/v)): 1H NMR (400 MHz, CDCl3) δ 7.11 (d, J = 3.4 Hz), 6.51 (dd, J = 3.5, 0.8 Hz), 5.53 (s), 4.36 (t, J = 6.7 Hz), 3.65 (t, J = 6.6 Hz), 1.80 – 1.63 (m, CH2), 1.63 – 1.50 (m, CH2), 1.48 – 1.31 (m, CH2), 1.00 – 0.86 (m, CH3); F2: 12 mg of n-butyl-α-L-gulofuranosidurono-6.3-lactone (4α) and (n-butyl) n-butyl-β-L-gulofuranosiduronate (5β). (Rf=0.42, CH2Cl2/MeOH (95/5, v/v)) : 1H NMR (400 MHz, CDCl3) δ 4.98 (t, J = 5.6 Hz, H3-4α), 4.95 (d, J = 4.5 Hz, H1-4α), 4.91 (s, H1-5β), 4.69 (dd, J = 5.5, 0.8 Hz, H4-4α), 4.52 (dd, J = 8.0, 1.3 Hz, H4-5β), 4.38 (d, J = 1.3 Hz, H5-5β), 4.28 (d, J = 0.7 Hz, H5-4α), 4.26 – 4.18 (m, OCH2), 4.15 (t, J = 5.1 Hz, H2-4α), 3.91 (d, J = 5.4 Hz, H2-5β), 3.71 (dt, J = 9.6, 6.7 Hz, OCH2), 3.58 (dt, J = 9.7, 6.7 Hz, OCH2), 3.43 (dt, J = 9.6, 6.7 Hz, OCH2), 1.73 – 1.59 (m, CH2), 1.58 – 1.47 (m, CH2), 1.46 – 1.24 (m, CH2), 0.99 – 0.86 (m, CH3); F3: 65 mg of n-butyl-β-D-mannofuranosidurono-6.3-lactone (3β), (n-butyl) n-butyl-α-D-mannofuranosiduronate (2α), n-butyl-α-L-gulofuranosidurono-6.3-lactone (4α), n-butyl-β-L-gulofuranosidurono-6.3-lactone (4β) and (n-butyl) n-butyl-β-L-gulofuranosiduronate (5β). (Rf=0.38, CH2Cl2/MeOH (95/5, v/v)): 1H NMR (400 MHz, CDCl3) δ 5.14 (dd, J = 7.4, 4.7 Hz, H3-4β), 5.06 (s, H1-4β), 5.03 (d, J = 4.6 Hz, H1-3β), 4.97 (s, H1-2α), 4.95 (d, J = 4.5 Hz, H1-4α), 4.90 (t, J = 5.0 Hz, H3-3β), 4.79 (dd, J = 6.8, 4.8 Hz, H4-3β), 4.72 (dd, J = 7.6, 4.0 Hz, H4-4β), 4.69 (d, J = 5.5 Hz, H4-4α), 4.56 (d, J = 4.0 Hz, H5-4β), 4.52 (d, J = 8.0 Hz, H4-5β), 4.43 (d, J = 2.9 Hz, H5-2α), 4.39 (d, J = 7.4 Hz, H5-3β), 4.30 – 4.19 (m, OCH2), 3.93 (d, J = 4.7 Hz, H2-2α), 3.79 (dt, J = 9.5, 6.7 Hz, OCH2), 3.66 (dt, J = 9.6, 6.7 Hz, OCH2), 3.54 – 3.38 (m, OCH2), 1.73 – 1.47 (m, CH2), 1.45 – 1.24 (m, CH2), 0.93 (m, CH3); F4: 49 mg of n-butyl-β-D-mannofuranosidurono-6.3-lactone (3β), n-butyl-α-D-mannofuranosidurono-6.3-lactone (3α) and n-butyl-β-L-gulofuranosidurono-6.3-lactone(4β). (Rf=0.29, CH2Cl2/MeOH (95/5, v/v)): 1H NMR (400 MHz, CDCl3) δ 5.13 (dd, J = 7.4, 4.7 Hz, H3-4β), 5.05 (d, J = 2.0 Hz, H1-3α), 5.03 (d, J = 4.6 Hz, H1-3β), 5.00 (t, J = 4.8 Hz, H3-3α), 4.90 (t, J = 4.8 Hz, H3-3β), 4.79 (dd, J = 6.8, 4.8 Hz, H4-3β), 4.76 (dd, J = 6.0, 4.5 Hz, H4-3α), 4.72 (dd, J = 7.6, 4.0 Hz, H4-4β), 4.27 – 4.17 (m, OCH2), 3.74 – 3.61 (m, OCH2), 3.50 – 3.35 (m, OCH2), 1.70 – 1.46 (m, CH2), 1.42 – 1.27 (m, CH2), 0.96 – 0.86 (m, CH3); F5: 183 mg of (n-butyl) n-butyl-α-D-mannopyranosiduronate (1α). (Rf=0.22, CH2Cl2/MeOH (95/5, v/v)) : 1H NMR (400 MHz, CDCl3) δ 4.90 (d, J = 1.7 Hz, H1-1α), 4.28 – 4.15 (m, OCH2), 4.28 – 4.15 (m, OCH2), 4.08 (d, J = 9.5 Hz, H5-1α), 4.00 (t, J = 9.3 Hz, H4-1α), 3.93 (dd, J = 3.4, 1.8 Hz, H2-1α), 3.86 (dd, J = 9.0, 3.4 Hz, H3-1α), 3.72 (dt, J = 9.7, 6.7 Hz, OCH2), 3.50 – 3.43 (m, OCH2), 1.72 – 1.62 (m, CH2), 1.62 – 1.51 (m, CH2), 1.44 – 1.30 (m, CH2), 0.93 (m, CH3).
