Submitted:
31 May 2023
Posted:
31 May 2023
You are already at the latest version
Abstract
Keywords:
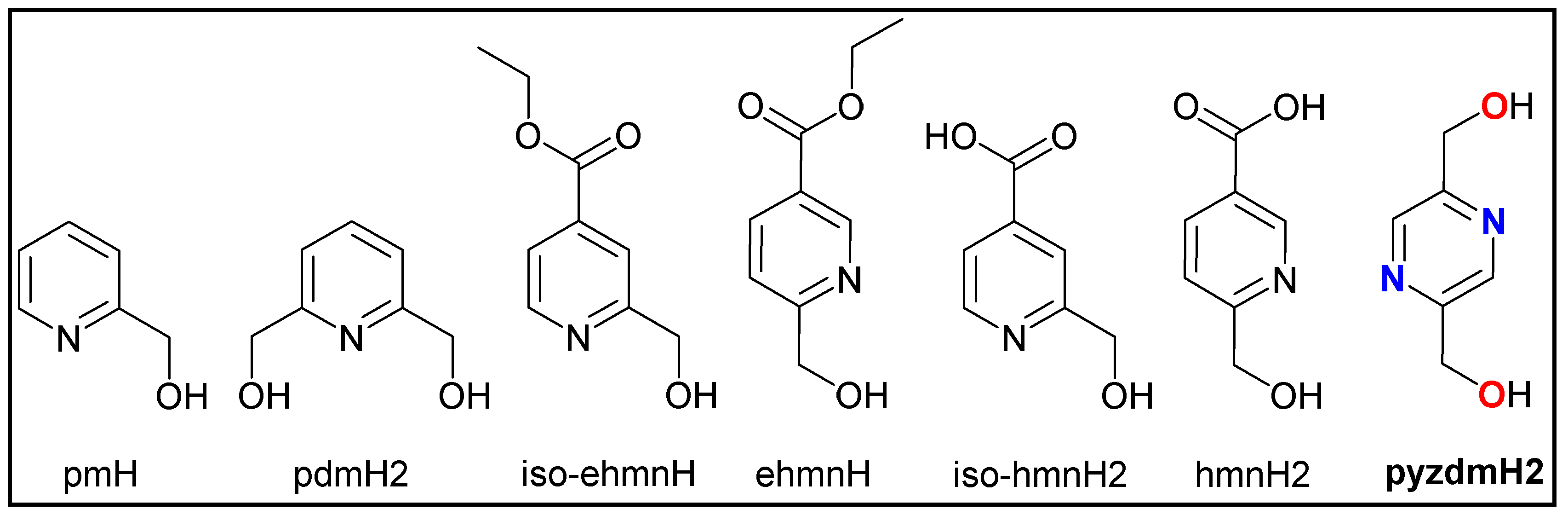
1. Introduction
2. Materials and Methods
2.1. Single X-ray crystallography
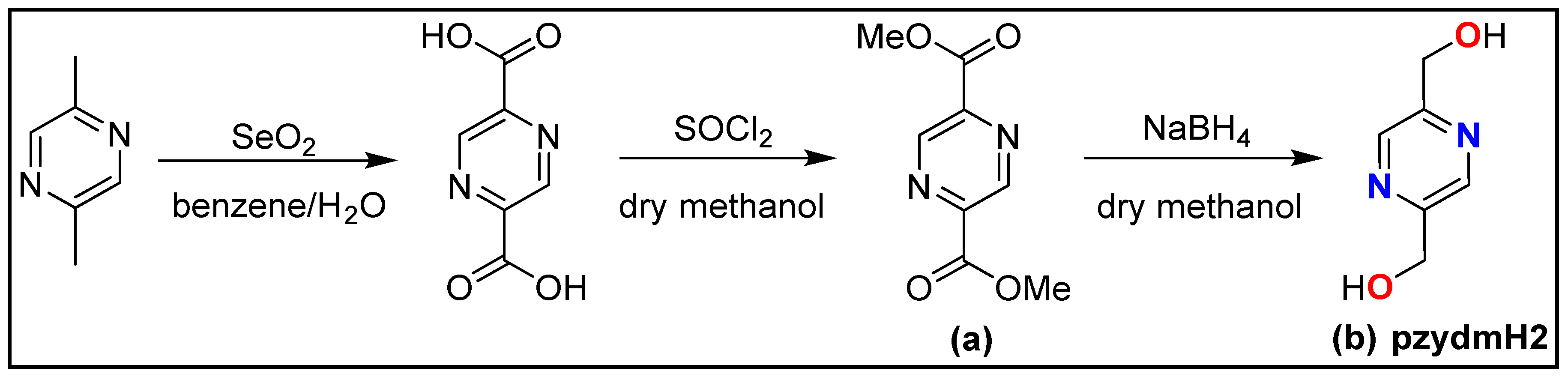
2.2. Synthetic procedures for pzydmH2
2.2.1. Preparation of dimethyl pyrazine-2,5-dicarboxylate (a)
2.2.2. Preparation of pyrazine-2,5-diyldimethanol (b)
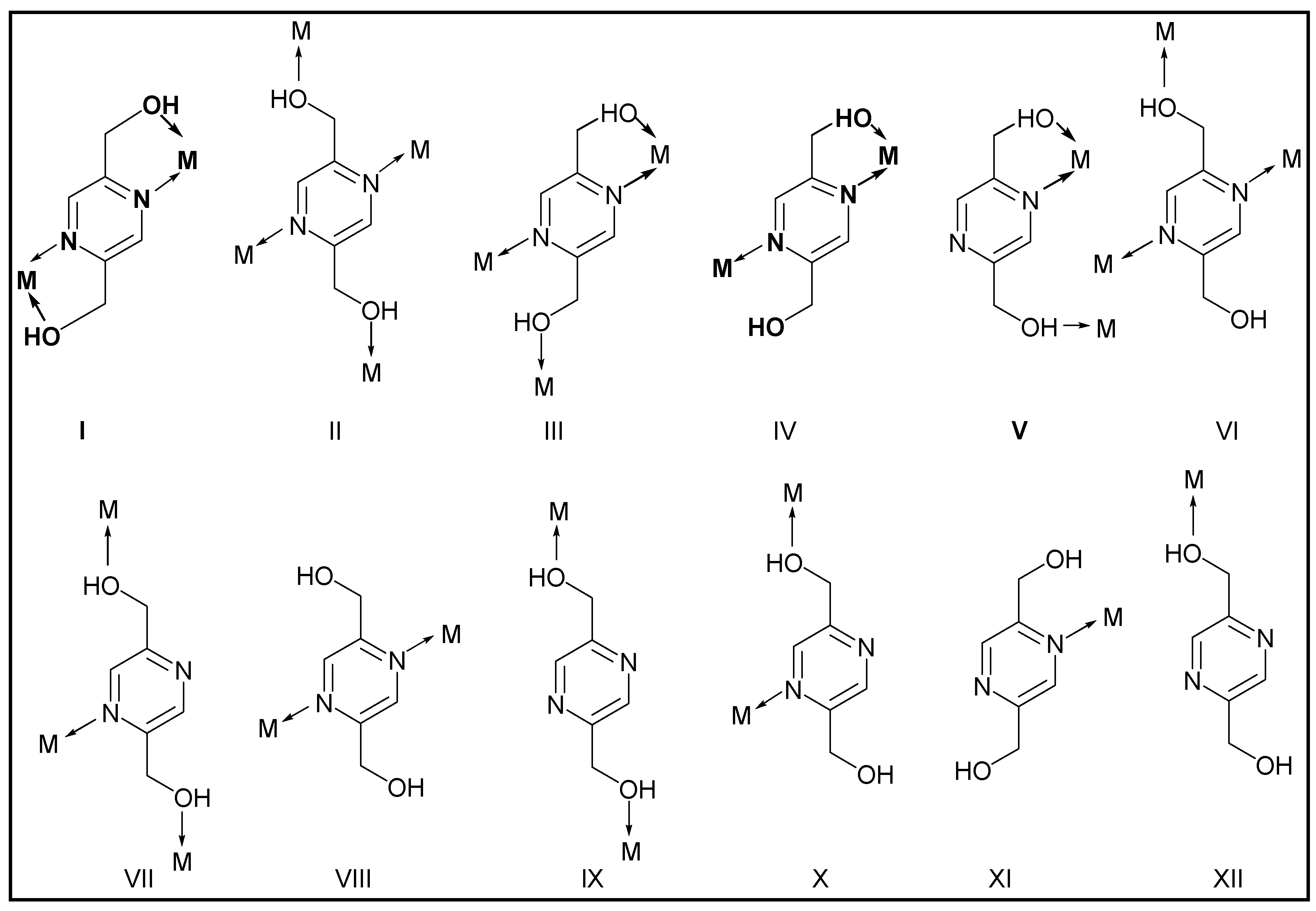
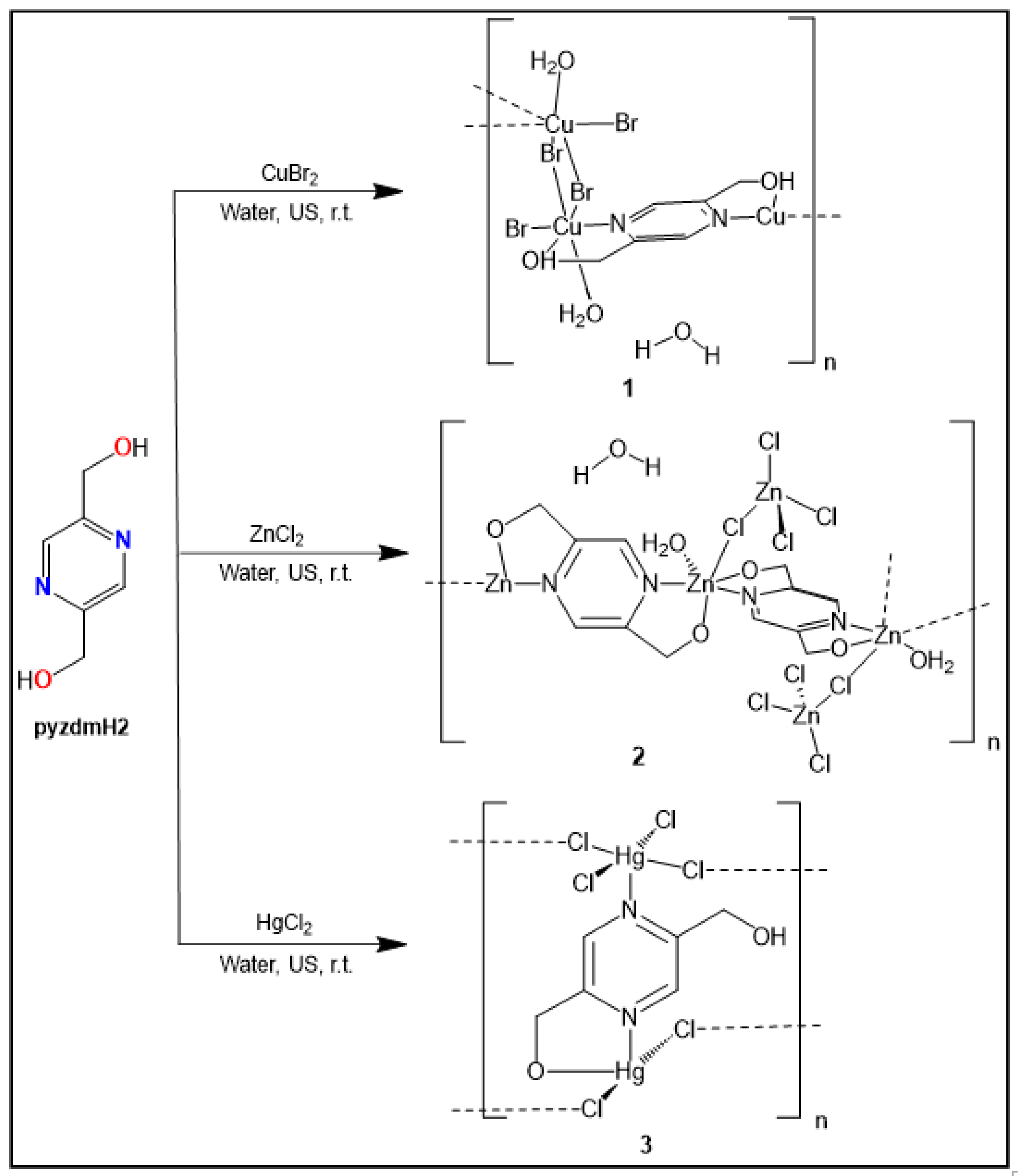
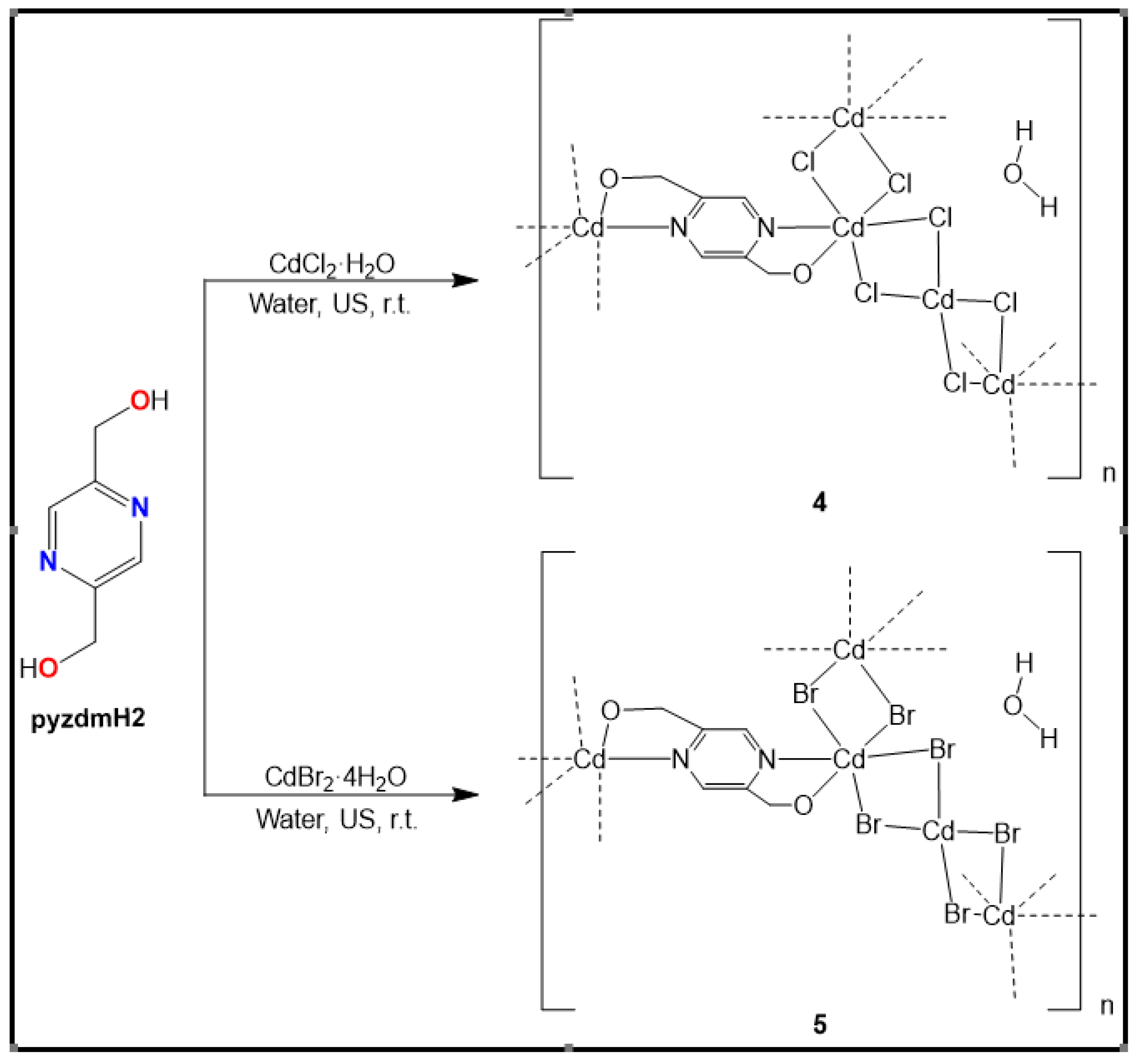
2.3. Syntheses of 1-5
3. Result and discussion
3.1. Synthesis organic linker and coordination polymers
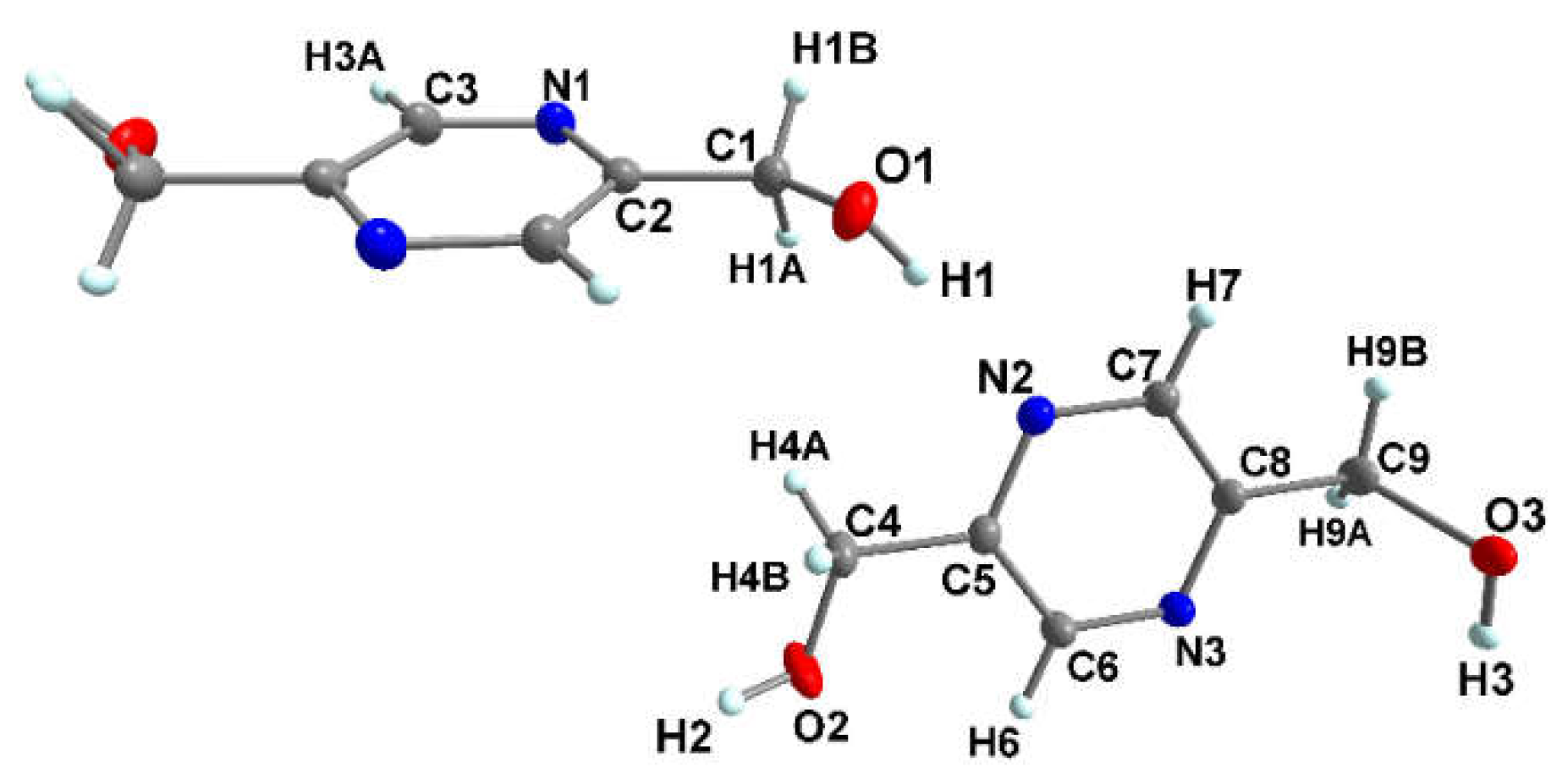
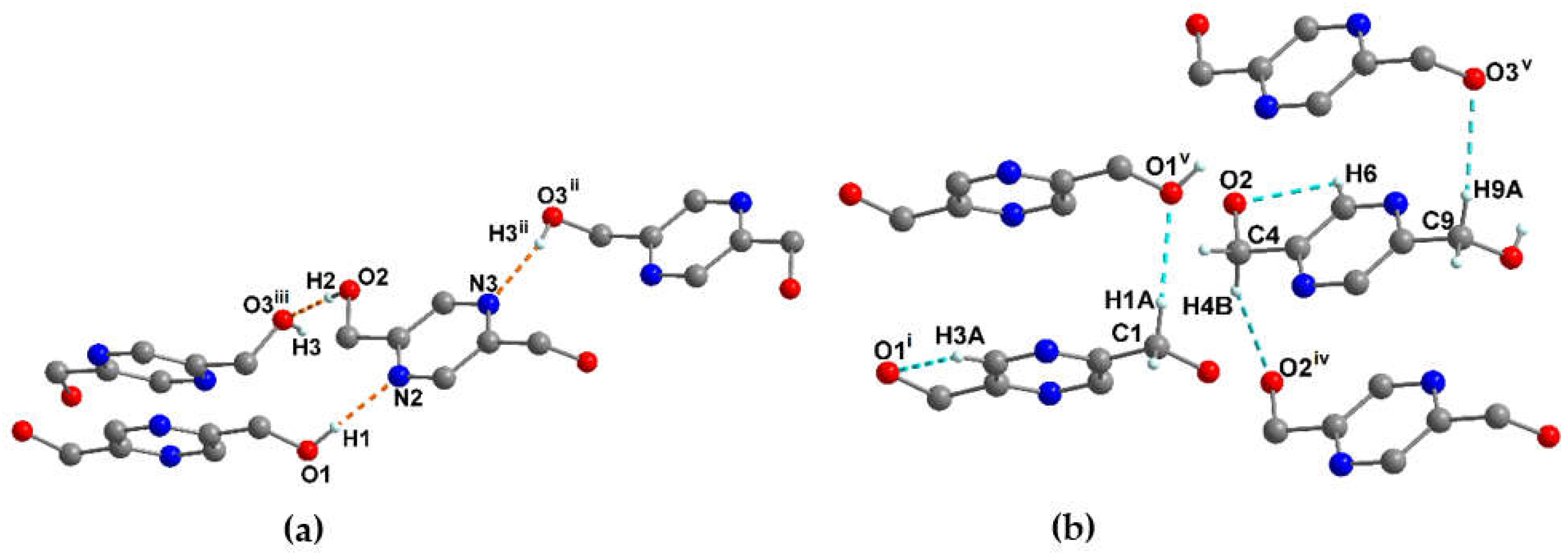
3.1.1. Crystal structure of pyrazine-2,5-diyldimethanol (pzydmH2)
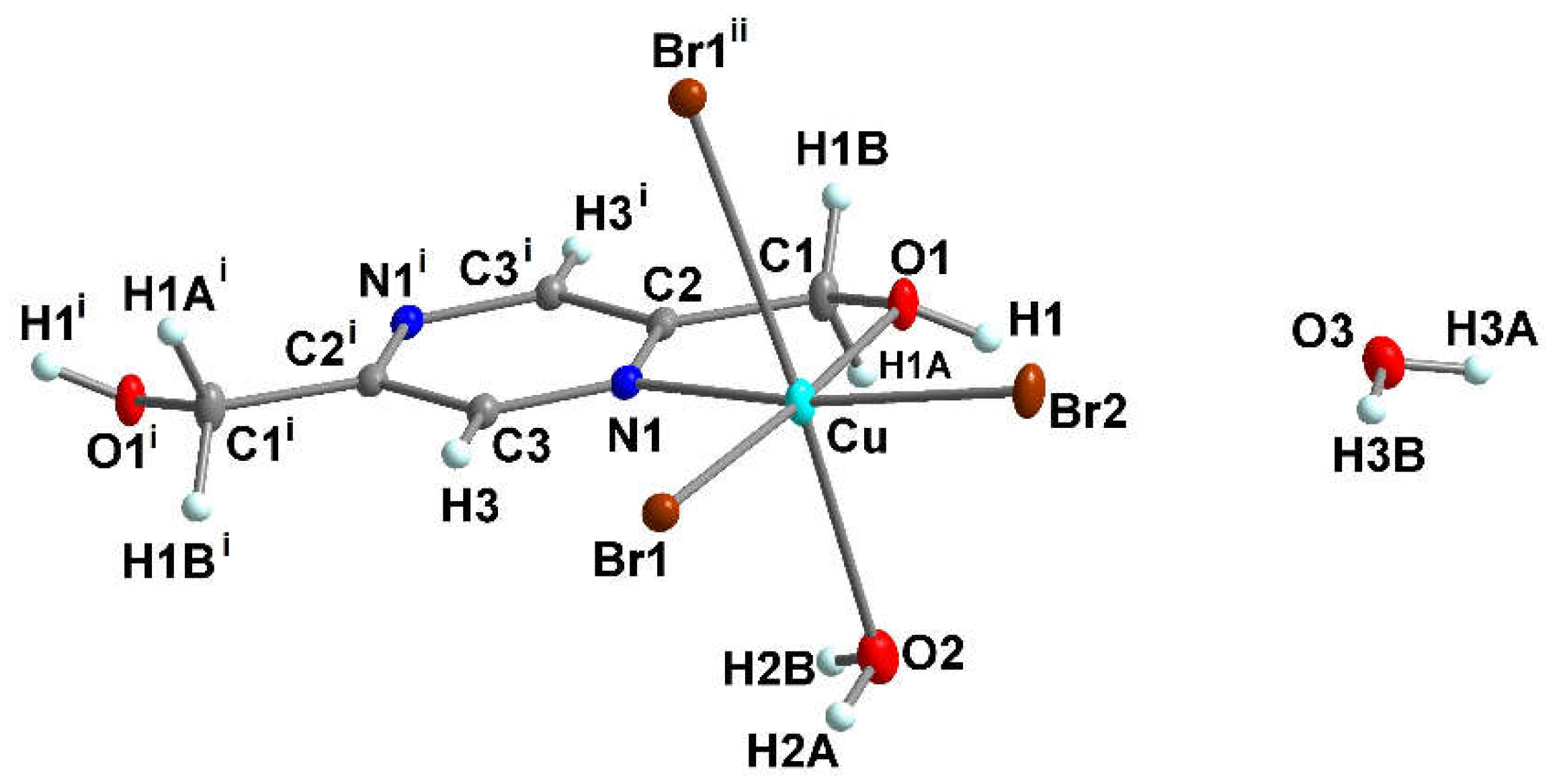
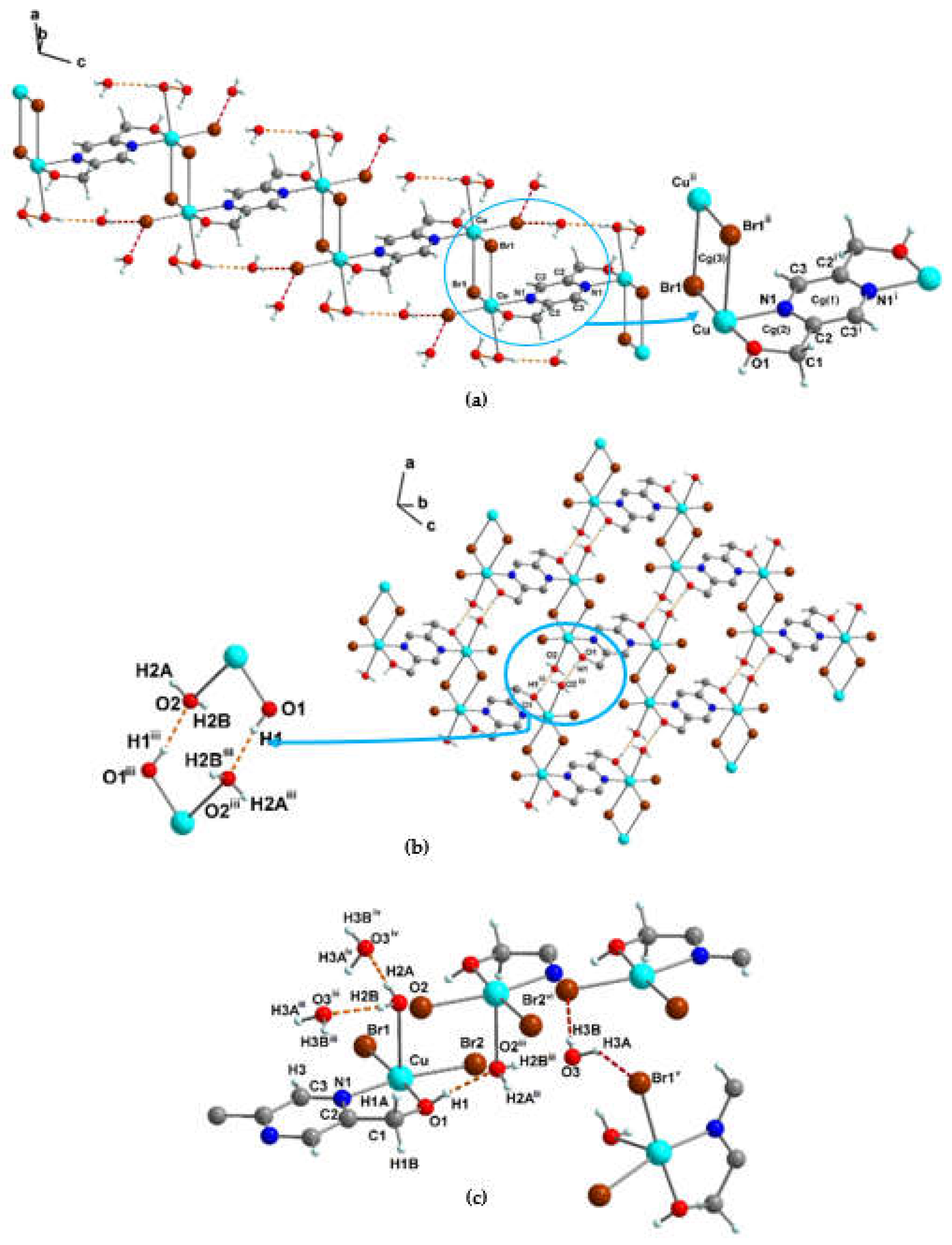
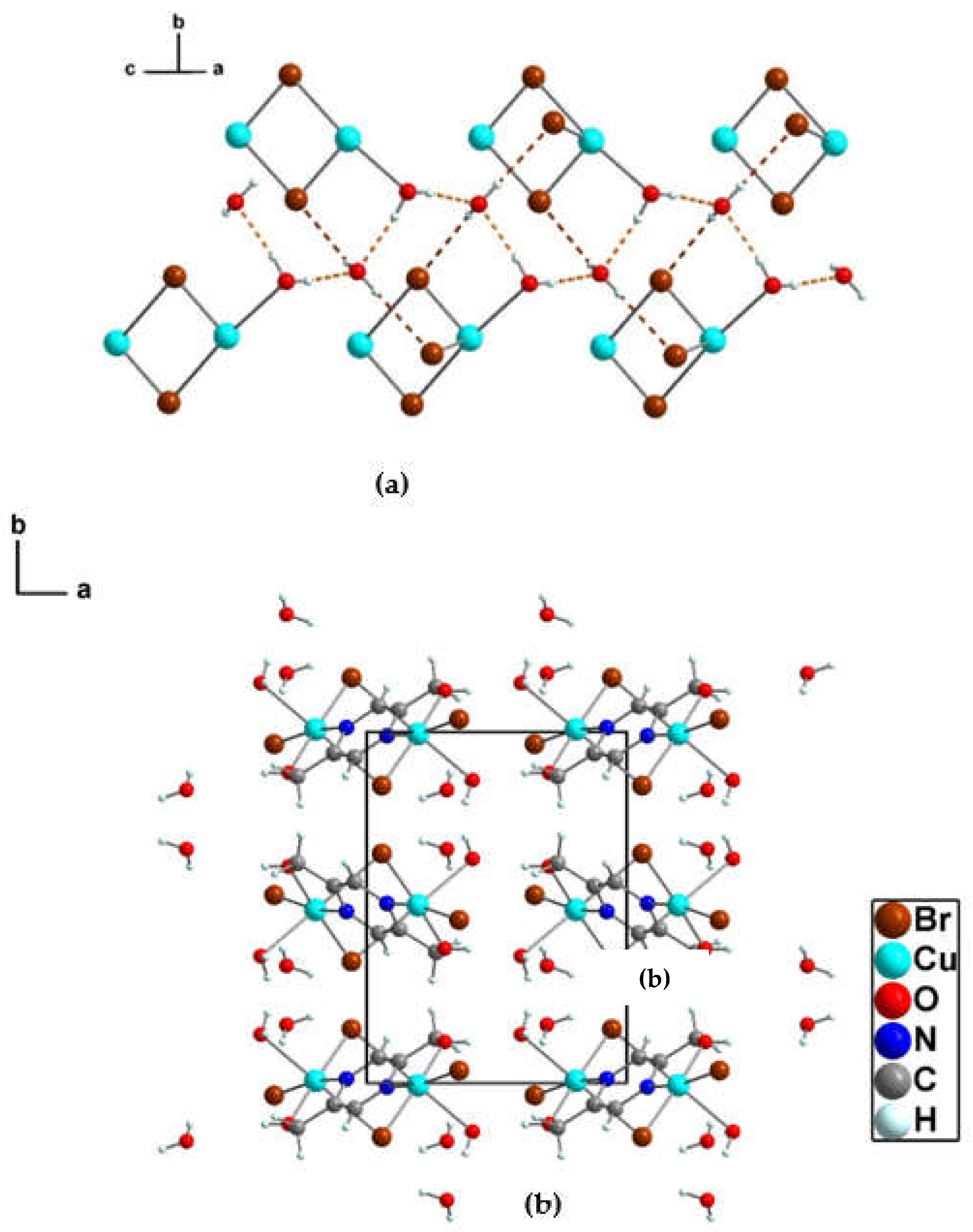
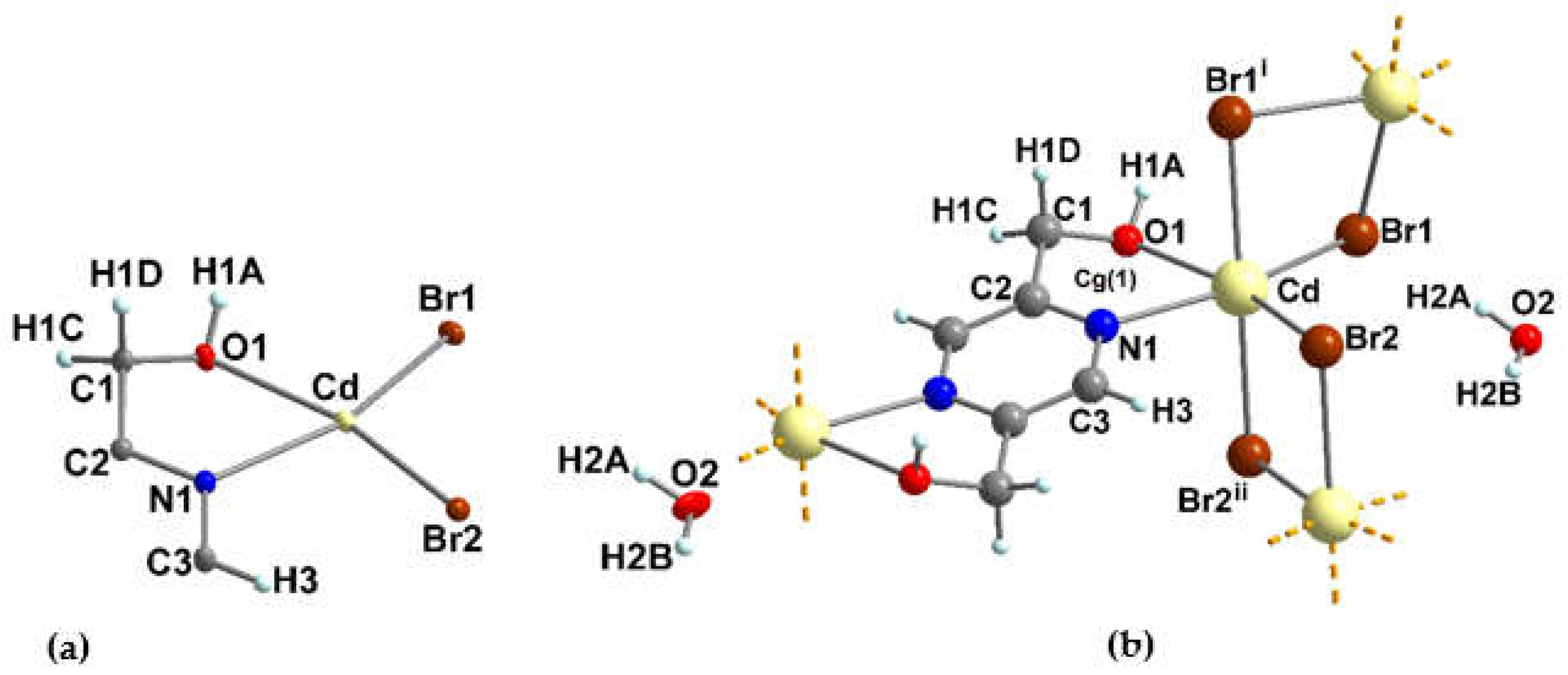
3.1.2. Crystal structure of {[Cu(pzydmH2)0.5(µ-Br)(Br)(H2O)]·H2O}n (1)
