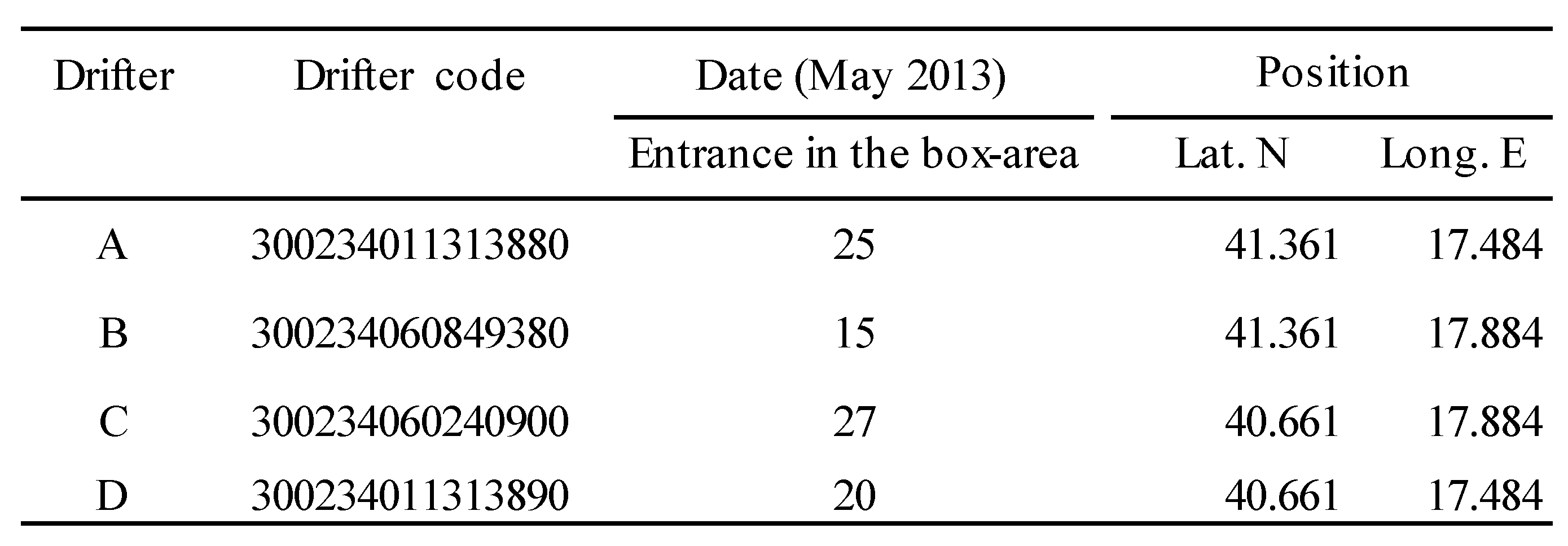1. Introduction
The existence of planktonic stages, generally larvae, in the life cycle of marine benthos species, has been considered as the main responsible of the observed geographic distribution and/or genetic inter-population connectivity, mainly for sessile neritic species [
1,
2]. The Supply Side Ecology [
3,
4] founded on larvae abundance and dispersal the interpretation of community dynamics and population connectivity along shorelines. However, only in few cases (the so called teleplanic larvae,
sensu [
5]) larvae justify high dispersal possibility for the species. More commonly, larvae have survival rates and persistence times in the planktonic stage (the Pelagic Larval Duration, PLD) which do not allow them to disperse on large spatial scales.
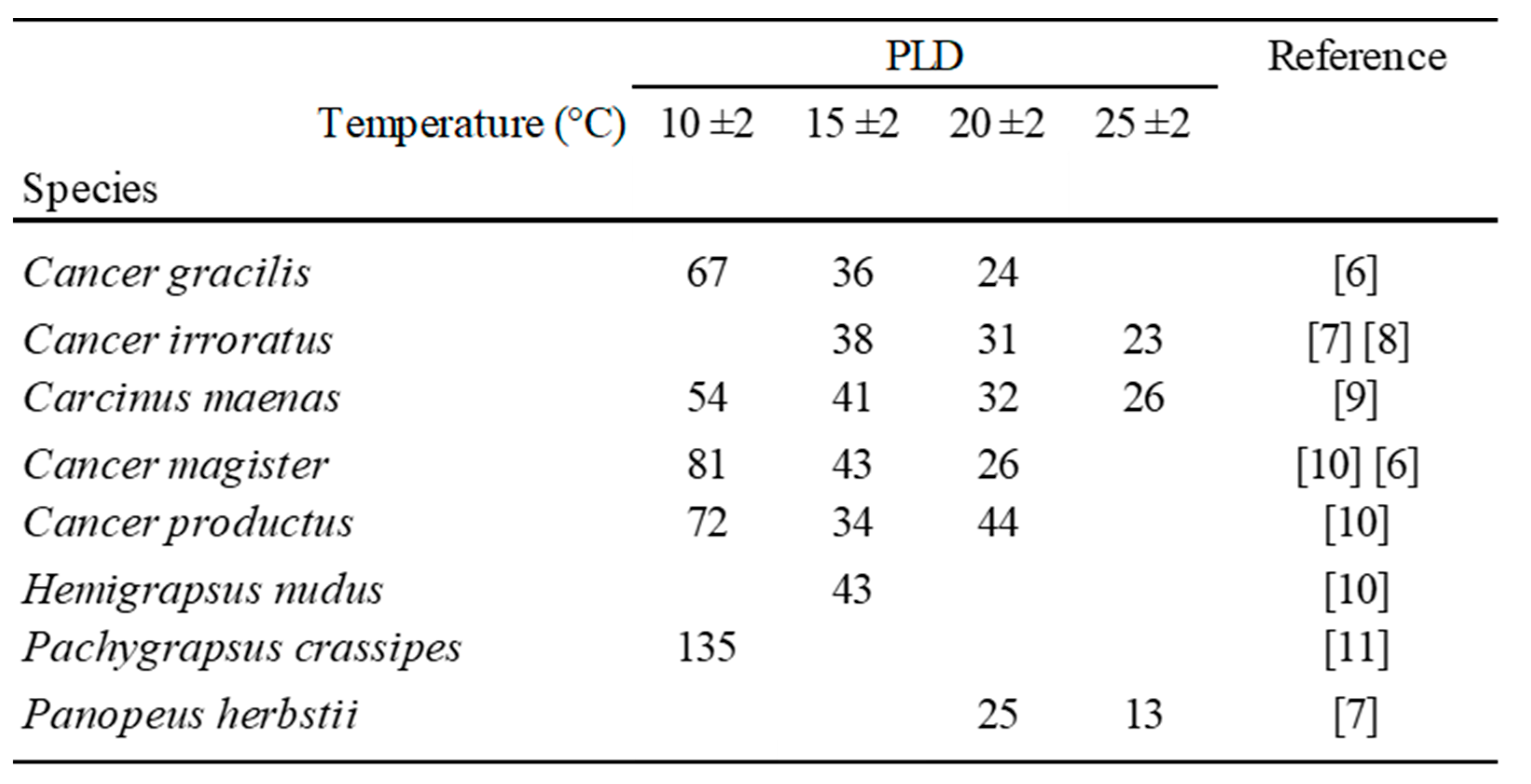
In laboratory conditions, the PLD of Brachyura larvae (zoea) has been observed to be inversely (if not linearly) correlated with water temperature (
Table 1).
The debate over degree to which marine larvae produced in a local population are likely to return to that population (self-recruitment, or retention), or migrate to another population (export), is open [
12]. Moreover, hydrodynamic models and genetic structure data indicate that the average scale of dispersal can vary widely even within a given species, at different locations in space and time [
12,
13,
14]. Dispersal prediction of larvae requires knowledge of the processes regulating larval dispersal and the spatial and temporal scales over which it occurs. Estimates of marine larval dispersal, which ranges from a few meters to hundreds of kilometers [
15,
16,
17,
18,
19], are well correlated with PLD for many organisms, including Decapoda, even though exceptions do exist [
20,
21,
22]. Furthermore, larval behavior significantly affects the dispersal: e.g., larvae occupying very near-bottom waters typically perform a short distance dispersal [
12].
Small basins could be interested by coast to coast exchanges of species propagules represented by planktonic larvae, more than large oceanic areas, also because generally corresponding to a more extended presence of the shelf and of its conditioning of water circulation [
23]. The case of the South Adriatic Sea could be interesting from this point of view because it is only 76 km wide in its narrower point (Otranto-Cape Linguetta), thus suggesting an enhancement of connectivity between benthic communities of the opposite sides. Bray et al. [
24] already predicted that larvae of coastal benthos in the Adriatic Sea are able to pass from the East to the West side of the basin following the surface currents, with Apulia (the South-West coast of the Basin) acting as a sink area.
Among Crustacea, Decapoda Brachyura represent a good candidate for studies on the dispersal capability of coastal benthos by means of larvae. These larvae (zoeae and megalopae) are reported as typical of the uppermost layer of the sea water (neuston) [
25], although performing daily vertical migrations, and this fact makes possible the prediction of their traveling routes inside surface currents. Dos Santos et al. [
26] well described a general tendency, among Decapoda larvae, to persist in the vicinity of their birth sites, with larvae of coastal species accumulating in coastal sites and those of the neritic species with a larger spatial distribution.
Inter-annual, cross-shore and alongshore differences on Decapoda larvae distribution have been established as closely affected by local hydrodynamic conditions of adult sites [
27] suggesting the existence of a strategy driven by the necessity to persist in the same area of adults, more than to disperse elsewhere by currents. Studies of Torres et al. [
28,
29] suggest that larvae of coastal/neritic species, living in shallow waters, perform daily vertical migrations (involving the neuston) smaller than those of mesopelagic and/or deep bottom species. From the standpoint of dispersion studies, the two-dimensional space represented by the sea surface is the migration field for those larvae which stay in the surface layer, at least for a part of the each day (generally nighttime). The frame is accomplished by the extension of the vertical migration behavior that typically this plankton show, with a pulsating presence, during the day, in different water layers which possibly move at different speeds and/or in different directions [
26].
The analysis of the neuston, and of pelagic Polychaeta of the whole water column, collected during the cruise has been already object of two papers [
30,
31]. Drifters tracks of the same cruise period are also available from Zambianchi et al. [
32]. The available data have been used as a term of discussion on the larvae distribution in south Adriatic Sea. The present study consists in the recording of a huge concentration of neritic Brachyura larvae in the field (a site in the south Adriatic Sea) and the possibility to investigate their distribution thank to a multidisciplinary monitoring of the marine environment. Here the attention has been focused on the swarm of Decapoda larvae which recorded in neuston of stations “Penna Grossa” (PGR) and S13 [
30]. This natural experiment is useful to clarify what role, if any, larvae play in the geographic distribution of species, and if they someway represent the best connection device between the populations inhabiting the opposite sides of a small marine basin.
