1. Introduction
Cordyceps militaris was an entomopathogenic fungus (an obligate parasite that grew on insects or insect larvae) distributed in Asia, particularly China. It was widely used in China as a raw medicine or dietary supplement to treat a variety of diseases [1-3]. Modern techniques showed that
C. militaris had remarkable healing properties involving antitumor, antioxidant, antibacterial, and immunomodulatory activities [
2]. Potent pharmacologically active compounds consisting of cordycepin, polysaccharides, fatty acids, mannitol, amino acids, trace elements, ash, fiber, and other chemical components were found in
C. militaris [
4].
Polysaccharides, which were substances capable of scavenging free radicals such as hydroxyl and superoxide anion radicals to prevent oxidative damage in living organisms, were found as pharmaceutical active ingredients in the highest concentrations in
C. militaris [
5,
6]. Several studies also proved that free radicals were closely related to the aging process [
7]. The free radical theory of aging focuses on the mitochondrial senescence hypothesis that was built on the work of Gerschman and Harman [
8,
9]. mtDNA was hypothetically mutated as reactive oxygen species (ROS) in mitochondria increase during aging, therefore it caused the breakdown of components of the mitochondria, dysfunction, and aging [
10]. Recently, the capability of ROS elimination and inhibition of mitochondrial swelling in mouse testing illustrated that polysaccharides from cultured
C. militaris fruit bodies (CMPs) were antioxidants with great potential for mitochondrial protection and anti-aging [
11]. In addition, CMPs also displayed their significant immunomodulatory and anti-aging activities in
Caenorhabditis elegans at the organismal level [
12]. In recent research works, polysaccharides possessed immunomodulatory properties [
13,
14,
15], hypoglycemic [
16,
17], anti-inflammatory [
18], antitumor, anti-metastatic [
19], anti-influenza virus [
20], and gram-positive bacteria inhibition such as
Staphylococcus aureus and
Micrococcus tetragenus [
21].
On the other hand, one of the potent antimicrobial components of
C. militaris fruiting bodies was a derivative of the nucleoside adenosine called cordycepin. It was first isolated from
C. militaris [
23], and presently synthesized owing to its insecticidal, antibacterial, and antitumor properties [
24,
25]. Cordycepin obtained from the liquid of cultured
C. militaris also brought several benefits for human health [
27], for instance, a potent activity of growth inhibitory against human enteric bacteria
Clostridium paraputrificum and
Clostridium perfringens [
26],
Fusarium oxysporum [
28],
Bipolaris maydis,
Mycosphaerella arachidicola,
Rhizoctonia solani and
Candida albicans [
29].
With our ambition on finding out new materials for cosmetic products,
C. militaris has been grown up, extracted, and used as an ingredient in skincare products in lieu of preservatives and antioxidants with respect also to inhibitor the growth of microbial and aging (
Figure 1). Antioxidant, antimicrobial, and serum stability effects have been evaluated to obtain the desired product that satisfied the cosmetically acceptable level [
22].
2. Materials and Methods
2.1. Fungus source and extraction
C. militaris fungus was supplied by the Research Center for Bioactive Natural Products, University of Science, VNU-HCM, Vietnam. The fungus was kept on solid media (peptone 5 g, yeast extract 10 g, glucose 40 g, MgSO4 0.5 g, KH2PO4 1 g, distilled water up to 1 L, 2% agar) at 4 oC. C. militaris was cultivated on broth media (without agar) to collect mycelia for this study. Mycelia were dried to constant weight, ground into powder, and extracted with methanol. 100 g mycelia powder was soaked in 1 L methanol absolute for 24 hours at room temperature. After filtration, the filtrate was concentrated by rotary vacuum evaporation to remove solvent and collect mycelium extract (ME). The broth media after cultivation was filtered and concentrated by rotary vacuum evaporation to collect broth extract (BE). The extracts were stored at 4 oC under dark conditions until used.
2.2. Microorganism
Microorganisms used in this study were supplied by the Research Center for Bioactive Natural Products, University of Science, VNU-HCM, Vietnam. The strains of Escherichia coli ATCC 8739 (E. coli), Pseudomonas aeruginosa ATCC 9027 (P. aeruginosa), Bacillus spizizenii ATCC 6633 (B. spizizenii), Staphylococcus aureus ATCC 6538 (S. aureus), Candida albicans ATCC 10231 (C. albicans) were used. Bacteria were cultured on Luria-Bertani (LB) agar, while C. albicans was cultured on a Sabouraud dextrose (SD) agar at 30 oC for one to three days. Microorganisms were also sub-cultured in broth media.
