Submitted:
29 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
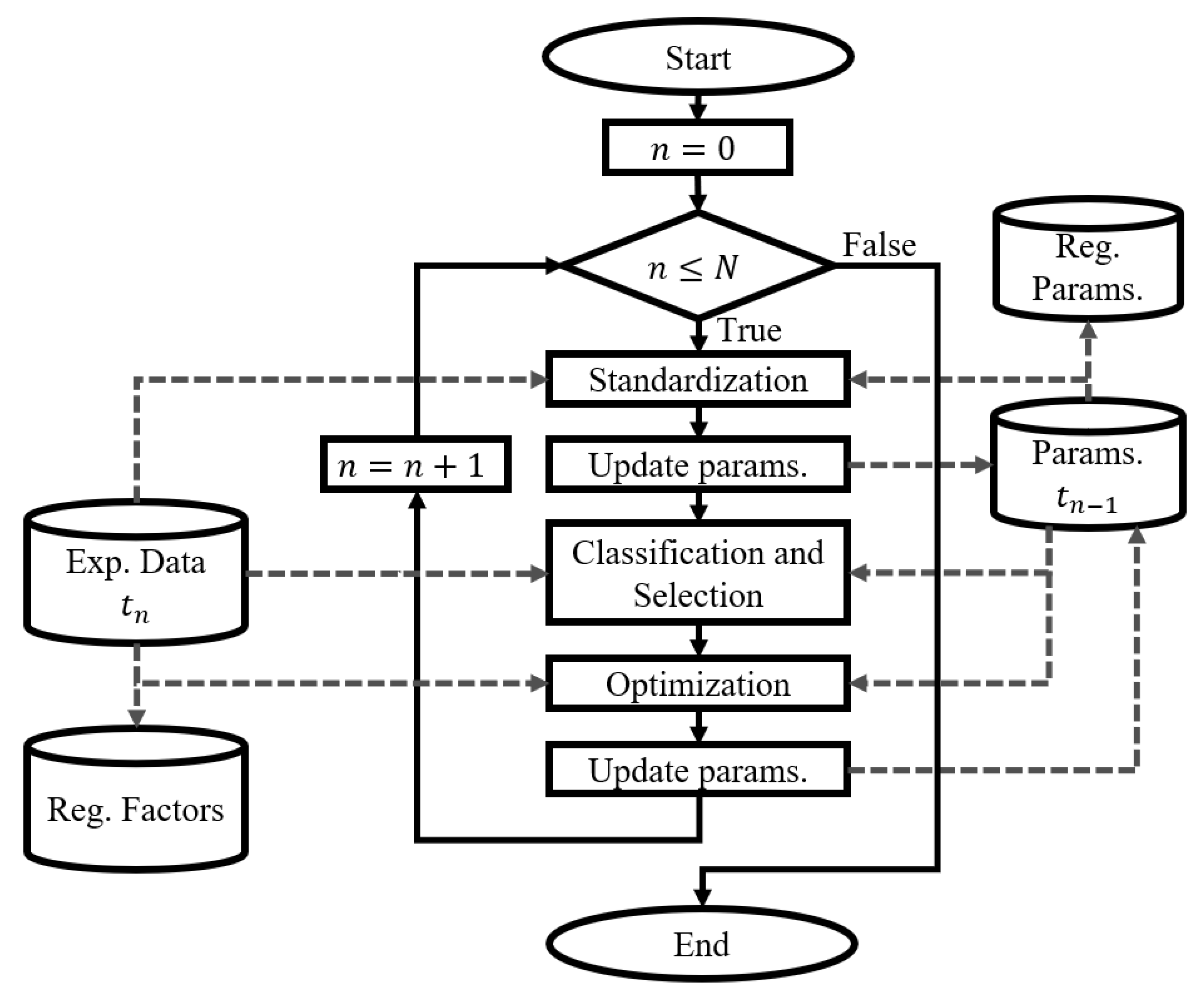
2.1. Dynamic Fitting Strategy
2.1.1. Dynamic Fitting of the Physiological Model
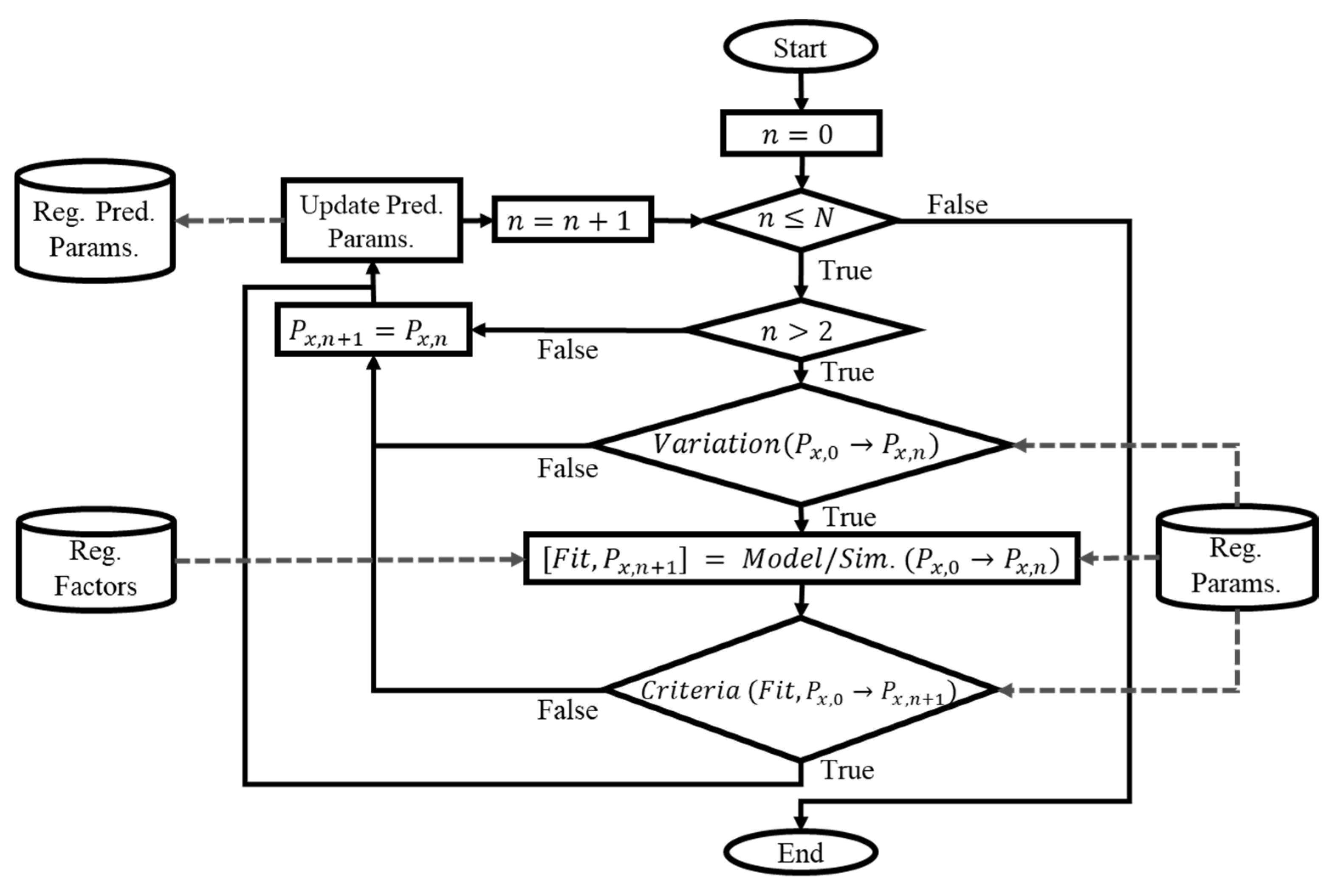
2.1.2. Dynamic Parameter Modeling
2.1.3. Validation
2.2. Case Study
2.2.1. Cardiorespiratory Model
2.2.2. Experimental Data
2.2.3. Computational Implementation and Data Analysis
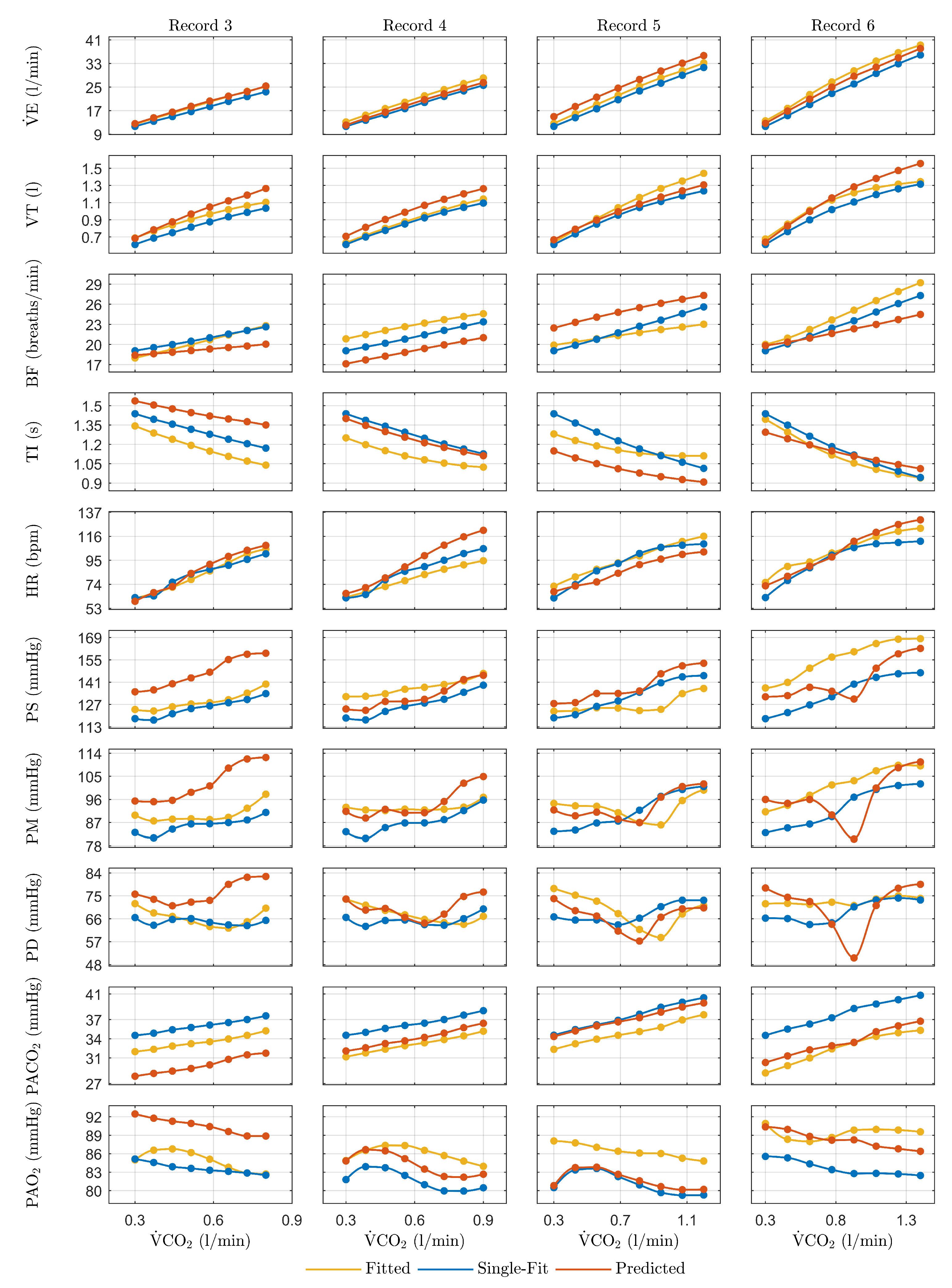
3. Results
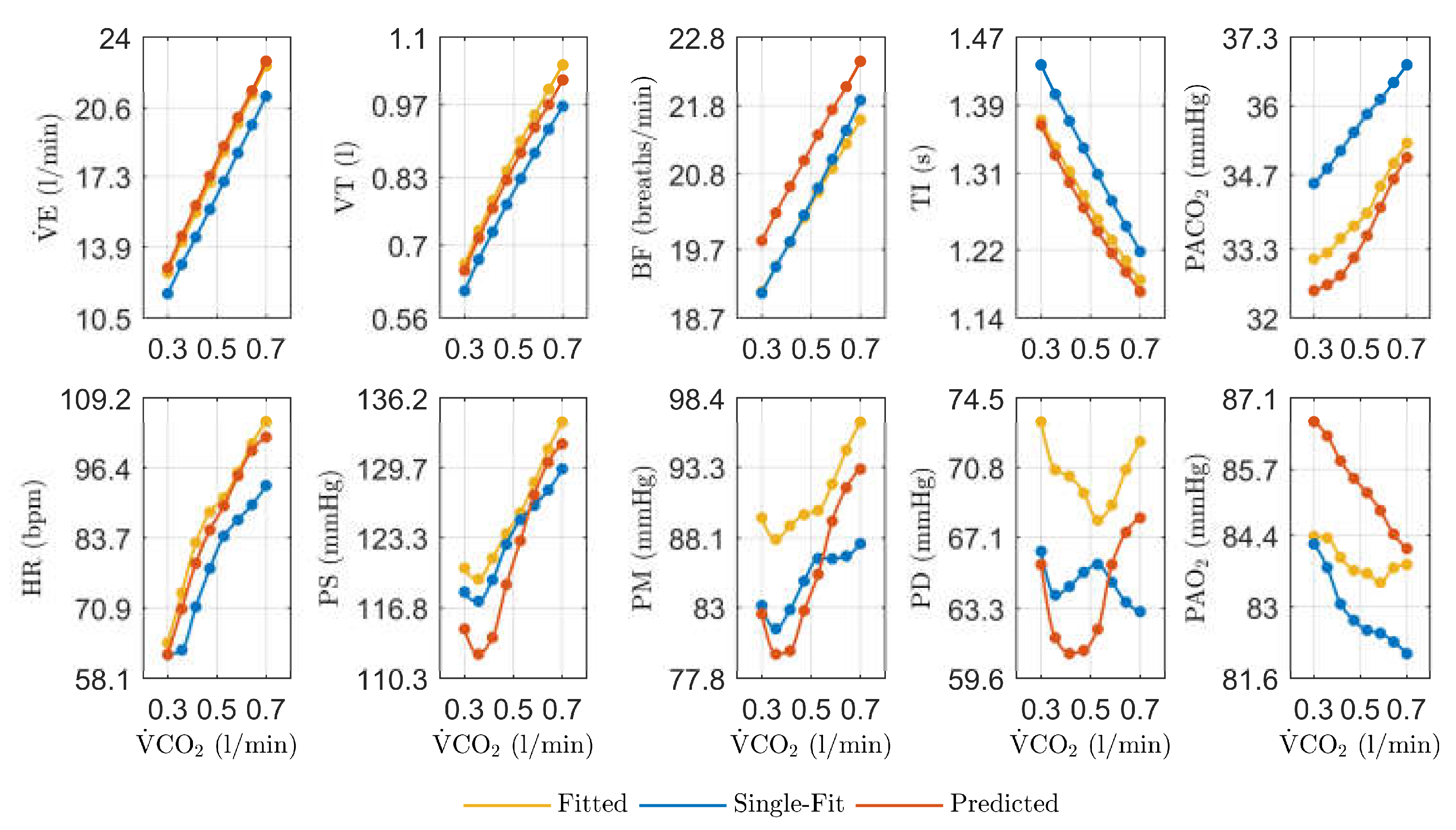
3.1. Dynamic Fitting of the Physiological Model
3.2. Dynamic Parameter Modeling
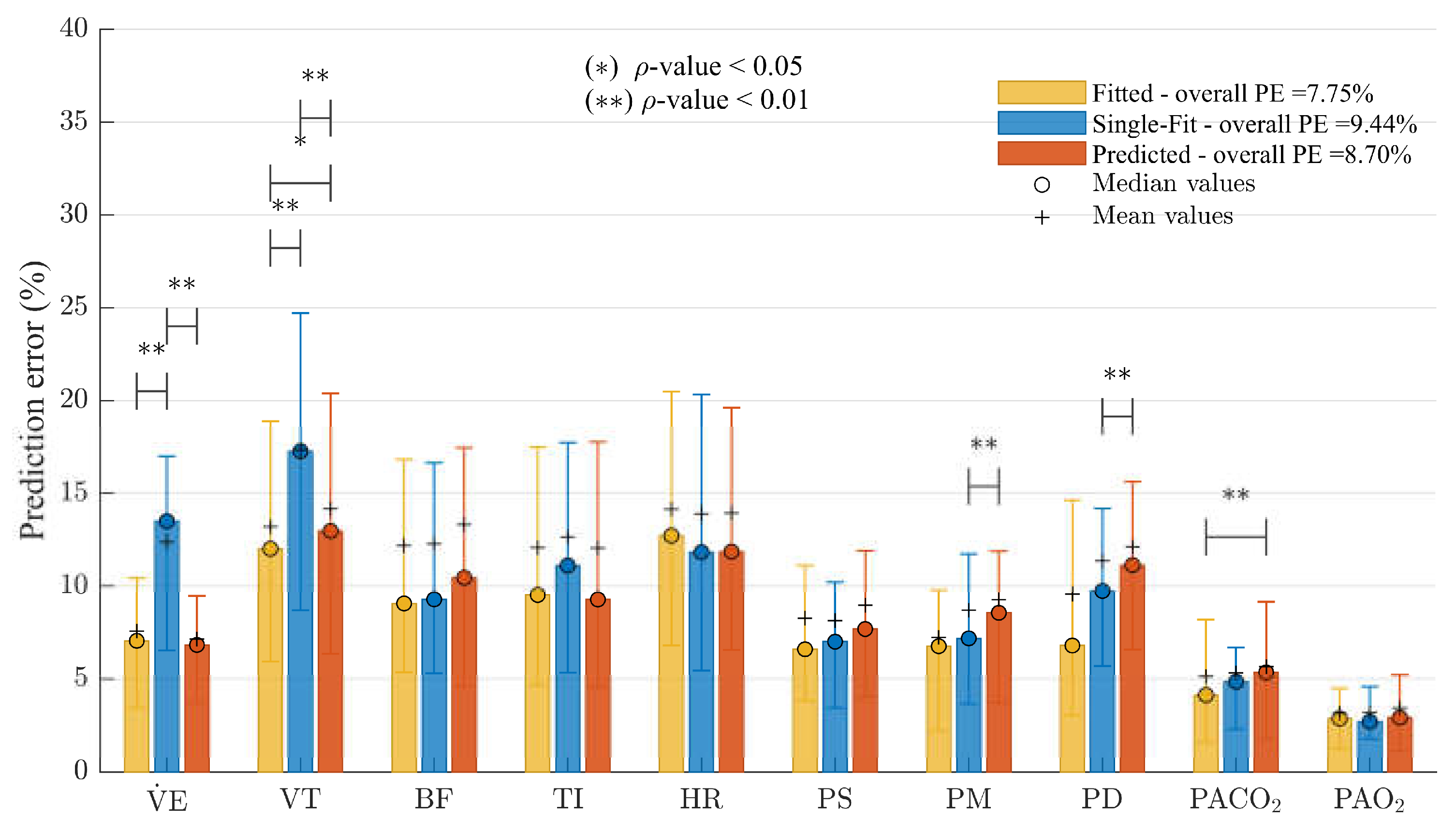
3.3. Validation
4. Discussion
4.1. Dynamic Model Fitting
4.2. Dynamic Parameter Modeling
4.3. Validation