Preparation of C12-C12 Man from OM
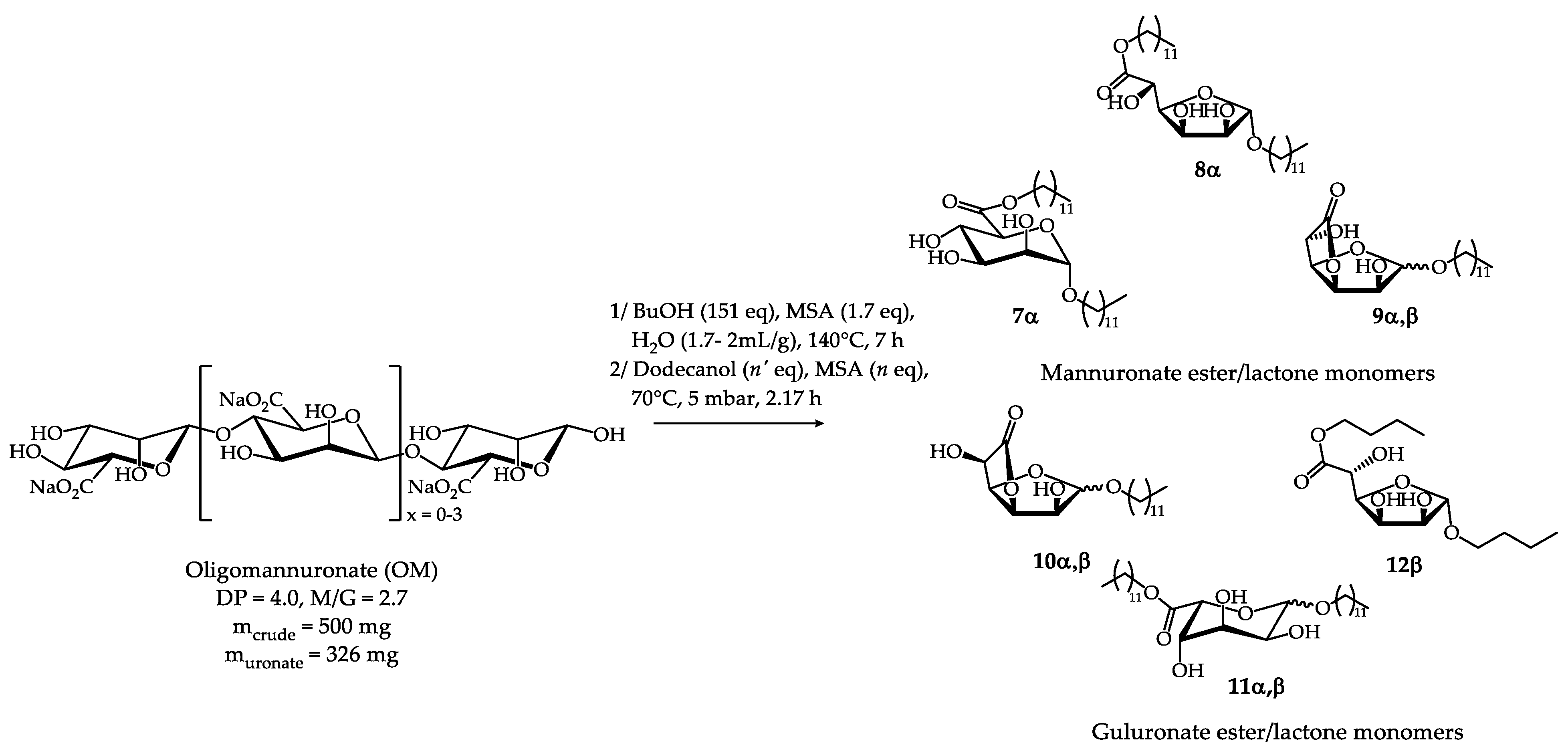
Oligomannuronate (500 mg, 1.81 mmol CO2-, 1 eq.) was dispersed in water (0.9 mL) and n-butanol (25 mL, 273 mmol, 151 eq.) in a round-bottom flask with a Dean-Stark apparatus. Methanesulfonic acid technical grade 70% wt (419 mg, 3.05 mmol, 1.7 eq.) was added and the mixture was refluxed under vigorous stirring. The water formed in the medium was gradually removed by azeotropic distillation. After 7 h, the mixture was cooled to ambient temperature. Dodecanol (1.6 mL, 7.2 mmol, 4 eq.) and the 70% methanesulfonic acid solution (246 mg, 1.8 mmol, 1 eq.) were added. The mixture was stirred at 70° C under reduced pressure (up to 5 mbar) using distillation apparatus. Once the butanol had been completely removed (2.17 h), water (16 mL) was added and the mixture was then neutralised with 1N NaOH solution (0.5 mL). The whole mixture was left to stir vigorously at 80° C for 1 h and the mixture was cooled to ambient temperature. The mixture was cooled at 0°C to solidify the organic phase which was isolated by solid-liquid extraction and dried under vacuum. The resulting mixture (1.684 g) was purified by a first silica gel chromatography with CH2Cl2/CH3OH, (100:0–96:4) to give crude mixture C12-C12 Man (504 mg). The molar composition of this mixture is as follows: 48% (n-dodecyl) n-dodecyl-α-D-mannopyranosiduronate (7α), 9% (n-dodecyl) n-dodecyl-α-D-mannofuranosiduronate (8α), 5% n-dodecyl-α-D-mannofuranosidurono-6.3-lactone (9α), 22% n-dodecyl-β-D-mannofuranosidurono-6.3-lactone (9β), 3% n-dodecyl-α-L-gulofuranosidurono-6.3-lactone (10α), 3% n-dodecyl-β-L-gulofuranosidurono-6.3-lactone (10β), 5% (n-dodecyl) n-dodecyl-β-L-gulopyranosiduronate (11β), 6% (n-dodecyl) n-dodecyl-β-L-gulofuranosiduronate (12β). 1H NMR (400 MHz, CDCl3) δ 5.07 (s, H1-10β), 5.05 (d, J = 2.0 Hz, H1-9α), 5.03 (d, J = 4.5 Hz, H1-9β), 4.99 (t, J = 4.7 Hz, H3-9α), 4.97 (t, J = 5.7 Hz, H3-10α), 4.97 (s, H1-8α, H2-9α), 4.95 (d, J = 4.5 Hz, H1-10α), 4.92 (d, J = 1.7 Hz, H1-7α), 4.91 (s, H1-12β), 4.90 (t, J = 5.0 Hz, H3-9β), 4.79 (dd, J = 6.8, 4.8 Hz, H4-9β), 4.72 (dd, J = 7.4, 4.0 Hz, H4-10β), 4.69 (d, J = 5.6 Hz, H4-10α), 4.58 (d, J = 4.0 Hz, H5-10β), 4.55 (d, J = 1.0 Hz, H5-11β), 4.53 (dd, J = 7.9, 1.4 Hz, H4-12β), 4.43 (d, J = 2.9 Hz, H5-8α), 4.39 (d, J = 7.0 Hz, H5-9β), 4.29 – 4.14 (m, OCH2), 4.09 (d, J = 9.6 Hz, H5-7α), 4.00 (t, J = 9.2 Hz, H4-7α), 3.95 (dd, J = 3.4, 1.7 Hz, H2-7α), 3.89 (dd, J = 8.9, 3.4 Hz, H3-7α), 3.82 – 3.68 (m, OCH2), 3.53 – 3.37 (m, OCH2), 1.74 – 1.64 (m, CH2), 1.63 – 1.53 (m, CH2), 1.38 – 1.20 (m, CH2), 0.91 – 0.85 (m, CH3). A second silica gel chromatography with CH2Cl2/CH3OH, (97:3) was made to help determine the molar composition. Five fractions F1-F5 were isolated and characterised by 1H NMR. F1 : 74 mg of (n-dodecyl) n-dodecyl-α-D-mannofuranosiduronate (8α), n-dodecyl-β-D-mannofuranosidurono-6.3-lactone (9β), n-dodecyl-α-L-gulofuranosidurono-6.3-lactone (10α) and (n-dodecyl) n-dodecyl-β-L-gulopyranosiduronate (12β). (Rf=0.47, CH2Cl2/MeOH (95/5, v/v)) : 1H NMR (400 MHz, CDCl3) δ 5.03 (d, J = 4.5 Hz, H1-9β), 4.98 (t, J = 5.8 Hz, H3-10α), 4.97 (s, H1-8α), 4.96 (d, J = 4.5 Hz, H1-10α), 4.91 (s, H1-12β), 4.89 (t, J = 5.0 Hz, H3-9β), 4.79 (dd, J = 6.8, 4.8 Hz, H4-9β), 4.70 (dd, J = 5.7, 0.8 Hz, H4-10α), 4.71 – 4.65 (m, H3-12β), 4.65 – 4.61 (m, H3-H4-8α), 4.52 (dd, J = 7.9, 1.3 Hz, H4-12β), 4.43 (d, J = 2.8 Hz, H5-8α), 4.37 (d, J = 1.3 Hz, H5-12β), 4.27 – 4.18 (m, OCH2), 3.92 (d, J = 4.5 Hz, H2-8α), 3.81 – 3.54 (m, OCH2), 1.76 – 1.63 (m, CH2), 1.62 – 1.48 (m, CH2), 1.39 – 1.20 (m, CH2), 0.91 – 0.85 (m, CH3); F2 : 64 mg of n-dodecyl-α-D-mannofuranosiduronate (8α), n-dodecyl-β-D-mannofuranosidurono-6.3-lactone (9β). (Rf=0.45, CH2Cl2/MeOH (95/5, v/v)) : 1H NMR (400 MHz, CDCl3) δ 5.03 (d, J = 4.6 Hz, H1-9β), 4.97 (s, H1-8α), 4.90 (t, J = 5.0 Hz, H3-9β), 4.79 (dd, J = 6.8, 4.8 Hz, H4-9β), 4.62 (m, H3-H4-8α), 4.42 (d, J = 2.9 Hz, H5-8α), 4.38 (d, J = 6.6 Hz, H5-9β), 4.29 – 4.18 (m, OCH2), 3.92 (d, J = 4.7 Hz, H2-8α), 3.78 (dt, J = 9.5, 6.8 Hz, OCH2), 3.65 (dt, J = 9.5, 7.3 Hz, OCH2), 3.53 – 3.35 (m, OCH2) ), 1.74 – 1.63 (m, CH2), 1.63 – 1.50 (m, CH2), 1.38 – 1.19 (m, CH2), 0.91 – 0.85 (m, CH3); F3: 30 mg of (n-dodecyl) n-dodecyl-α-D-mannopyranosiduronate (7α), (n-dodecyl) n-dodecyl-α-D-mannofuranosiduronate (8α), n-dodecyl-α-D-mannofuranosidurono-6.3-lactone (9α), n-dodecyl-β-D-mannofuranosidurono-6.3-lactone (9β), n-dodecyl−β-L-gulofuranosidurono-6.3-lactone (10β), (n-dodecyl) n-dodecyl-α-L-gulopyranosiduronate (11α), (n-dodecyl) n-dodecyl-β-L-gulopyranosiduronate (11β), and (n-dodecyl) n-dodecyl-β-L-gulofuranosiduronate (12β). (Rf=0.38, CH2Cl2/MeOH (95/5, v/v)): 1H NMR (400 MHz, CDCl3) δ 5.14 (dd, J = 7.4, 4.7 Hz, H3-10β), 5.07 (s, H1-10β), 5.05 (d, J = 2.0 Hz, H1-9α), 5.03 (d, J = 4.5 Hz, H1-9β), 4.99 (t, J = 4.7 Hz, H3-7α), 4.97 (s, H2-9α), 4.92 (d, J = 1.7 Hz, H5-7α), 4.91 (s, H1-12β), 4.89 (t, J = 5.0 Hz, H3-9β), 4.78 (dt, J = 5.8, 4.6 Hz, H4-9α,β), 4.72 (dd, J = 7.5, 4.0 Hz, H4-10β), 4.65 – 4.61 (m, H3-H4-8α), 4.63 (d, J = 7.8 Hz, H1-11β), 4.60 (d, J = 1.7 Hz, H5-11α), 4.57 (d, J = 4.0 Hz, H5-10β), 4.55 (d, J = 1.5 Hz, H5-11β), 4.52 (dd, J = 7.9, 1.0 Hz, H4-12β), 4.43 (d, J = 2.9 Hz, H5-8α), 4.40 (d, J = 1.3 Hz, H5-12β), 4.39 (d, J = 6.3 Hz, H5-9β), 4.27 – 4.18 (m, OCH2), 4.13 (dd, J = 4.0, 1.7 Hz, H4-11β), 4.09 (d, J = 9.5 Hz, H5-7α), 3.80 – 3.64 (m, OCH2), 3.55 – 3.37 (m, OCH2), 1.73 – 1.48 (m, CH2), 1.42 – 1.18 (m, CH2), 0.93 – 0.84 (m, CH3); F4 : 240 mg of (n-dodecyl) n-dodecyl-α-D-mannopyranosiduronate (7α). (Rf=0.3, CH2Cl2/MeOH (95/5, v/v)) : 1H NMR (400 MHz, CDCl3) δ 4.91 (d, J = 1.7 Hz, H1-7α), 4.21 (m, OCH2), 4.08 (d, J = 9.6 Hz, H5-7α), 4.01 (t, J = 9.3 Hz, H4-7α), 3.94 (dd, J = 3.4, 1.8 Hz, H2-7α), 3.88 (dd, J = 8.9, 3.4 Hz, H3-7α), 3.72 (dt, J = 9.7, 6.8 Hz, OCH2), 3.47 (dt, J = 9.7, 6.5 Hz, OCH2), 1.73 – 1.64 (m, CH2), 1.64 – 1.52 (m, CH2), 1.38 – 1.22 (m, CH2), 0.92 – 0.84 (m, CH3); F5 : 9 mg of a mixture of products (numerous 1H NMR signals).