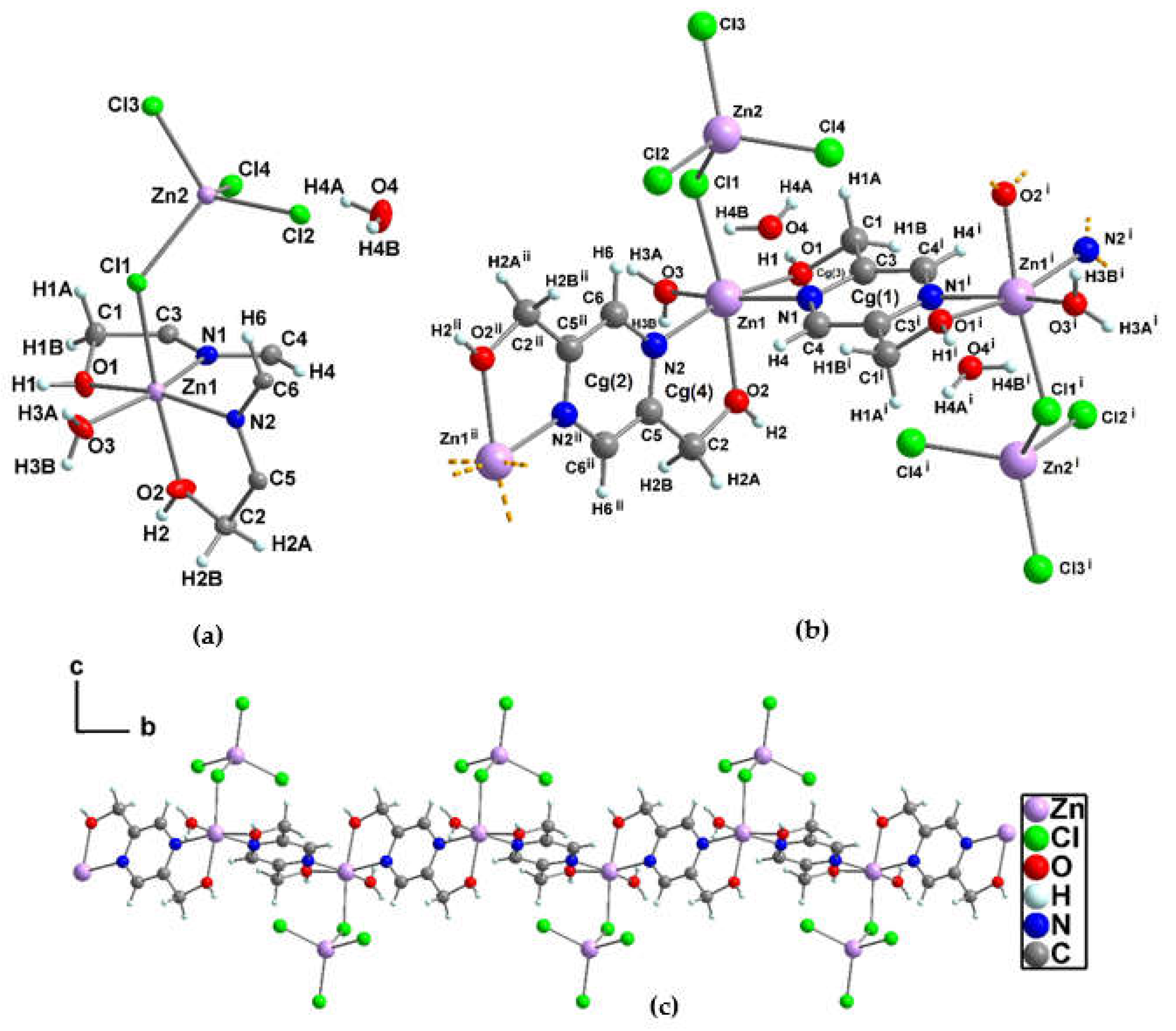
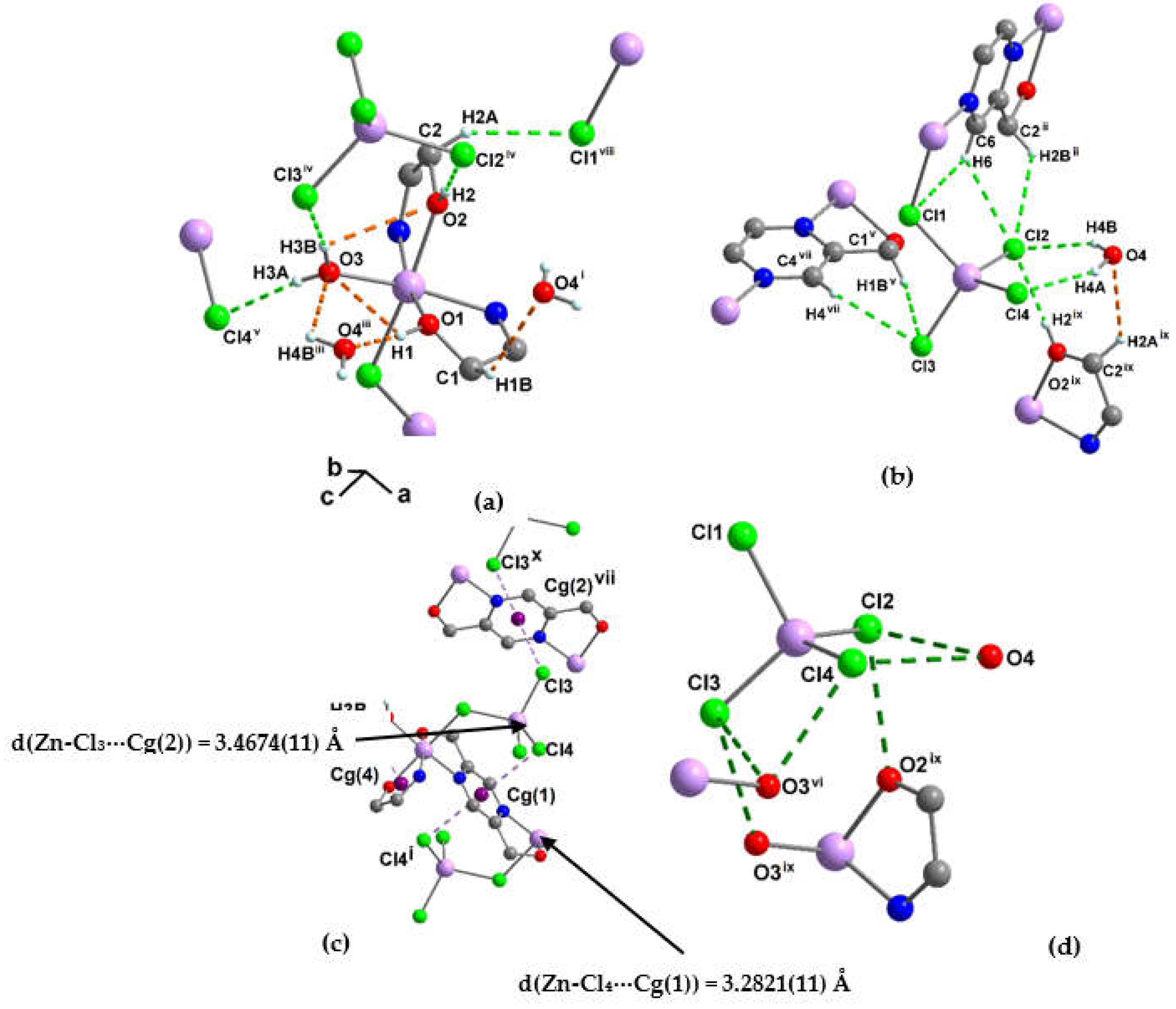
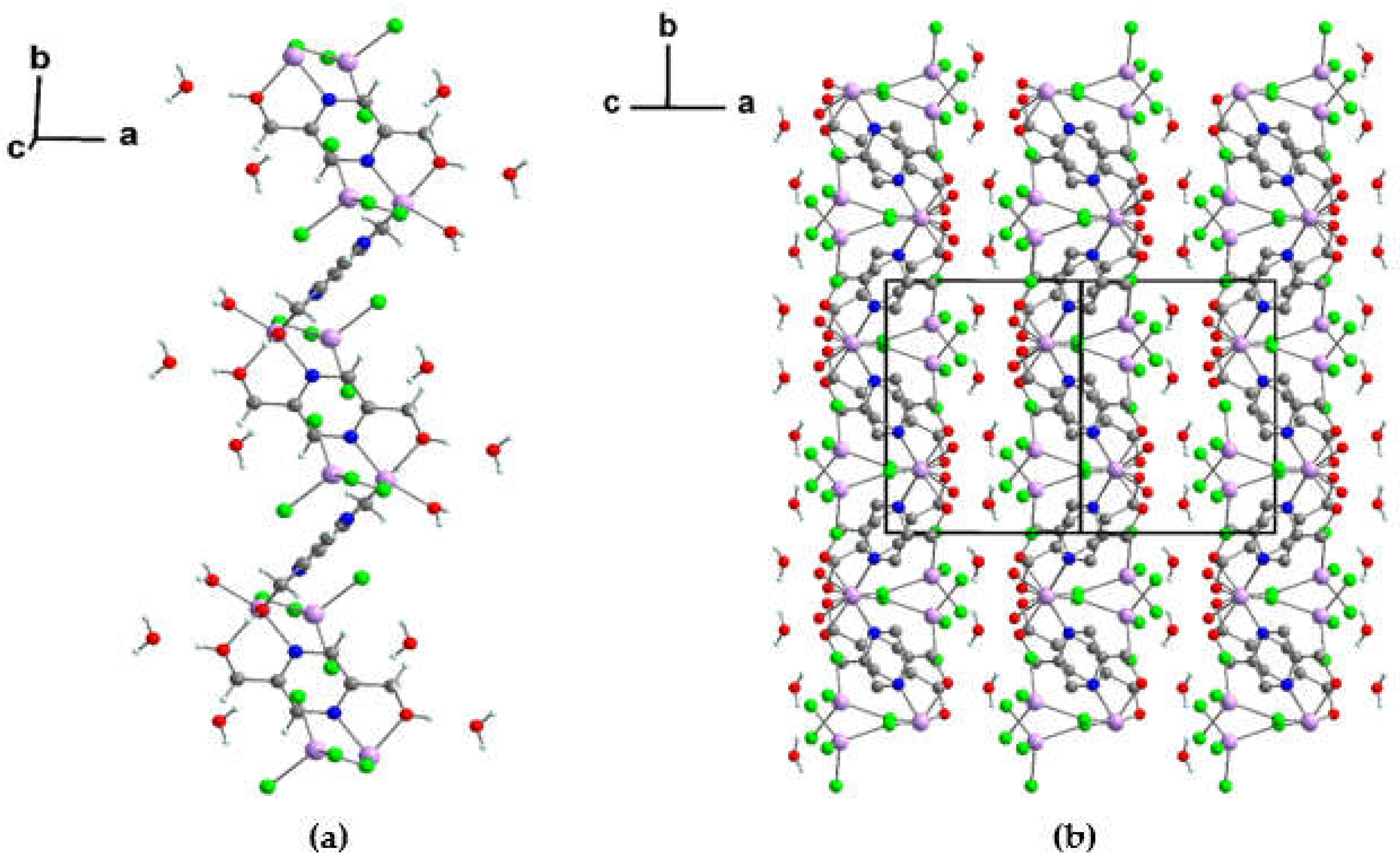
3.1.3. Crystal structure of {[Zn2(pzydmH2)(µ-Cl)(Cl)3(H2O)] ·H2O}n (2)
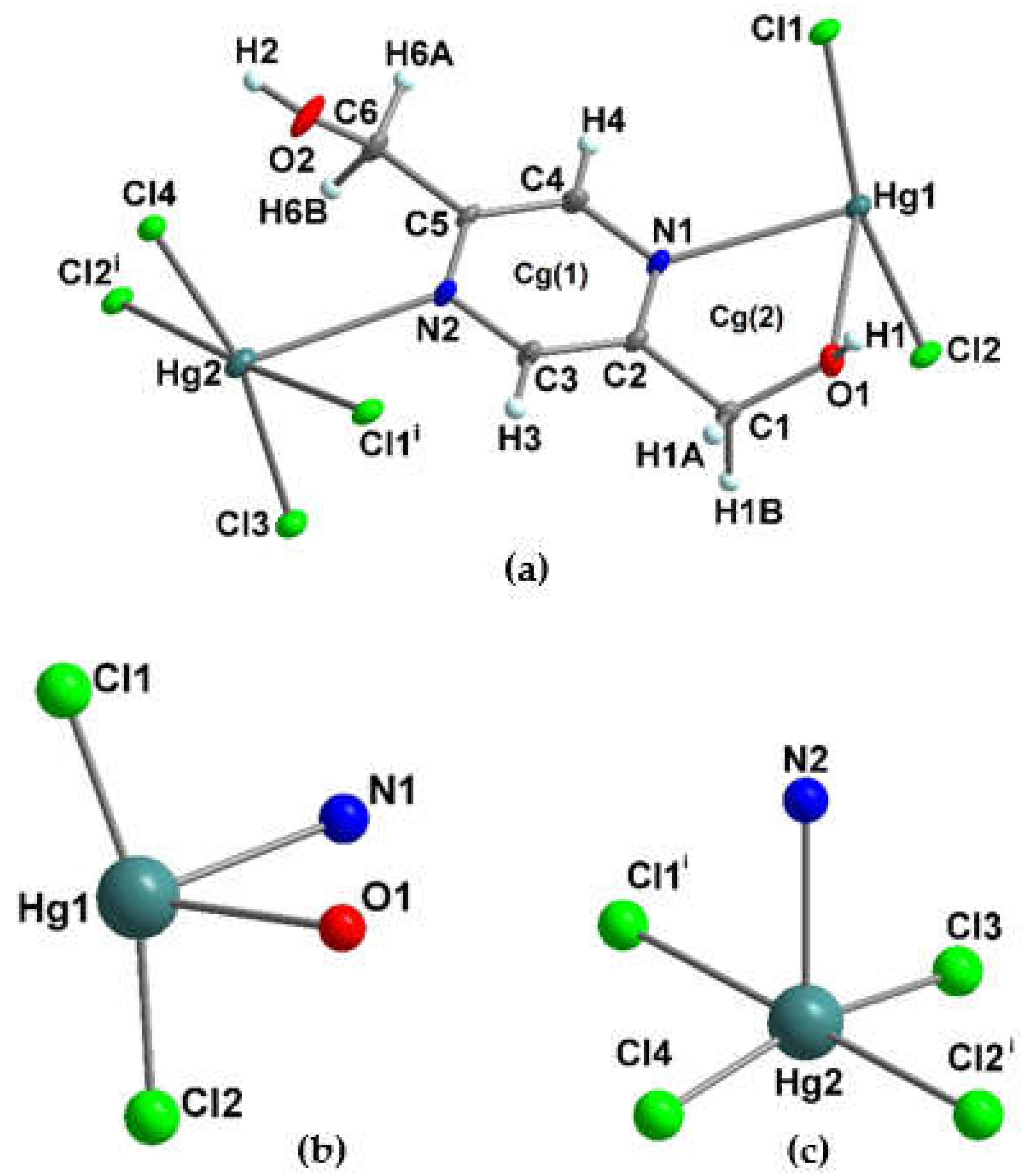
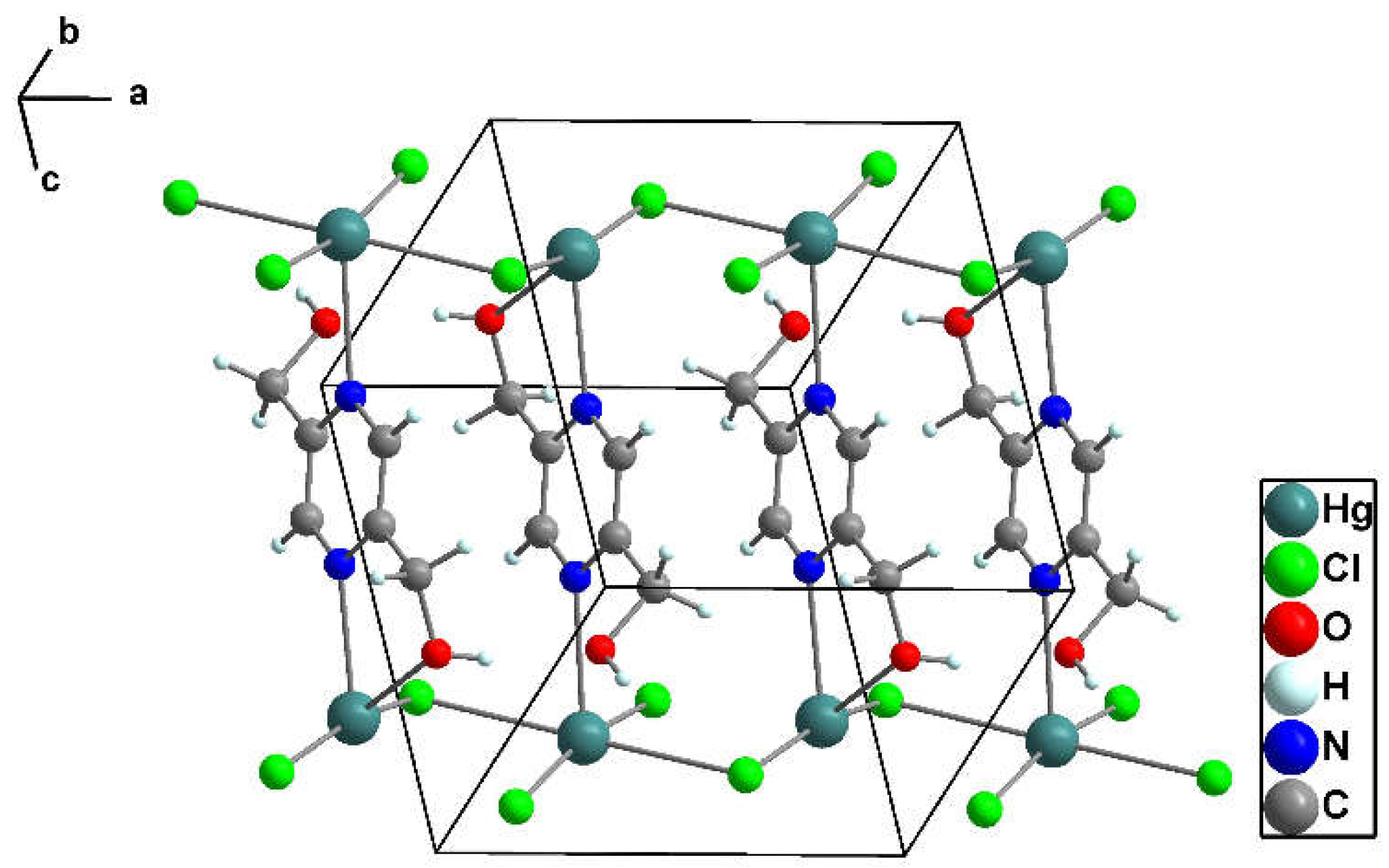
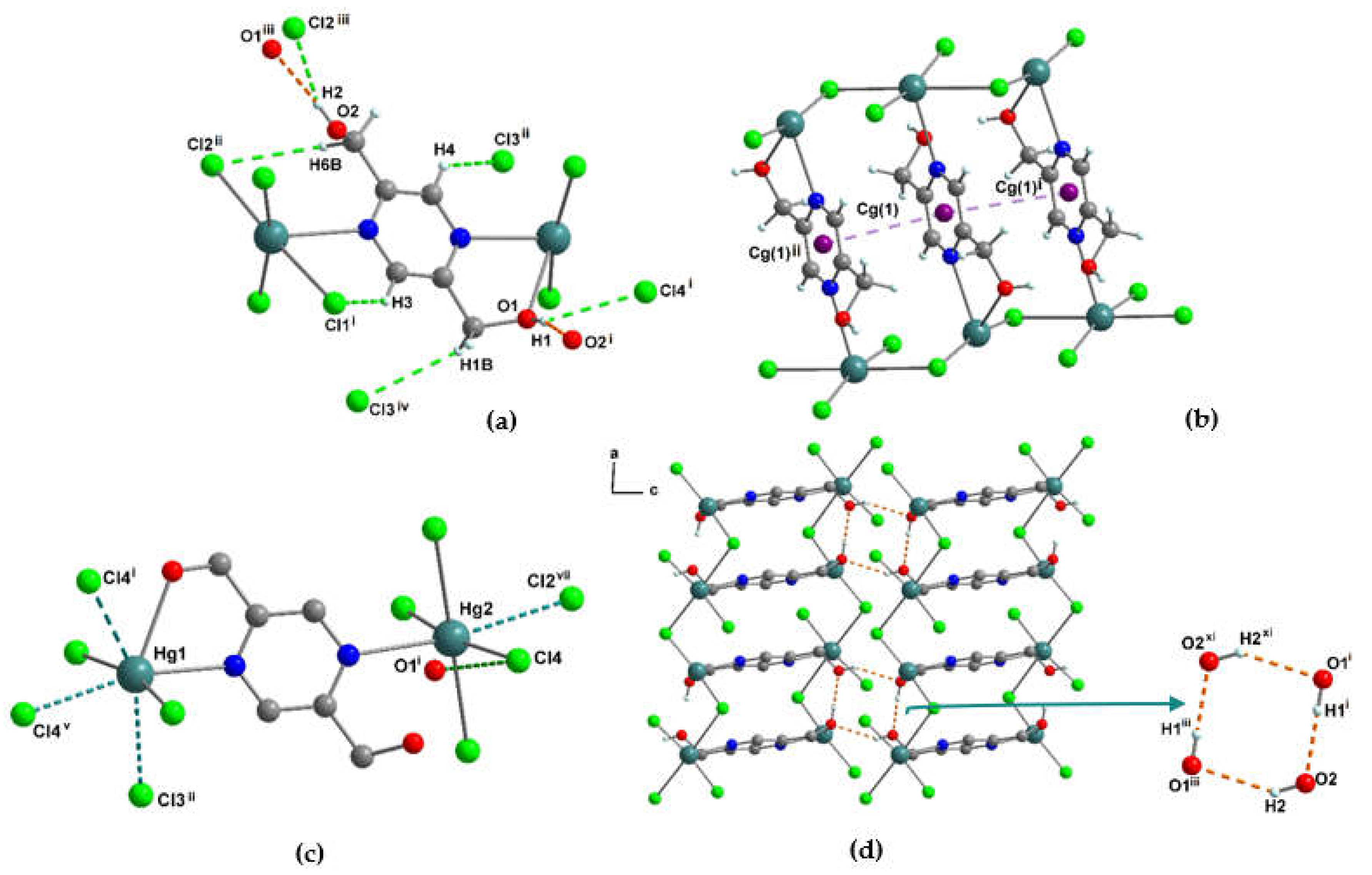
3.1.4. Crystal structure of [Hg2(pzydmH2)(µ-Cl)2(Cl)2]n (3)
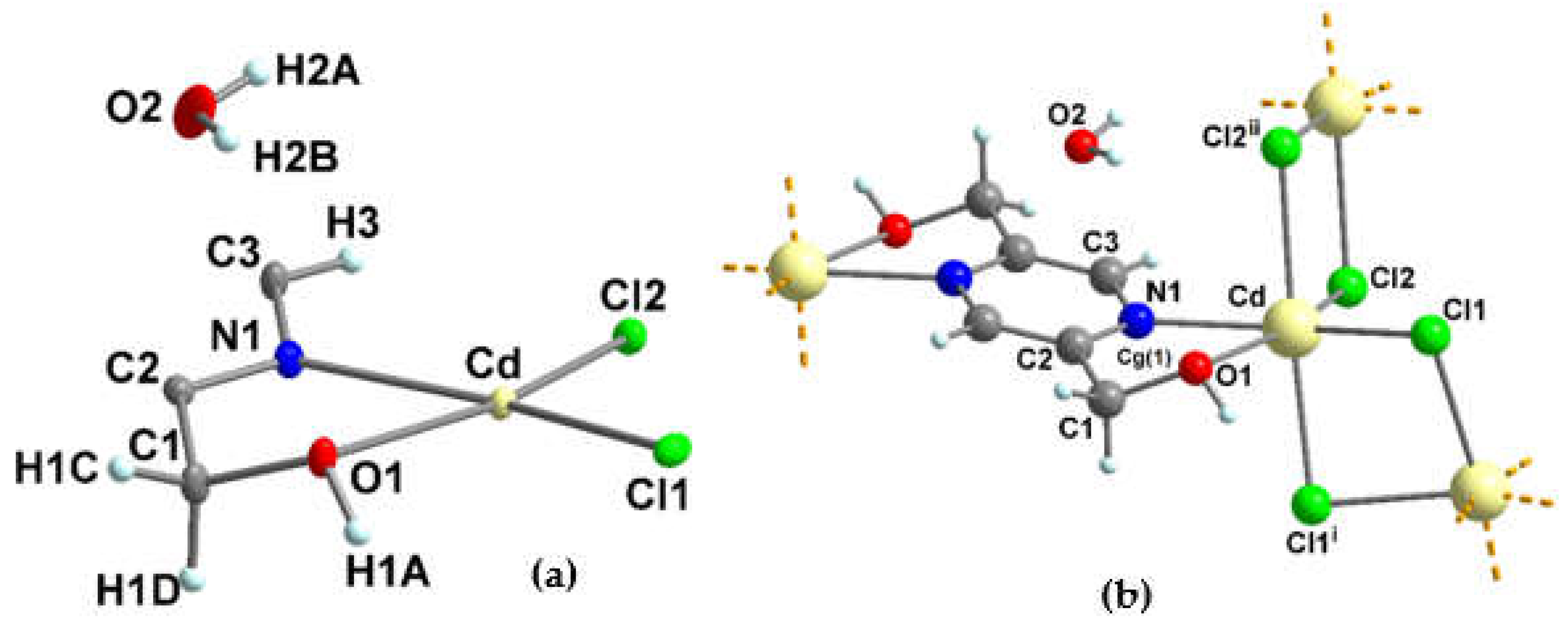
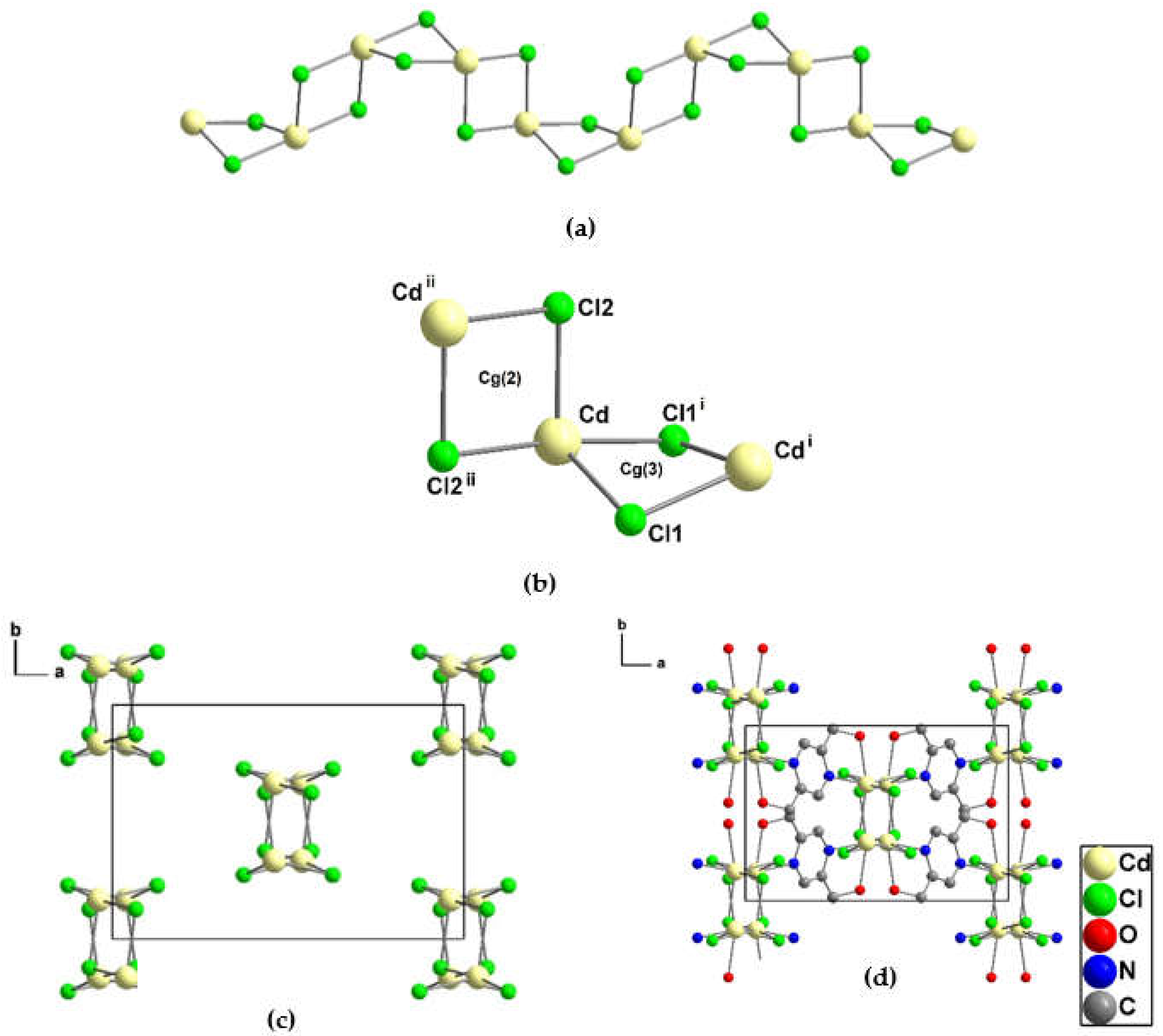
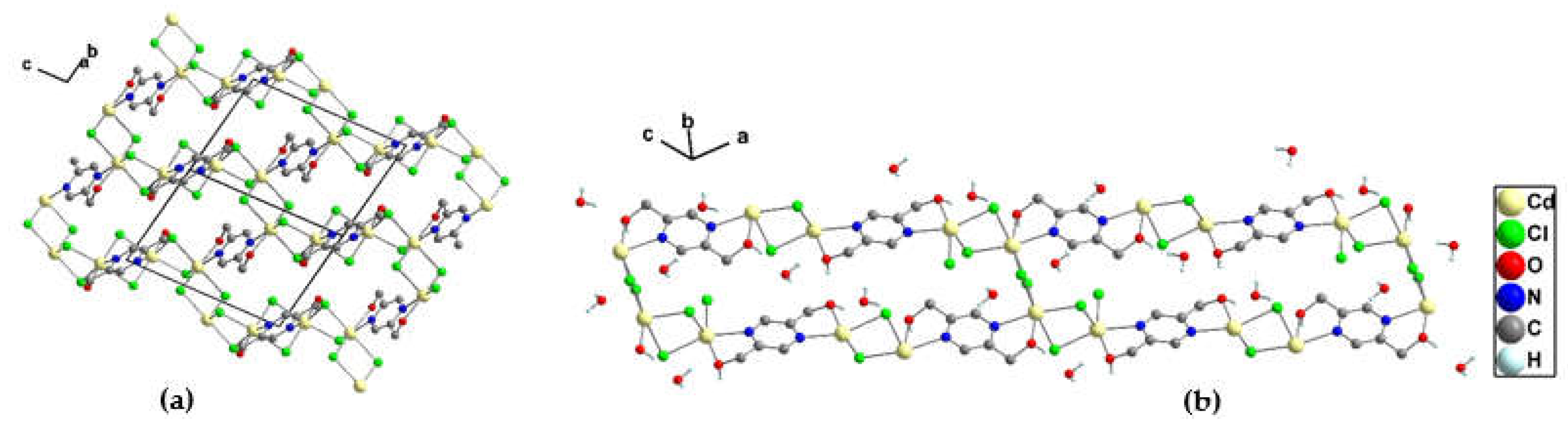
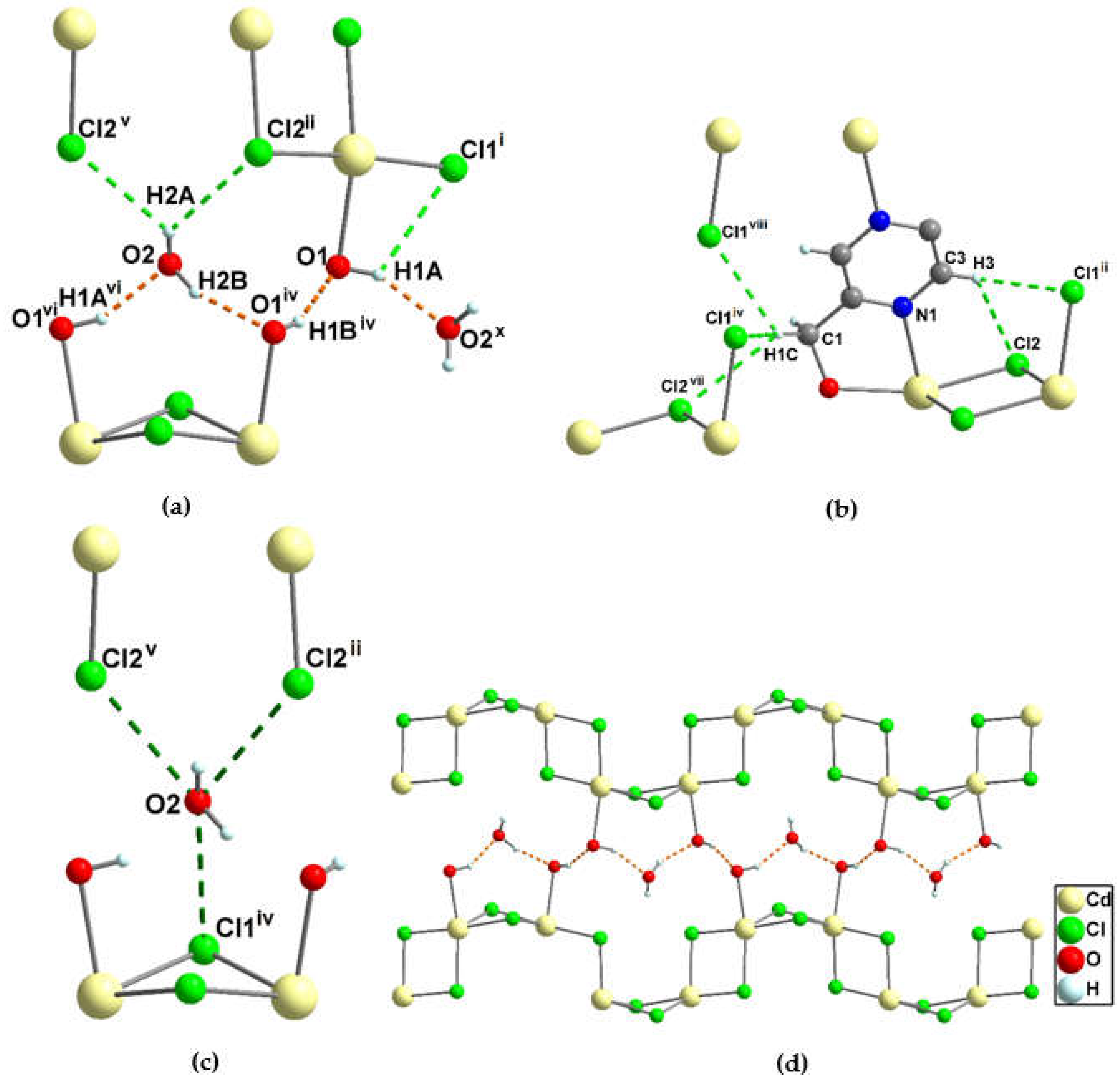
3.1.5. Crystal structure of {[Cd2(pzydmH2)(µ-Cl)4]·H2O}n (4)
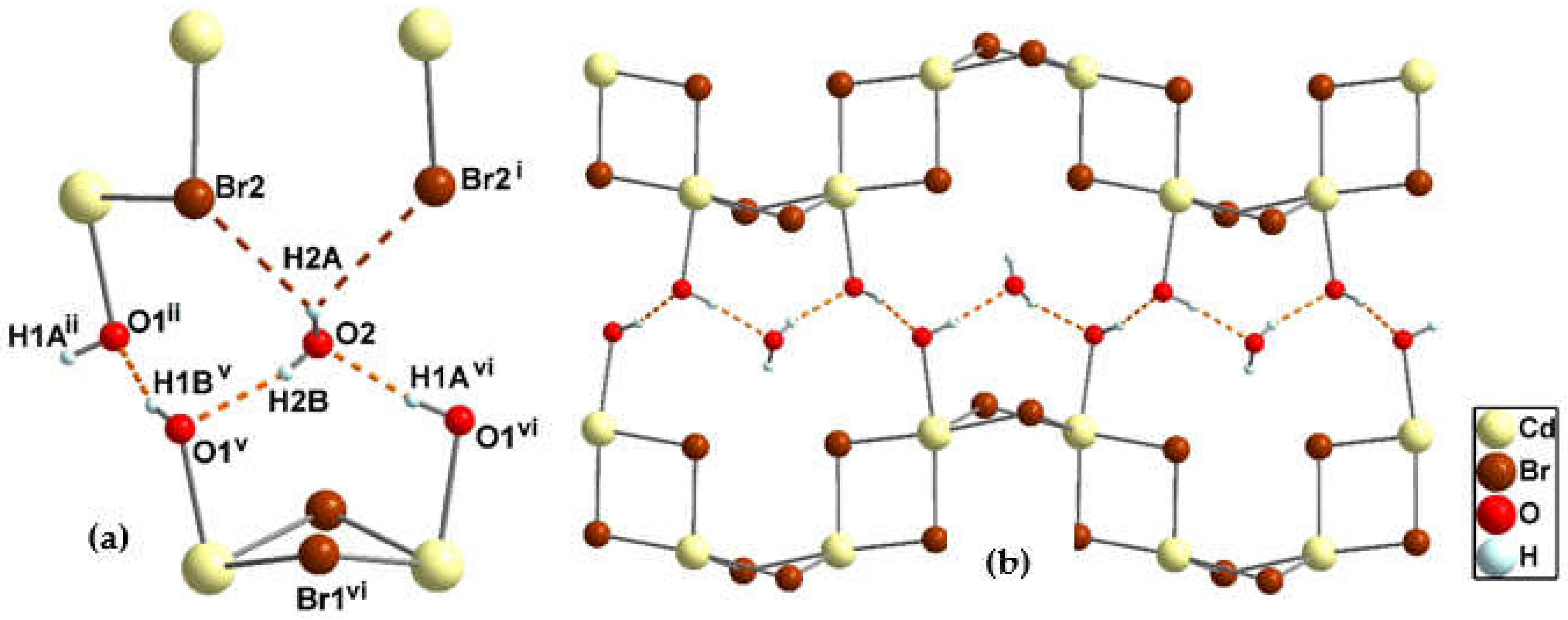
3.1.6. Crystal structure of {[Cd2(pzydmH2)(µ-Br)4].H2O}n (5)
3.3. IR studies
4. Conclusion
Supplementary Materials
Funding
Acknowledgements
Conflicts of Interest
References
- Armaghan M, Niu RJ, Liu Y, Zhang WH, Hor TA, Lang JP. Zn-based metal–organic frameworks (MOFs) of pyridinemethanol–carboxylate conjugated ligands: Deprotonation-dependent structures and CO2 adsorption. Polyhedron. 2018, 153, 218-25. [https://doi.org/10.1016/j.poly.2018.07.029]. [CrossRef]
