Submitted:
29 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
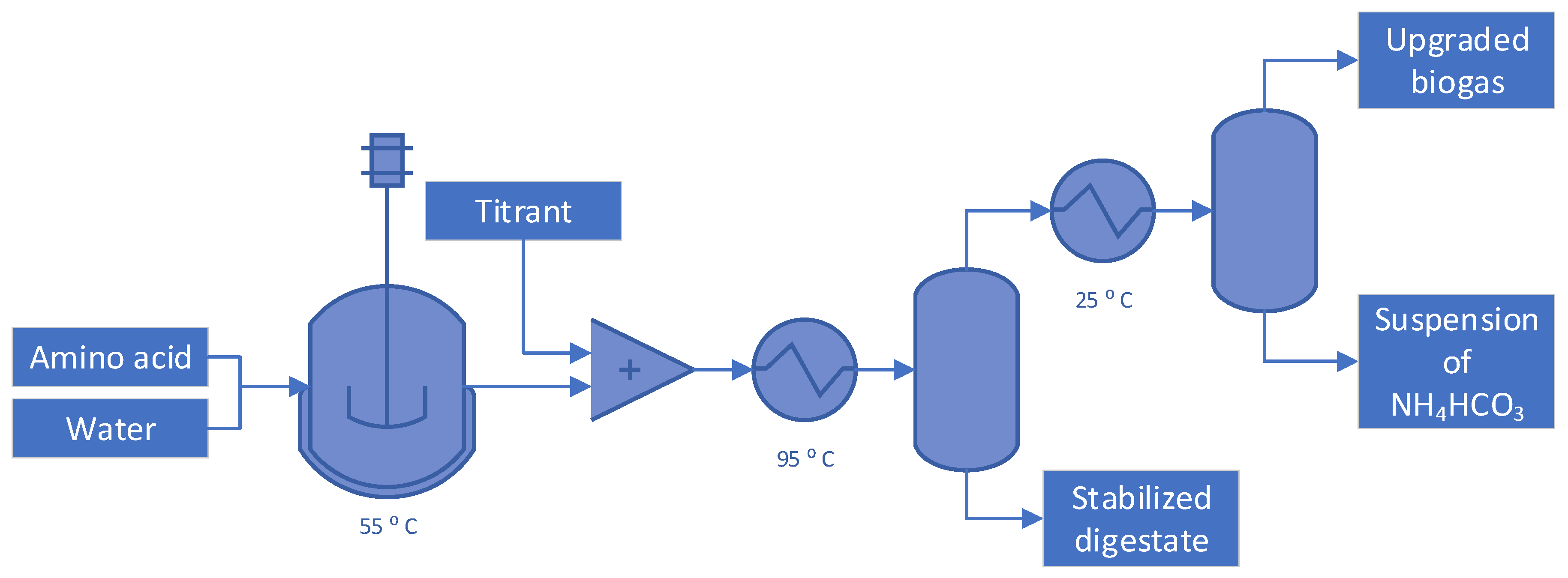
2. Materials and methods
3. Results
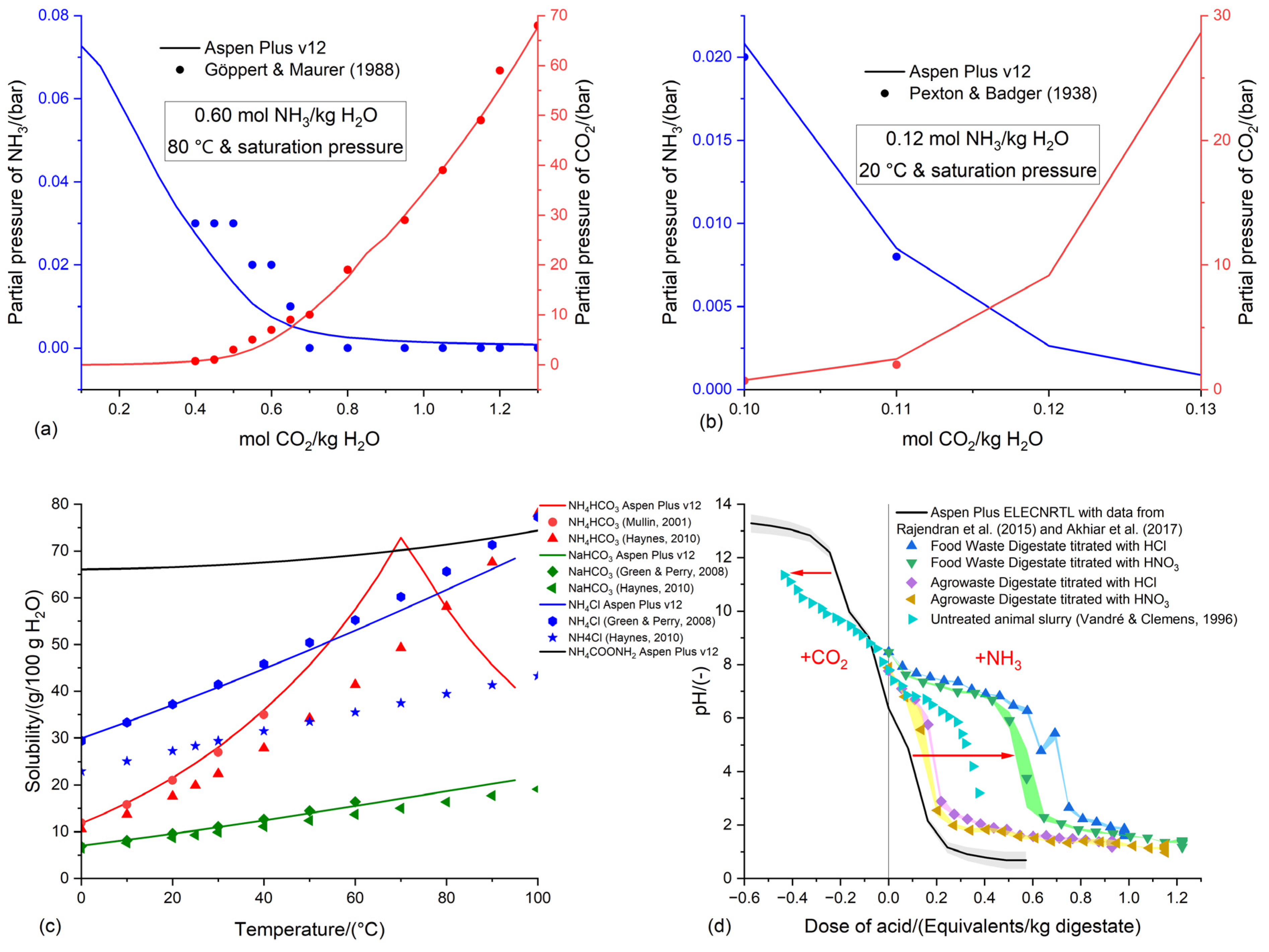
3.1. Preliminary validation of the Aspen Plus v12 ELECNRTL methodology
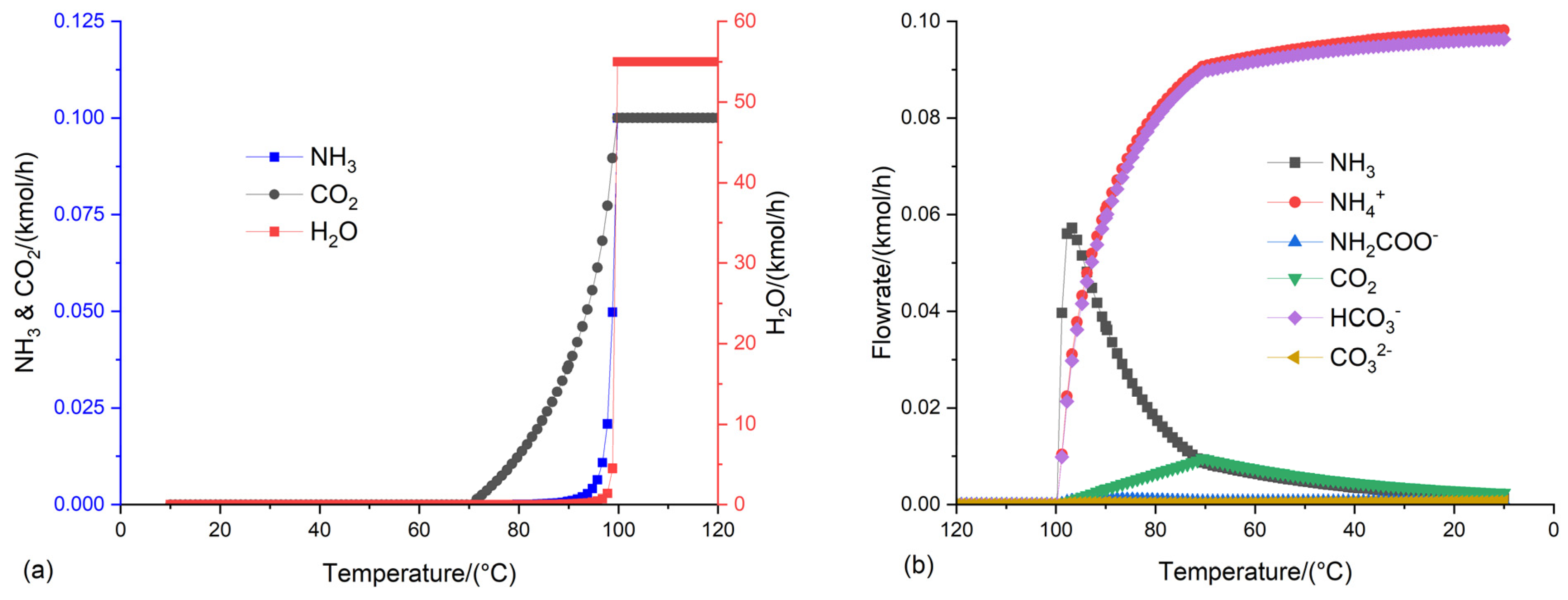
3.2. Optimization of the conditions of the flash distillation to produce NH4HCO3