4.4. Application, Limitations, and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Healthcare in overview across the globe. EPMA J. 2011, 2, 71–89. [CrossRef]
- Golubnitschaja, O.; Kinkorova, J.; Costigliola, V. Predictive, Preventive and Personalised Medicine as the hardcore of ‘Horizon 2020’: EPMA position paper. EPMA J. 2014, 5, 6–6. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-L.; Tsai, F.-J. Personalized medicine: A paradigm shift in healthcare. Biomed. Pharmacother. 2013, 3, 66–72. [Google Scholar] [CrossRef]
- Habetha, J. The myheart project - Fighting cardiovascular diseases by prevention and early diagnosis. Conference Proceedings. Annual International Conference of the IEEE Engineering in Medicine and Biology Society. LOCATION OF CONFERENCE, United StatesDATE OF CONFERENCE; pp. 6746–6749.
- Lai, L.; Wang, Z.; Wu, X. The Progress of Research on Prediction Model for Acute Hypotensive Episodes. In Proceedings of the 2010 3rd International Conference on Biomedical Engineering and Informatics; IEEE, October 2010; Vol. 3; pp. 1067–1070. [Google Scholar]
- Pantelopoulos, A.; Bourbakis, N. A Health Prognosis Wearable System with Learning Capabilities Using NNs. In Proceedings of the 2009 21st IEEE International Conference on Tools with Artificial Intelligence; 2009; pp. 243–247. [Google Scholar]
- Batzel, J.J.; Bachar, M.; Karemaker, J.M.; Kappel, F. Mathematical Modeling and Validation in Physiology; Batzel, J.J. , Bachar, M., Kappel, F., Eds.; Lecture Notes in Mathematics; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; ISBN 978-3-642-32881-7. [Google Scholar]
- Batzel, J.J.; Kappel, F.; Schneditz, D.; Tran, H.T. Cardiovascular and Respiratory Systems : Modeling, Analysis, and Control; 1st ed.; SIAM: Philadelphia, United States, 2007; ISBN 9780898716177. [Google Scholar]
- Das, A.; Menon, P.P.; Hardman, J.G.; Bates, D.G. Optimization of Mechanical Ventilator Settings for Pulmonary Disease States. IEEE Trans. Biomed. Eng. 2013, 60, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Marmarelis, V.Z. Nonlinear Dynamic Modeling of Physiological Systems; 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; ISBN 9780471679370.
- Ellwein, L.M. Cardiovascular and Respiratory Regulation, Modeling and Parameter Estimation, North Carolina State University: Ann Arbor, United States, 2008.