2. Materials and Methods
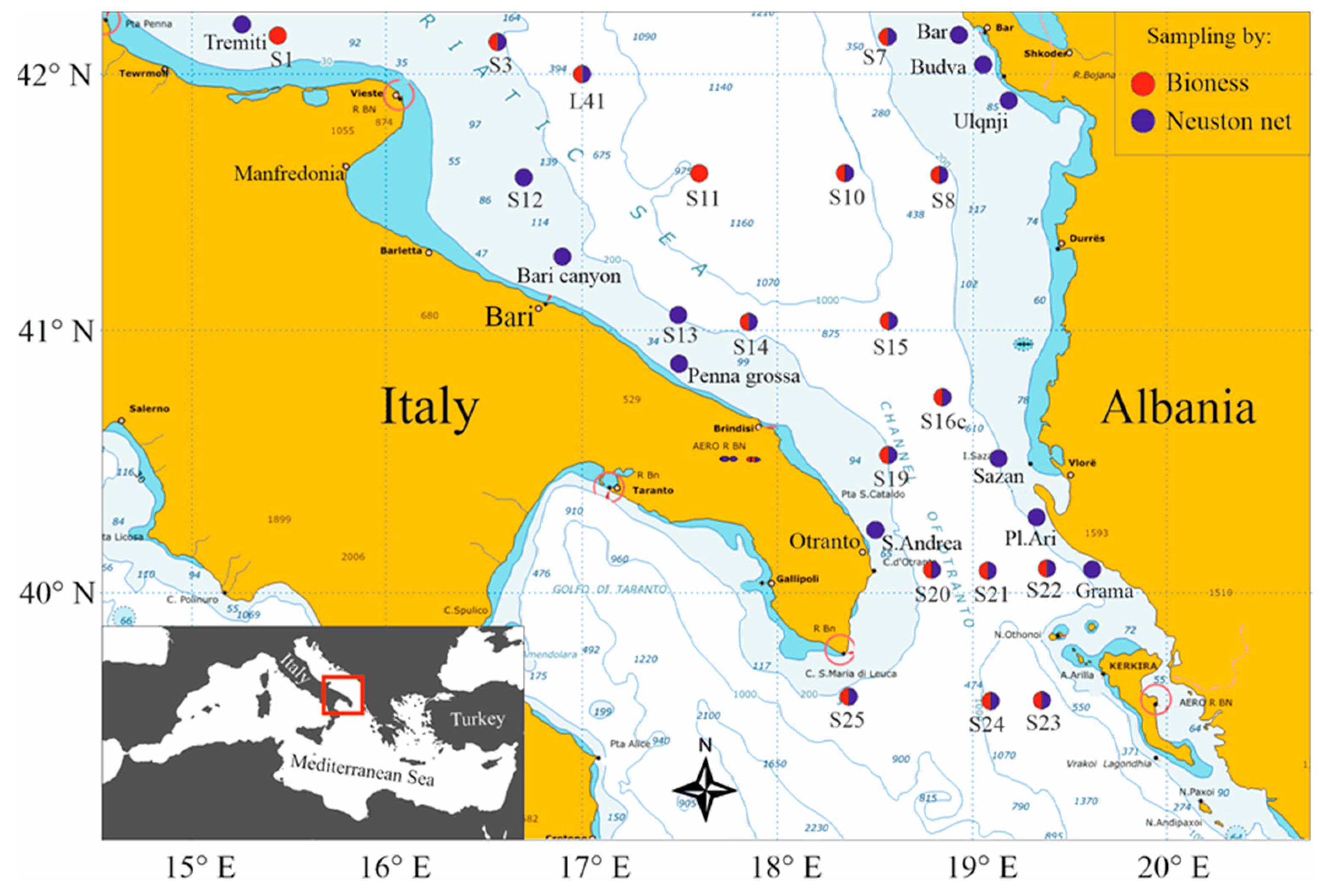
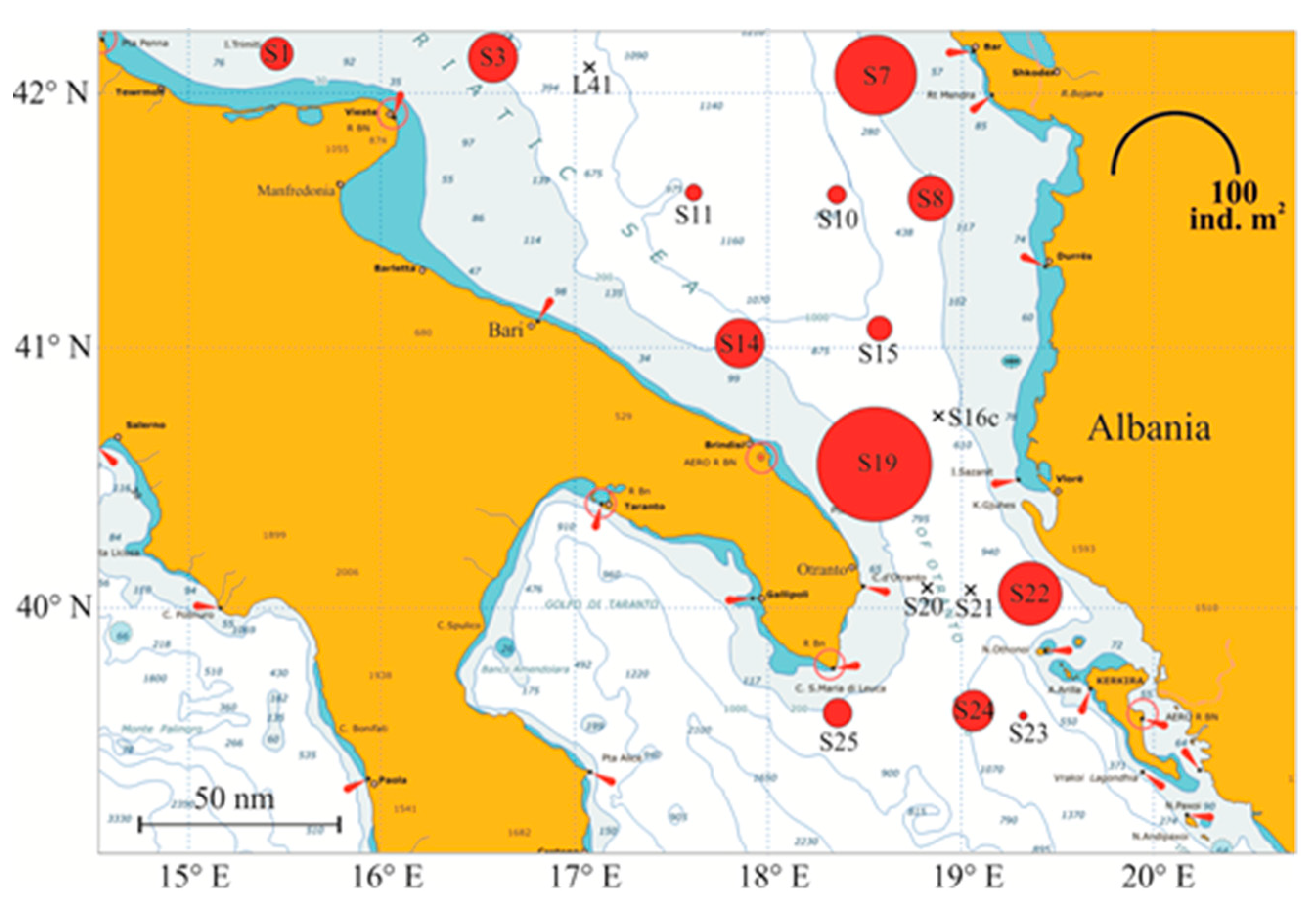
The study area was the southern Adriatic and northern Ionian Seas in the Eastern Mediterranean, between the Italian Apulian and Albanian-Greece coasts (
Figure 1). An oceanographic cruise was carried out aboard
R/V Urania from 8 to 21 May 2013 in the framework of the EU FP7 CoCoNET project [
33].
2.1. Abiotic Parameters
The water column values of Temperature, Salinity and dissolved Oxygen were obtained by a multiparametric probe incorporated into a carousel of Niskin bottles. Another multiparametric unit, (equipped with a Chlorophyll a fluorometer) was integrated to a BIONESS multinet sampler. Data collected were used to identify main water layers.
For the aim of the present study, due the mostly superficial presence of Brachyura larvae, only surface water characteristics (over the thermocline) were used to calculate pelagic life duration (PLD) of larvae.
2.2. Neuston Collection
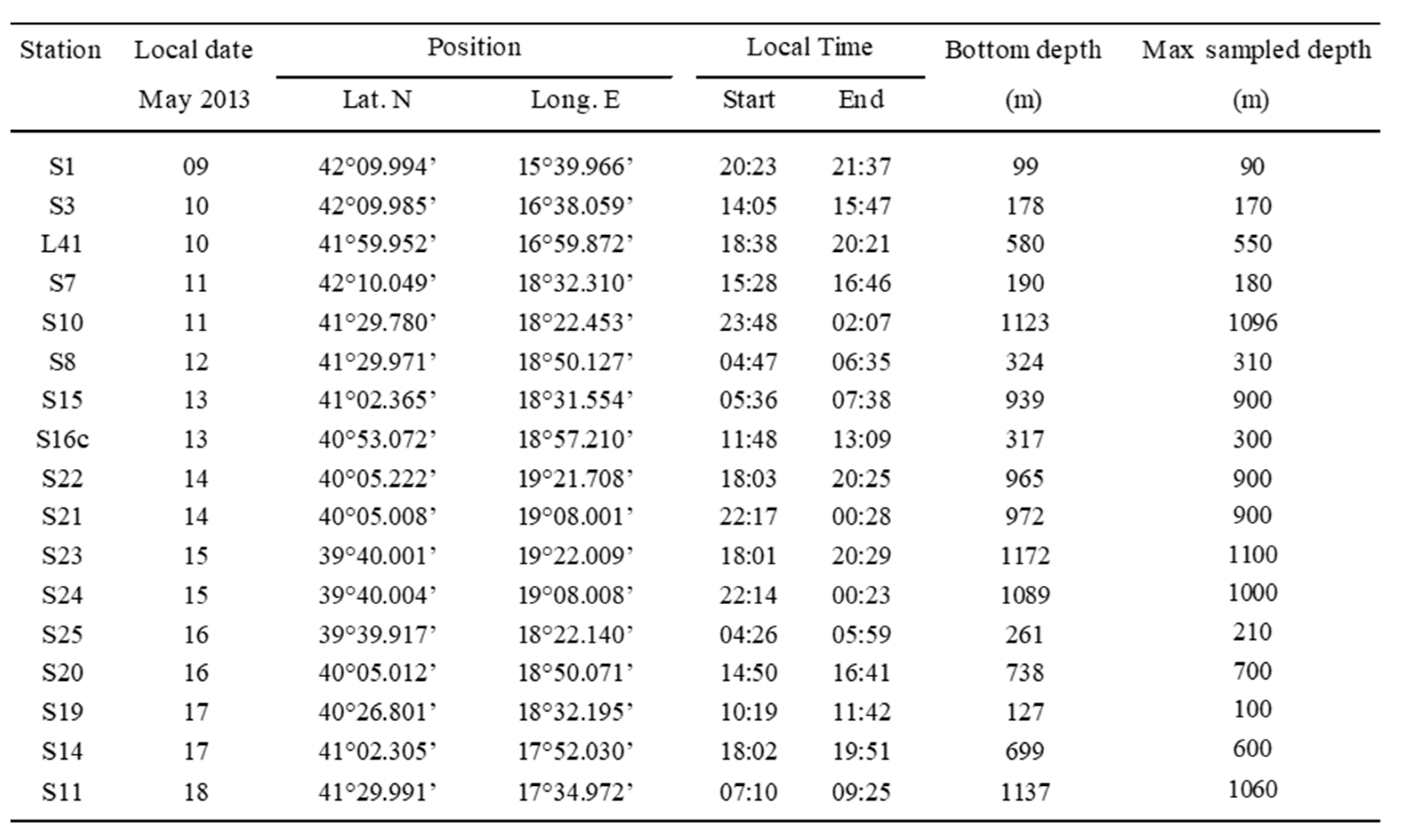
A neuston net (1 x 0.5 m rectangular mouth opening and a 200 µm mesh size), equipped with lateral buoys to float on the sea surface with the upper border at 5 cm above the sea level, was used to collect neuston in a total of 27 stations (
Figure 1). The net was towed at the speed of 1 knot. The presence of a flowmeter at the center of the mouth allowed us to measure the volume of filtered water at each sample collection. The filtered volume (m³) for each sample was calculated by approximately correcting the value by an average of 90% of the net mouth surface (on the basis of the non-complete submersion of the net mouth). The abundance of neuston categories were expressed as ind. m
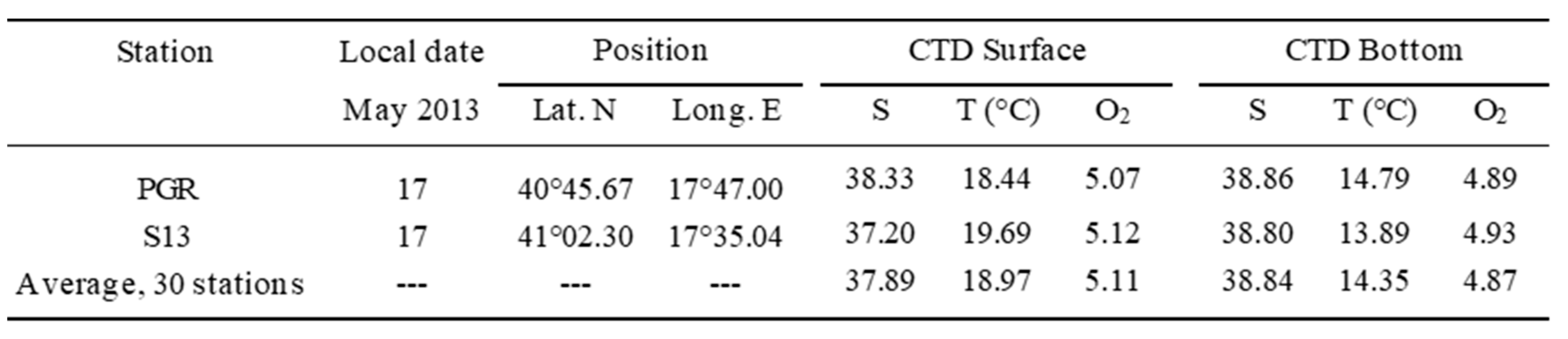
-3. At each sample collection, the neuston was immediately stored in 50 ml Falcon tubes with 95% ethanol (final concentration, 80-90%). At each station, abundance data represent the average of two neuston collections. At the stations PGR and S13 (
Table 2), Brachyura larvae (zoeae and megalopae) were found as dominant on the remaining zooplankton community [
30]). Such abundant populations were chosen for the study of Brachyura dispersion and, consequently, the larvae were identified at the species level.
