Submitted:
31 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Hypothesis
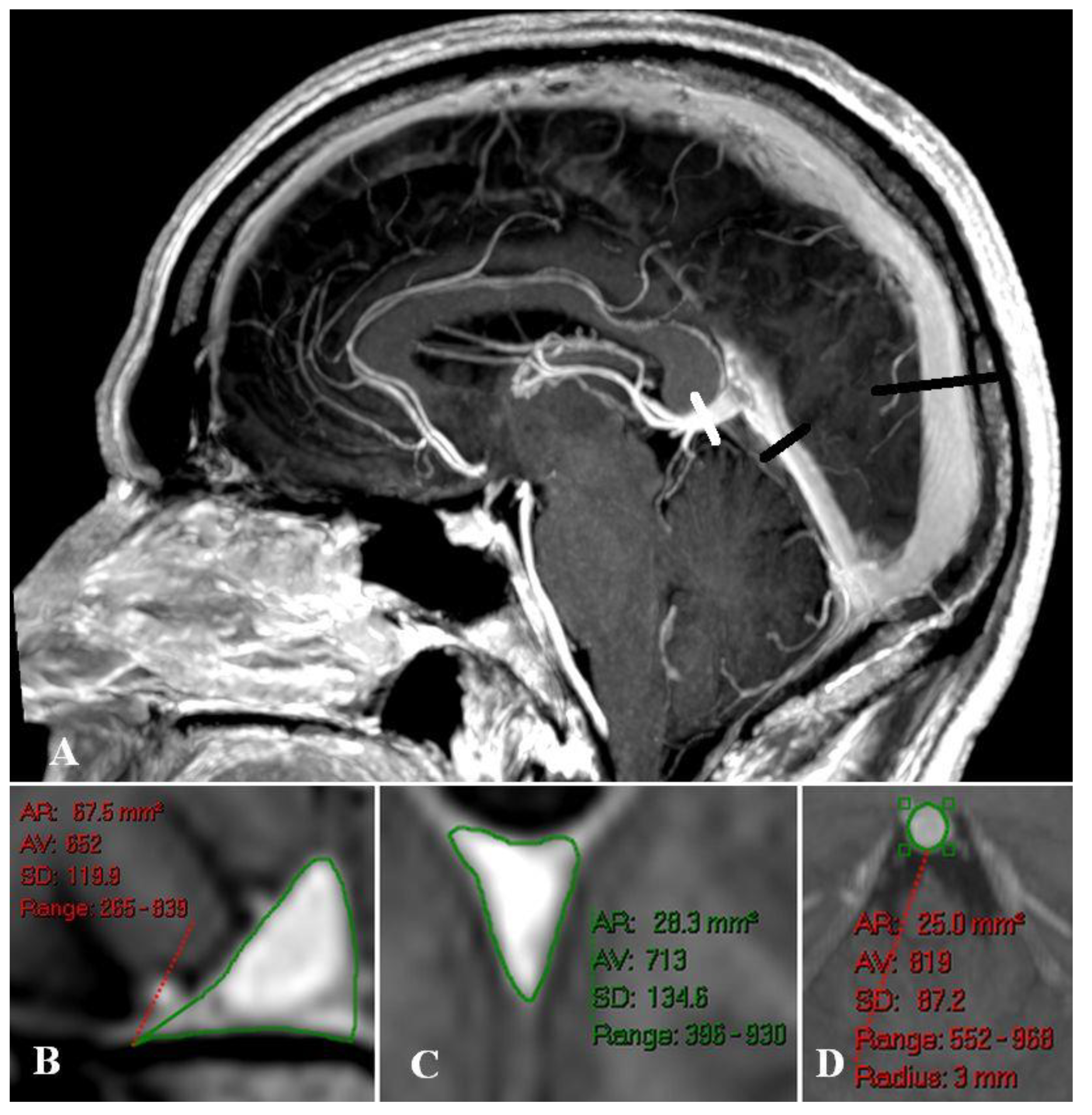
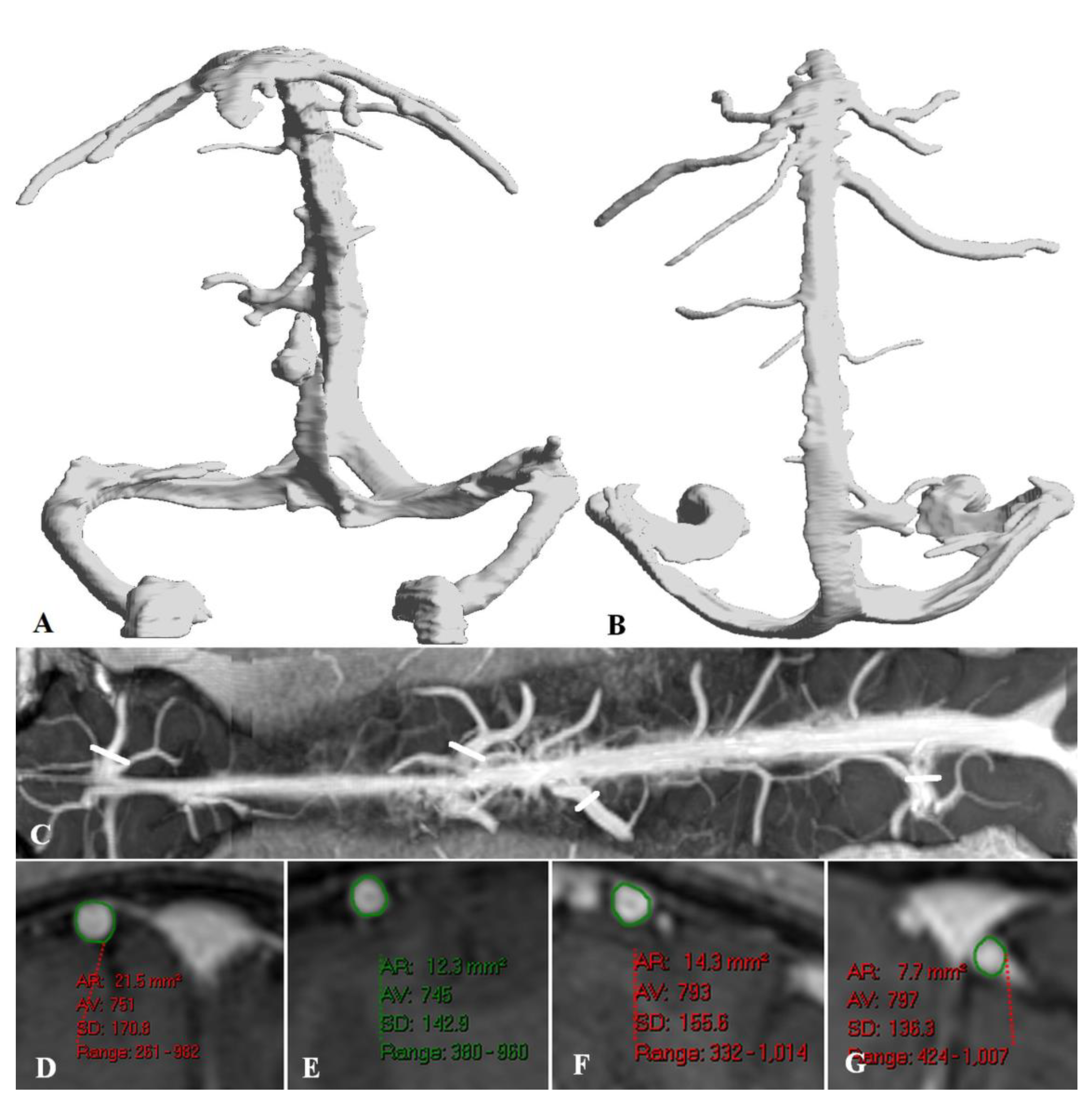
Illustrating Cases
Discussion
Sagittal and straight sinus dilatation
Bridging vein dilatation
Effect of elevated venous pressure on the brain.