Preparation of H-C12 Man from OM
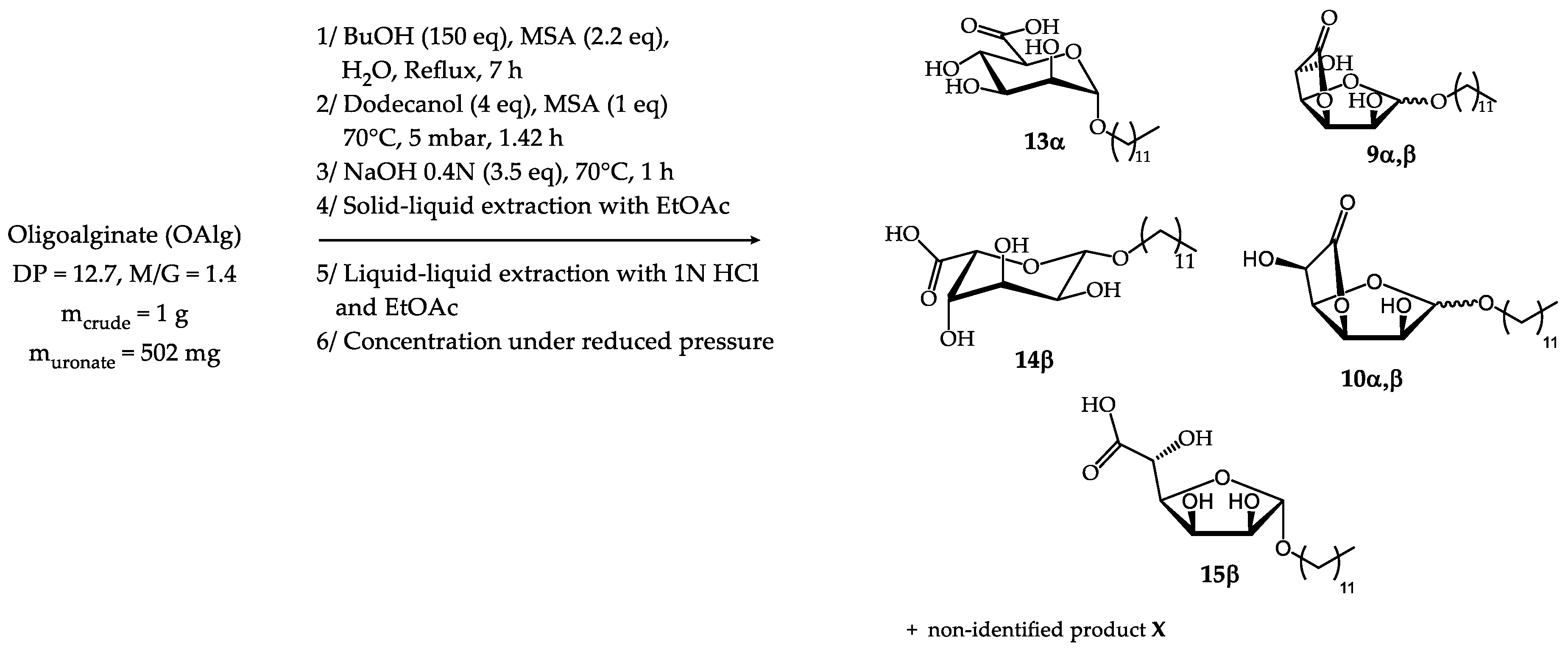
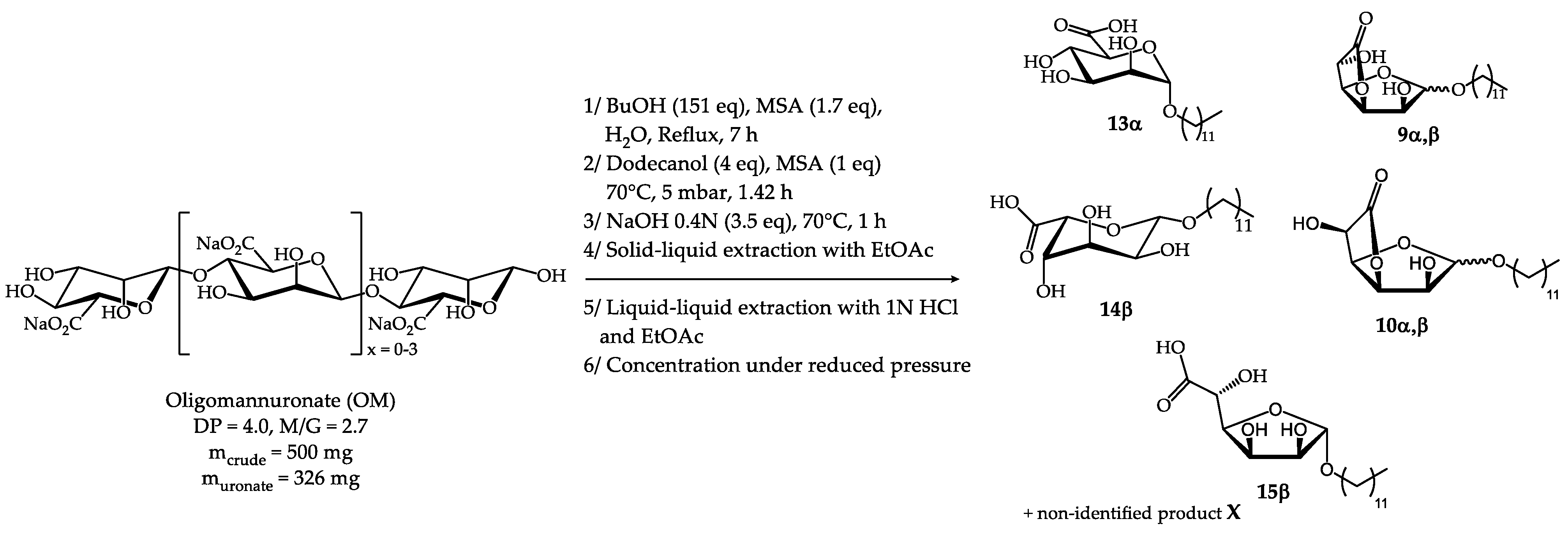
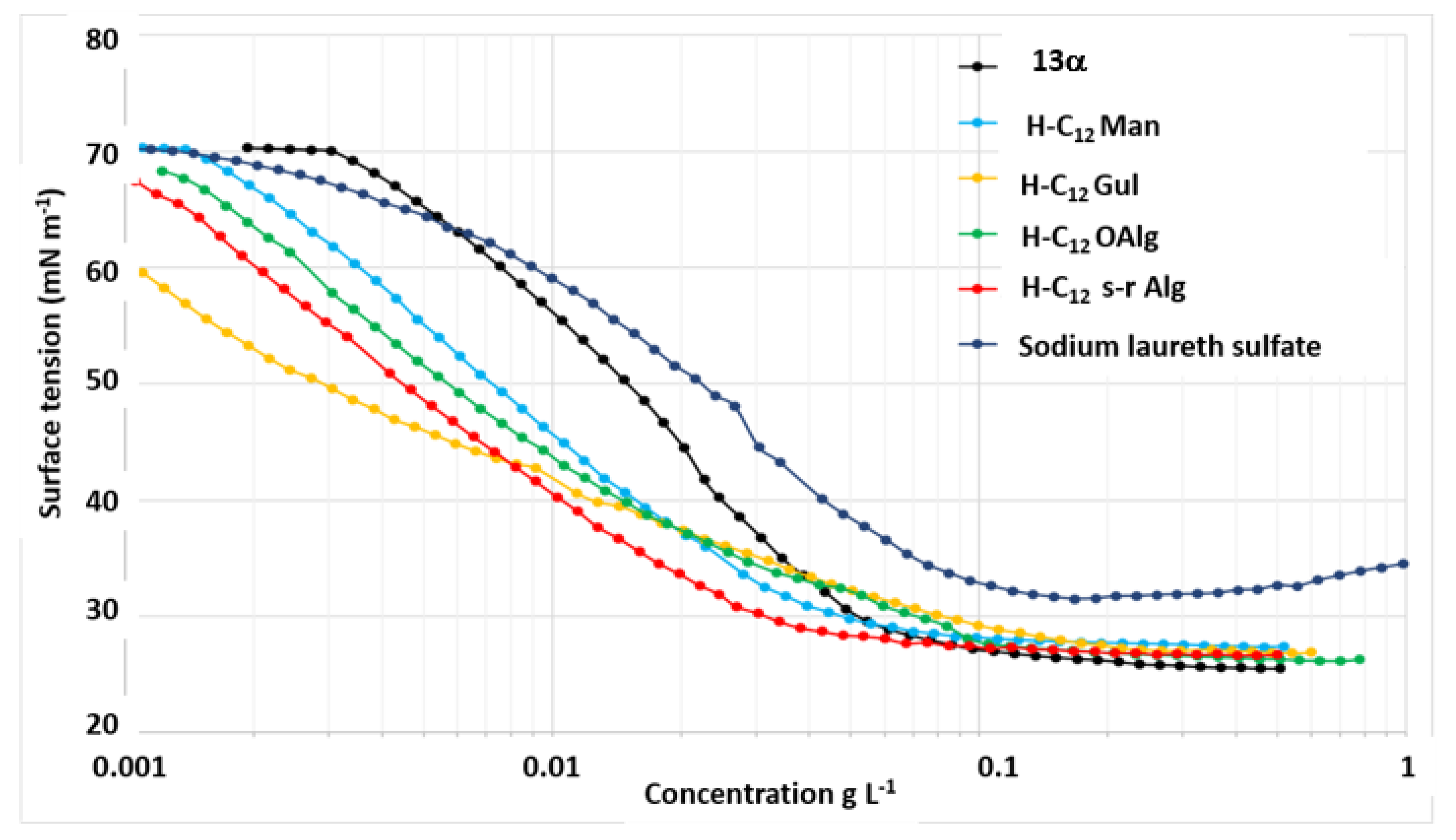
Oligomannuronate (500 mg, 1.81 mmol CO2-, 1 eq.) was dispersed in water (1 mL) and n-butanol (25 mL, 273 mmol, 151 eq.) in a round-bottom flask with a Dean-Stark apparatus. Methanesulfonic acid technical grade 70% wt (422 mg, 3.08 mmol, 1.7 eq.) was added and the mixture was refluxed under vigorous stirring. The water formed in the medium was gradually removed by azeotropic distillation. After 7 h, the mixture was cooled to ambient temperature. Dodecanol (1.6 mL, 7.2 mmol, 4 eq.) and the 70% methanesulfonic acid solution (248 mg, 1.81 mmol, 1 eq.) were added. The mixture was stirred at 70° C under reduced pressure (up to 5 mbar) using distillation apparatus. Once the butanol had been completely removed (1.42 h), a 0.4N NaOH solution (13.5 mL, 5.4 mmol, 3.0 eq.) was added and the mixture was left to stir vigorously at 70° C. for 1 h. The water was then removed by freeze-drying or by azeotropic distillation with butanol. The excess dodecanol present in the crude product was remove by solid-liquid extraction with EtOAc. At the end of this treatment, the mixture of products was dissolved in ice-cold water (15 mL) and EtOAc (22.5 mL), then a 1N hydrochloric acid solution (3.1 mL) was added. The aqueous solution was extracted with EtOAc (5x7.5 mL). The organic phases were combined and washed with a saturated NaC1 solution (30 mL) and a 1N hydrochloric acid solution (150 µL). The organic phase was dried with MgSO4 and then concentrated under vacuum. A mixture of products H-C12 Man was obtained (341 mg), the molar composition of which is: 47% n-dodecyl-α-D-mannopyranosiduronic (13α), 4% n-dodecyl-α-D-mannofuranosidurono-6.3-lactone (9α), 12% n-dodecyl-β-D-mannofuranosidurono-6.3-lactone (9β), 4% n-dodecyl-β-L-gulopyranosiduronic (14β), 9% n-dodecyl-α-L-gulofuranosidurono-6,3-lactone (10α), 9% n-dodecyl-β-L-gulofuranosidurono-6,3-lactone (10β), 4% n-dodecyl-β-L-gulofuranosiduronic acid (15β), 10% non-identified molecule X. 1H NMR (400 MHz, CD3OD) δ 5.11 (d, J = 6.5 Hz, X), 5.07 (dd, J = 7.6, 4.7 Hz, H3-10β), 5.00 (d, J = 1.6 Hz, H1-9α), 4.98 (s, H1-10β), 4.97 (t, J = 5.7 Hz, H3-10α), 4.96 (d, J = 3.8 Hz, H1-9β), 4.92 (d, J = 4.4 Hz, H1-10α), 4.80 (d, J = 2.1 Hz, H1-13α), 4.73 (dt, J = 5.0, 2.3 Hz, H4-9α,β), 4.62 (d, J = 8.3 Hz, H1-14β), 4.62 – 4.56 (m, H4-10α,β), 4.55 (d, J = 4.9 Hz, H-X), 4.49 (s, H5-14β), 4.47 (d, J = 6.6 Hz, H5-9β), 4.43 – 4.38 (m), 4.35 (d, J = 5.1 Hz, H-X), 4.29 (d, J = 2.2 Hz, H5-15β), 4.18 (t, J = 3.9 Hz, H3-14β), 4.14 – 4.08 (m, H2-10β/H2-10α/H4-14β), 4.05 (t, J = 4.8 Hz, X), 4.00 (d, J = 9.4 Hz, H5-13α), 3.89 (t, J = 9.2 Hz, H4-13α), 3.79 (dd, J = 3.4, 2.1 Hz, H2-13α), 3.76 – 3.63 (m, OCH2), 3.56 – 3.38 (m, OCH2), 1.67 – 1.51 (m, CH2), 1.46 – 1.22 (m, CH2), 0.94 – 0.87 (m, CH3).