2.3. BIONESS Sampling
To investigate the horizontal and vertical distribution of zooplankton, and decapod larvae particularly, 17 stations on the Apulian and Albanian shelves and offshore waters, including the Strait of Otranto, were sampled (
Figure 1). Samples were taken by the by the electronic multinet BIONESS (Bedford Institute of Oceanography Net and Environmental Sampling System [
34], a multiple-opening and closing-net sampler equipped with ten nets (200 µm) with a mouth area of 0.25 m
2. By a multi-parametric probe (SBE 911 plus, Seabird Electronics) and a fluorescence sensor (Seapoint Chlorophyll Fluorometer, Seapoint Sensors) mounted on its frame, data of depth (m), temperature (°C), salinity and Chl
a (μg/l) were processed with Ocean Data View (ODV) software to obtain real time vertical profiles. Flow velocity and filtration efficiency were monitored by internal and external flowmeters (GO2031H). The BIONESS was towed at a speed of 1.5-2 m s
-1 and slowly lowered along an oblique path, allowing very detailed resolution of the zooplankton vertical distribution. During each tow, a msximum of nine depth intervals was sampled. During the first downcast, the thermocline, pycnocline and halocline depths, and the depth and thickness of the Deep Chlorophyll Maximum (DCM) layer were analysed in order to decide upon the sampling layers. A total of 136 zooplankton samples were collected in several layers between the surface and few meters above the seabed, along a 0–1100 m water column (
Table 3). Five 20 m thick layers (0–20 m, 20–40 m, 40–60 m, 60–80 m, 80–100 m) in the first 100 m were sampled, followed by more wide up to the maximum reached depth
. Filtered water volume varied between 25 and 108 m
3, with volume increasing, generally, with depth. On board, all zooplankton samples were preserved in 4% buffered formaldehyde and seawater solution. Sampling details are shown in
Table 3. Sunrise and sunset times were 05.49 and 20:45 (GMT + 2:00).
2.4. Laboratory Analysis
In the laboratory, a qualitative–quantitative analyses of both neuston and mesozooplankton samples were performed. Sub-samples ranging from 1/10 to 1/25 were analyzed for species identification and specimens counts, depending on the total sample richness, while identification of rare species was carried out on the entire sample. For the neuston, all organisms of each taxon were counted and classified at higher taxonomic levels; the Brachyura larvae in stations PGR and S13, were identified at species-level. Larvae derived from BIONESS collections where roughly identified as Decapoda, containing not only Brachyura but, in less extend, also Anomura and shrimp larvae. BIONESS samples were not collected in PGR and S13 coastal stations (those interested by the Brachyura swarm), but their results have been used to have a general picture of Decapoda larvae abundance (ind.m-3) and distribution in the whole south Adriatic basin.
2.5. Statistical Analysis
To assess the vertical partitioning of the species by daytime and nighttime abundances, the Weighted Mean Depth (WMD) of decapod larvae was calculated according to the equation: WMD = Σ(ni × zi × di) / Σ(ni × zi), where ni is the number of ind.100 m
-3 in the i layer, di is the depth of a sample i (centre of the depth interval), and zi the thickness of the layer [
35,
36].
Based on the vertical profiles of temperature and salinity at each station, the thickness of the thermocline and halocline layers were visually estimated. Likewise, the depth at which the fluorescence reached the maximum was considered as the Deep Chlorophyll Maximum (DCM) depth. Then the correlations between these parameters and the abundance of decapod larvae in the 17 stations were tested using the Kendall rank-based test, which does not rely on any assumptions on the distributions of the dependent and independent variables.
2.6. Surface Drifters
Lagrangian, i.e. water-following, instruments are best suited to study transport and dispersion in the marine environment [
37,
38]. A total of 26 CODE drifters (for a description see Pisano et al., [
39], or Zambianchi et al. [
32]) have been employed to reconstruct the surface water circulation in the south Adriatic, during the period of the cruise. They were equipped with batteries and transmit a radio signal. Their 2-D motion has been tracked by satellite, for a maximum period of 6 months (corresponding to the maximum duration of the batteries). The present study only took into account the tracks of 4 drifters transiting in the same area of Brachyura larvae swarm (stations PGR and S13) at the same period of their localization, i.e. from 2 days before to 10 days after their collection (
Table 4). Based on the water temperature, a PLD of 40 days has been established for the larvae (zoeae, mostly of the 1
st instar) found in May 2013 at stations PGR and S13, (surface water temperature 18.44 and 19.69 °C; average of the whole basin, 18.97°C, and never more than 21.15 °C in the other stations).
CODE drifter motion represents the uppermost 1 m of the sea surface circulation; their GPS positions were accurate to a 10 m order of magnitude and were transmitted/recorded every 15 min. Raw data were edited to remove spikes and errors and are available at
http://www.coconet-fp7.eu. An area (rectangular box) has been individuated around the two selected stations (PGR and S13), considering them as the starting points for the larvae drift. The selected box-area had size of 0.7° Lat N x 0.4° Long E (
Table 4).
3. Results
3.1. BIONESS Environmental Profiles
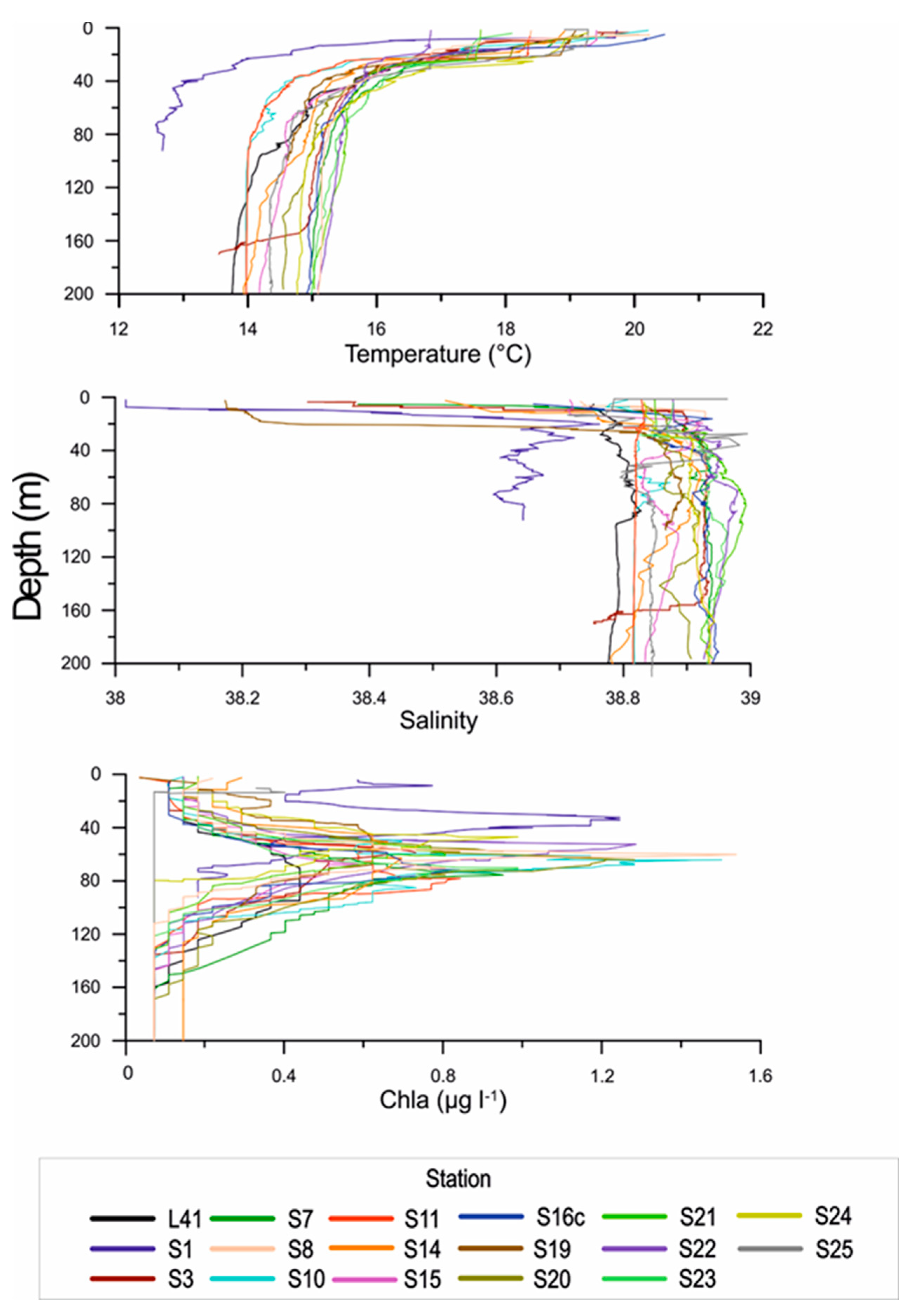
Considering the vertical BIONESS profiles of temperature, salinity and fluorescence, we can extrapolate some general considerations for both the entire study area and the different coastal and pelagic sampled sites. Temperature and salinity profiles provide an overall hydrographical picture of the all investigated area (
Figure 2), showing an evident stratified temperature and a marked thermocline between 20 and 40 m that separates the upper layer from the underlaying layers, with a difference of about 4-5° C between the upper (warmer) and the lower (colder) layer. Also a marked alocline was evident deeper (18-26 m) on the Italian side than on the Albanian side (5-15 m). Horizontal and vertical variability of thermohaline characteristics in the area evidenced some marked differences both among stations and along the sampled water column (
Figure 3).