2.3. Assessment of anti-microorganism properties
Anti-microorganism properties screening of the extracts was evaluated using disc diffusion assay [
30]. Briefly, each microorganism was grown separately on suitable agar media for one to three days. Colonies were resuspended into sterile physiological saline until the turbidity reached 0.5 McFarland standard (approximate count of 10
8 CFU/mL). A volume of 100 µL of each microorganism suspension was spread onto LB agar plates (for bacteria) and SD agar plates (for yeast). The individual extracts, mixed extracts (
Table 1), and serum samples (
SS) were dissolved in appropriate solvents (methanol for mycelium extract or distilled water for broth extract, and serum samples) at a concentration of 100 mg/mL. 6-mm sterilized filter paper discs were infused with 20 µL of each extract, allowed to dry, and placed onto the inoculated plates. Then, the plates were left for incubation at 37 °C for 24 to 48 hours. The anti-microorganism activity was evaluated by inhibition zones. The diameters of the inhibition zones were measured to the closest whole millimeter. Each assay was finalized in triplicate. Mean values (± SD) are reported in this study. Gentamycin 500 µg/mL (5 µg/disc) and cycloheximide 15 mg/mL (0.15 mg/disc) were used as positive controls to compare anti-microorganism activity. Filter discs infused with 20 µL of distilled water and absolute methanol were used as negative controls.
2.4. Assessment of antioxidants by using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging assay
The free radical-scavenging activities of extracts and serum samples were assessed by using the method of Devi
et al [
31]. Briefly, 100 µL of each sample with different concentrations (0.25 mg/mL, 0.5 mg/mL, 1.0 mg/mL, 1.25 mg/mL, 2.5 mg/mL, 5.0 mg/mL) for extracts and 100 mg/mL for serum samples were mixed with 100 µL of DPPH solution in methanol (0.5 mM). The mixture was shaken thoroughly and left to stand for 30 minutes at room temperature in the dark and measured at 517 nm by a UV-visible spectrophotometer to get the absorbance. Each assay was repeated three times. A low absorbance of the reaction mixture stated a high free-radical-scavenging activity. Vitamin C was used as a positive control. The ability to scavenge the DPPH radical was determined based on the equation (
1):
where Ablank and Asample are the absorbances of the control reaction (containing all reagents except the test samples) and the absorbance of the test samples, respectively.
2.5. Materials and preparation of serum
Each material of mixture
D was weighed, dissolved in MiliQ water at a ratio of 1:100 (
w/
w), and stirred continuously until the mixture became homogeneous completely at room temperature, at 250 rpm speed in 30 minutes. Then, each material of mixture
E was also added in the priority and mixed at 70-75
oC, at 250 rpm speed for 15 minutes. Similarly, the orderly component of mixture
F was weighed and agitated in MiliQ water at 70-75
oC for 15 minutes. After that, mixture
E was poured into mixture
F available in the main mixer and continuously mixed at 70-75
oC for 20 minutes. Subsequently, mixture
D was poured into the main mixer and followed the above conditions for 15 minutes. After cooling down to 45
oC, the components of mixture
G were added to the main mixer. The stirring speed at 150 rpm for 15 minutes was maintained till cooling down the room temperature. Finally, mixture
H consisting of fragrance and extract in proportion (all extracts were dissolved at a concentration of 10% in ethanol 70%) was added and mixed for 15 minutes (
Table 2).
2.6. Stability test for serum
The necessary amount of serum (3.0 g) was put into a clean and dry glass jar. Several serum samples were stored at different temperatures (4 oC, room temperature, and 40 oC) for 0, 14, and 28 days to determine the stability of the physicochemical and organoleptic properties (color, scent, homogeneity, pH, appearance). The serum samples’ characteristics in different conditions were monitored and recorded.
2.7. Microbial contamination test for serum
Firstly, a necessary amount of serum sample (1.0 g) was dissolved in 1 mL of sterile distilled water. Then, several concentrations of serum samples such as 0.05 g/mL and 0.005 g/mL were also obtained from the dilution of the above serum solutions. Subsequently, 100 µL of diluted serum samples were spread onto
LB agar plates and
PGA (Potato Glucose Agar) plates. Each test was finalized in triplicate. After that, the plates were left for incubation at 37 °C for 24 to 48 hours. Subsequently, the results were recorded after three days for
LB plates and five days for
PGA plates (
Table 3).
3. Results
3.1. Extraction yields
Based on the above protocol, 54 g of ME was collected from 160 g mycelia powder, and 5.9 g of BE was collected from 500 mL of broth media, with yields of 33.8 g/L and 11.8 g/L, respectively. The dried extracts were resuspended in suitable solvents for the next experiments.