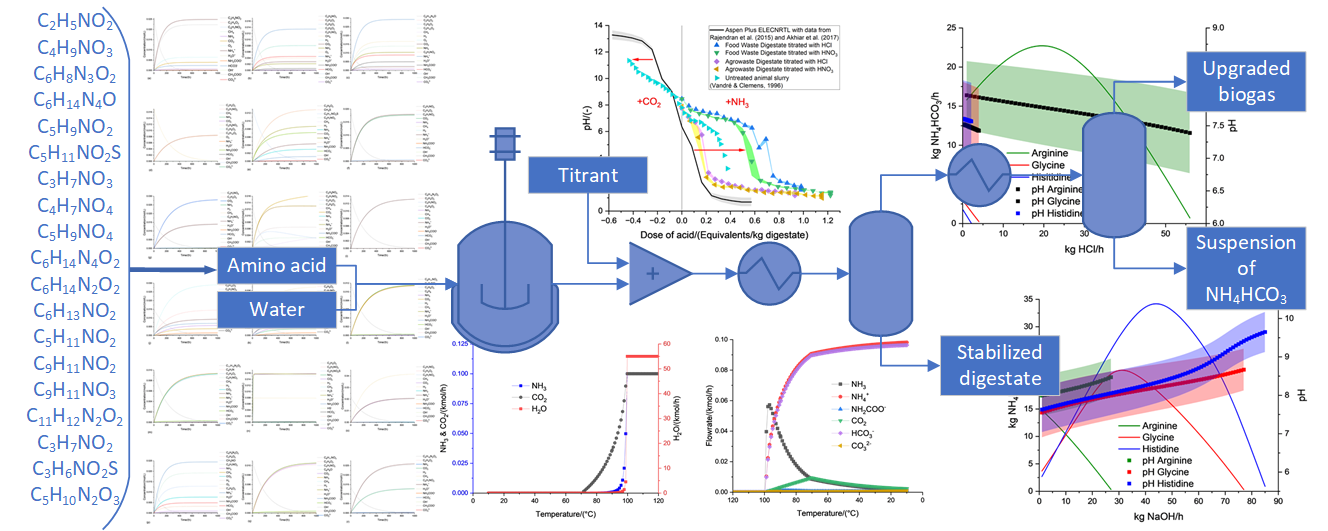
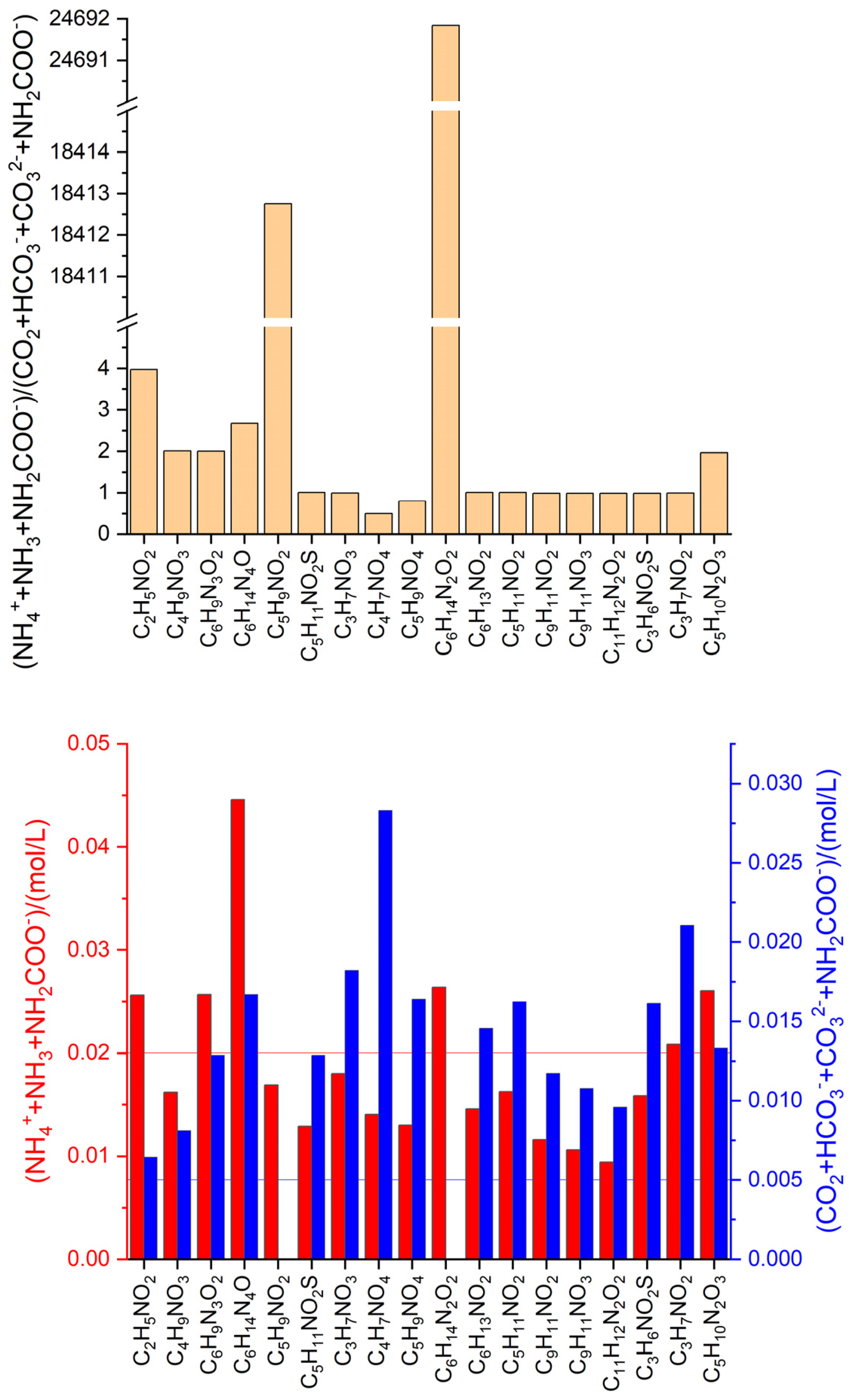
3.3. Anaerobic fermentation of amino acids
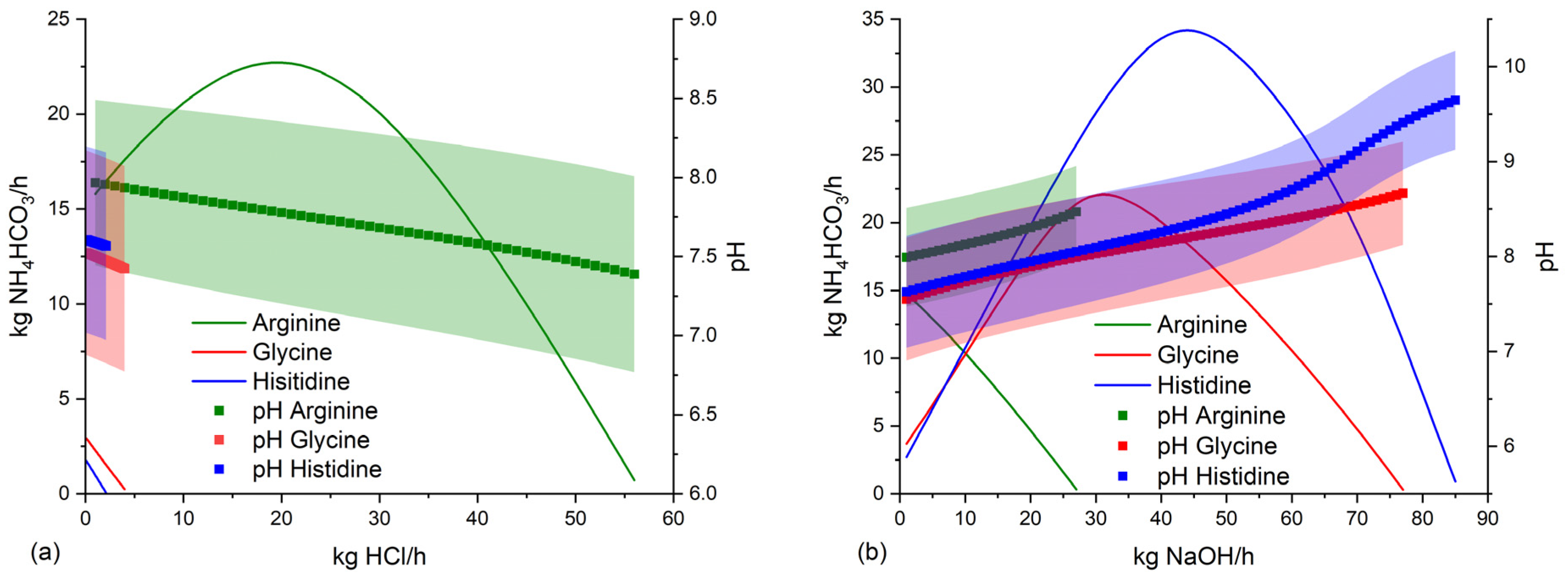
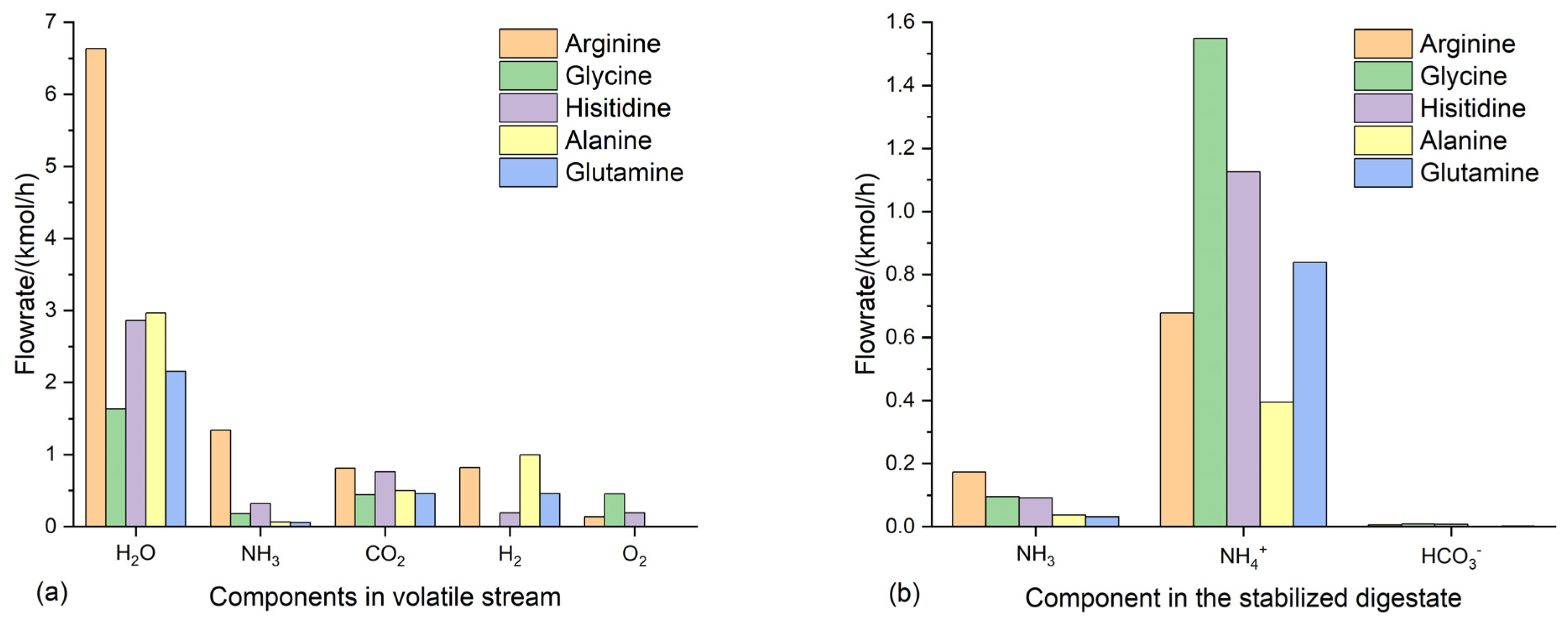
3.4. Suitable conditions for NH4HCO3-stabilization of anaerobic digestate
4. Discussion
5. Conclusions
Data Availability Statement
Acknowledgements
References