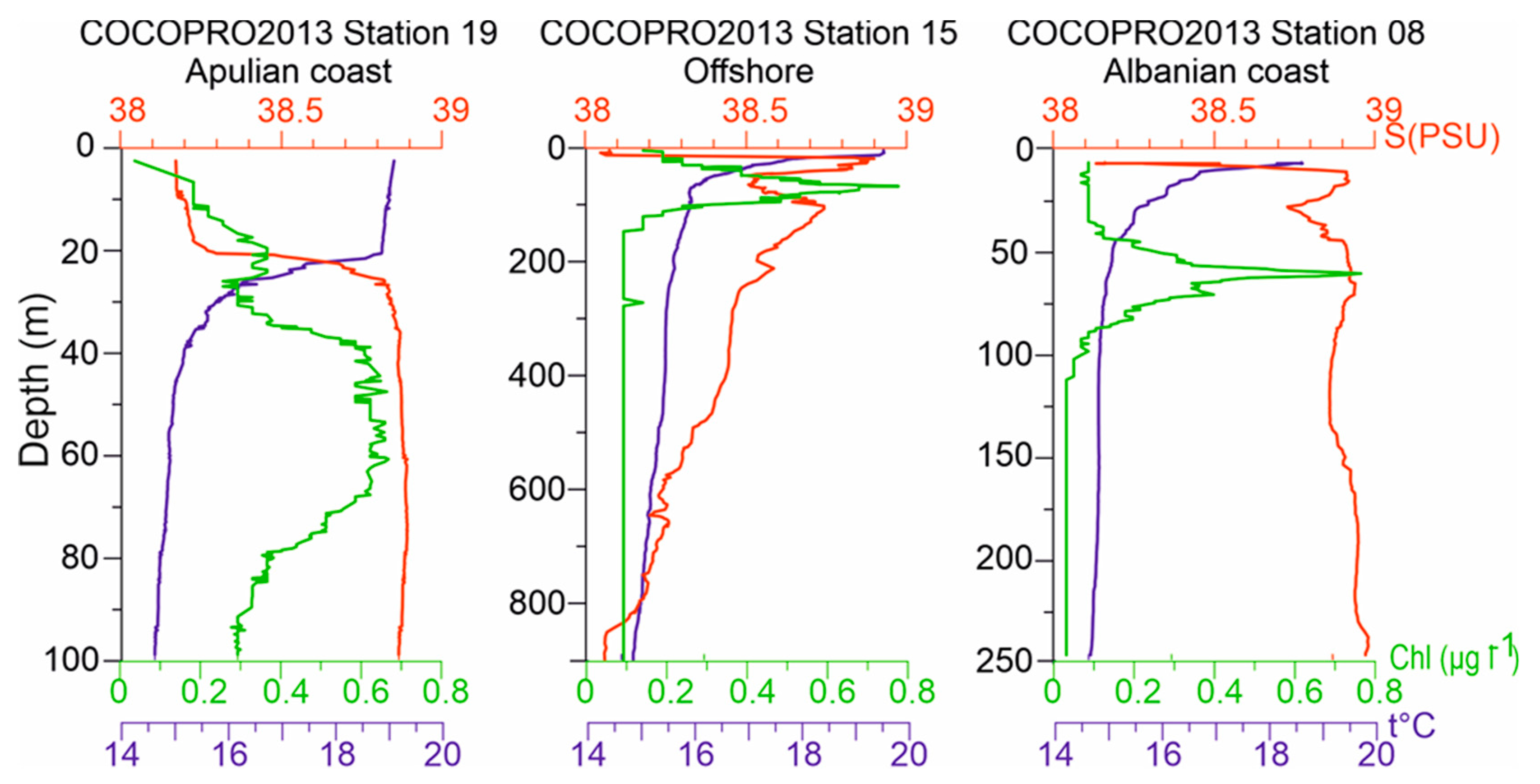
Fluorescence profiles showed maxima in the layer between 50 m and 80 m in depth for all the stations (
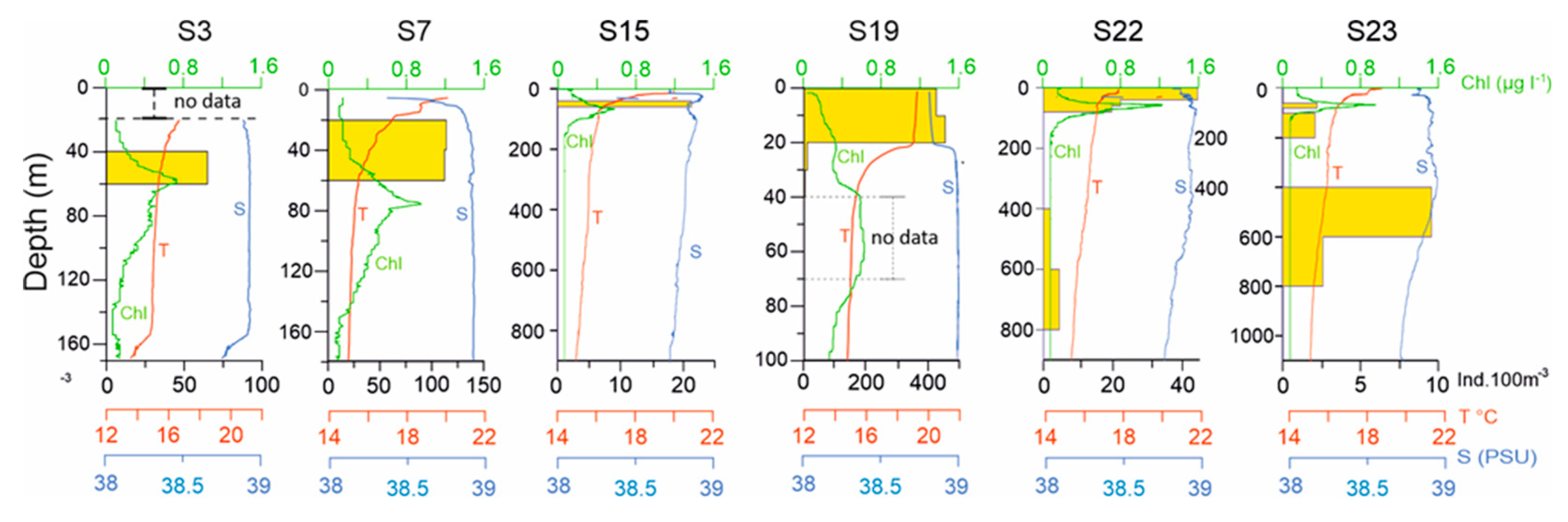
Figure 2), except for St. S1 that showed highest chlorophyll
a concentration- at about 35 m (1.17 mg m
−3). A different depth of the Deep Chlorophyll Maximum (DCM) among sites was detected.. Generally, in the areas close to the coast and in Otranto Channel this maximum was found at about 60 m, but with very different max chlorophyll
a values: 0.696 mg m
−3, 1.54 mg m
−3 and 1.12 mg m
−3, in the Apulian, Albanian side and Otranto Channel, respectively (
Figure 3).
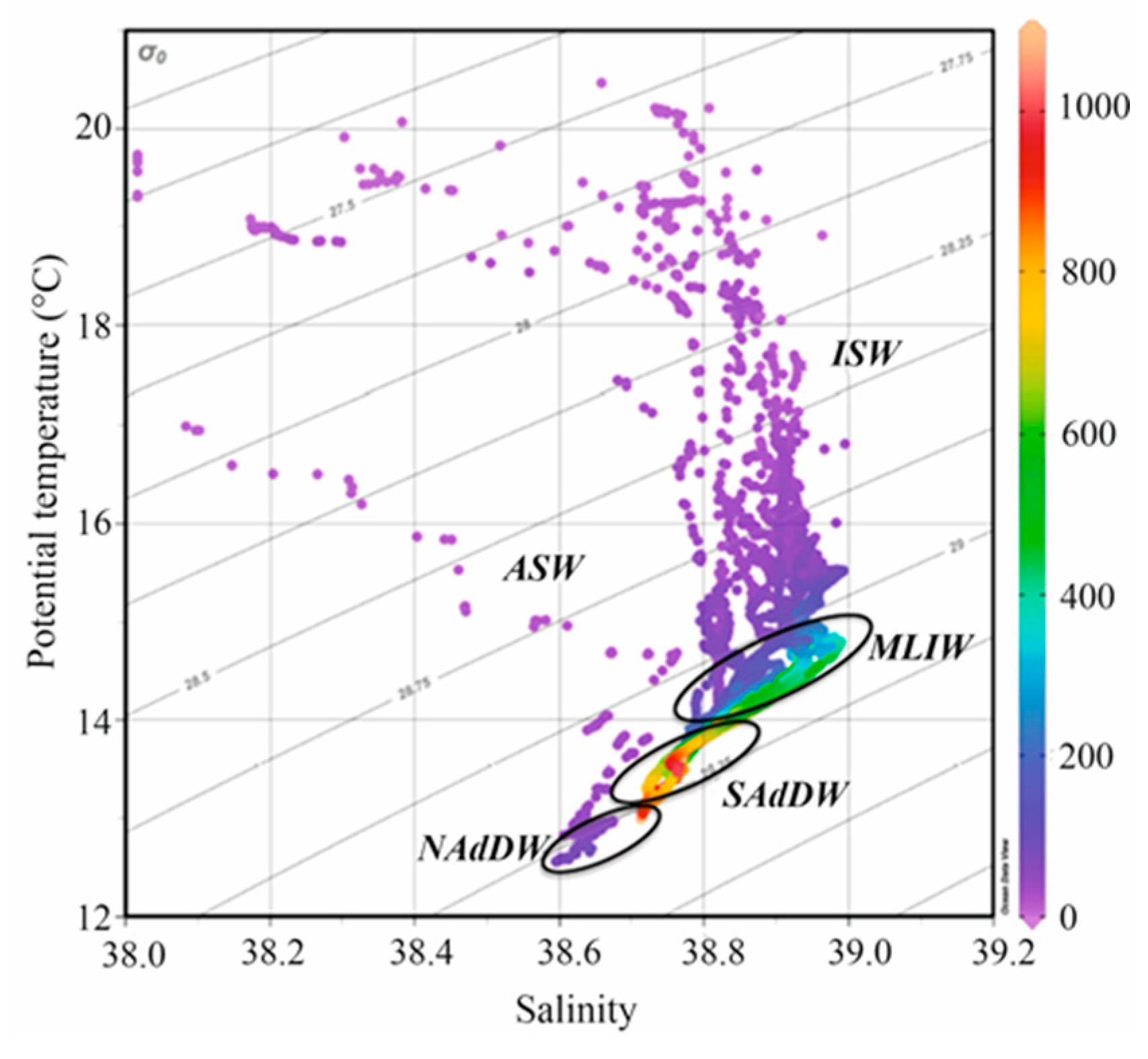
A different vertical structure of the water masses between Apulian, offshore and Albanian stations characterized the oceanographic conditions of the mid-May spring sampling period (
Figure 4). In particular, the upper layer up to 100–150 m is dominated by ISW except along the Italian coast, where ASW can be found. The intermediate layer hosts the MLIW and the deep layer is occupied by ADW originated in the Southern basin (SAdDW) or formed in the Northern basin (NAdDW).
3.2. BIONESS Zooplankton Distribution
Mean total zooplankton abundance was 442 ± SD 258 ind. m-3. Copepod was the most abundant taxon representing from the 72 to 91% of the total zooplankton, with a mean abundance of 401 ± 236 ind.m-3. Zooplankton abundance and biomass (Dry Mass) were higher on the Italian (408±811.7 ind. m-3, 9.7±15.5 mg m-3, respectively) than Albanian coasts (219±53.0 ind. m-3, 5.4±2.7 mg m-3). Spring holoplankton accounted for the main part of the zooplankton (85-98%). Abundance peaks of the most representative species occurred at the chlorophyll maximum depth between 20-40 m and 60-80 m depth.
3.3. Spatial and Vertical Distribution of Decapod Larvae
Meroplankton percentage increased along the Albanian coasts, mostly due to bivalve and polychaete larvae. Decapoda larvae represented less than 0.7% of the zooplankton community and about 7.2% of the meroplankton. Decapod larvae densities were higher along the coastal and continental shelf waters, rather than in offshore pelagic waters (Figure.5). In the integrated 0-100 m layer abundance values were highly variable, 0.44 to 88.89 ind. m-2 in the stations 23 and 19, respectively. In decreasing abundance order follow the stations S7 (45.16 ind.m2), S22 (26.11), S14 (16.89) and S3 (16.22). In four stations (L41, S16c, S20, S21) no Decapoda larvae were found.
Decapod larvae vertical distribution of is shown in
Figure 6. Among the nine stations with a depth greater than 700 m, only in two (S22 and S23) very few decapod larvae were found in the layers between 400 and 800 m depth. More than 95% of the larvae occupied the 100 m surface layer. Higher larvae concentrations occurred in the 0-20 m in station 19 along the Italian coast (about 436 ind.100 m
-3) just over the thermocline, and in the layer 20-60 m in the S7 on the Albanian side (113 ind.100 m
-3) below the thermocline and over the DCM. In the other stations the concentration of Decapoda larvae was lower than 70 ind.100 m
-3.