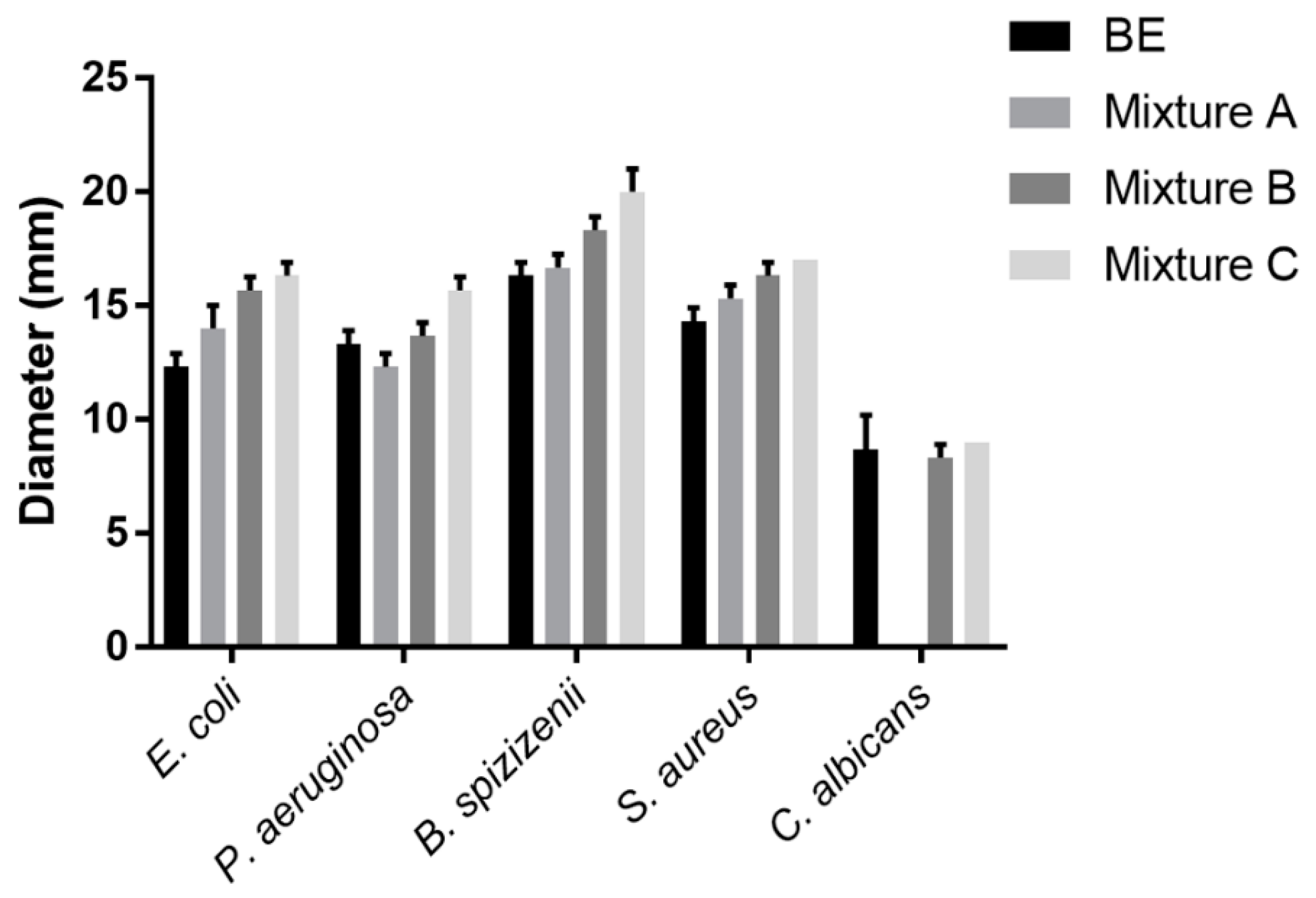
3.2. Antimicrobial properties of extracts
The ability of the extracts to repress the growth of microorganisms was determined by using a disc diffusion assay with 2 mg of each extract per disc (
Figure 2). The
ME showed that antimicrobial resistance did not appear. Meanwhile, the
BE was resistant to five tested strains, for instance, moderately resistant to two Gram-negative strains (
E. coli and
P. aeruginosa) or yeast strain (
C. albicans), and strongly resistant to two Gram-positive strains (
B. spizizenii and
S. aureus). Three prepared samples of extracts (
A,
B,
C) also displayed moderate activity against
P. aeruginosa and strong resistance to other three bacterial strains including
E. coli,
B. spizizenii, and
S. aureus. Especially, only samples
B and
C had the capability to inhibit
C. albicans. Overall, the mean diameters of inhibition zones for
E. coli, P. aeruginosa, B. spizizenii, S. aureus, and
C. albicans achieving respectively 16.3 mm, 15.7 mm, 20.0 mm, 17.0 mm, and 9.0 mm illustrated that sample
C was the best resistant.