- Armaghan M, Lu WJ, Wu D, Wei Y, Yuan FL, Ng SW, Amini MM, Zhang WH, Young DJ, Hor TA, Lang JP. Isolation of first row transition metal-carboxylate zwitterions. RSC Adv. 2015, 5, 42978-89. [https://doi.org/10.1039/C5RA05564D. [CrossRef]
- Armaghan M, Shang XJ, Yuan YQ, Young DJ, Zhang WH, Hor TA, Lang JP. Metal–Organic Frameworks via Emissive Metal-Carboxylate Zwitterion Intermediates. ChemPlusChem. 2015, 80, 1231-4. [https://doi.org/10.1002/cplu.201500134]. [CrossRef]
- Ma PP, Hao ZM, Wang P, Zhang WH, Young DJ. trans-[Ni(pdm)2]2+(pdm= 2-pyridinemethanol) as a reliable synthon for isoreticular metal–organic frameworks of linear dicarboxylates. J. Solid State Chem. 2023, 317, 123721. [https://doi.org/10.1016/j.jssc.2022.123721]. [CrossRef]
- Liu Y, Lin SX, Niu RJ, Liu Q, Zhang WH, Young DJ. Zinc and cadmium complexes of pyridinemethanol carboxylates: Metal carboxylate zwitterions and metal–organic frameworks. ChemPlusChem. 2020, 85, 832-7. [https://doi.org/10.1002/cplu.202000175]. [CrossRef]
- Telfer SG, Kuroda R, Lefebvre J, Leznoff DB. Boxes, helicates, and coordination polymers: A structural and magnetochemical investigation of the diverse coordination chemistry of simple pyridine-alcohol ligands. Inorg. chem. 2006, 45, 4592-601. [https://doi.org/10.1021/ic0517218]. [CrossRef]
- Taguchi T, Stamatatos TC, Abboud KA, Jones CM, Poole KM, O’Brien TA, Christou G. New Fe4, Fe6, and Fe8 clusters of iron (III) from the use of 2-pyridyl alcohols: structural, magnetic, and computational characterization. Inorg. chem. 2008, 47, 4095-108. [https://doi.org/10.1021/ic701756p]. [CrossRef]
- Telfer SG, Sato T, Kuroda R. Noncovalent Ligand Strands for Transition-Metal Helicates: The Straightforward and Stereoselective Self-Assembly of Dinuclear Double-Stranded Helicates Using Hydrogen Bonding. Angew. Chem. Int. Ed. 2004, 43, 581-4. [https://doi.org/10.1002/anie.200352833]. [CrossRef]