Preparation of H-C12 Gul from OG
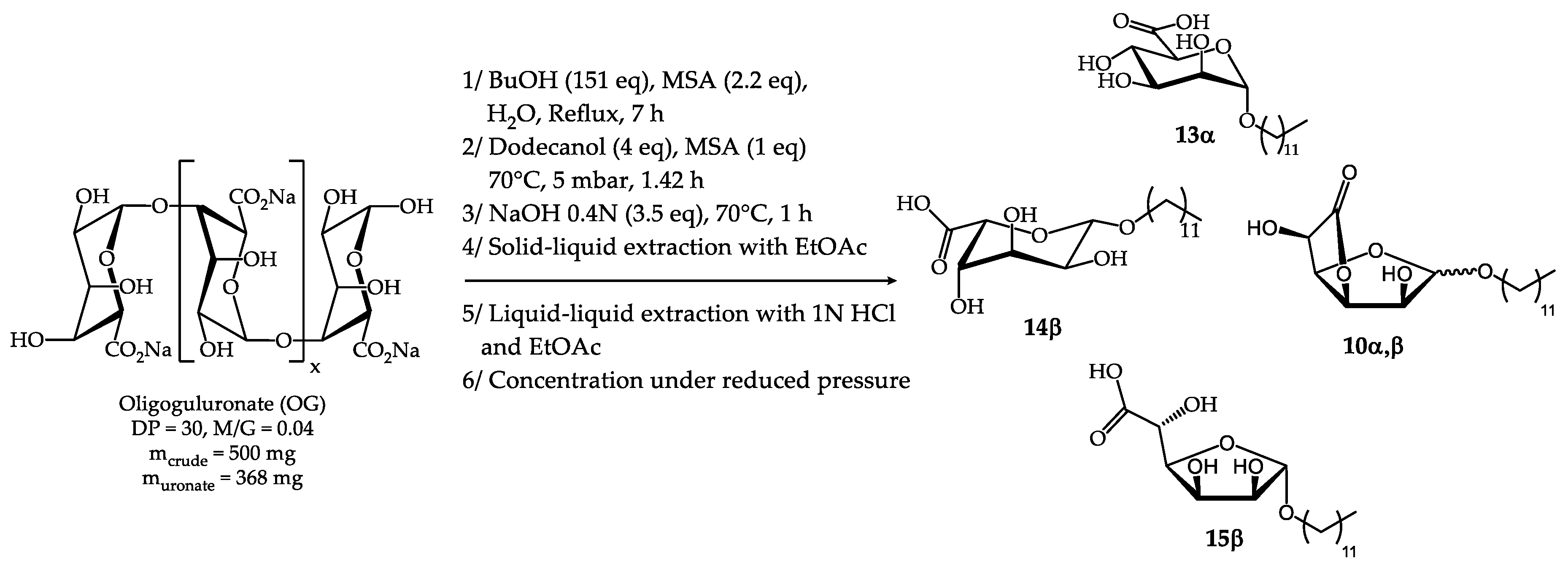
Oligoguluronate (500 mg, 2.1 mmol CO2-, 1 eq.) was dispersed in water (1.5 mL) and n-butanol (29 mL, 317 mmol, 151 eq.) in a round-bottom flask with a Dean-Stark apparatus. Methanesulfonic acid technical grade 70% wt (634 mg, 4.62 mmol, 2.2 eq.) was added and the mixture was refluxed under vigorous stirring. The water formed in the medium was gradually removed by azeotropic distillation. After 7 h, the mixture was cooled to ambient temperature. Dodecanol (1.8 mL, 8.3 mmol, 4 eq.) and the 70% methanesulfonic acid solution (288 mg, 2.1 mmol, 1 eq.) were added. The mixture was stirred at 70° C under reduced pressure (up to 5 mbar) using distillation apparatus. Once the butanol had been completely removed (1.42 h), a 0.4N NaOH solution (18.5 mL, 7.4 mmol, 3.5 eq.) was added and the mixture was left to stir vigorously at 70° C. for 1 h. The water was then removed by freeze-drying or by azeotropic distillation with butanol. The excess dodecanol present in the crude product was removed by solid-liquid extraction with EtOAc. At the end of this treatment, the mixture of products was dissolved in ice-cold water (27.5 mL) and EtOAc (27.5 mL), then a 1N hydrochloric acid solution (3.6 mL) was added. The aqueous solution was extracted with EtOAc (3x15 mL). The organic phases were combined and washed with a saturated NaC1 solution (35 mL). The organic phase was dried with MgSO4 and then concentrated under vacuum. A mixture of products H-C12 Gul was obtained (356 mg), the molar composition of which is: 15% n-dodecyl α-D-mannopyranosiduronic acid (13α), 13% n-dodecyl β-L-gulopyranosiduronic acid (14β), 26% n-dodecyl α-L-gulofuranosidurono-6,3-lactone (10α), 26% n-dodecyl β-L-gulofuranosidurono -6,3 –lactone (10β), 21% n-dodecyl-β-L-gulofuranosiduronic acid (15β). 1H NMR (400 MHz, CD3OD) δ 5.07 (dd, J = 7.5, 4.7 Hz, H3-10β), 4.98 (s, H1-10β), 4.96 (t, J = 5.5 Hz, H3-10α), 4.92 (d, J = 4.5 Hz, H1-10α), 4.80 (d, J = 2.1 Hz, H1-13α), 4.62 (d, J = 8.4 Hz, H1-14β), 4.51 (dd, J = 7.6, 2.1 Hz, H4- 15β), 4.49 (d, J = 1.7 Hz, H5-14β), 4.39 (d, J = 4.3 Hz, H5-10β), 4.29 (d, J = 2.1 Hz, H5- 15β), 4.02 (dd, J = 3.6, 1.4 Hz, H4-14β), 4.00 (d, J = 9.7 Hz, H5-13α), 3.89 (t, J = 9.2 Hz, H4-13α), 3.84 (d, J = 5.0 Hz, H2- 15β), 3.79 (dd, J = 3.4, 2.1 Hz, H2-13α), 3.76 – 3.59 (m, OCH2), 3.56 – 3.36 (m, OCH2), 1.69 – 1.50 (m, CH2), 1.45 – 1.25 (m, CH2), 0.97 – 0.87 (m, CH3).