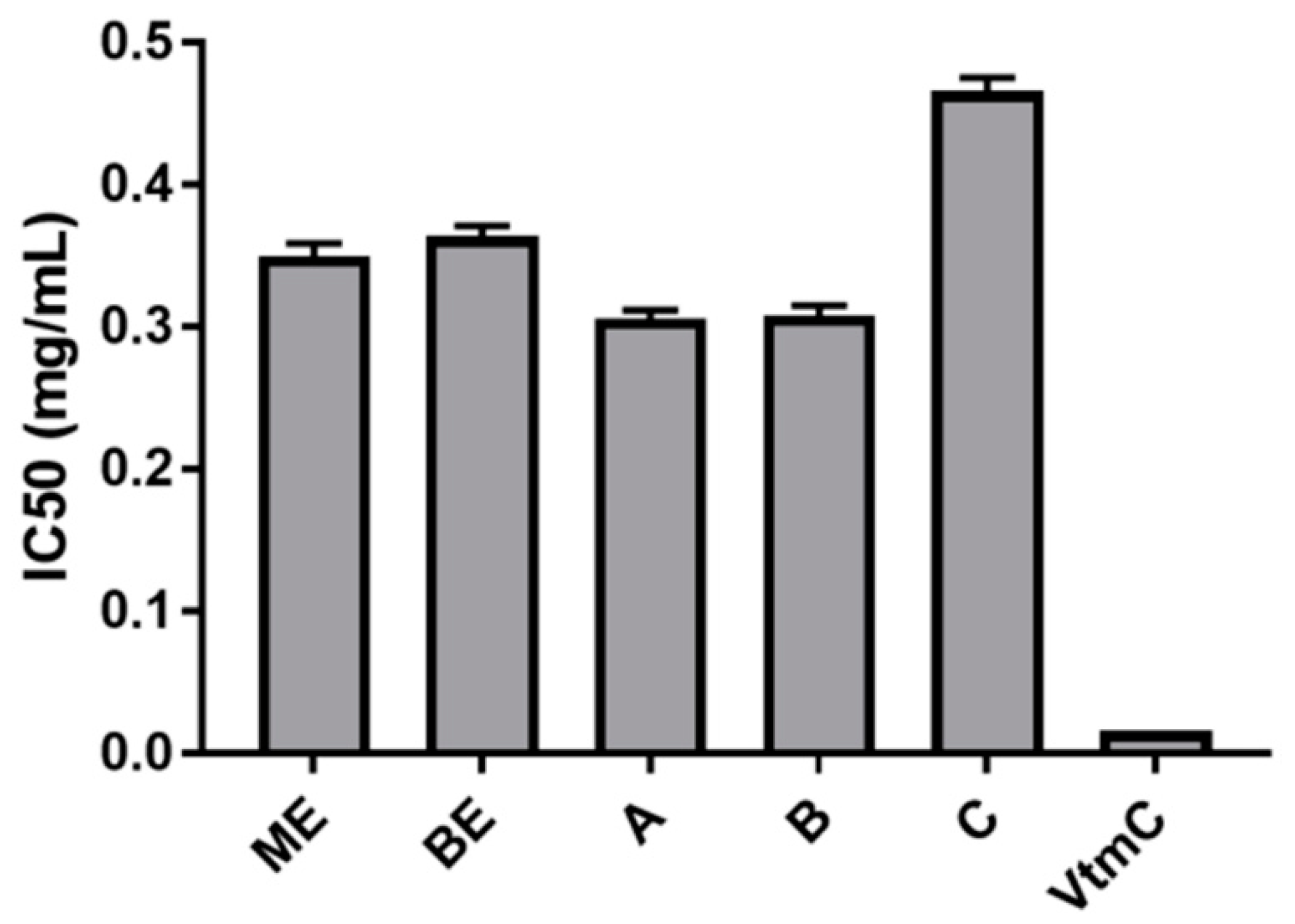
3.3. Antioxidant properties of extracts by DPPH assay
DPPH is a stable radical, which can receive an electron or hydrogen radical to become a stable and diamagnetic molecule, a purple solution in methanol, and becomes a pale liquid when reacting with antioxidant molecules. The low value of optical density at 517 nm (OD
517) displayed the high radical-scavenging activities of the extract. The results indicated that all five samples of extracts (
ME,
BE,
A,
B, and
C) possessed good DPPH radical-scavenging activity at the concentration of 0.25-1.00 mg/mL. When the concentration of samples was increased over 1.00 mg/mL, the radical scavenging capacity was not changed considerably because DPPH was completely neutralized. The lowest IC
50 value (0.306 mg/mL) of sample
A indicated that the best antioxidant capacity measured by DPPH-scavenging was found in sample
A, the mixture of
BE and
ME with the weight ratio 1:2. Subsequently, the values of IC
50 were obtained in the increasing order such as 0.306 mg/mL for the sample
A, 0.308 mg/mL for the sample
B, 0.350 mg/mL for the sample
ME, 0.364 mg/mL for the sample
BE, and 0.466 mg/mL for the sample
C (
Figure 3).
3.4. Anti-microorganism properties of serum product
The ability of the serum samples to inhibit microorganism growth was determined using a disc diffusion assay with 2 mg of each serum sample per disc (
Table 4). All serum samples showed moderate activity against
P. aeruginosa, S. aureus, and especially
B. spizizenii, however, all of them displayed fuzzy inhibition zones against
E. coli and
C. albicans. In addition, the serum base also possessed antimicrobial activity, but in general, the serum samples containing the extracts brought better outcomes.