- Olguín J, Brooker S. Spin crossover active iron (II) complexes of selected pyrazole-pyridine/pyrazine ligands. Coord. Chem. Rev. 2011, 255, 203-40. [https://doi.org/10.1016/j.ccr.2010.08.002]. [CrossRef]
- Wang JF, Feng T, Li YJ, Sun YX, Dong WK, Ding YJ. Novel structurally characterized Co (II) metal-organic framework and Cd (II) coordination polymer self-assembled from a pyridine-terminal salamo-like ligand bearing various coordination modes. J. Mol. Struct. 2021,1231, 129950. [https://doi.org/10.1016/j.molstruc.2021.129950]. [CrossRef]
- Telfer SG, Kuroda R. The Versatile, Efficient, and Stereoselective Self-Assembly of Transition-Metal Helicates by Using Hydrogen-Bonds. Chem. Eur. J. 2005,11, 57-68. [https://doi.org/10.1002/chem.200400485]. [CrossRef]
- Zhang J, Teo P, Pattacini R, Kermagoret A, Welter R, Rogez G, Hor TA, Braunstein P. Structural Effects of Sodium Cations in Polynuclear, Multicubane-Type Mixed Na–Ni Complexes. Angew. Chem. 2010, 122, 4545-8. [https://doi.org/10.1002/ange.201001412]. [CrossRef]