- Ellwein, L.; Pope, S.; Xie, A.; Batzel, J.; Kelley, C.; Olufsen, M. Patient-specific modeling of cardiovascular and respiratory dynamics during hypercapnia. Math. Biosci. 2013, 241, 56–74. [Google Scholar] [CrossRef]
- Cardona-Morrell, M.; Prgomet, M.; Turner, R.M.; Nicholson, M.; Hillman, K. Effectiveness of continuous or intermittent vital signs monitoring in preventing adverse events on general wards: a systematic review and meta-analysis. Int. J. Clin. Pr. 2016, 70, 806–824. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Albanese, A.; Ursino, M.; Chbat, N.W. An integrated mathematical model of the human cardiopulmonary system: model validation under hypercapnia and hypoxia. Am. J. Physiol. Circ. Physiol. 2016, 310, H922–H937. [Google Scholar] [CrossRef] [PubMed]
- Haouzi, P.; Poon, C.-S.; Secher, N.H.; Keslacy, S.; Matecki, S.; Carra, J.; Borrani, F.; Candau, R.; Prefaut, C.; Ramonatxo, M.; et al. Ventilatory control in hypercapnia and exercise: optimization hypothesis. J. Appl. Physiol. 1987, 62, 2447–2459. [Google Scholar] [CrossRef]
- Serna, L.Y.; Mañanas, M.A.; Hernández, A.M.; Rabinovich, R.A. An Improved Dynamic Model for the Respiratory Response to Exercise. Front. Physiol. 2018, 9, 69. [Google Scholar] [CrossRef]
- Tehrani, F.T. Optimal Control of Respiration in Exercise. In Proceedings of the Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society.; pp. 2020009836286200263211–3214.
- Magosso, E.; Cavalcanti, S.; Ursino, M. Theoretical analysis of rest and exercise hemodynamics in patients with total cavopulmonary connection. Am. J. Physiol. Circ. Physiol. 2002, 282, H1018–H1034. [Google Scholar] [CrossRef]
- Cheng, L.; Ivanova, O.; Fan, H.-H.; Khoo, M.C. An integrative model of respiratory and cardiovascular control in sleep-disordered breathing. Respir. Physiol. Neurobiol. 2010, 174, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.M. Sistema de Control Respiratorio Ante Estímulos y Patologías: Análisis, Modelado y Simulación; OmniScript.; Publicia, 2014; ISBN 978-3-639-55914-9.
- Karamolegkos, N.; Albanese, A.; Isaza, F.; Chbat, N.W. Patient Emulator: A Tool for Testing Mechanical Ventilation Therapies. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE, August 2016; Vol. 2016; pp. 4321–4324. [Google Scholar]
- Tehrani, F.T. Automatic Control of Mechanical Ventilation and the Inspired Fraction of Oxygen in the Premature Infant: A Simulation Study. In Proceedings of the Proceedings of the First Joint BMES/EMBS Conference.; p. 1999211999993701519991339.
- Magosso, E.; Ursino, M. Cardiovascular response to dynamic aerobic exercise: A methematical model. Med Biol. Eng. Comput. 2002, 40, 660–674. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.C.; Monteiro, W.D.; Farinatti, P.d.T.V. Avaliação da capacidade máxima de exercício: uma revisão sobre os protocolos tradicionais e a evolução para modelos individualizados. Rev. Bras. de Med. do Esporte 2011, 17, 363–369. [Google Scholar] [CrossRef]
- Fonoberova, M.; Mezić, I.; Buckman, J.F.; Fonoberov, V.A.; Mezić, A.; Vaschillo, E.G.; Mun, E.-Y.; Vaschillo, B.; Bates, M.E. A computational physiology approach to personalized treatment models: the beneficial effects of slow breathing on the human cardiovascular system. Am. J. Physiol. Circ. Physiol. 2014, 307, H1073–H1091. [Google Scholar] [CrossRef]
- Serna, L.Y.; Mañanas, M. .; Marín, J.; Hernández, A.M.; Benito, S. Optimization techniques in respiratory control system models. Appl. Soft Comput. 2016, 48, 431–443. [Google Scholar] [CrossRef]
- Kovacs, F.; Hosszu, G. A proposed method for dynamic fitting of MOS model parameters. IEEE Trans. Comput. Des. Integr. Circuits Syst. 1993, 12, 1503–1507. [Google Scholar] [CrossRef]
- Lingxiong Shao, L.; Smith, R.; Freifelder, R.; Karp, J.S. Practical Approaches for Oxygen Flow Studies in PET. In Proceedings of the Proceedings of 1994 IEEE Nuclear Science Symposium - NSS’94; pp. 199431416–1420.
- Shande, S.; Shouzhen, Z.; Bo, H. Identification of Parameters of Synchronous Machine and Excitation System by On-Line Test. In Proceedings of the International Conference on Advances in Power System Control, Operation and Management; IET; 1991; pp. 716–719. [Google Scholar]
- Yanjun Yan, Y.; Osadciw, L.A.; Benson, G.; White, E. Inverse Data Transformation for Change Detection in Wind Turbine Diagnostics. In Proceedings of the 2009 Canadian Conference on Electrical and Computer Engineering; IEEE, May 2009; pp. 944–949. [Google Scholar]
- Khoo, M.C.K. Physiological Control Systems: Analysis, Simulation, and Estimation; 2nd ed.; Wiley-IEEE Press: New Jersey, 2018; ISBN 978-1-119-05880-9. [Google Scholar]
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: the challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef]
- Eslami, O.; Zarei, M.; Shidfar, F. The association of dietary patterns and cardiorespiratory fitness: A systematic review. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1442–1451. [Google Scholar] [CrossRef]
- Jerath, R.; Harden, K.; Crawford, M.; Barnes, V.A.; Jensen, M. Role of cardiorespiratory synchronization and sleep physiology: effects on membrane potential in the restorative functions of sleep. Sleep Med. 2014, 15, 279–288. [Google Scholar] [CrossRef]
- Myers, J.; McAuley, P.; Lavie, C.J.; Despres, J.-P.; Arena, R.; Kokkinos, P. Physical Activity and Cardiorespiratory Fitness as Major Markers of Cardiovascular Risk: Their Independent and Interwoven Importance to Health Status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef]
- World Health Organization: Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 February 2023).