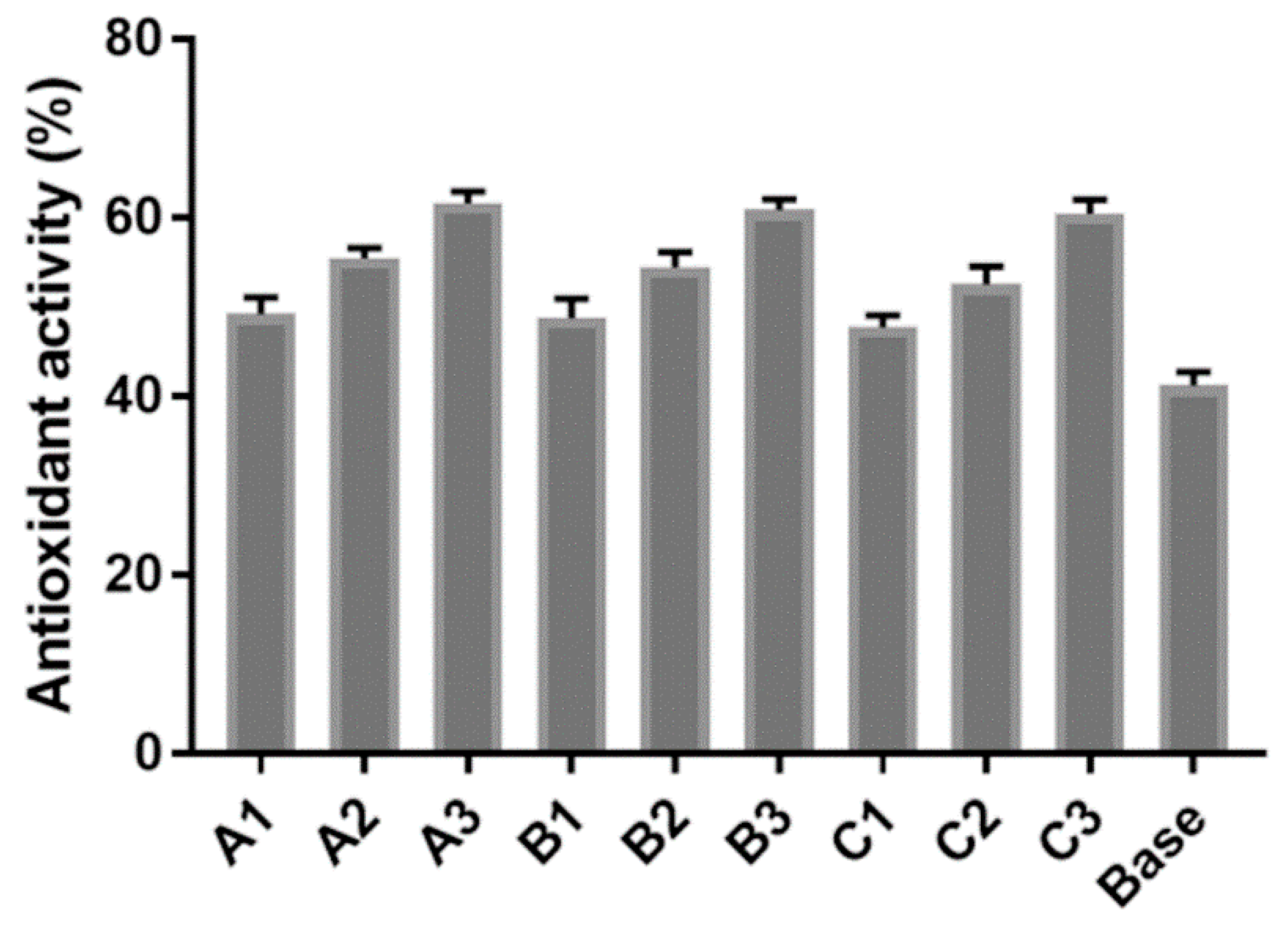
3.5. Antioxidant properties of serum product
The finished base was a milky white, smooth, and homogeneous serum with a pleasant flavor, suitable pH (6.0-6.5), and expected viscosity (800-880 cPs). The capacity of DPPH scavenging illustrated the potential antioxidant activity of serum. Consequently, the more amount of extract added into the serum, the more antioxidation obtained, in which, the highest activity of antioxidants (62%) was recognized in sample
A3 containing 3% of extract
A, contrarily the lowest activity of anti-oxidant (48%) belongs to the sample
C1 containing 1% of extract
C. Moreover, the serum base also possessed a good antioxidant activity owing to the starting ingredients (
Figure 4).