- Stamatatos TC, Abboud KA, Wernsdorfer W, Christou G. High-Nuclearity, High-Symmetry, High-Spin Molecules: A Mixed-Valence Mn10 Cage Possessing Rare T symmetry and an S=22 Ground State. Angew. Chem. 2006, 118, 4240-3. [https://doi.org/10.1002/ange.200600691]. [CrossRef]
- Ramírez J, Brelot L, Osinska I, Stadler AM. CH…O hydrogen bond in the crystal structure of a pyrazine-based ligand and determination of the amplitude of the ligand conformational change induced by Cu (II) coordination. J. Mol. Struct. 2009, 931, 20-4. [https://doi.org/10.1016/j.molstruc.2009.05.021]. [CrossRef]
- Ferreira SB, Kaiser CR. Pyrazine derivatives: a patent review (2008–present). Expert Opin. Ther. Patents. 2012, 22,1033-51. [https://doi.org/10.1517/13543776.2012.714370]. [CrossRef]
- Juhas M, Zitko J. Molecular interactions of pyrazine-based compounds to proteins. J. Med. Chem. 2020, 63, 8901-16. [https://doi.org/10.1021/acs.jmedchem.9b02021]. [CrossRef]
- Riel AM, Rowe RK, Ho EN, Carlsson AC, Rappé AK, Berryman OB, Ho PS. Hydrogen bond enhanced halogen bonds: a synergistic interaction in chemistry and biochemistry. Acc. Chem. Res. 2019, 52, 2870-80. [https://doi.org/10.1021/acs.accounts.9b00189]. [CrossRef]
- Azbell TJ, Pitt TA, Bollmeyer MM, Cong C, Lancaster KM, Milner P. Ionothermal Synthesis of Metal-Organic Frameworks Using Low-Melting Metal Salt Precursors. Angew. Chem. 2023, 135, e202218. [https://doi.org/10.26434/chemrxiv-2022-00xd7]. [CrossRef]
- Guerah NE, Zerrouki K, Benslama O, Daran JC, Bouacida S, Bouchene R. New polymorph for Cd (II) chloro-bridged coordination polymer based on 3-aminopyrazin-2-carboxylic acid: Synthesis, structural characterization, Hirshfeld surface analysis, thermal properties and molecular docking study on the antifungal activity. J. Mol. Struct. 2022, 1258, 132681. [https://doi.org/10.1016/j.molstruc.2022.132681]. [CrossRef]
- Bruker AXS, APEX2, Bruker AXS Inc., Madison, Wisconsin, USA, 2012.