- Sarmiento, C.A.; Serna, L.Y.; Hernández, A.M.; Mañanas, M. . A Novel Strategy to Fit and Validate Physiological Models: A Case Study of a Cardiorespiratory Model for Simulation of Incremental Aerobic Exercise. Diagnostics 2023, 13, 908. [Google Scholar] [CrossRef]
- Burth, M.; Verghese, G.; Velez-Reyes, M. Subset selection for improved parameter estimation in on-line identification of a synchronous generator. IEEE Trans. Power Syst. 1999, 14, 218–225. [Google Scholar] [CrossRef]
- Ginsburg, G.S.; Willard, H.F. Genomic and personalized medicine: foundations and applications. Transl. Res. 2009, 154, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.T. A challenging drug development process in the era of personalized medicine. Drug Discov. Today 2011, 16, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.L. Personalized medicine: A near future or yet miles to go? Adv. Integr. Med. 2015, 2, 112–113. [Google Scholar] [CrossRef]
- Tutton, R.; Jamie, K. Personalized medicine in context: social science perspectives. Drug Discov. Today: Ther. Strat. 2013, 10, e183–e187. [Google Scholar] [CrossRef]
- Strand, L.B.; Laugsand, L.E.; Wisløff, U.; Nes, B.M.; Vatten, L.; Janszky, I. Insomnia Symptoms and Cardiorespiratory Fitness in Healthy Individuals: The Nord-Trøndelag Health Study (HUNT). Sleep 2013, 36, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Kubo, H.; Mori, T.; Sato, T. Pulmonary and Cardiovascular Integrated Model Controlled with Oxygen Consumption. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference; IEEE, 2005; Vol. 7 VOLS; pp. 304–307. [Google Scholar]
- Mananas, M.A.; Hernandez, A.M.; Romero, S.; Grino, R.; Rabinovich, R.; Benito, S.; Caminal, P. Analysis of Respiratory Models at Different Levels of Exercise, Hypercapnia and Hypoxia. In Proceedings of the Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat.; pp. 0337439200332754–2757.
- Poon, C.-S.; Lin, S.L.; Knudson, O.B.; Secher, N.H.; Racca, F.; Appendini, L.; Gregoretti, C.; Stra, E.; Patessio, A.; Donner, C.F.; et al. Optimization character of inspiratory neural drive. J. Appl. Physiol. 1992, 72, 2005–2017. [Google Scholar] [CrossRef]
- Sarmiento, C.A.; Hernández, A.M.; Serna, L.Y.; Mañanas, M. . An integrated mathematical model of the cardiovascular and respiratory response to exercise: model-building and comparison with reported models. Am. J. Physiol. Circ. Physiol. 2021, 320, H1235–H1260. [Google Scholar] [CrossRef]
- American Thoracic Society. American College of Chest Physicians ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Baker, R.J.; Kozoll, D.D.; A Meyer, K. The use of surface area as a basis for establishing normal blood volume. . 1957, 104, 183–9. [Google Scholar] [PubMed]
- Mosteller, R.D. Simplified Calculation of Body-Surface Area. New Engl. J. Med. 1987, 317, 1098–1098. [Google Scholar] [CrossRef] [PubMed]
- Hansen, N.; Ostermeier, A. Completely Derandomized Self-Adaptation in Evolution Strategies. Evol. Comput. 2001, 9, 159–195. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; White, R.D. Exercise Stress Testing for Primary Care and Sports Medicine; Evans, C.H. , White, R.D., Eds.; Springer New York: New York, NY, 2009; ISBN 978-0-387-76596-9. [Google Scholar]

| Registered information | DB1 records | DB2 records | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Environmental conditions | |||||||||
| Ambient temperature (ºC) | 26.6 ± 1.5 | 27.0 ± 1.6 | 25.9 ± 1.3 | 25.0 | 26.0 | 28.0 | 25.0 | 27.0 | 26.0 |