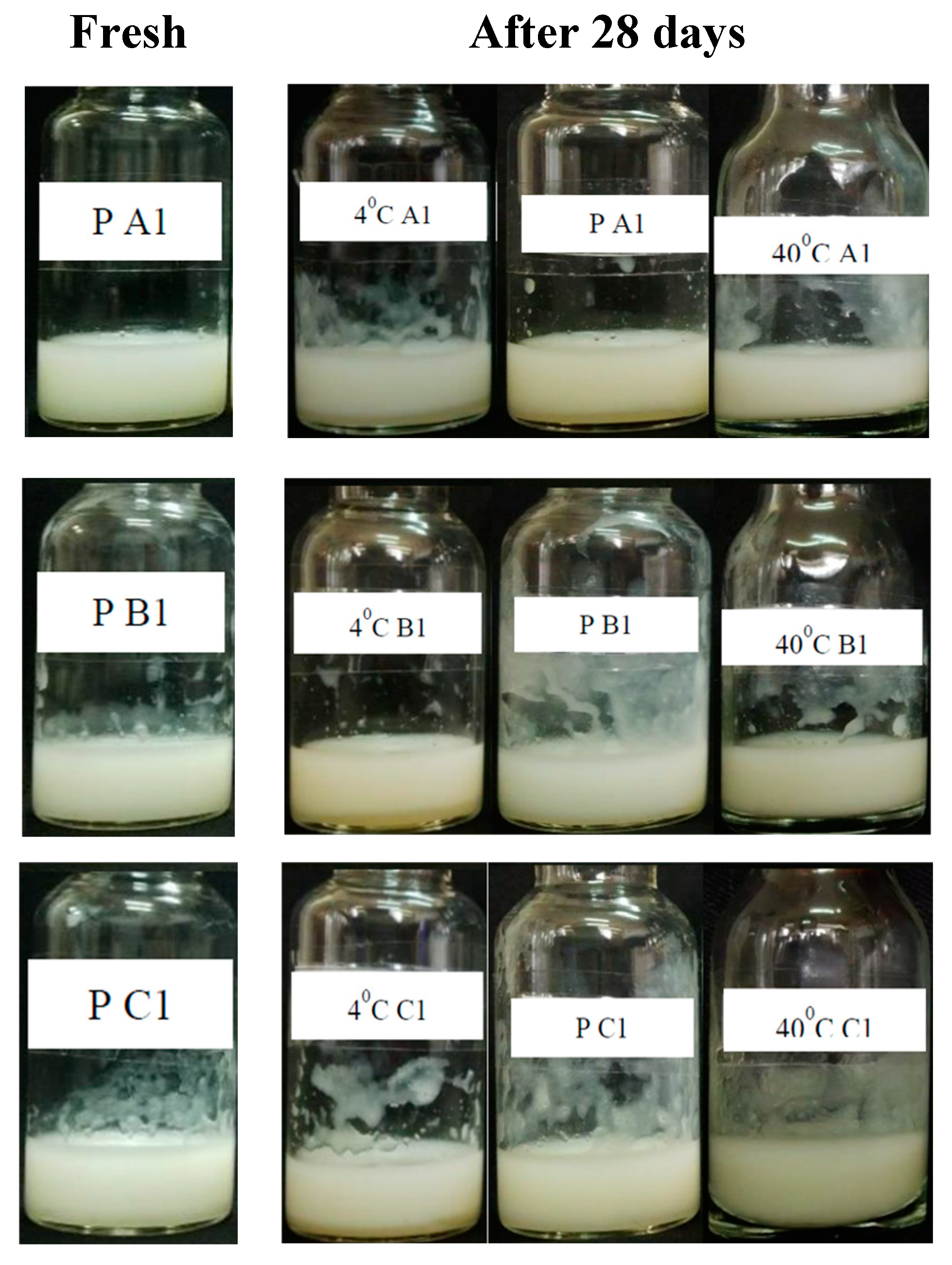
3.6. Stability of serum product
In this work, the extracts played the role of nutrient and bio-active ingredients in the serum. The amount of extract added to the product would affect the serum structure, therefore, the product stability had to be tested for each percentage of extract used. The serum samples were produced based on the formula, in which, only the amount of extract varied with a reasonable ratio, for instance, 1%, 2%, and 3% respectively (
Table 3). A finished serum must be a homogeneous appearance, pleasant odor, milky color, suitable pH, expected bio-activities, and stability under changing temperature conditions.
After the testing period, three serum samples
A1,
B1, and
C1 showed better stability at 4
oC, room temperature (P ~ 28-30
oC), and 40
oC during 0, 14, and 28 days than the others. Although the color of nine samples changed a little bit, the changes in the flavor and pH as well as the emulsion separation did not occur significantly in comparison with the fresh sample kept at room temperature (
Figure 5). This matter could be explained due to the balance between the amount of extract (1%) and the other ingredients in the serum formula. The higher amount of extract such as 2% or 3%, the more unstable serum after 14 days.
3.7. Serum microbial contamination
Serum samples were diluted and tested for microbial contamination on a suitable media. Based on the colonies’ characteristics, the total viable count for aerobic bacteria and molds in the serum samples were respectively determined as follows:
A1: 0 and 20;
B1: 80 and 10;
C1: 30 and 20 (
Table 5), not exceed 102-103 CFU/mL of the product, incompatible with the previous literature [
32]. Aerobic bacteria grown up in the
LB plate at a spreading concentration of 0.5 g/mL appeared with white, convex, glossy colonies in the range of size from 1 to 10 mm. On the
PGA plates, the colonies with a diameter ranging from 2 to 30 mm appeared with white silk and colored centers. At concentrations of 0.05 and 0.005 g/mL, no characteristic colonies were detected.
4. Discussion
All mixed extracts showed higher activity than the ME and their activity increased gradually in the sample following the increased amount of the BE in the composition because the antimicrobial activity of the ME component was recognized not to be strong enough as BE component. Consequently, the extracts were the potential to be utilized as an antibacterial ingredient in cosmetics in order to inhibit the contamination of microorganisms, as well as repress their activity on the skin.
In the next experiments, the antioxidant capability was checked by DPPH assay. The testing results of the DPPH assay indicated that the antioxidant activity of the ME was higher than that of the BE owing to a higher amount of cordycepic acid. Obviously, the content of extracts depended on the extraction procedure, for instance, the ME was extracted with absolute MeOH, a suitable solvent for cordycepic acid extraction but not for polysaccharides, therefore the BE, the remaining broth, had to contain more polysaccharides and less cordycepic acid than the ME. Consequently, the DPPH scavenging capacity of the ME performed more efficiently than that of the BE. In the mixed extracts, the antioxidant activity increased gradually from C to A, corresponding to the weight increase of ME.