Preparation of H-C12 OAlg from OAlg
Oligoalginate (1.0 g, 2.84 mmol CO2-, 1 eq.) was dispersed in water (2.0 mL) and n-butanol (39 mL, 426 mmol, 150 eq.) in a round-bottom flask with a Dean-Stark apparatus. Methanesulfonic acid technical grade 70% wt (857 mg, 6.24 mmol, 2.2 eq.) was added and the mixture was refluxed under vigorous stirring. The water formed in the medium was gradually removed by azeotropic distillation. After 7 h, the mixture was cooled to ambient temperature. Dodecanol (2.5 mL, 11.2 mmol, 4 eq.) and the 70% methanesulfonic acid solution (391 mg, 2.85 mmol, 1 eq.) were added. The mixture was stirred at 70° C under reduced pressure (up to 5 mbar) using distillation apparatus. Once the butanol had been completely removed (1.42 h), a 0.4N NaOH solution (25 mL) was added and the mixture was left to stir vigorously at 70° C. for 1 h. The water was then removed by freeze-drying or by azeotropic distillation with butanol. The excess dodecanol present in the crude product was removed by solid-liquid extraction with EtOAc. At the end of this treatment, the mixture of products was dissolved in ice-cold water (30 mL) and EtOAc (45 mL), then a 1N hydrochloric acid solution (6 mL) was added. The aqueous solution was extracted with EtOAc (8x15 mL). The organic phases were combined and washed with a saturated NaC1 solution (60 mL) and a 1N hydrochloric acid solution (300 µL). The organic phase was dried with MgSO4 and then concentrated under vacuum. A mixture of products H-C12 OAlg was obtained (759 mg), the molar composition of which is: 23% n-dodecyl-α-D-mannopyranosiduronic (13α), 9% n-dodecyl-β-L-gulopyranosiduronic (14β), 20% n-dodecyl-α-L-gulofuranosidurono-6,3-lactone (10α), 20% n-dodecyl-β-L-gulofuranosidurono -6,3 –lactone (10β), 9% n-dodecyl-β-L-gulofuranosiduronic acid (15β), 4% n-dodecyl-α-D-mannofuranosidurono-6.3-lactone (9α), 9% n-dodecyl-β-D-mannofuranosidurono-6.3-lactone (9β), 6% non-identified molecule X. 1H NMR (400 MHz, CD3OD) δ 5.11 (d, J = 6.5 Hz, X), 5.07 (dd, J = 7.5, 4.7 Hz, H3-10β), 4.97 (s, H1-10β), 4.95 (t, J = 5.6 Hz, H3-10α), 4.95 (d, J = 4.1 Hz, H1-9β), 4.91 (d, J = 4.5 Hz, H1-10α), 4.80 (d, J = 2.1 Hz, H1-13α), 4.72 (td, J = 4.7, 1.5 Hz, H4-9α,β), 4.61 (d, J = 8.2 Hz, H1-14β), 4.60 – 4.55 (m, H4-10α,β), 4.54 (d, J = 5.0 Hz, X), 4.51 (s (br), H4-15β), 4.48 (d, J = 1.6 Hz, H5-14β), 4.46 (d, J = 6.5 Hz, H5-9β), 4.38 (d, J = 4.3 Hz, H5-10β), 4.35 (d, J = 5.0 Hz, X), 4.29 (d, J = 2.0 Hz, H5- 15β), 4.12 – 4.08 (m, H2-10β/H4-10α), 3.88 (t, J = 9.3 Hz, H4-13α), 3.78 (dd, J = 3.4, 2.1 Hz, H2-13α), 3.75 – 3.61 (m, OCH2), 3.55 – 3.35 (m, OCH2), 1.70 – 1.49 (m, CH2), 1.45 – 1.23 (m, CH2), 0.95 – 0.86 (m, CH3).
Preparation of H-C12 s-r Alg from s-r Alg
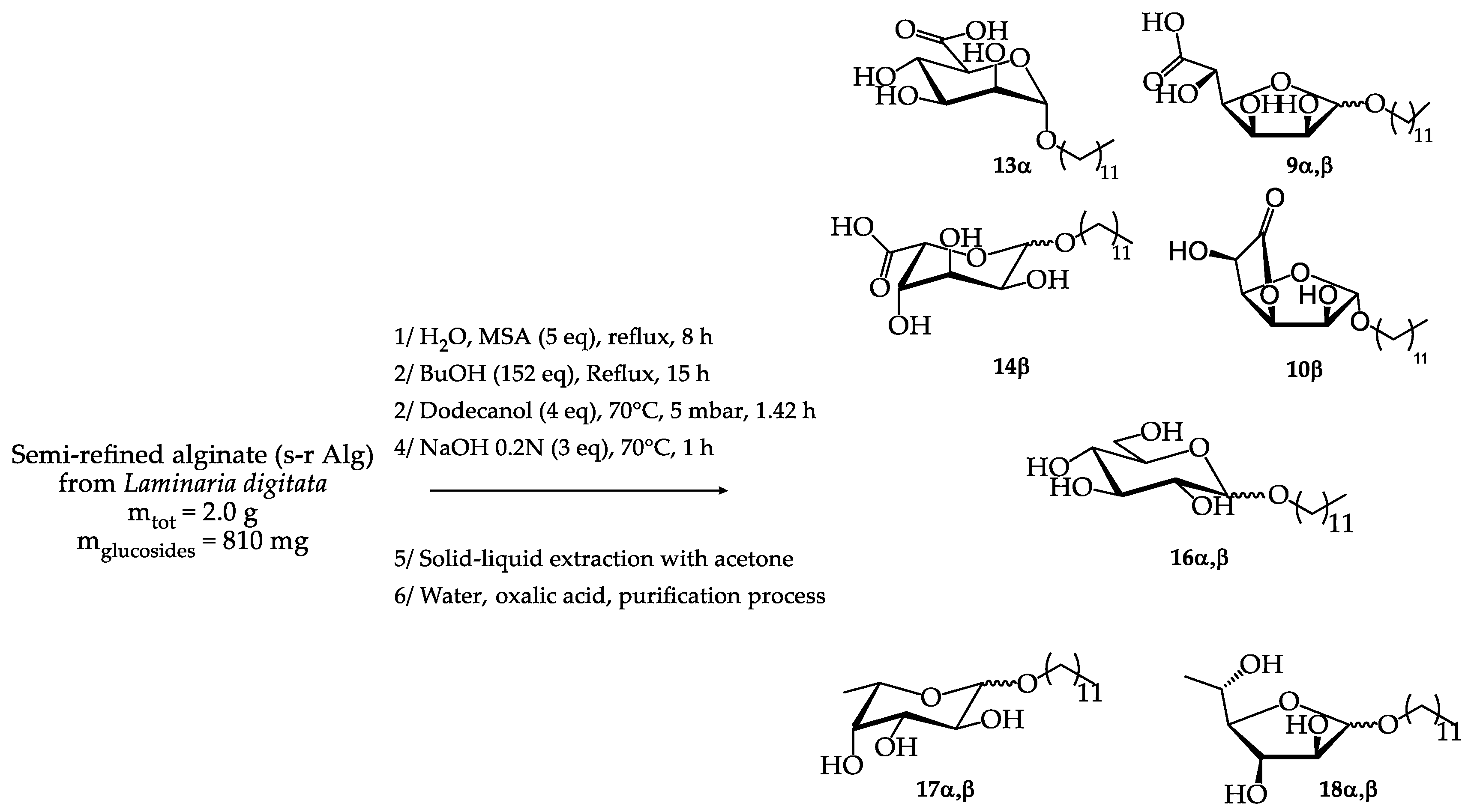