- C. Da Costa Gomez, Biogas as an energy option: an overview, in: A. Wellinger, J.D. Murphy, D. Baxter (Eds.), Biogas Handb., 1st ed., Elsevier, Cambridge, UK, 2013: pp. 1–16. [CrossRef]
- T. Al Seadi, C. Lukehurst, Quality management of digestate from biogas plants used as fertiliser, 2012. http://www.iea-biogas.net/files/daten-redaktion/download/publi-task37/digestate_quality_web_new.pdf (accessed 29 May 2023).
- C. Lukehurst, P. Frost, T. Al Seadi, Utilisation of digestate from biogas plants as biofertiliser, 2010. https://task37.ieabioenergy.com/wp-content/uploads/sites/32/2022/02/Digestate_Brochure_Revised_12-2010.pdf (accessed 29 May 2023).
- S. Astals, M. Martínez-Martorell, S. Huete-Hernández, V.B. Aguilar-Pozo, J. Dosta, J.M. Chimenos, Nitrogen recovery from pig slurry by struvite precipitation using a low-cost magnesium oxide, Sci. Total Environ. 768 (2021) 144284. [CrossRef]
- B. Drosg, W. Fuchs, T. Al Seadi, M. Madsen, B. Linke, Nutrient Recovery by Biogas Digestate Processing, (2015). https://task37.ieabioenergy.com/wp-content/uploads/sites/32/2022/02/NUTRIENT_RECOVERY_RZ_web2.pdf (accessed 29 May 2023).
- W. Fuchs, B. Drosg, Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters, Waster Sci. Technol. 67 (2013) 1984–1993. [CrossRef]
- K. Möller, T. Müller, Effects of anaerobic digestion on digestate nutrient availability an crop growth: A review, Eng. Life Sci. 12 (2012) 242–257. [CrossRef]
- D.A. Burke, Removal of Ammonia from Fermentation Effluent and Sequestration as Ammonium Bicarbonate and/or Carbonate, 2010. https://patents.google.com/patent/US7811455B2/en (accessed 29 May 2023).