After extracts were applied to cosmetic products, the anti-microorganism ability of serum samples was existed and quite compatible with the anti-microorganism ability of the investigated extracts. Consequently, the anti-microorganism activity of the serum base was enhanced via the addition of extract and depended on the amount of extract added. In addition, all serum samples exhibited higher antioxidant percentages than the serum base. They indicated that the supplemented extracts had promoted antioxidant activity quite well in the serum samples. As increasing the amount of extracts used for the serum, they could enhance the antioxidant activity, however, they also affected the balance of ingredients in the serum and reduced the stability of the product.
Additionally, one of the important items in the standard of cosmetic products is microbial contamination. The results concluded that the antibacterial properties of serum A1 were better than those of B1 and C1 owing to the lowest microbial contamination. Although the antibacterial activity of sample A was lower than those of sample B and sample C, sample A1 had the lowest microbial contamination, followed by sample C1 and then sample B1. The combination of microbial capacity between the extracts and the other ingredients in the formula of serum increased the microbial efficiency of the extracts to limit the microbial contamination of the product.
5. Conclusions
Comprehensive work on the application of the extract from Cordyceps militaris as a natural antioxidant and antimicrobial agent in the serum formula has been introduced. The extracts prepared from mycelium extract ME and broth extract BE in different weight ratios have been used for serum and tested the antioxidant and antimicrobial capacity. Interestingly, the suitable extract produced from the ratio of 2 ME with 1 BE has been selected and added to serum with the appropriate percentage (1%) of the serum sample. A finished serum sample was tested the antioxidant ability, antimicrobial capacity, and stability in order to satisfy commercial production and long-term use with high durability in varied temperature conditions, with antibacterial and antioxidant capabilities at an acceptable level.
Author Contributions
T. X. T. L. and B.-H. T. N conceived and designed the experiments; H.-V. V.-N. mainly performed the experiments and wrote the original draft preparation; G.T. D.-P. analyzed the data and wrote the original draft preparation; T. X. T. L. wrote and edited the paper.
Funding
Please add: This research received no external funding
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
Acknowledgments
We respectfully acknowledge Prof. Ngo Le-Van, M.Sc Minh-Thoa Thi Nguyen, M.Sc Minh-Quan Hoang-Trong (Research Center for Bioactive Natural Products, University of Science, Ho Chi Minh City) for chemicals and technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.; Liu, C.C.; Wang, Y.Y.; Xu, H.; Su, H.; Cheng, X. Antibacterial activities of the novel silver nanoparticles biosynthesized using Cordyceps militaris extract. Curr. Appl. Phys. 2016, 16, 969–973. [Google Scholar] [CrossRef]
- Lin, Q.; Long, L.; Wu, L.; Zhang, F.; Wu, S.; Zhang, W.; Sun, X. Evaluation of different agricultural wastes for the production of fruiting bodies and bioactive compounds by medicinal mushroom Cordyceps militaris. J. Sci. Food Agric. 2017, 97, 3476–3480. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Barros, L.; Calhelha, R.C.; Ciric, A.; van Griensven, L.J.L.D.; Sokovic, M.; Ferreira, I.C.F.R. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal, and antihuman tumor cell lines properties. Food Chem. Toxicol. 2013, 62, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Leung, P. H.; Zhang, Q.X.; Wu, J.Y. Mycelium cultivation, chemical composition and antitumour activity of a tolypocladium sp. fungus isolated from wild Cordyceps sinensis. J. Appl. Microbiol. 2006, 101, 275–283. [Google Scholar]
- Wang, L.; Wang, G.; Zhang, J.; Zhang, G.; Jia, L.; Liu, X.; Deng, P.; Fan, K. Extraction optimization and antioxidant activity of intracellular selenium polysaccharide by Cordyceps sinensis SU-02. Carbohydr. Polym. 2011, 86, 1745–1750. [Google Scholar] [CrossRef]
- Zhan, Y.; Dong, C.; Yao, Y. Antioxidant Activities of Aqueous Extract from Cultivated Fruit-bodies of Cordyceps militaris (L.) link in vitro. J. Integr. Plant Biol. 2006, 48, 1365–1370. [Google Scholar] [CrossRef]
- Hekimi, S.; Lapointe, J.; Wen, Y. Taking a ‘good’ look at free radicals in the aging process. Trends Cell Biol. 2011, 21, 569–576. [Google Scholar] [CrossRef]
- Viña, J.; Sastre, J.; Pallardó, F.; Borrás, C. Mitochondrial theory of aging: Importance to explain why females live longer than males. Antioxidants Redox Signal. 2003, 5, 549–556. [Google Scholar] [CrossRef]
- Harman, D. Free radical theory of aging: An update - Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef]