| Relative humidity (%) | 63.7 ± 5.0 | 58.7 ± 4.5 | 61.8 ± 7.1 | 56.0 | 56.0 | 64.0 | 49.0 | 70.0 | 49.0 |
| Subject data | |||||||||
| Age (years) | 28.3 ± 6.8 | 29.0 ± 6.9 | 29.9 ± 6.9 | 25.2 | 26.0 | 29.9 | 30.8 | 31.5 | 63.1 |
| Height (cm) | 173.6 ± 6.3 | 173.6 ± 6.3 | 173.6 ± 6.3 | 163.0 | 163.0 | 167.4 | 167.4 | 167.4 | 165.0 |
| Weight (kg) | 76.7 ± 9.6 | 76.6 ± 10.8 | 77.6 ± 11.4 | 71.8 | 70.9 | 83.3 | 79.2 | 81.6 | 71.3 |
| ) | 25.5 ± 2.8 | 25.4 ± 3.0 | 25.7 ± 3.2 | 27.0 | 26.7 | 29.7 | 28.3 | 29.1 | 26.2 |
| Average sleep (hours per day) | 6.6 ± 0.9 | 6.6 ± 1.0 | 6.7 ± 1.0 | 7.0 | 7.0 | 6.5 | 7.0 | 8.0 | 6.0 |
| Average physical activity (hours per week) | 6.4 ± 4.5 | 4.4 ± 4.5 | 3.3 ± 3.5 | 5.0 | 2.0 | 0.0 | 4.0 | 0.0 | 0.0 |
| Parameter | DB1 | DB2 | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | |
| (ml) | 5025.9 ± 454.6 | 5022.2 ± 497.4 | 5062.4 ± 526.0 | 4642.0 | 4604.7 | 5185.1 | 5023.7 | 5118.7 | 4657.5 |
| 0.9 ± 0.5 | 0.7 ± 0.3 | 0.8 ± 0.6 | 0.9 | 0.8 | 1.0 | 1.2 | 1.4 | 1.4 | |
| (ml) | 0.72 ± 0.12 | 0.76 ± 0.13 | 0.78 ± 0.13 | 0.60 | 0.73 | 0.77 | 0.67 | 0.66 | 0.74 |
| PE | DB1 | DB2 | |||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Overall | 7.57 | 8.42 | 8.03 | 8.51 | 6.13 | 7.24 | 5.25 | 5.13 | 6.84 |
| 7.40 ± 7.90 | 7.37 ± 9.81 | 7.19 ± 7.47 | 10.27 ± 2.63 | 15.07 ± 5.47 | 17.07 ± 9.93 | 14.89 ± 7.15 | 16.21 ± 2.73 | 17.05 ± 5.94 | |
| 11.10 ± 12.26 | 11.81 ± 15.53 | 12.56 ± 13.56 | 12.96 ± 16.26 | 1.76 ± 5.86 | 5.52 ± 9.69 | 12.99 ± 3.79 | 4.56 ± 11.39 | 16.23 ± 8.75 | |
| 10.08 ± 13.58 | 12.06 ± 13.12 | 9.83 ± 12.46 | 16.19 ± 14.23 | 10.31 ± 6.47 | 13.52 ± 14.61 | 4.64 ± 6.27 | 6.68 ± 5.97 | 3.49 ± 4.44 | |
| 10.41 ± 12.46 | 11.88 ± 13.57 | 10.03 ± 13.36 | 8.56 ± 10.17 | 7.83 ± 7.02 | 10.67 ± 11.78 | 3.88 ± 9.62 | 6.42 ± 5.06 | 6.03 ± 2.72 | |
| 10.50 ± 11.19 | 13.44 ± 14.18 | 12.81 ± 14.81 | 8.69 ± 5.84 | 11.05 ± 4.42 | 6.77 ± 13.87 | 2.26 ± 5.71 | 1.73 ± 2.85 | 3.79 ± 3.71 | |
| 5.08 ± 7.35 | 5.36 ± 6.04 | 6.41 ± 7.57 | 9.67 ± 9.54 | 2.45 ± 1.79 | 4.22 ± 4.25 | 2.70 ± 6.86 | 2.11 ± 2.88 | 3.91 ± 6.81 | |
| 5.47 ± 7.23 | 5.15 ± 7.10 | 7.00 ± 7.89 | 5.62 ± 4.87 | 2.31 ± 1.17 | 6.18 ± 3.84 | 3.46 ± 3.13 | 3.89 ± 8.28 | 4.80 ± 2.22 | |
| 8.94 ± 12.64 | 10.39 ± 10.05 | 7.49 ± 11.29 | 10.45 ± 10.12 | 8.54 ± 1.06 | 6.48 ± 5.31 | 5.23 ± 6.21 | 6.12 ± 10.60 | 3.74 ± 1.89 | |
| 4.29 ± 4.83 | 4.43 ± 4.37 | 4.38 ± 6.67 | 1.06 ± 1.43 | 1.80 ± 0.66 | 0.90 ± 0.96 | 2.04 ± 1.18 | 2.63 ± 1.22 | 7.44 ± 4.81 | |
| 2.48 ± 3.10 | 2.33 ± 3.05 | 2.62 ± 3.34 | 1.65 ± 1.06 | 0.17 ± 0.13 | 1.09 ± 1.73 | 0.38 ± 1.31 | 0.98 ± 0.78 | 1.98 ± 1.28 | |
| Parameter | DB1 | |||
| 1 | 2 | 3 | ||
| Fitted | Fitted | Fitted | Pred. | |
| Cardiovascular Controller | ||||
| 0.0792 | 0.0886 | 0.0840 | 0.0967 | |
| 0.6704 | 0.6712 | 0.6712 | 0.6709 | |
| 0.4266 | 0.4266 | 0.3845 | * | |
| 0.650 | 0.461 | 0.461 | 0.550 | |
| 41.5 | 41.5 | 39.0 | * | |
| 0.180 | 0.173 | 0.162 | 0.172 | |
| Cardiovascular System | ||||
| 0.000437 | 0.000448 | 0.000475 | 0.000463 | |
| 0.000180 | 0.000181 | 0.000175 | 0.000176 | |
| 0.000220 | 0.000184 | 0.000236 | 0.000256 | |
| 0.0478 | 0.0488 | 0.0476 | 0.0479 | |
| Gas Exchange System | ||||
| 0.0559 | 0.0559 | 0.0609 | * | |
| 8.80 | 9.21 | 8.84 | 9.02 | |
| 194.40 | 207.10 | 207.10 | 196.29 | |
| 0.00074 | 0.00074 | 0.00106 | 0.00092 | |
| Respiratory Controller | ||||
| 17.42 | 17.69 | 18.97 | 17.99 | |
| 0.2792 | 0.2967 | 0.2989 | 0.2923 | |