- D.A. Burke, Reclaiming Ammonia From Anaerobic Digestate As A Profitable Product, Proc. Water Environ. Fed. 2015 (2015) 1–12. [CrossRef]
- Z. Jamaludin, S. Rollings-Scattergood, K. Lutes, C. Vaneeckhaute, Evaluation of sustainable scrubbing agents for ammonia recovery from anaerobic digestate, Bioresour. Technol. 270 (2018) 596–602. [CrossRef]
- D. Drapanauskaite, R.M. Handler, N. Fox, J. Baltrusaitis, Transformation of Liquid Digestate from the Solid-Separated Biogas Digestion Reactor Effluent into a Solid NH4HCO3 Fertilizer: Sustainable Process Engineering and Life Cycle Assessment, ACS Sustain. Chem. Eng. 9 (2021) 580–588. [CrossRef]
- W.M. Budzianowski, Benefits of biogas upgrading to biomethane by high-pressure reactive solvent scrubbing, Biofuels, Bioprod. Biorefining. 6 (2012) 12–20. [CrossRef]
- R. Sander, Compilation of Henry’s law constants (version 4.0) for water as solvent, Atmos. Chem. Phys. 15 (2015) 4399–4981. [CrossRef]
- M. Walker, C. Banks, S. Heaven, J. Frederickson, Residual biogas potential test for digestates, 2010. https://www.ktbl.de/fileadmin/user_upload/Allgemeines/Download/Ringversuch-Biogas/Residual-Biogas-Potential.pdf (accessed 29 May 2023).
- H. Saveyn, P. Eder, End-of-waste criteria for biodegradable waste subjected to biological treatment (compost & digestate): Technical proposals, 2014. https://publications.jrc.ec.europa.eu/repository/handle/JRC87124 (accessed 29 May 2023).
- WRAP, BSI PAS 110:2014 Specification for whole digestate, separated liquor and separated fibre derived from the anaerobic digestion of source-segregated biodegradable materials, 2014. https://wrap.org.uk/sites/default/files/2021-03/PAS110_2014.pdf (accessed 29 May 2023).
- K. Rajendran, H.R. Kankanala, M. Lundin, M.J. Taherzadeh, A novel process simulation model (PSM) for anaerobic digestion using Aspen Plus, Bioresour. Technol. 168 (2014) 7–13. [CrossRef]
- J.C. Centorcelli, D. Drapanauskaite, R.M. Handler, J. Baltrusaitis, Solar Steam Generation Integration into the Ammonium Bicarbonate Recovery from Liquid Biomass Digestate: Process Modeling and Life Cycle Assessment, ACS Sustain. Chem. Eng. 9 (2021) 15278–15286. [CrossRef]
- J.C. Centorcelli, W.L. Luyben, C.E. Romero, J. Baltrusaitis, Dynamic Control of Liquid Biomass Digestate Distillation Combined with an Integrated Solar Concentrator Cycle for Sustainable Nitrogen Fertilizer Production, ACS Sustain. Chem. Eng. 10 (2022) 7409–7417. [CrossRef]
- Akhiar, A. Battimelli, M. Torrijos, H. Carrere, Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion, Waste Manag. 59 (2017) 118–128. [CrossRef]
- Moure Abelenda, K.T. Semple, A.J. Lag-Brotons, B.M.J. Herbert, G. Aggidis, F. Aiouache, Strategies for the production of a stable blended fertilizer of anaerobic digestates and wood ashes, Nature-Based Solut. 2 (2022) 100014. [CrossRef]
- UK Government, Annex 3: FETF 2023 Productivity and Slurry eligible Items, Guidance. (2023). https://www.gov.uk/government/publications/farming-equipment-and-technology-fund-fetf-2023/annex-3-fetf-2023-productivity-and-slurry-eligible-items (accessed 29 May 2023).
- F. García-Ochoa, V.E. Santos, L. Naval, E. Guardiola, B. López, Kinetic model for anaerobic digestion of livestock manure, Enzyme Microb. Technol. 25 (1999) 55–60. [CrossRef]
- P. Canu, M. Pagin, Biogas upgrading by 2-steps methanation of its CO2 – Thermodynamics analysis, J. CO2 Util. 63 (2022) 102123. [CrossRef]
- V. Darde, CO₂ capture using aqueous ammonia, PhD Thesis, Technical University of Denmark, 2011. https://www.cere.dtu.dk/-/media/centre/cere/publications/phd-thesis/2011/victor_darde_phd.pdf?la=da&hash=8A67D4D04AFF063B772D6A90CC6D88B9BEBAEABD (accessed 29 May 2023).
- V. Darde, W.J.M. van Well, E.H. Stenby, K. Thomsen, Modeling of Carbon Dioxide Absorption by Aqueous Ammonia Solutions Using the Extended UNIQUAC Model, Ind. Eng. Chem. Res. 49 (2010) 12663–12674. [CrossRef]
- J.W. Mullin, Crystallization, 4th ed., Butterworth-Heinemann, Oxford ; Boston, 2001, pp. 478–535. [CrossRef]
- W.M. Haynes, CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data, 91st ed., CRC Press, Boca Raton, Fla., 2010, pp. 8-121–8-126.