3.4. Diel Vertical Migration
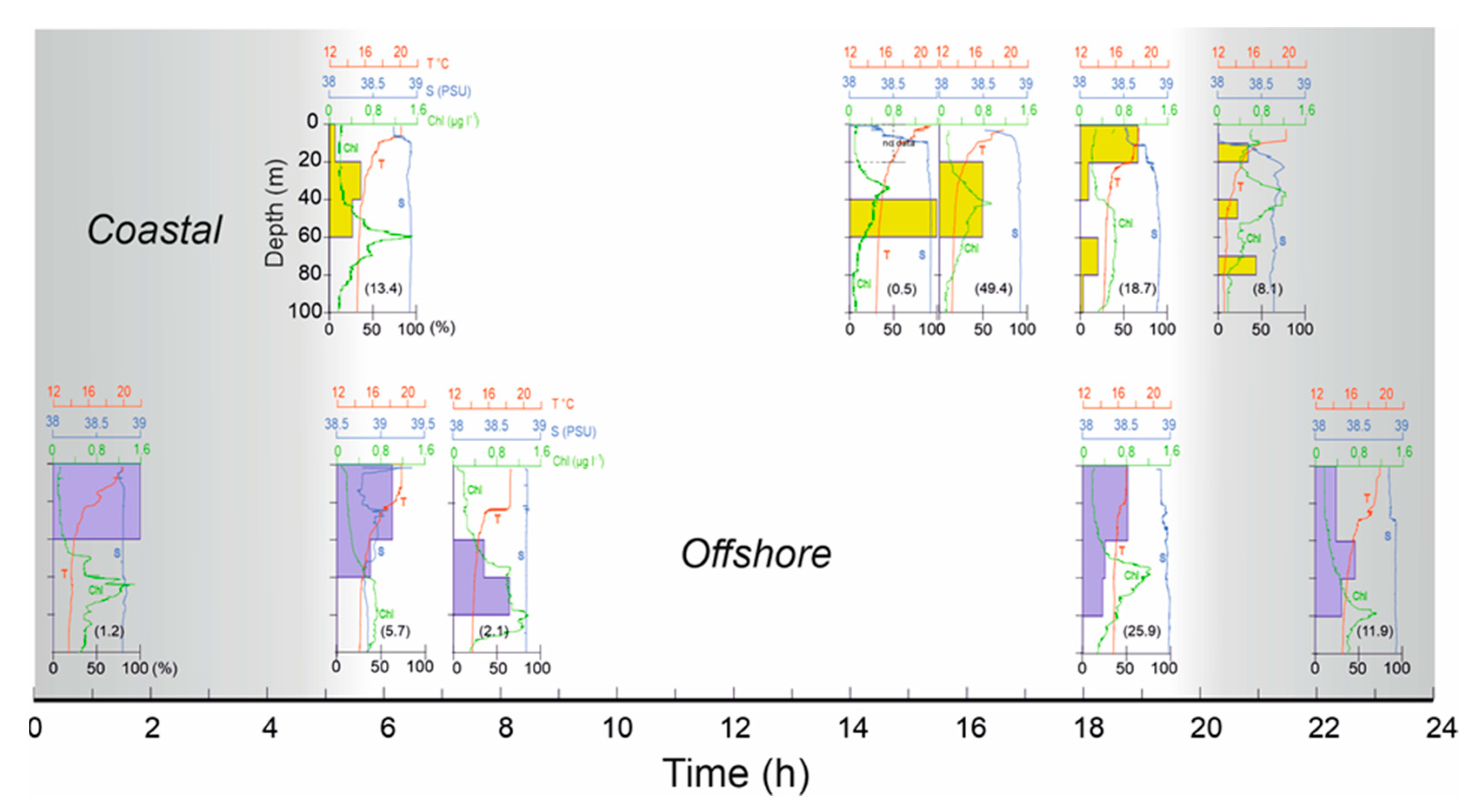
In order to examine temporal changes in the vertical distribution of decapod larvae abundance (%), in the absence of daily vertical catches in a fixed station, ten coastal and offshore BIONESS samples were chosen, and sorted according to the daily sampling time (
Figure 7). Decapod larvae showed clear diel vertical migration, that did not appear to be affected by the difference among inshore and shelf stations physical conditions.
Sunrise and sunset times were 05.49 and 20:45 (GMT + 2:00), respectively. In the morning, between 07:00 and 08:00 h, about one hour after sunrise, the whole community of decapod larvae is distributed between 40 and 80 m depth, with the greatest percentage between 60 and 80 m. At the beginning of the afternoon, between 14:00 and 16:00 h, the community remains compact between 40 and 60 m, even if a part is distributed up to 20 m. Between 18:00 and 21:00 h, in the period prior to night, their distribution is almost bimodal, with the highest percentage in the first 20 m (coastal) and up to 40 m (offshore) and low number of individuals up to at 80 m. Between 22:00 and 24:00, at the beginning of the nocturnal period, the community occupies almost the whole water column between surface and 80 m depth, with about 50% between 60 and 80 m. Between midnight and 02:00 h, their distribution entirely occupies the layer between the surface and 40 m. Before sunrise, between 05:00 and 06:00 a migration to the deeper layers is evident and ends after sunrise.
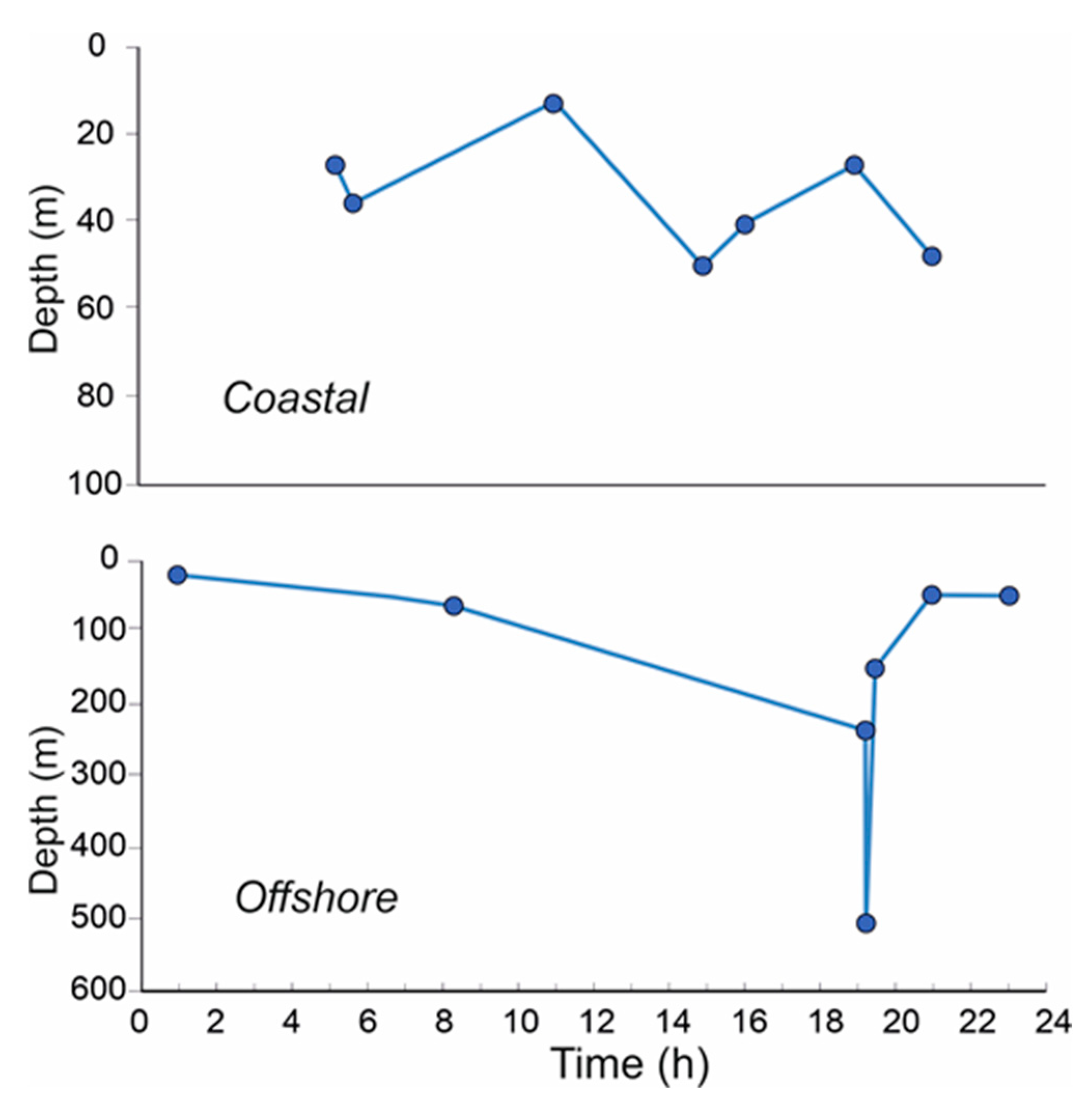
Figure 8 shows the WMD trend in coastal stations (bottom 100 m depth) and in offshore stations (bottom 600 m depth). It seems that the migratory behavior of decapod larvae in coastal stations is quite regular between 20 and 60 m depth and daily time independent. In offshore stations, on the other hand, migration is classically compatible with the day-night cycle, where a minimum WMD value is evident at about 20m at night which gradually becomes deeper up to late afternoon (about 19.00-20.00 h) when the migration towards the surface layers begins.
3.5. Vertical Distribution of Larvae in Relation to Environmental Variables
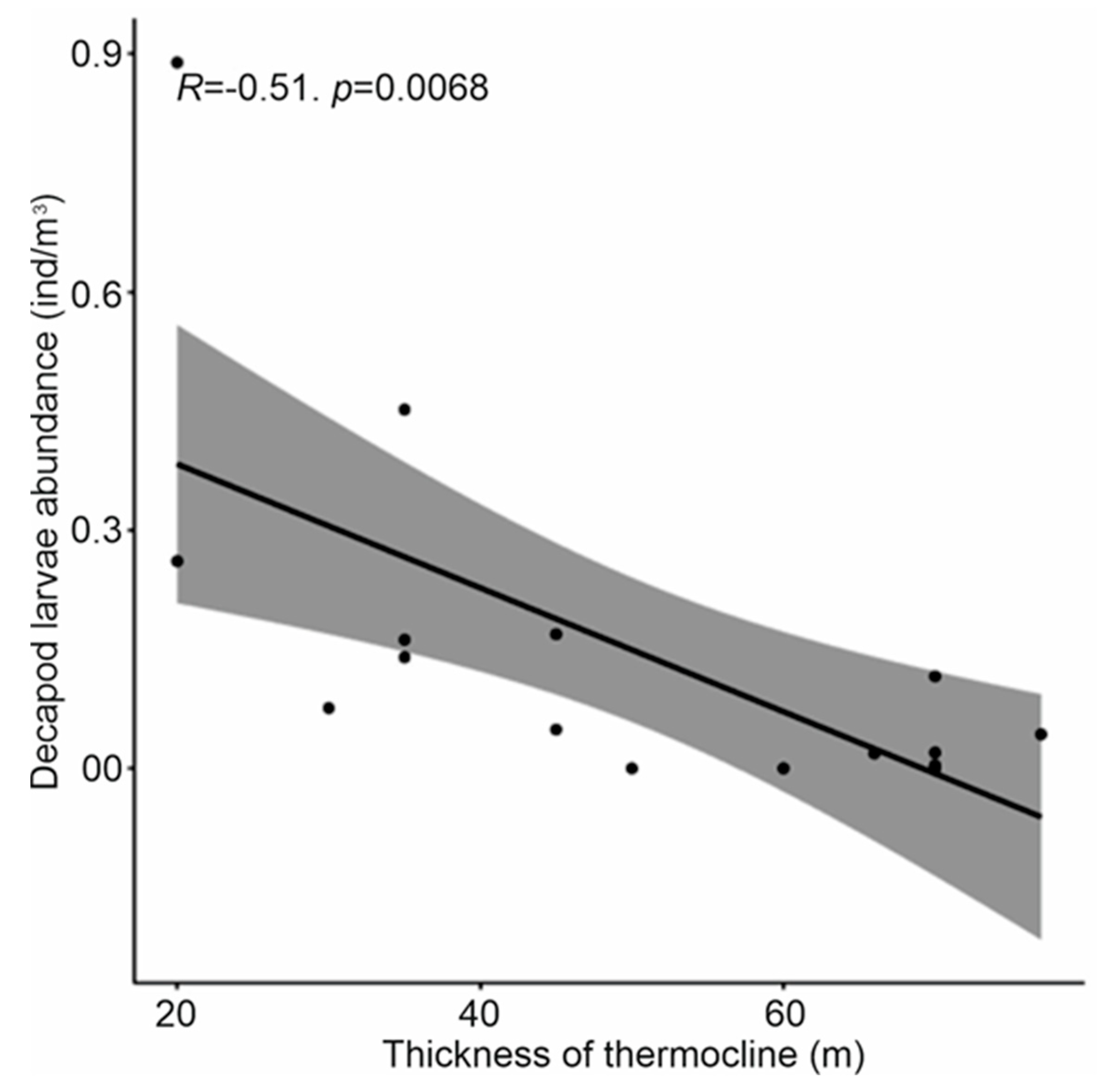
The abundance of decapod larvae appears significantly and inversely correlated to the thickness of the thermocline layer (Tau =-0.51, Kendall rank-based test, p<0.01), and is therefore lower in offshore waters than in coastal waters (
Figure 9). However, this abundance is not significantly correlated either with the depth of the chlorophyll maximum (DCM) or with the thickness of the halocline (p>0.05 Kendall rank based test).