Funding
Institutional Review Board Statement
Data Availability Statement
Conflict of Interest
Abbreviations
References
- R. Baker and E.J. Shaw, Diagnosis and management of chronic fatigue syndrome or myalgic encephalomyelitis (or encephalopathy): summary of NICE guidance, BMJ. 2007, 335, 446–448. [CrossRef]
- B. M. Carruthers, Definitions and aetiology of myalgic encephalomyelitis: how the Canadian consensus clinical definition of myalgic encephalomyelitis works, J Clin Pathol. 2007, 60, 117–119. [CrossRef]
- A. R. Valdez, E.E. Hancock, S. Adebayo, et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning, Front Pediatr. 2018, 6, 412. [CrossRef]
- H. Lassmann, Multiple sclerosis pathology: evolution of pathogenetic concepts, Brain Pathol. 2005, 15, 217–222. [CrossRef]
- I. J. Bakken, K. Tveito, N. Gunnes, et al. Two age peaks in the incidence of chronic fatigue syndrome/myalgic encephalomyelitis: a population-based registry study from Norway 2008-2012, BMC Med. 2014, 12, 167. [CrossRef]
- F. Gilli, K.D. DiSano and A.R. Pachner, SeXX Matters in Multiple Sclerosis, Front Neurol. 2020, 11, 616. [CrossRef]
- D. S. Reich, C.F. Lucchinetti and P.A. Calabresi, Multiple Sclerosis, N Engl J Med. 2018, 378, 169–180. [CrossRef]
- B. M. Carruthers, M.I. van de Sande, K.L. De Meirleir, et al. Myalgic encephalomyelitis: International Consensus Criteria, J Intern Med. 2011, 270, 327–338. [CrossRef]
- N. Sepulveda, J. Malato, F. Sotzny, et al. Revisiting IgG Antibody Reactivity to Epstein-Barr Virus in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Its Potential Application to Disease Diagnosis, Front Med (Lausanne). 2022, 9, 921101. [CrossRef]
- K. Bjornevik, M. Cortese, B.C. Healy, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis, Science. 2022, 375, 296–301. [CrossRef]
- G. Morris and M. Maes, Myalgic encephalomyelitis/chronic fatigue syndrome and encephalomyelitis disseminata/multiple sclerosis show remarkable levels of similarity in phenomenology and neuroimmune characteristics, BMC Med. 2013, 11, 205. [CrossRef]
- S. Rooney, L. Wood, F. Moffat and L. Paul, Prevalence of fatigue and its association with clinical features in progressive and non-progressive forms of Multiple Sclerosis, Mult Scler Relat Disord. 2019, 28, 276–282. [CrossRef]
- M. Hulens, R. Rasschaert, G. Vansant, I. Stalmans, F. Bruyninckx and W. Dankaerts, The link between idiopathic intracranial hypertension, fibromyalgia, and chronic fatigue syndrome: exploration of a shared pathophysiology, J Pain Res. 2018, 11, 3129–3140. [CrossRef]
- N. Higgins, J. Pickard and A. Lever, Lumbar puncture, chronic fatigue syndrome and idiopathic intracranial hypertension: a cross-sectional study, JRSM Short Rep. 2013, 4, 1–7. [CrossRef]
- Ragauskas, L. Bartusis, I. Piper, et al. Improved diagnostic value of a TCD-based non-invasive ICP measurement method compared with the sonographic ONSD method for detecting elevated intracranial pressure, Neurol Res. 2014, 36, 607–614. [Google Scholar] [CrossRef]
- Bragee, A. Michos, B. Drum, M. Fahlgren, R. Szulkin and B.C. Bertilson, Signs of Intracranial Hypertension, Hypermobility, and Craniocervical Obstructions in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome, Front Neurol. 2020, 11, 828. [Google Scholar] [CrossRef]