- M. Brondi, M. Eisa, R. Bortoletto-Santos, D. Drapanauskaite, T. Reddington, C. Williams, C. Ribeiro, J. Baltrusaitis, Recovering, Stabilizing, and Reusing Nitrogen and Carbon from Nutrient-Containing Liquid Waste as Ammonium Carbonate Fertilizer, Agriculture. 13 (2023) 909. [CrossRef]
- R. Vandré, J. Clemens, Studies on the relationship between slurry pH, volatilization processes and the influence of acidifying additives, Nutr. Cycl. Agroecosystems. 47 (1996) 157–165. [CrossRef]
- Z.X. Mu, C.S. He, J.K. Jiang, J. Zhang, H.Y. Yang, Y. Mu, A modified two-point titration method for the determination of volatile fatty acids in anaerobic systems, Chemosphere. 204 (2018) 251–256. [CrossRef]
- D.W. Green, R.H. Perry, Perry’s chemical engineers’ handbook., 8th ed./, McGraw-Hill, New York ; London, 2008, pp. 2-125–2-129.
- Limoli, M. Langone, G. Andreottola, Ammonia removal from raw manure digestate by means of a turbulent mixing stripping process, J. Environ. Manage. 176 (2016) 1–10. [CrossRef]
- S. Astals, V. Nolla-Ardèvol, J. Mata-Alvarez, Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate, Bioresour. Technol. 110 (2012) 63–70. [CrossRef]
- S. Astals, V. Nolla-Ardèvol, J. Mata-Alvarez, Thermophilic co-digestion of pig manure and crude glycerol: Process performance and digestate stability, J. Biotechnol. 166 (2013) 97–104. [CrossRef]
- European Sustainable Phosphorus Platform, 1st White Ammonia Research Meeting (WARM) - 7th June 2023, N-Recovery. (2023). https://www.phosphorusplatform.eu/activities/conference/n-recovery (accessed 29 May 2023).
- A.T. Ukwuani, W. Tao, Developing a vacuum thermal stripping – acid absorption process for ammonia recovery from anaerobic digester effluent, Water Res. 106 (2016) 108–115. [CrossRef]
- E. Martín-Hernández, A.M. Sampat, V.M. Zavala, M. Martín, Optimal integrated facility for waste processing, Chem. Eng. Res. Des. 131 (2018) 160–182. [CrossRef]
- L. Morey, B. Fernández, L. Tey, C. Biel, A. Robles-Aguilar, E. Meers, J. Soler, R. Porta, M. Cots, V. Riau, Acidification and solar drying of manure-based digestate to produce improved fertilizing products, J. Environ. Manage. 336 (2023) 117664. [CrossRef]
- Moure Abelenda, K.T. Semple, G. Aggidis, F. Aiouache, Dataset on the solid-liquid separation of anaerobic digestate by means of wood ash-based treatment, Data Br. 44 (2022) 108536. [CrossRef]
- K. Meixner, W. Fuchs, T. Valkova, K. Svardal, C. Loderer, M. Neureiter, G. Bochmann, B. Drosg, Effect of precipitating agents on centrifugation and ultrafiltration performance of thin stillage digestate, Sep. Purif. Technol. 145 (2015) 154–160. [CrossRef]
- F.M. Baena-Moreno, E. Le Saché, C.A. Hurd Price, T.R. Reina, B. Navarrete, From biogas upgrading to CO2 utilization and waste recycling: A novel circular economy approach, J. CO2 Util. 47 (2021) 101496. [CrossRef]
- Moure Abelenda, A. Ali, K.T. Semple, F. Aiouache, Aspen Plus® Process Simulation Model of the Biomass Ash-Based Treatment of Anaerobic Digestate for Production of Fertilizer and Upgradation of Biogas, Energies. 16 (2023) 3039. [CrossRef]
- [dataset] A. Moure Abelenda, Aspen Plus models of the flash distillation process for stabilization of anaerobic digestate and synthesis of ammonium bicarbonate, (2023). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