- APEX4 v2021.4-1, 2003, 2004 Bruker Nonius.
- APEX4 v2021.4-1, 2005-2018 Bruker AXS.
- APEX4 v2021.4-1, 2019, 2020 Bruker Nano.
- APEX4 v2021.4-1, 2021 Bruker AXS.
- SMART, data collection program for the CCD Area-Detector System; SAINT, data reduction and frame integration program for the CCD area-detector system. Bruker analytical X-ray systems, Madison, Wisconsin, USA, 1997.
- G. M. Sheldrick, in Program SADABS: Area-detector absorption correction, University of Göttingen, Germany, 1996.
- Sheldrick GM. SHELXT–Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3-8. [https://doi.org/10.1107/S2053273314026370]. [CrossRef]
- Sheldrick GM. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3-8. [https://doi.org/10.1107/S2053229614024218]. [CrossRef]
- Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JA, Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. appl. cryst. 2009, 42, 339-41. [https://doi.org/10.1107/S0021889808042726]. [CrossRef]
- Hübschle CB, Sheldrick GM, Dittrich B. ShelXle: a Qt graphical user interface for SHELXL. J. appl. cryst. 2011, 44, 1281-4. [https://doi.org/10.1107/S0021889811043202]. [CrossRef]
- K. Brandenburg, DIAMOND (Version 4.6.8), Crystal and Molecular Structure Visualization, Crystal Impact, Dr. H. Putz & Dr. K. Brandenburg GbR, Bonn Germany, 1997–2022, http://www.crystalimpact. com/diamond.
- Macrae CF, Sovago I, Cottrell SJ, Galek PT, McCabe P, Pidcock E, Platings M, Shields GP, Stevens JS, Towler M, Wood PA. Mercury 4.0: From visualization to analysis, design and prediction. J. appl. cryst. 2020, 53, 226-35. [https://doi.org/10.1107/S1600576719014092]. [CrossRef]
- Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, Streek JV, Wood PA. Mercury CSD 2.0–new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466-70. [https://doi.org/10.1107/S0021889807067908]. [CrossRef]
- Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, Streek JV. Mercury: visualization and analysis of crystal structures. Journal of applied crystallography. 2006, 39, 453-7.
- Steiner T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48-76. [https://onlinelibrary.wiley.com/doi/10.1002/1521-3773(20020104)41:1%3C48::AID-ANIE48%3E3.0.CO;2-U].
- Riel AM, Rowe RK, Ho EN, Carlsson AC, Rappé AK, Berryman OB, Ho PS. Hydrogen bond enhanced halogen bonds: a synergistic interaction in chemistry and biochemistry. Acc. Chem. Res. 2019, 52, 2870-80. [https://doi.org/10.1021/acs.accounts.9b00189]. [CrossRef]
- Prasanna MD, Row TG. C–halogen···π interactions and their influence on molecular conformation and crystal packing: A database study. Cryst. eng. 2000, 3, 135-54. [https://doi.org/10.1016/S1463-0184(00)00035-6]. [CrossRef]
- Dolai G, Roy S, Sen S, Giri RS, Mandal B. Crystal structure of 1-(2, 4, 6-trichlorobenzoyloxy) benzotriazole (TCB-OBt): observation of uncommon intermolecular oxygen–oxygen interaction and synthetic application in amidation. New J. Chem. 2021, 45, 19804-11. [https://doi.org/10.1039/D1NJ04048K]. [CrossRef]
- Groizard T, Kahlal S, Dorcet V, Roisnel T, Bruneau C, Halet JF, Gramage-Doria R. Nonconventional Supramolecular Self-Assemblies of Zinc (II)–Salphen Building Blocks. Eur. J. Inorg. Chem. 2016, 32, 5143-51. [https://doi.org/10.1002/ejic.201600866]. [CrossRef]
- Sharma C, Singh AK, Joy J, Jemmis ED, Awasthi SK. Experimental and theoretical study of intramolecular O⋯O interaction in structurally rigid β-keto carboxylic esters. RSC adv. 2016, 6, 91689-93. [https://doi.org/10.1039/C6RA20483J]. [CrossRef]
- Gleiter R, Haberhauer G, Werz DB, Rominger F, Bleiholder C. From noncovalent chalcogen–chalcogen interactions to supramolecular aggregates: experiments and calculations. Chem. Rev. 2018, 118, 2010-4. [https://doi.org/10.1021/acs.chemrev.7b00449]. [CrossRef]
- C. Janiak, A critical account on pi-pi stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc., Dalton Trans. 2000, 3885-3896. [https://doi.org/10.1039/B003010O]. [CrossRef]
- Alvarez S. Distortion pathways of transition metal coordination polyhedra induced by chelating topology. Chem. Rev. 2015, 115, 13447-83. [https://doi.org/10.1021/acs.chemrev.5b00537]. [CrossRef]
- Molčanov K, Kojić-Prodić B. Towards understanding π-stacking interactions between non-aromatic rings. IUCrJ. 2019, 6, 156-66. [https://doi.org/10.1107/S2052252519000186]. [CrossRef]
- Spek AL. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148-55. [https://doi.org/10.1107/S090744490804362X]. [CrossRef]
- Spek AL. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr., 2003, 36, 7-13. [https://doi.org/10.1107/S0021889802022112]. [CrossRef]
- Spek AL. What makes a crystal structure report valid?. Inorganica Chim. Acta., 2018, 470, 232-7. [https://doi.org/10.1016/j.ica.2017.04.036]. [CrossRef]
- Spackman MA, Jayatilaka D. Hirshfeld surface analysis. CrystEngComm. 2009, 11, 19-32. [https://doi.org/10.1039/B818330A]. [CrossRef]
- Spackman MA, McKinnon JJ. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm. 2002, 4, 378-92. [https://doi.org/10.1039/B203191B]. [CrossRef]
- Hogue RW, Dhers S, Hellyer RM, Luo J, Hanan GS, Larsen DS, Garden AL, Brooker S. Self-Assembly of Cyclohelicate [M3L3] Triangles Over [M4L4] Squares, Despite Near-Linear Bis-terdentate L and Octahedral M. Chem. Eur. J. 2017; 23, 14193-9. [https://doi.org/10.1002/chem.201702153]. [CrossRef]
- Turner MJ, McKinnon JJ, Jayatilaka D, Spackman MA. Visualisation and characterisation of voids in crystalline materials. CrystEngComm. 2011, 13, 1804-13. [https://doi.org/10.1039/C0CE00683A]. [CrossRef]
- Nibbering ET, Dreyer J, Kühn O, Bredenbeck J, Hamm P, Elsaesser T. Vibrational dynamics of hydrogen bonds. Analysis and control of ultrafast photoinduced reactions. Kühn O, Wöste L, Eds.; Springer series in chemical physics: Germany, 2007, pp. 619-87.
| D-H···A | d(D-H) | d(H···A) | d(D···A) | <(DHA) |
|---|---|---|---|---|
| pzydmH2 | ||||
| O(1)-H(1)···N(2) | 0.87(16) | 1.97(16) | 2.84(12) | 171 |
| O(2)-H(2)···O(3)#iii | 0.85(16) | 1.87(16) | 2.72(11) | 174 |
| O(3)-H(3)···N(3)#ii | 0.85(16) | 1.99(16) | 2.80(11) | 159 |
| Symmetry transformations used to generate equivalent atoms: ii = -x+2, -y+2, -z+1 #iii = x, -y+3/2, z-1/2. | ||||
| 1 | ||||
| O(1)-H(1)···O(2)#iii | 0.82(16) | 1.82(2) | 2.63(18) | 172 |
| O(2)-H(2A)···O(3)#iv | 0.82(2) | 1.96(2) | 2.77(21) | 173 |
| O(2)-H(2B)···O(3)#iii | 0.78(2) | 2.06(2) | 2.83(20) | 169 |
| O(3)-H(3A)···Br(1)#v | 0.87(2) | 2.55(2) | 3.23(16) | 135 |
| O(3)-H(3B)···Br(2)#vi | 0.80(2) | 2.49(2) | 3.29(15) | 178 |
| Symmetry transformations used to generate equivalent atoms: iii = 1-x, 1-y, 1-z, iv = 1-x, -1/2+y, 1/2-z, v = 1-x, -1/2+y, 1/2-z, vi = 1-x, 1-y, -z. | ||||
| 2 | ||||
| O(1)-H(1)···O(4)#iii | 0.87(2) | 1.72(2) | 2.59(3) | 175 |
| O(2)-H(2)···Cl(2)#iv | 0.83(3) | 2.25(3) | 3.08(19) | 176 |
| O(3)-H(3B)···Cl(3)#iv | 0.82(4) | 2.38(4) | 3.16(2) | 160 |
| O(3)-H(3A)···Cl(4)#v | 0.82(4) | 2.29(4) | 3.10(2) | 170 |
| O(4)-H(4A)···Cl(4) | 0.85(3) | 2.38(3) | 3.16(2) | 153 |
| O(4)-H(4B)···Cl(2) | 0.84(3) | 2.55(4) | 3.29(2) | 148 |
| Symmetry transformations used to generate equivalent atoms: iii = x-1, y, z, iv = x-1/2, -y+3/2, z+1/2, v = -x+1/2, y+1/2, -z+1/2. | ||||
| 3 | ||||
| Intra O(1)-H(1)···O(2)#i | 0.82(19) | 1.93(2) | 2.73(3) | 165 |
| Intra O(1)-H(1)···Cl(4)#i | 0.82(19) | 2.75(2) | 3.12(3) | 109 |
| O(2)-H(2)···O(1)#iii | 0.83(19) | 2.25(3) | 2.96(3) | 143 |
| O(2)-H(2)···Cl(2)#iii | 0.83(19) | 2.82(4) | 3.45(3) | 136 |
| Symmetry transformations used to generate equivalent atoms: i = -x+2, -y+1, -z+1, iii = x, y, z-1. | ||||
| 4 | ||||
| O(1)-H(1A)···O(2)#x | 0.85(6) | 1.83(10) | 2.62(10) | 154 |
| O(1)-H(1A)···O(2)#iv | 0.85(6) | 1.99(10) | 2.69(10) | 138 |
| O(1)-H(1B)···O(1)#iv | 0.85(8) | 1.82(10) | 2.62(5) | 156 |
| O(2)-H(2B)···O(1)#iv | 0.93 | 1.85 | 2.69(10) | 149 |
| O(2)-H(2A)···Cl(2)#ii | 0.80 | 2.65(13) | 3.26(9) | 134 |
| O(2)-H(2A)···Cl(2)#v | 0.80 | 2.68(13) | 3.18(9) | 122 |
| Symmetry transformations used to generate equivalent atoms: ii = -x+1,-y+1,-z+1, iv = -x+1,-y,-z+1, v = x, 1-y, 1/2+z, x = x, -y, z-1/2. | ||||
| 5 | ||||
| O(1)-H(1A)#vi···O(2) | 0.85(6) | 1.83(7) | 2.68(10) | 172(8) |
| O(1)-H(1B)#v···O(1)#ii | 0.85(6) | 1.81(9) | 2.64(5) | 165(12) |
| O(2)-H(2B)···O(1)#v | 0.72 | 1.99 | 2.70(10) | 165 |
| O(2)-H(2A)···Br(2) | 0.84 | 2.78 | 3.39(9) | 131 |
| O(2)-H(2A)···Br(2)#i | 0.84 | 2.88 | 3.33(9) | 115 |
| Symmetry transformations used to generate equivalent atoms: i = -x+1, y, -z+3/2, ii = -x+1, -y+1, -z+1, v = x, y+1, z, vi = 1-x, y+1, 3/2-z,. | ||||
| The hydrogen bond lengths highlighted in bold and used to indicate hydrogen bonds correspond to a bond that is typically between 0.45 and 0.20 Å shorter than the sum of the van der Waals radii of the atoms participating in the hydrogen bond. The bond angle that is highlighted in bold indicates angle that are close to 180 °. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