| 0.20250 | 0.20238 | 0.20238 | 0.20241 | |
| 6.79x10-9 | 8.69x10-9 | 7.80x10-9 | 1.14x10-8 | |
| 0.876 | 0.825 | 0.819 | 0.838 | |
| 0.368 | 0.343 | 0.397 | 0.334 | |
| 1.722 | 1.878 | 1.676 | 1.730 | |
| 94.9 | 82.1 | 82.1 | 93.0 | |
| Respiratory Mechanics | ||||
| 27.47 | 24.37 | 25.56 | 28.08 | |
| 3.53 | 2.97 | 3.08 | 3.15 | |
| Parameter | DB2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||||
| Fitted | Fitted | Fitted | Pred. | Fitted | Pred. | Fitted | Pred. | Fitted | Pred. | |
| Cardiovascular Controller | ||||||||||
| -0.130 | -0.130 | -0.130 | -0.130 | -0.092 | * | -0.092 | ** | -0.092 | -0.113 | |
| 0.102 | 0.108 | 0.081 | 0.109 | 0.081 | 0.066 | 0.045 | 0.041 | 0.054 | ** | |
| 1.940 | 1.940 | 1.940 | 1.940 | 1.876 | * | 1.876 | 2.432 | 1.613 | ** | |
| 0.588 | 0.588 | 0.549 | * | 0.581 | 0.542 | 0.571 | 0.631 | 0.571 | ** | |
| 0.55254 | 0.55254 | 0.55360 | * | 0.55360 | 0.55384 | 0.55360 | 0.55503 | 0.55360 | 0.55360 | |
| 0.180 | 0.182 | 0.182 | 0.189 | 0.182 | 0.181 | 0.182 | 0.178 | 0.182 | 0.182 | |
| 40.0 | 40.0 | 46.2 | * | 46.2 | ** | 55.4 | 51.8 | 39.8 | 58.3 | |
| 117.3 | 96.2 | 74.5 | ** | 92.6 | ** | 92.6 | 68.4 | 92.3 | 90.7 | |
| 22.03 | 22.03 | 26.00 | * | 26.00 | 26.47 | 26.00 | 25.25 | 26.00 | 26.00 | |
| Cardiovascular System | ||||||||||
| 0.0105 | 0.0098 | 0.0098 | 0.0068 | 0.0098 | 0.0099 | 0.0098 | 0.0109 | 0.0098 | 0.0097 | |
| 0.0116 | 0.0078 | 0.0078 | ** | 0.0079 | 0.0078 | 0.0087 | 0.0071 | 0.0109 | ** | |
| 0.000477 | 0.000469 | 0.000486 | 0.000434 | 0.000363 | 0.000488 | 0.000488 | ** | 0.000431 | 0.000233 | |
| 0.0014 | 0.0014 | 0.0014 | 0.0014 | 0.0013 | * | 0.0013 | ** | 0.0013 | ** | |
| 0.053 | 0.054 | 0.053 | 0.055 | 0.053 | 0.053 | 0.046 | 0.052 | 0.090 | ** | |
| Gas Exchange System | ||||||||||
| 1.819 | 1.819 | 1.819 | 1.819 | 1.819 | 1.819 | 1.819 | 1.819 | 2.365 | * | |
| 0.05591 | 0.05591 | 0.05591 | 0.05591 | 0.06029 | * | 0.06029 | ** | 0.06029 | 0.05792 | |
| 11.10 | 11.10 | 10.65 | 10.68 | 10.14 | 10.54 | 9.64 | ** | 10.05 | 9.64 | |
| 87.00 | 93.77 | 93.77 | 88.29 | 93.77 | 93.76 | 93.77 | ** | 93.77 | ** | |
| 194.40 | 194.40 | 149.91 | * | 149.91 | ** | 149.91 | 149.91 | 149.91 | ** | |
| 0.00098 | 0.00081 | 0.00075 | ** | 0.00063 | 0.00076 | 0.00066 | 0.00053 | 0.00065 | ** | |
| 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.1 | * | 15.1 | ** | |
| Respiratory Controller | ||||||||||
| 25.13 | 22.98 | 22.73 | 24.65 | 20.72 | 21.33 | 21.76 | 21.95 | 20.33 | ** | |
| 0.4385 | 0.4573 | 0.4481 | 0.5398 | 0.4559 | 0.4167 | 0.4664 | 0.4593 | 0.4664 | 0.5005 | |
| 0.20250 | 0.17739 | 0.16777 | ** | 0.16777 | 0.16555 | 0.15523 | ** | 0.19200 | ** | |
| 2.69x10-9 | 3.76x10-9 | 3.85x10-9 | ** | 2.36x10-9 | ** | 4.80x10-9 | 3.06x10-9 | 3.54x10-9 | ** | |
| 0.458 | 0.752 | 0.899 | ** | 0.609 | 0.933 | 0.609 | ** | 1.099 | ** | |
| 0.468 | 0.245 | 0.691 | ** | 0.348 | 0.794 | 0.348 | ** | 0.348 | ** | |
| 1.362 | 0.834 | 0.899 | ** | 1.123 | 1.794 | 1.016 | ** | 1.348 | ** | |
| 1055.4 | 1055.4 | 1069.8 | * | 1069.8 | 1072.9 | 1069.8 | 1069.8 | 1069.8 | 1069.8 | |
| 72.2 | 47.7 | 51.2 | 68.5 | 63.5 | 90.1 | 93.9 | ** | 58.7 | ** | |
| 0.2381 | 0.2278 | 0.2381 | 0.1831 | 0.2273 | 0.2358 | 0.2273 | 0.3339 | 0.2319 | 0.2057 | |
| Respiratory Mechanics | ||||||||||
| 19.86 | 29.00 | 29.00 | 21.60 | 26.72 | 27.14 | 29.00 | 29.01 | 26.21 | ** | |
| 3.06 | 3.06 | 3.50 | * | 3.50 | 3.60 | 3.50 | 4.10 | 3.50 | 3.50 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