- Manczak, M.; Jung, Y.; Park, B.S.; Partovi, D.; Reddy, P.H. Time-course of mitochondrial gene expressions in mice brains: Implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J. Neurochem. 2005, 92, 494–504. [Google Scholar] [CrossRef]
- Li, X.T.; Li, H.C.; Bin Li, C.; Dou, D.Q.; Gao, M.B. Protective effects on mitochondria and anti-aging activity of polysaccharides from cultivated fruiting bodies of Cordyceps militaris. Am. J. Chin. Med. 2010, 38, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, Y.; Chen, Y.; Cao, Y. Partial structural characterization, as well as immunomodulatory and anti-aging activities of CP2-c2-s2 polysaccharide from: Cordyceps militaris. RSC Adv. 2016, 6, 104094–104103. [Google Scholar] [CrossRef]
- Lee, J.S.; Hong, E.K. Immunostimulating activity of the polysaccharides isolated from Cordyceps militaris. Int. Immunopharmacol. 2011, 11, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kwon, J.S.; Yun, J.S.; Park, J.W.; Shin, W.C.; Lee, S.Y.; Hong, E.K. Structural characterization of immunostimulating polysaccharide from cultured mycelia of Cordyceps militaris. Carbohydr. Polym. 2010, 80, 1011–1017. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, J.S.; Won, D.P.; Lee, K.E.; Shin, W.C.; Hong, E.K. Study on macrophage activation and structural characteristics of purified polysaccharide from the liquid culture broth of Cordyceps militaris. Carbohydr. Polym. 2010, 82, 982–988. [Google Scholar] [CrossRef]
- Li, S.P.; Zhang, G.H.; Zeng, Q.; Huang, Z.G.; Wang, Y.T.; Dong, T.T.X.; Tsim, K.W.K. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine. 2006, 13, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, Y.; Bian, Y.; Wong, J.H.; Ng, T. B.; Wang, H. Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozotocin-induced diabetic rats. Appl. Microbiol. Biotechnol. 2006, 72, 1152–1156. [Google Scholar] [CrossRef]
- Yu, R.; Song, L.; Zhao, Y.; Bin, W.; Wang, L.; Zhang, H.; Wu, Y.; Ye, W.; Yao, X. Isolation and biological properties of polysaccharide CPS-1 from cultured Cordyceps militaris. Fitoterapia. 2004, 75, 465–472. [Google Scholar] [CrossRef]
- Ng, T.B.; Wang, H.X. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharm. Pharmacol. 2010, 57, 1509–1519. [Google Scholar] [CrossRef]
- Patel, K.J.; Ingalhalli, R.S. Cordyceps militaris (L.: Fr.) Link – An important medicinal mushroom. J. Pharmacogn. Phytochem. 2013, 2, 315–319. [Google Scholar]
- Dong, C.; Yang, T.; Lian, T. A comparative study of the antimicrobial, antioxidant, and cytotoxic activities of methanol extracts from fruit bodies and fermented mycelia of caterpillar medicinal mushroom Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms. 2014, 16, 485–495. [Google Scholar] [CrossRef]
- Amaral, L.F.B.; Camilo, N.S.; Pereda, M.D.C.V.; Levy, C.E.; Moriel, P.; Mazzola, P.G. Evaluation of antimicrobial effectiveness of C-8 xylitol monoester as an alternative preservative for cosmetic products. Int. J. Cosmet. Sci. 2011, 33, 391–397. [Google Scholar] [CrossRef]
- Cunningham, K.G.; Hutchinson, S.A.; Manson, W.; Spring, F.S. Cordycepin, a metabolic product from cultures of Cordyceps. Militaris (Linn.) Link. Part I. Isolation and characterisation. J. Chem. Soc. 1951, 1951, 2299–2300. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Cordyceps - A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry. 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Urabe, E.; Honda, H.; Sakurai, A.; Sakakibara, M. Enhanced production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzyme Microb. Technol. 2007, 40, 1199–1205. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Park, S.J.; Lee, S.G.; Shin, S.C.; Choi, D.H. Cordycepin: Selective growth inhibitor derived from liquid culture of Cordyceps militaris against Clostridium spp. J. Agric. Food Chem. 2000, 48, 2744–2748. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia. 2010, 81, 961–968. [Google Scholar] [CrossRef]
- Park, B.T.; Na, K.H. , Jung, E C., Park, J.W., and Kim, H.H. Antifungal and anticancer activities of a protein from the mushroom Cordyceps militaris. Korean J. Physiol. Pharmacol. 2009, 13, 49–54. [CrossRef]
- Wong, J.H.; Ng, T.B; Wang, H.; Sze, S.C.W.; Zhang, K.Y.; Li, Q.; Lu, X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine. 2011, 18, 387–392. [Google Scholar] [CrossRef]
- Phi-Ly, T.T. Research on chemical composition and biological activity of essential oil from tangerine (Litsea cubeba (Lour.) Pers.). Dissertation of Master degree, Nong Lam University, Ho Chi Minh City, 2012. [Google Scholar]
- Devi, K.P.; Suganthy, N.; Kesika, P.; Pandian, S.K. Bioprotective properties of seaweeds: in vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC. Complement. Altern. Med. 2008, 8, 38. [Google Scholar] [CrossRef]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules. 2018, 23, 1571. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).