The semi-refined alginate derived from Laminaria digitata (2.0 g, 4.3 mmol sugar units, 1 eq.) was dispersed in water (60 mL) and the 70% methane-sulfonic acid solution (2.95 g, 21.5 mmol, 5 eq.) was added in a round-bottom flask with a Dean-Stark apparatus. The mixture was refluxed with vigorous stirring. At 8 h of reaction, butanol (60 mL, 656 mmol, 152 eq.) was added and the mixture was left at reflux with vigorous stirring. The water present in the medium was gradually removed by azeotropic distillation. After a further 15 h of reaction, and once the mixture had returned to ambient temperature, dodecanol (3.8 mL, 17 mmol, 4 eq.) was added. The mixture was stirred at 70° C under reduced pressure (up to 5 mbar) using distillation apparatus. Once the butanol had been completely removed (1.42 h), a 0.2N NaOH solution (60 mL) was added and the mixture was left to stir vigorously at 70° C. for 1 h. The water was then removed by freeze-drying or by azeotropic distillation with butanol. The excess dodecanol present in the crude product was removed by solid-liquid extraction with acetone. At the end of this treatment, the mixture of products was dissolved in ice-cold water (75 mL) and then a 0.5M oxalic acid solution (8.0 mL) was added. The water was then removed by freeze-drying. The crude product was purified by solid-liquid extraction with acetone (20 mL + 8x10 mL). The filtrate was concentrated under vacuum. At the end of this treatment, a mixture of products was obtained (628 mg), the weight composition of which is: 23% n-dodecyl-α-D-mannopyranosiduronic (13α), 5% n-dodecyl-α-D-mannofuranosidurono-6.3-lactone (9α), 13% n-dodecyl-β-D-mannofuranosidurono-6.3-lactone (9β), 11% n-dodecyl-β-L-gulopyranosiduronic (14β), 20% n-dodecyl-β-L-gulofuranosidurono -6,3 –lactone (10β), 5% n-dodecyl-α−L-fucopyranosides 17α, 8% n-dodecyl-β-L-fucopyranosides 17β and 15% n-dodecyl-α,β-L-fucofuranosides 18α,β. 1H NMR (400 MHz, acetone-d6) δ 5.08 (dd, J = 7.5, 4.7 Hz, H3-10β), 5.03 (s, H1-10β), 4.95 (d, J = 4.7 Hz, H1-9β), 4.89 (t, J = 4.8 Hz, H3-9β), 4.85 (d, J = 2.0 Hz, H1-13α), 4.83 (d, J = 3.8 Hz, H1-17α), 4.80 (td, J = 4.9, 1.4 Hz, H4-9α,β), 4.75 (d, J = 3.6 Hz, H1-18α,β), 4.65 (dd, J = 7.3, 4.4 Hz, H4-10β), 4.56 (d, J = 5.4 Hz), 4.51 (d, J = 1.7 Hz, H5-14β), 4.43 (d, J = 4.4 Hz, H5-10β), 4.27 (d, J = 7.7 Hz, H1-17β), 4.25 (t, J = 4.4 Hz), 4.18 (d, J = 4.7 Hz, H2-10β), 4.09 (dd, J = 3.6, 1.6 Hz, H4-14β), 3.83 (dd, J = 3.4, 2.0 Hz, H2-13α), 3.79 – 3.63 (m, OCH2), 3.51 – 3.31 (m, OCH2), 1.68 – 1.49 (m, CH2), 1.46 – 1.26 (m, CH2), 1.24 (d, J = 6.6 Hz, CH3 17 or 18), 1.22 (d, J = 6.8 Hz, CH3-17 or 18), 1.21 (d, J = 6.7 Hz, CH3-17 or 18), 0.97 – 0.86 (m, CH3).
Preparation of H-C8 crude Alg from crude Alg
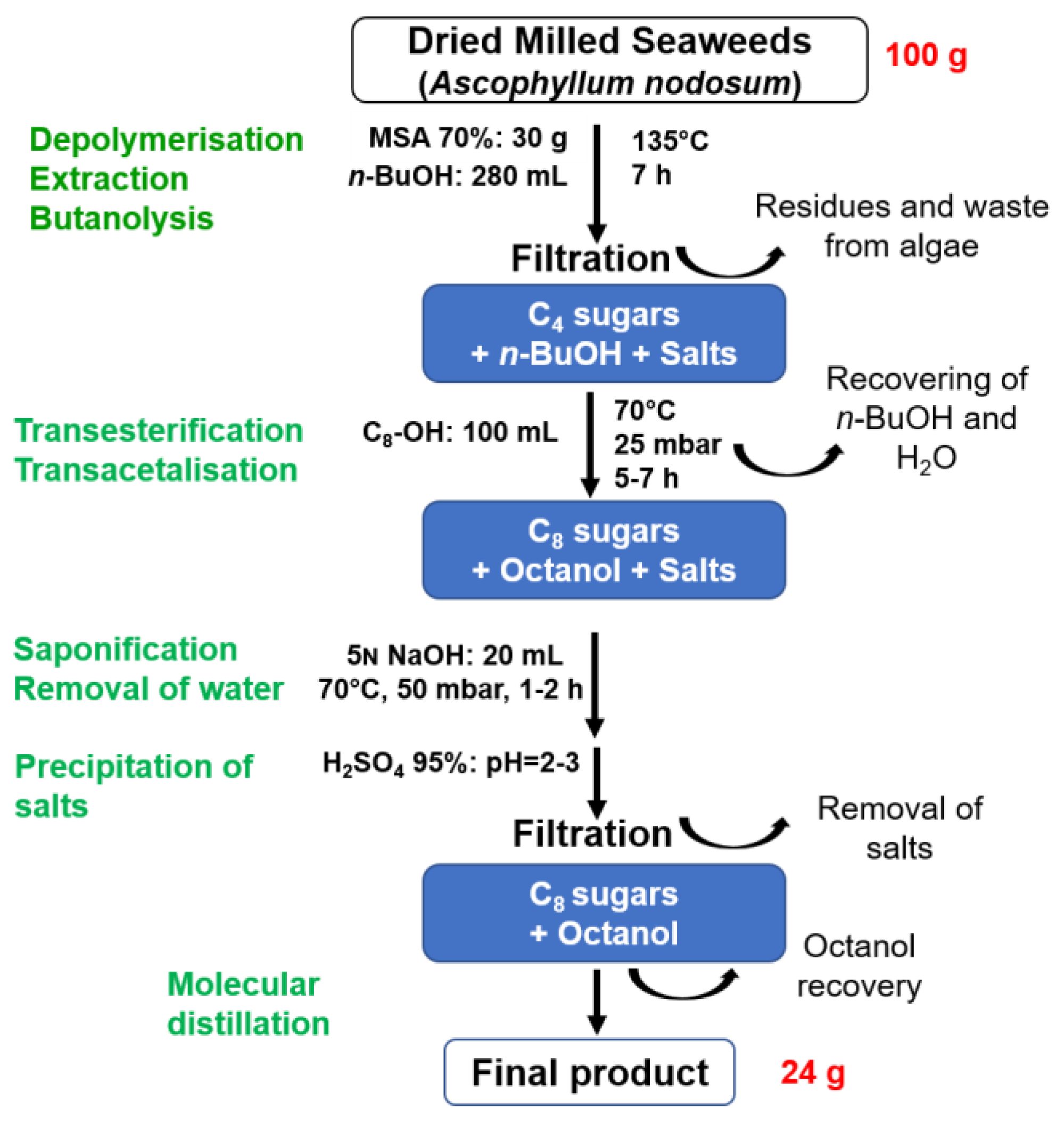
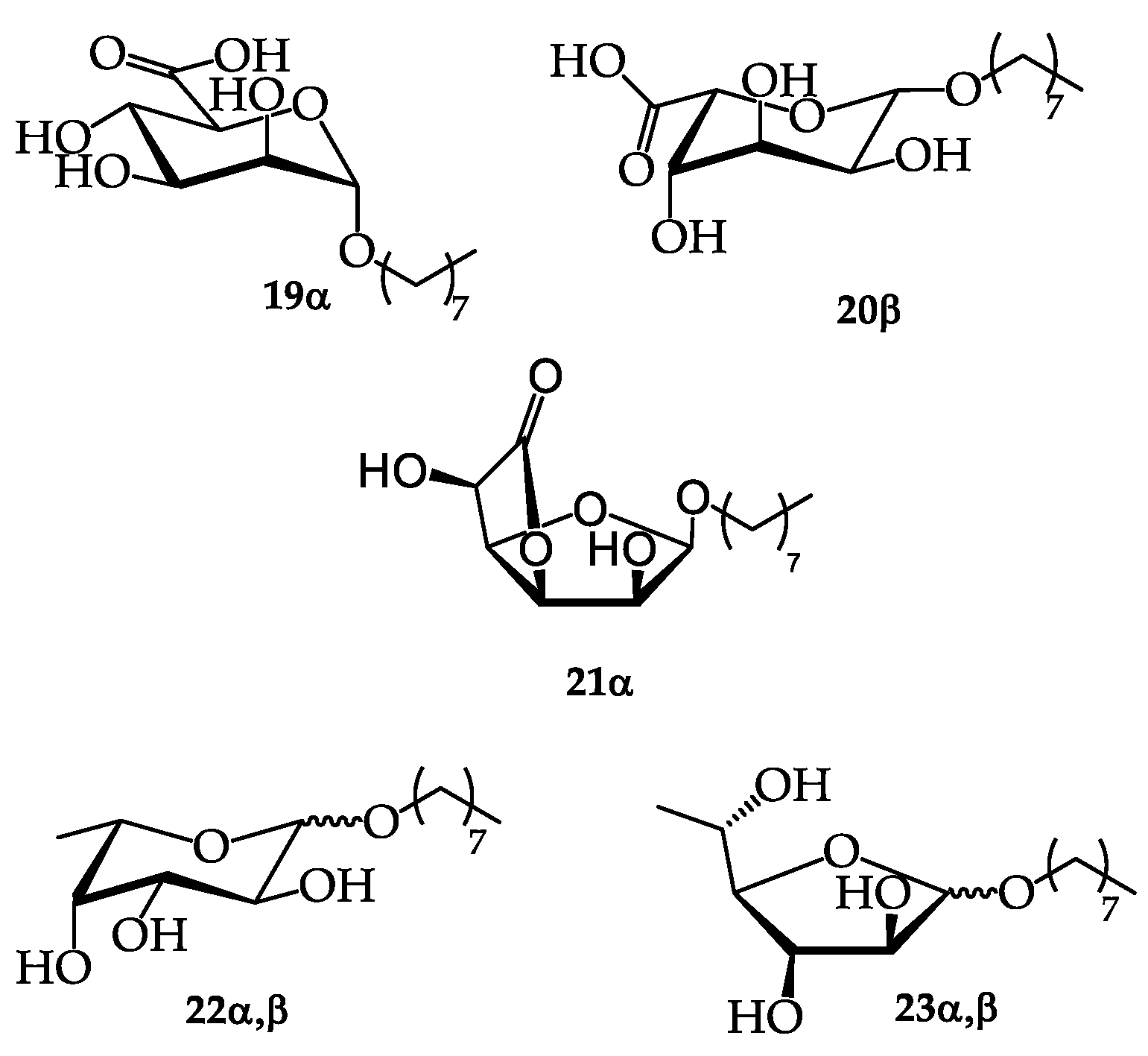