- J. Vilisaar, S. Harikrishnan, M. Suri and C.S. Constantinescu, Ehlers-Danlos syndrome and multiple sclerosis: a possible association, Mult Scler. 2008, 14, 567–570. [CrossRef]
- G. A. Bateman, J. Lechner-Scott and A.R. Bateman, Modelling of the dilated sagittal sinuses found in multiple sclerosis suggests increased wall stiffness may be a contributing factor, Sci Rep. 2022, 12, 17575. [CrossRef]
- G.A. Bateman, A.R. G.A. Bateman, A.R. Bateman and J. Lechner-Scott, Dilatation of the Bridging Cerebral Veins in Multiple Sclerosis Correlates with Fatigue and Suggests an Increase in Pressure, Research Square PREPRINT (Version 1) (2022). [CrossRef]
- K. Shapiro, A. Marmarou and K. Shulman, Characterization of clinical CSF dynamics and neural axis compliance using the pressure-volume index: I. The normal pressure-volume index, Ann Neurol. 1980, 7, 508–514. [CrossRef]
- M. E. Wagshul, P.K. Eide and J.R. Madsen, The pulsating brain: A review of experimental and clinical studies of intracranial pulsatility, Fluids Barriers CNS. 2011, 8, 5. [CrossRef]
- C. Fjeldstad, C. Frederiksen, A.S. Fjeldstad, M. Bemben and G. Pardo, Arterial compliance in multiple sclerosis: a pilot study, Angiology. 2010, 61, 31–36. [CrossRef]
- G. A. Bateman, J. Lechner-Scott and R.A. Lea, A comparison between the pathophysiology of multiple sclerosis and normal pressure hydrocephalus: is pulse wave encephalopathy a component of MS?, Fluids Barriers CNS. 2016, 13, 18. [CrossRef]
- S. ElSankari, O. Baledent, V. van Pesch, C. Sindic, Q. de Broqueville and T. Duprez, Concomitant analysis of arterial, venous, and CSF flows using phase-contrast MRI: a quantitative comparison between MS patients and healthy controls, J Cereb Blood Flow Metab. 2013, 33, 1314–1321. [CrossRef]
- G. A. Bateman, J. Lechner-Scott, A.R. Bateman, J. Attia and R.A. Lea, The Incidence of Transverse Sinus Stenosis in Multiple Sclerosis: Further Evidence of Pulse Wave Encephalopathy, Mult Scler Relat Disord. 2020, 46, 102524. [CrossRef]
- V. A. Spence, G. Kennedy, J.J. Belch, A. Hill and F. Khan, Low-grade inflammation and arterial wave reflection in patients with chronic fatigue syndrome, Clin Sci (Lond). 2008, 114, 561–566. [CrossRef]
- P. C. Rowe, C.L. Marden, S. Heinlein and C.C. Edwards, 2nd, Improvement of severe myalgic encephalomyelitis/chronic fatigue syndrome symptoms following surgical treatment of cervical spinal stenosis, J Transl Med. 2018, 16, 21. [CrossRef]
- J. R. Houston, M.S. Eppelheimer, S.H. Pahlavian, et al. A morphometric assessment of type I Chiari malformation above the McRae line: A retrospective case-control study in 302 adult female subjects, J Neuroradiol. 2018, 45, 23–31. [CrossRef]
- V. G. Xydis, A.K. Zikou, V. Kostadima, L.G. Astrakas, P. Kosta and M.I. Argyropoulou, The association between multiple sclerosis and spondylosis: When and why, Eur J Radiol. 2017, 91, 47–51. [CrossRef]
- D. Gratch, D. Do, P. Khankhanian, M. Schindler, J.E. Schmitt and J.R. Berger, Impact of cervical stenosis on multiple sclerosis lesion distribution in the spinal cord, Mult Scler Relat Disord. 2020, 45, 102415. [CrossRef]
- Finkelmeyer, J. He, L. Maclachlan, A.M. Blamire and J.L. Newton, Intracranial compliance is associated with symptoms of orthostatic intolerance in chronic fatigue syndrome, PLoS One. 2018, 13, e0200068. [Google Scholar] [CrossRef]