3.6. Neuston Collection
Decapoda larvae have been collected in the neuston of many stations of the South Adriatic in the same cruise (see Liparoto et al. [
30]). Among Decapoda (
Table 5), Brachyura larvae (zoeae and megalopae) were considerably abundant in the neuston (about 0-50 cm below the sea surface) of isolated coastal stations. In May 2013, their abundance ranged between 0 and 3.75 ind.m
-3 in the whole neuston collection, but PGR and S13 (Italian side of the basin) showed concentrations of 93.02 and 231.00 ind. m
-3, respectively. In the same station 13 the highest number of Natantia larvae was recorded (593.97 ind. m
-3). In total the average mean of the four groups of decapods was 34.82 ind. m
-3±150.29. Average abundance values of Decapoda larvae obtained by 15 neuston stations, 23 mesozooplankton (WP2) stations and 17 multilayered (BIONESS) collections, gave results of 1.87 and 0.34 ind. m
-3 in neuston and water column, respectively.
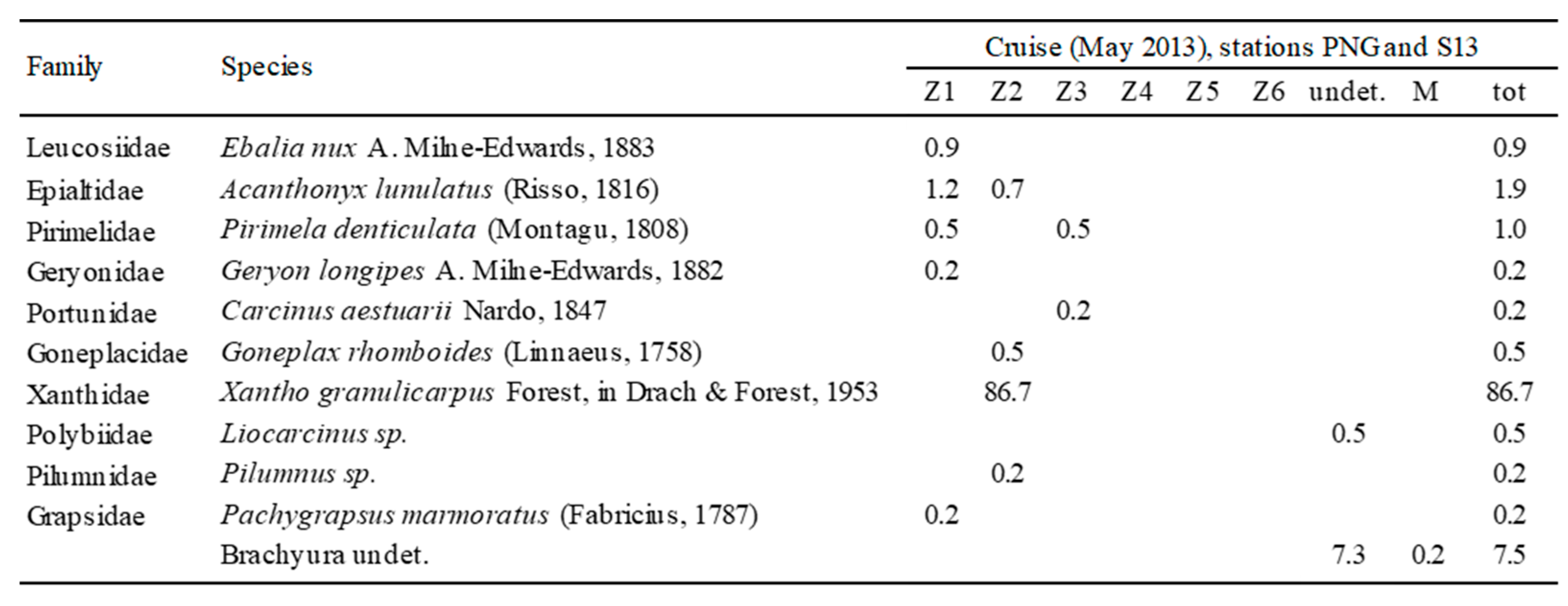
The detailed analysis of data from the two rich neuston stations (S13 and PNG) allowed the recognition of Brachyura (
Table 6) as the main components of the whole Decapoda assemblage. The identified larvae were mainly zoea-l instar belonging to a total of 11 species, largely (87%) represented by
Xantho granulicarpus.
3.7. Transport by Surface Currents
The basin- and sub basin-wide circulation described by CODE drifters deployed in the framework of the 2013 cruise has been discussed in two recent papers [
40,
41]. Lagrangian surface trajectories accurately depict an overall cyclonic circulation, dominated by a northwestward coastal current along the Balkan coast, commonly reported to as EAC (East Adriatic Current) and a southeastward one along the Italian coasts (WAC, or West Adriatic Current). West-East connections are guaranteed by three cyclonic recirculations localised in correspondence of the three morphologically distinct sub-basins of the Adriatic Sea (northern, central and southern).
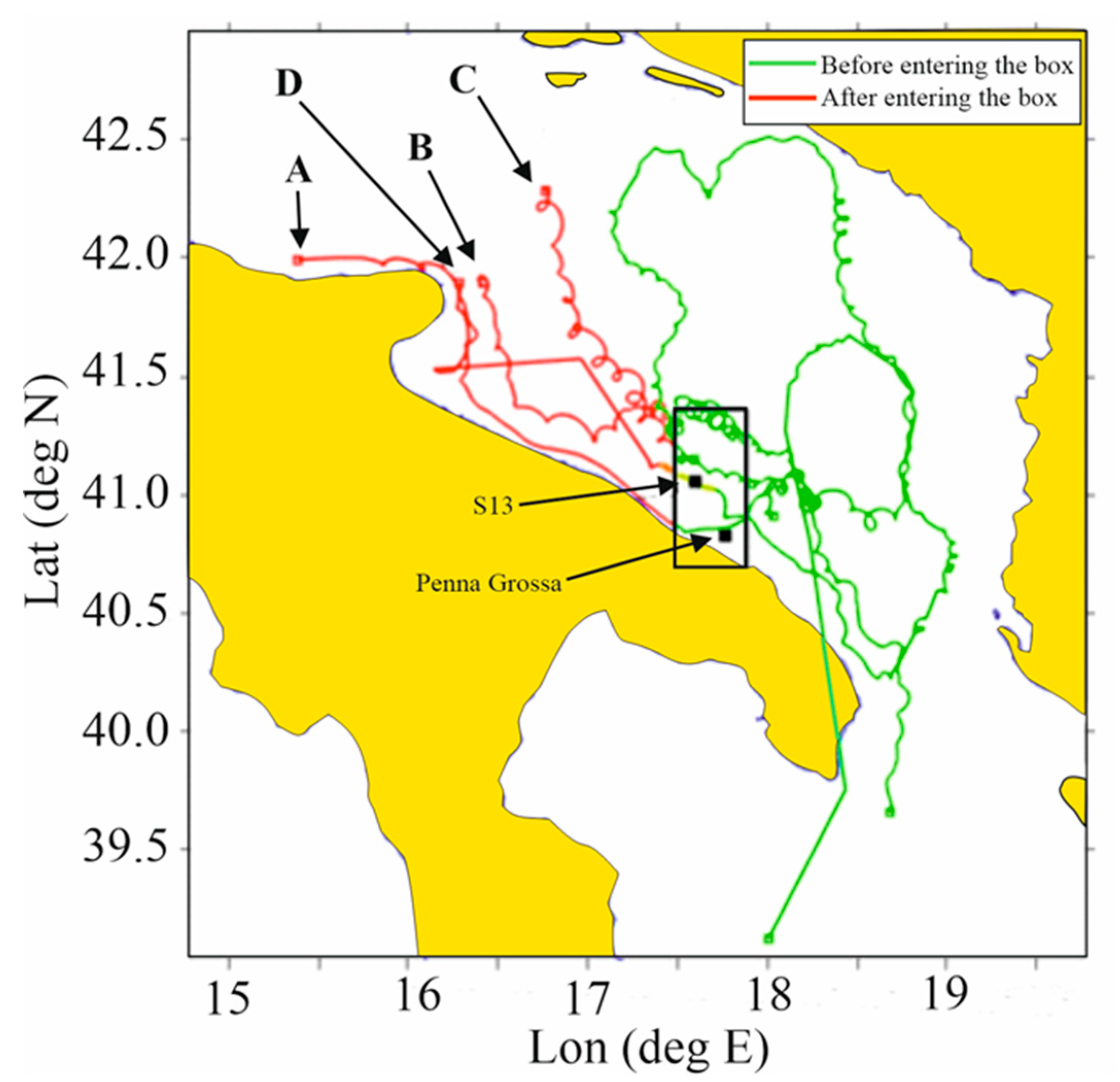
In consideration of the approximate PLDs evaluated as above described, we considered the backward and forward destiny of surface drifters passing by stations with high concentration of larvae, for a drifting time of 40 days (as often done in physical-biological studies [
42,
43]. Within our set, data suggest that the traveling zoeae are mostly (in 3 but 4 cases) directed South Eastwards along the Italian coast and had the possibility to reach the continental platform area on the opposite side of the basin in one out of four cases (
Figure 10).
4. Discussion
The vertical profiles of temperatures in May 2013 show a thermocline between 20 and 40 m depth and generally above the DCM. Larvae of Decapoda (collected with BIONESS in the whole basin) appeared strongly linked to such a layer, suggesting that they are involved in surface water circulation. Among Decapoda, larvae of Brachyura are reported as typical components of the neuston [
25], and well persisting in the first 50 m if deriving from coastal benthic species [
26]. Also in the present study, multilayer samplings carried out over the whole studied area, suggested that Decapoda larvae preferred the uppermost water layers (0-40 m). The aggregation of such a rich plankton component at the sea surface justifies a prediction of their horizontal distribution with time, based on surface drifter movements. The availability of a set of surface drifters deployed during the same cruise when zooplankton and neuston have been collected, allowed us to assess the destiny of surface water masses and indirectly the destiny of their content in terms of larval populations. In fact, although possible exceptions exist, water masses above the thermocline can be considered as homogenous from the oceano-dynamic point of view. Our assessment has been tuned on a definition of the PLD derived from the literature (even though for different species). The maximum PLD relative to the larval stage/age and to the sea surface temperature of May 2013 resulted to be 45 days (mainly zoeae). Surface drifters transiting through the area containing the sampling stations were followed backward and forward in time for a total of 40 days, in order to reconstruct a possible larvae dispersal path. Even though PLD is not known for the species found in abundance during the considered period (
Xantho granulicarpus), literature data suggest as possible a cross of the South Adriatic Sea from west to east. The species mainly represented in the samples of the present study is common along the Mediterranean coastline and inter-population genetic connections among crab species is documented It is known that the littoral crab
Pachygrapsus marmoratus is genetically uniform in the whole Mediterranean Sea [
44] even if compared populations are separated by thousands of kilometers. Schiavina et al. [
45]), however, established that coastal crabs of different species are genetically related and grouped in three areas of the Adriatic Sea: North, Central, and South, independently from their collocation on the Italian or Balkan coastline. This mirrors the circulation of the Adriatic, which can be summarized in an overall cyclonic circuit, further subdivided, both morphologically and dynamically, into three sub-basins and three corresponding cyclonic re-circulations. In terms of transport of passive particles, an asymmetry has been observed in the zonal exchange, with a preferential East to West surface connection with respect to the opposite, West to East one [
40]. This is witnessed by the successive results by Bray et al. [
24] who assessed a preferential transfer of larvae from the eastern to the western coast, with the southwestern coast (i.e. the Apulian one) functioning mainly as a sink area. Fraser et al. [
46] demonstrated that for many coastal taxa transoceanic transport and landfall occur thanks to passive rafting of adults on buoyant objects, more than larvae drift. On this basis, Treml et al. [
47] predicted that for 95% of coral reef species, the larval settlement occurs within 155 Km of source population and/or within 13 days.