The dried milled Ascophyllum nodosum seaweed (100 g) was introduced into the reactor (1 L). Butanol (140 ml) and the methane-sulfonic acid solution (30 g of 70% MSA in 140 mL of butanol) were added. The mixture was refluxed and stirred with a four-blade Teflon paddle at 400 rpm. For the double envelope, the temperature was fixed at 135°C. After 7 h of reaction, the mixture was recovered hot through the lower tap and then filtered or centrifuged to remove residues. The presence of products in the reaction medium was monitored by TLC with CH2Cl2/MeOH (95/5, v/v) as the eluent and vanillin solution as the staining reagent. The filtrate/supernatant was transferred into the reactor (1 L) and octanol (100 ml) was added. The reaction mixture is pulled under vacuum at 70° C for 5-7 h to evaporate butanol and perform the transesterification/transglycosylation reactions. The mixture was made alkaline with a concentrated sodium hydroxide solution (5N NaOH, 20 mL). After 1-2 h at 70°C, only nonionic surfactants were visible by TLC (eluent: CH2Cl2/MeOH (95/5, v/v)). The anionic molecules were revealed by TLC (AcOEt/iPrOH/H2O (6/3/1, v/v/v)). The mixture was pulled under vacuum to evaporate the water. At room temperature, 95% of H2SO4 was added to acidify to pH 2-3 and precipitate the salts (counter-ions of the anionic surfactants). Surfactants were miscible in octanol. This oil phase was filtered to remove the salts which were insoluble. The oily filtrate was injected into a molecular distillation apparatus to eliminate octanol. At 100° C under reduced pressure (1 mbar), 24 g of the surfactant composition was recovered in the form of a viscous syrup, and consequently in a mass yield of approximately 24% relative to the starting algae. At room temperature, the product is a pasty and hygroscopic solid. The mixture was submitted to thermogravimetric analysis, which showed that it consisted of 83.2% organic matter; 10.5% water; and 6.75% ash. 1H NMR analysis revealed the presence of 30 mol% n-octyl α-D-mannopyranosiduronic acid 19α , 6 mol% n-octyl β-L-gulopyranosiduronic acid 20β, n-octyl α-L-gulofuranosidurono-6,3-lactone 21α, 13 mol% n-octyl-α-L-fucopyranoside 22α, 9 mol% n-octyl-β-L-fucopyranosides 22β, 9 mol% n-ocyl-α-L-fucofuranosides 23α and 28 mol% n-octyl-β-L-fucofuranoside 23β. 1H NMR (400 MHz, CD3OD) δ 5.05 (d, J = 3.2 Hz), 4.97 (t, J = 5.5 Hz, H5-21α), 4.93 (d, J = 4.6 Hz, H1-21α), 4.82 (d, J = 2.0 Hz, H1-19α), 4.78 (d, J = 3.8 Hz, H1-22α), 4.75 (d, J = 2.2 Hz, H1-23β), 4.72 (d, J = 3.7 Hz, H1-23α), 4.64 (d, J = 8.1 Hz, H1-20β), 4.61 (d, J = 4.6 Hz), 4.50 (d, J = 1.6 Hz, H5-20β), 4.43 (t, J = 4.8 Hz), 4.26 (d, J = 7.8 Hz, H1-22β), 3.88 (t, J = 9.4 Hz, H4-19α), 3.80 (dd, J = 3.5, 1.9 Hz, H2-19α), 3.77 – 3.63 (m, OCH2), 3.53 – 3.35 (m, OCH2), 1.72 – 1.50 (m, CH2), 1.48 – 1.30 (m, CH2), 1.27 (d, J = 6.4 Hz, CH3 22 or 18), 1.26 (d, J = 6.5 Hz, CH3-22 or 23), 1.22 (d, J = 6.6 Hz, CH3-22 or 23), 1.19 (d, J = 6.5 Hz, CH3-22 or 23), 1.01 – 0.88 (m, CH3).