- J. N.P. Higgins and J.D. Pickard, A paradigm for chronic fatigue syndrome: caught between idiopathic intracranial hypertension and spontaneous intracranial hypotension; caused by cranial venous outflow obstruction, Fatigue. 2021, 9, 139–147. [CrossRef]
- M. Hulens, R. Rasschaert, W. Dankaerts, I. Stalmans, G. Vansant and F. Bruyninckx, Spinal fluid evacuation may provide temporary relief for patients with unexplained widespread pain and fibromyalgia, Med Hypotheses. 2018, 118, 55–58. [CrossRef]
- C. W. Adams, R.N. Poston, S.J. Buk, Y.S. Sidhu and H. Vipond, Inflammatory vasculitis in multiple sclerosis, J Neurol Sci. 1985, 69, 269–283. [CrossRef]
- Y. Yu, J. Chen, Z. Si, et al. The hemodynamic response of the cerebral bridging veins to changes in ICP, Neurocrit Care. 2010, 12, 117–123. [CrossRef]
- G. A. Bateman, Magnetic resonance imaging quantification of compliance and collateral flow in late-onset idiopathic aqueductal stenosis: venous pathophysiology revisited, J Neurosurg. 2007, 107, 951–958. [CrossRef]
- H. D. Portnoy, C. Branch and M.E. Castro, The relationship of intracranial venous pressure to hydrocephalus, Childs Nerv Syst. 1994, 10, 29–35. [CrossRef]
- J. Chen, X.M. Wang, L.M. Luan, et al. Biological characteristics of the cerebral venous system and its hemodynamic response to intracranial hypertension, Chin Med J (Engl). 2012, 125, 1303–1309.
- G. A. Bateman, A.R. Bateman and G.M. Subramanian, Dilatation of the bridging cerebral cortical veins in childhood hydrocephalus suggests a malfunction of venous impedance pumping, Sci Rep. 2022, 12, 13045. [CrossRef]
- E. Z. Papadaki, V.C. Mastorodemos, E.Z. Amanakis, et al. White matter and deep gray matter hemodynamic changes in multiple sclerosis patients with clinically isolated syndrome, Magn Reson Med. 2012, 68, 1932–1942. [CrossRef]
- S. Viola, P. Viola, L. Fiorelli, M.P. Buongarzone and P. Litterio, Transcranial brain photoplethysmography to study the venules of cerebral cortex in patients with multiple sclerosis, Phlebology. 2015, 30, 119–126. [CrossRef]
- G. A. Fulop, C. Ahire, T. Csipo, et al. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice, Geroscience. 2019, 41, 575–589. [CrossRef]
- K. Thapaliya, S. Marshall-Gradisnik, D. Staines and L. Barnden, Diffusion tensor imaging reveals neuronal microstructural changes in myalgic encephalomyelitis/chronic fatigue syndrome, Eur J Neurosci. 2021, 54, 6214–6228. [CrossRef]
- C.T. Primiani, M. C.T. Primiani, M. Lawton, A.E. Hillis and F.K. Hui, Pearls & Oy-sters: Cerebral Venous Congestion Associated With Cognitive Decline Treated by Jugular Release, Neurology (2022). [CrossRef]
- N. Higgins, J. Pickard and A. Lever, Borderline Intracranial Hypertension Manifesting as Chronic Fatigue Syndrome Treated by Venous Sinus Stenting, J Neurol Surg Rep. 2015, 76, e244–e247. [CrossRef]
- J. S. Cheng, J. Nash and G.A. Meyer, Chiari type I malformation revisited: diagnosis and treatment, Neurologist. 2002, 8, 357–362. [CrossRef]
- W. S. Wilke, Can fibromyalgia and chronic fatigue syndrome be cured by surgery?, Cleve Clin J Med. 2001, 68, 277–279. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