To obtain an indication of the vertical distribution of Brachyura larvae the multilayer samplings of the BIONESS were used as a reference, although they did not interest the stations rich in Brachyura larvae. Larvae of BIONESS samples in general appeared as scantly concentrated confirming the exceptionality of the result coming from PGR and S13 stations. From the analysis of the whole sample set collected it is evident that Decapoda larvae swarm interested heavily only and just stations PGR and S13
. The present study show that Brachyura larvae generated at level of the stations PGR and S13 mostly disperse alongshore in South-East direction. The West-East coast connection for neritic crab species based on larvae dispersal is possible, but weak, because based on only ¼ of the individuated dispersal paths, and because the survival rate of Brachyura larvae after 40 days should be very low. It is possible that Brachyura use other solutions than planktonic larvae to disperse in large geographic areas. Discontinuous geographic presence of corals in isolated Pacific atolls has not been justified with larvae dispersal, but with rafting of the benthic phase on buoyant pumice [
48]. The geographic distribution of Hydrozoa in the Mediterranean Sea does not correspond to the existence and duration of the planktonic stage (the medusa) in the life cycle of each species [
49]. The role of no planktonic stages in the geographic distribution of neritic benthic organisms, and in the connectivity of distant populations, has been investigated in further depth taking into consideration viable fragments (the so called asexually produced propagules). These are sometimes more abundant than larval stages in coastal plankton [
50,
51,
52]. Additionally, also resting stages might allow species to perform long travels and/or to be relatively insensitive to ecological barriers [
53]. Whatever the nature of the propagules, their dispersal mechanisms represent an open question, the main problem being a quantitative assessment of the phenomenon. Such alternative dispersal strategies justify species distribution and genetic flow between populations, more than that attributable to larvae.
5. Conclusion
The present study proposes a general framework for Brachyura larvae circulation (
Xantho granulicarpus, in detail). The PLD obtained from literature data, based on larval age and on water temperature, and the study of drifter motion in the southern Adriatic suggested that zonal coast to coast crossing from Italian to Balkan side by larvae is possible in the studied period and at the investigated latitude, but it appears as not sufficiently reliable to ensure inter-population connectivity. Particularly, most drifters (here considered as proxies for larvae in the surface layer) moved mostly along shore (southeastwards) and crossed the basin in only one case among 4, in agreement with Carlson et al.’s experimental and numerical findings [
40]. Finally, the high mortality which affects crab larvae, further should reduce the drifting survivors down to a negligible number. All these considerations suggest that the recorded huge swarm of
Xantho granulicarpus larvae on the Apulian side of the southern Adriatic, is probably not enough to justify a genetic connectivity of the two opposite populations, and to push for alternative solutions for maintaining such a connectivity.
The genetic connection of Adriatic benthic populations has been ascertained (see the case of Fratini et al. [
44]). This notwithstanding, the limited possibility of
X. granulicarpus larvae to cross the south Adriatic during their PLD reduces their importance in the framework of genetic connection of opposite side populations, and confines them mostly to the renewal of very close (adjacent) populations. Weersing and Toonen [
54] already found that in the marine environment average PLD is poorly correlated with connectivity calculated on the genetic structure of populations. This was confirmed, among others, by a successive study by Treml et al. [
47] where the different role of PLD in local- and broad-scale connectivity is discussed and suggested that 95% of the connectivity based on larvae occurs within the first 13 days and 155 km from the source population.
The present study, conducted directly in the field, adds information to many others, with newly considered species, areas, and/or seasons. Connectivity of Brachyura populations results not reliable if exclusively linked to planktonic larvae, and other distributional strategies are probably available for every species.
Author Contributions
Conceptualization, A.G., G.B. and L.G.; Laboratory analysis, R.M., A.G., S.V. and F.V.; Statistical analysis, A.B. and V.B.; Oceanography, P.C. and E.Z.; Writing—original draft preparation, A.G., R.M., G.B., L.G. and E.Z.; Writing—review and editing, all authors.; All authors have read and agreed to the published version of the manuscript.
Funding
No funds are currently available for this research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available by Genuario Belmonte and Roberta Minutoli for Neuston and BIONESS, respectively.
Acknowledgments
The research has been carried out in the frame of the FP7 Ocean 11 Program project “towards COast to COast NETworks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential” (COCONET, responsible Ferdinando Boero). A special thank is addressed to the crew of RV Urania, to the colleagues who shared with us the cruise experience, and particularly to Giuseppe Arena (UNIME) for the BIONESS management during the oceanographic campaign, Stefano Aliani and Mireno Borghini (CNR La Spezia, Italy) who organized and assumed the responsibility of the cruise. Special thanks are due to Prof. Daniela Pessani and Dr. Giorgia Di Muzio of the University of Turin for the diagnosis of the decapod Brachyura larvae.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scheltema, R.S. Passive dispersal of planktonic larvae and the biogeography of tropical sublittoral invertebrate species. In Marine Eutrophication and Population Dynamics; Colombo, G., Ferrari, I., Ceccherelli, V.U., Rossi, R., Eds.; Olsen & Olsen: Fredensborg, Denmark, 1992; pp. 195–202. [Google Scholar]
- Giangrande, A.; Geraci, S.; Belmonte, G. Life-cycle and life-history diversity in marine invertebrates and the implications in community dynamics. Oceanography and Marine Biology, Annual Review 1994, 32, 305–333. [Google Scholar]
- Gaines, S.; Roughgarden, J. Larval settlement rate: a leading determinant of structure in an ecological community of the marine intertidal zone. Proceedings of the National Academy of Sciences 1985, 83, 3707–3711. [Google Scholar] [CrossRef] [PubMed]
- Lewin, R. Supply-side ecology. Science 1986, 234, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, R.S. On dispersal and planktonic larvae of benthic invertebrates: an eclectic overview and summary of problems. Bulletin of Marine Science 1986, 39, 290–322. [Google Scholar]
- Sulkin, S.D.; Mckeen, G.L. Laboratory study of survival and duration of individual zoeal stages as a function of temperature in the brachyuran crab. Cancer magister Marine Biology 1989, 103, 31–37. [Google Scholar] [CrossRef]
- Sastry, A. Culture of brachyuran crab larvae using a re-circulating sea-water system in the laboratory. Helgolander wiss. Meeresunters 1970, 20, 406–416. [Google Scholar] [CrossRef]
- Bliss, D.E.; Vernberg, F.; Vernberg, W. Biology of the Crustacea. 7: Behaviour and Ecology; Academic Press: London, UK, 1983. [Google Scholar]
- Nagaraj, M. Combined effects of temperature and salinity on the zoeal development of then green crab, Carcinus maenas (Linnaeus, 1758) (Decapoda: Portunidae). Scientia Marina 1993, 57, 1–8. [Google Scholar]
- Lough, R.G. Dynamics of crab larvae (Anomura, Brachyura) off the central Oregon coast, 1969-1971. Thesis of the Oregon State University 1975. [Google Scholar]
- Miller, S.H. Larval behavior and natural trace element signatures as indicators of crustacean population connectivity. Thesis of the University of California 2011. [Google Scholar]
- Kinlan, B.P.; Gaines, S.D.; Lester, S.E. Propagule dispersal and the scales of marine community process. Diversity and Distributions 2005, 11, 139–148. [Google Scholar] [CrossRef]
- Cowen, R.K.; Paris, C.B.; Olson, D.B.; Fortuna, J.L. The role of long distance dispersal versus local retention in replenishing marine populations. Gulf and Caribbean Research 2003, 14, 129–137. [Google Scholar] [CrossRef]
- Sotka, E.E.; Wares, J.P.; Barth, J.A.; Grosberg, R.K.; Palumbi, RS.R. Strong genetic clines and geographical variation in gene flow in the rocky intertidal barnacle Balanus glandula. Molecular Ecology 2004, 13, 2143–2156. [Google Scholar]
- Scheltema, R.S. Larval dispersal as a means of genetic exchange between geographically separated populations of shallow-water benthic marine gastropods. Biological Bulletin 1971, 140, 284–322. [Google Scholar] [CrossRef]
- Roughgarden, J.; Gaines, S.; Possingham, H.P. Recruitment dynamics in complex life cycles. Science 1988, 241, 1460–1466. [Google Scholar] [CrossRef]
- Botsford, L.W.; Moloney, C.L.; Hastings, A.; Largier, J.L.; Powell, T.M.; Higgins, K.; Quinn, J.F. The influence of spatially and temporally varying oceanographic conditions on meroplanktonic metapopulations. Deep Sea Research II 1994, 41, 107–145. [Google Scholar] [CrossRef]
- Cowen, R.K.; .Lwiza, K.M.M.; Sponaugle, S.; Paris, C.B.; Olson, D.B. Connectivity of Marine.
- Populations: Open or Closed? Science 2000, 287, 857–859. [CrossRef] [PubMed]
- Kinlan, B.P.; Gaines, S.D. Propagule dispersal in marine and terrestrial environments: a community perspective. Ecology 2003, 84, 2007–2020. [Google Scholar] [CrossRef]
- Shanks, A.L.; Grantham, B.A.; Carr, M. Propagule dispersal distance and the size and spacing of marine reserves. Ecological Applications 2003, 13, S159–S169. [Google Scholar] [CrossRef]
- Shanks, A. L. Pelagic larval duration and dispersal distance revisited. Biological Bulletin 2009, 216, 373–385. [Google Scholar] [CrossRef]
- Siegel, D. A.; Kinlan B., P.; Gaylord, D.; Gaines S., D. Lagrangian descriptions of marine larval dispersion. Marine Ecology Progress Series 2003, 260, 83–96. [Google Scholar] [CrossRef]
- Pires, R.F.T.; Pan, M.; Santos, A.M.P.; Peliz, Á.; Boutov, D.; dos Santos, A. Modelling the variation in larval dispersal of estuarine and coastal ghost shrimp: Upogebia congeners in the Gulf of Cadiz. Marine Ecology Progress Series 2013, 492, 153–168. [Google Scholar] [CrossRef]
- Bray, L.; Kassis, D.; Hall-Spencer, J.M. Assessing larval connectivity for marine spatial planning in the Adriatic. Marine Environmental Research 2017, 125, 73–81. [Google Scholar] [CrossRef]
- Zaitsev, Y. P. Neuston of Seas and Oceans. In The sea surface and the global change; Liss, P.S., Duce, R.A., Eds.; Cambridge, University Press, 1997; pp. 371–382. [Google Scholar]
- dos Santos, A.; Santos, A.M.P.; Conway, D.V.P.; Bartilotti, C.; Lourenço, P.; Queiroga, H. Diel vertical migration of decapod larvae in the Portuguese coastal upwelling ecosystem: implications for offshore transport. Marine Ecology Progress Series 2008, 359, 171–183. [Google Scholar] [CrossRef]
- Pochelon, P.N.; Pires, R.F.T.; Dubert, J.; Nolasco, R.; Santos, A.M.P.; Queiroga, H.; dos Santos, A. Decapod larvae distribution and species composition off the southern Portuguese coast. Continental Shelf Research 2017, 151, 53–61. [Google Scholar] [CrossRef]
- Torres, A.P.; Dos Santos, A. Balbín, R.; Alemany, F.; Massutí, E.; Reglero, P. Decapod crustacean larval communities in the Balearic Sea (western Mediterranean): seasonal composition, horizontal and vertical distribution patterns. Journal of Marine Systems 2014, 138, 112–126. [CrossRef]
- Torres, A. P.; Reglero P.; Hidalgo M.; Abello’P.; Simao D.S.; Alemany, F.; Massutı´, E.; Dos Santos, A. Contrasting patterns in the vertical distribution of decapod crustaceans throughout ontogeny. Hydrobiologia 2018, 808, 137–152. [CrossRef]
- Liparoto, A.; Mancinelli, G.; Belmonte, G. Spatial variation in biodiversity patterns of neuston in the Western Mediterranean and Southern Adriatic Seas. Journal of Sea Research 2016, 129, 12–21. [Google Scholar] [CrossRef]
- Guglielmo, R.; Bergamasco, A.; Minutoli, R.; Patti, F.P.; Belmonte, G.; Spanò, N.; Zagami, G.; Bonanzinga, V.; Guglielmo, L.; Granata, A. – The Otranto Channel (South Adriatic Sea), a hot spot area of plankton biodiversity: pelagic polychaetes. Scientific Reports 2019, 9, 19490. [Google Scholar] [CrossRef]
- Zambianchi, E.; Trani, M.; Falco, P. Lagrangian Transport of Marine Litter in the Mediterranean Sea. Frontiers Environmental Science 2017, 5. [Google Scholar] [CrossRef]
- Boero, F.; Foglini, F.; Fraschetti, S.; Goriup, P.; Macpherson, E.; Planes, S. COCONET CONSORTIUM. CoCoNet: Towards Coast to Coast Networks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential. SCIRES-IT 2016, 6, 1–95. [Google Scholar]
- Sameoto, D.D.; Saroszynsky, L.O.; Fraser, W.B. BIONESS, a new design in multiple net zooplankton sampler. Journal. Fishery Research. Board Canada 1980, 3, 722–724. [Google Scholar] [CrossRef]
- Barange, M. Vertical migration and habitat partitioning of six euphausiid species in the northern Benguela upwelling system. Journal Plankton Research 1990, 12, 1223–1237. [Google Scholar] [CrossRef]
- Andersen, V.; Sardou, J. The die1 migrations and vertical distributions of zooplankton and micronekton in the Northwestern Mediterranean Sea, 1. Euphausiids, mysids, decapod and fishes. Journal Plankton Research 1992, 14, 1129–1554. [Google Scholar] [CrossRef]
- Davis, R.E. Observing the general circulation with floats. Deep Sea Research, Part A. Oceanographic Research Papers 1991, 38, S531–S571. [Google Scholar] [CrossRef]
- Corrado, R.; Lacorata, G.; Palatella, L.; Santoler, i.R.; Zambianchi, E. General characteristics of relative dispersion in the ocean. Scientific Reports 2017, 7, 46291. [Google Scholar] [CrossRef]
- Pisano, A.; De Dominicis, M.; Biamino, W.; Bignami, F.; Gherardi, S.; Colao, F.; Coppini, G.; Marullo, S.; Sprovieri, M.; Trivero, P.; Zambianchi, E.; Santoleri, R. An oceanographic survey for oil spill monitoring and model forecasting validation using remote sensing and in situ data in the Mediterranean Sea. Deep Sea Research Part II: Topical Studies in Oceanography 2016, 133, 132–145. [Google Scholar] [CrossRef]
- Carlson, D. F.; Griffa, A.; Zambianchi, E.; Suaria, G.; Corgnati, L.; Magaldi, M.G.; Poulain, P.-M.; Russo, A.; Bellomo, L.; Mantovani, C.; Celentano, P.; Molchard, A.; Borghini, M. Observed and modeled surface Lagrangian transport between coastal regions in the Adriatic Sea with implications for marine protected areas. Continental Shelf Research 2016, 118, 23–48. [Google Scholar] [CrossRef]
- Sciascia, R.; Berta, M.; Carlson, D.F.; Griffa, A.; Panfili, M.; La Mesa, M.; Corgnati, L.; Mantovani, C.; Domenella, E.; Fredj, E.; Magaldi, M.G.; D’Adamo, R.; Pazienza, G.; Zambianchi, E.; Poulain, P.-M. Linking sardine recruitment in coastal areas to ocean currents using surface drifters and HF radar: a case study in the Gulf of Manfredonia, Adriatic Sea. Ocean Science 2018, 14, 1461–1482. [Google Scholar] [CrossRef]
- Batchelder, H. P. Forward-in-time-/backward-in-time-trajectory (FITT/BITT) modeling of particles and organisms in the coastal ocean. Journal of Atmospheric and Oceanic Technology 2006, 23, 727–741. [Google Scholar] [CrossRef]
- Cianelli, D.; D’Alelio, D.; Uttieri, M.; Sarno, D.; Zingone, A.; Zambianchi, E.; Ribera D’alcalà, M. Disentangling physical and biological drivers of phytoplankton dynamics in a coastal system. Scientific Reports 2017, 7, 15868. [Google Scholar] [CrossRef]
- Fratini, S.; Ragionieri, L.; Deli, T.; Harrer, A.; Marino, I.A.M.; Cannicci, S.; Zane, L.; Schubart, C.D. Unravelling population genetic structure with mitochondrial DNA in a notional panmictic coastal crab species: sample size makes the difference. BMC Evolutionary Biology 2016. [Google Scholar] [CrossRef] [PubMed]
- Schiavina, M.; Marino, I.A.M.; Zane, L.; Melià, P. Matching oceanography and genetics at the basin scale. Seascape connectivety of the Mediterranean shore crab in the Adriatic. Molecular Ecology 2014, 23, 5496–5507. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.I.; Nikula, R.; Waters, G.M. Oceanic rafting by a coastal community. Proceedings Royal Society 2011, B 278, 649–55. [Google Scholar] [CrossRef]
- Treml, E.A.; Roberts, J.J.; Chao, Y.; Halpin, P.N.; Possingham, H.P.; Rigin, C. Reproductive output and Duration of the Pelagic Larval Stage determine seascape-wide connectivity of marine populations. Integrative and Comparative Biology 2012, 52, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Jokiel, P.L. Rafting of reef corals and other organisms at Kwajalein Atoll. Marine Biology 1989, 101, 483–493. [Google Scholar] [CrossRef]
- Boero, F.; Bouillon, J. Zoogeography and life cycle patterns of Mediterranean hydromedusae (Cnidaria). Biology Journal of the Linnean Society 1993, 48, 239–266. [Google Scholar] [CrossRef]
- Pati, A.C.; Belmonte, G.; Fanelli, G.; Giangrande, A.; Gravili, C.; Saracino, O.; Boero, F. Interkingdom convergence in the architecture of asexual propagules. Biologia Marina Mediterranea 1998, 5, 365–366. [Google Scholar]
- Moscatello, S.; Belmonte, G. The plankton of a shallow submarine cave (‘Grotta di Ciolo’, Salento Peninsula, SE Italy). Marine Ecology 2007, 28 Suppl.1, 47–59. [Google Scholar] [CrossRef]
- Pati, A.C.; Belmonte, G. Asexually Generated Propagules from Subtidal Sessile Benthic Organisms. Progress in Aqua Farming and Marine Biology 2018, 1, 180002. [Google Scholar]
- Belmonte, G.; Rubino, F. Resting cysts from coastal marine plankton. Oceanography and Marine Biology Annual Review 2019, 57, 1–88. [Google Scholar]
- Weersing, K.; Toonen, R.J. Population genetics, larval dispersal, and connectivity in marine systems. Marine Ecology Progress Series 2009, 393, 1–12. [Google Scholar] [CrossRef]
Figure 1.
Map of the south Adriatic Sea, with CoCoNET sampling locations. Red and blue circles indicate BIONESS and Neuston samples, respectively.
Figure 1.
Map of the south Adriatic Sea, with CoCoNET sampling locations. Red and blue circles indicate BIONESS and Neuston samples, respectively.
Figure 2.
Potential temperature, salinity and fluorescence vertical profiles at all sampled. Stations, from surface down to 200 m.
Figure 2.
Potential temperature, salinity and fluorescence vertical profiles at all sampled. Stations, from surface down to 200 m.
Figure 3.
Vertical profiles of Temperature (°C), Salinity, and Chla (μg L-1), at stations S19, S15, S08 in the CoCoNet cruise 2013 as representatives of Italian, central South Adriatic, and Balkan coast, respectively. Please note that depth scales vary.
Figure 3.
Vertical profiles of Temperature (°C), Salinity, and Chla (μg L-1), at stations S19, S15, S08 in the CoCoNet cruise 2013 as representatives of Italian, central South Adriatic, and Balkan coast, respectively. Please note that depth scales vary.
Figure 4.
Oceanographic context during the CoCoNet Cruise in Southern Adriatic Sea (May 2013). θ-S diagram of the collected CTD profiles. Colors indicate the depth (m). Water mass types are highlighted.
Figure 4.
Oceanographic context during the CoCoNet Cruise in Southern Adriatic Sea (May 2013). θ-S diagram of the collected CTD profiles. Colors indicate the depth (m). Water mass types are highlighted.
Figure 5.
Representation of Decapoda larvae abundance in the Southern Adriatic. Values come from the integration of 0-100 m surface layer, and they are referred to a water column of 1 m2 basis and 100 m of depth. Stations PGR and S13 (interested by a swarm of Decapoda larvae) were not interested by the BIONESS collection.
Figure 5.
Representation of Decapoda larvae abundance in the Southern Adriatic. Values come from the integration of 0-100 m surface layer, and they are referred to a water column of 1 m2 basis and 100 m of depth. Stations PGR and S13 (interested by a swarm of Decapoda larvae) were not interested by the BIONESS collection.
Figure 6.
Vertical distribution of Decapoda larvae in 5 selected stations of the cruise 2013.
Figure 6.
Vertical distribution of Decapoda larvae in 5 selected stations of the cruise 2013.
Figure 7.
Vertical distribution of the decapod larvae (as percentage of total numbers) from the BIONESS hauls at coastal and offshore five stations selected according to the daily sampling time. Numbers in brackets are average abundance in ind. m–3 for the entire water column at each sampling time.
Figure 7.
Vertical distribution of the decapod larvae (as percentage of total numbers) from the BIONESS hauls at coastal and offshore five stations selected according to the daily sampling time. Numbers in brackets are average abundance in ind. m–3 for the entire water column at each sampling time.
Figure 8.
Vertical profiles of decapod larvae WMD values in coastal and offshore stations, according to the daily sampling time.
Figure 8.
Vertical profiles of decapod larvae WMD values in coastal and offshore stations, according to the daily sampling time.
Figure 9.
Relationship between decapod larvae abundance and thermocline thickness.
Figure 9.
Relationship between decapod larvae abundance and thermocline thickness.
Figure 10.
Trajectories of 4 drifters passing by the area of Stations PGR, and S13 (black square in the rectangular area) at the time of the Brachyura larvae swarm. Different colors of the tracks indicate the drifter paths before (red) entering the area (rectangle) and after (green) exiting, for 40 days.
Figure 10.
Trajectories of 4 drifters passing by the area of Stations PGR, and S13 (black square in the rectangular area) at the time of the Brachyura larvae swarm. Different colors of the tracks indicate the drifter paths before (red) entering the area (rectangle) and after (green) exiting, for 40 days.
Table 1.
Pelagic Larval Duration PLD (days) of some Brachyura species reared in laboratory at salinity values > 33 ‰, and different temperatures.
Table 1.
Pelagic Larval Duration PLD (days) of some Brachyura species reared in laboratory at salinity values > 33 ‰, and different temperatures.
Table 2.
Hydrological parameters (Salinity, Temperature, dissolved Oxygen) of the sea water in the South Adriatic Sea, measured during the two CoCoNet oceanographic cruises (May 2013), at stations Penna Grossa (PGR), and S13, and as average values of the whole basin. CTD = Conductivity-Temperature-Dissolved Oxygen.
Table 2.
Hydrological parameters (Salinity, Temperature, dissolved Oxygen) of the sea water in the South Adriatic Sea, measured during the two CoCoNet oceanographic cruises (May 2013), at stations Penna Grossa (PGR), and S13, and as average values of the whole basin. CTD = Conductivity-Temperature-Dissolved Oxygen.
Table 3.
CoCoNet-WP11 May 2013 cruise in the South Adriatic Sea. Stations and BIONESS sampling data.
Table 3.
CoCoNet-WP11 May 2013 cruise in the South Adriatic Sea. Stations and BIONESS sampling data.
Table 4.
Identification code of the drifters entering the box-area containing the stations where Brachyura larvae were abundant in the neuston (date of collection, 17 May 2013). Only drifters entering the delimited area in the period 15-27 May have been considered.
Table 4.
Identification code of the drifters entering the box-area containing the stations where Brachyura larvae were abundant in the neuston (date of collection, 17 May 2013). Only drifters entering the delimited area in the period 15-27 May have been considered.
Table 5.
Decapod larvae abundance (ind. m-3) in the whole neuston collection.
Table 5.
Decapod larvae abundance (ind. m-3) in the whole neuston collection.
Table 6.
Larvae identified in the two cruises and relative instars (Z1–Z6 = zoea stage, instars 1-6; M = megalopa stage; undet. = not determined). Reported numbers indicate percentages on the total of larvae.
Table 6.
Larvae identified in the two cruises and relative instars (Z1–Z6 = zoea stage, instars 1-6; M = megalopa stage; undet. = not determined). Reported numbers indicate percentages on the total of larvae.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).