Submitted:
31 May 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
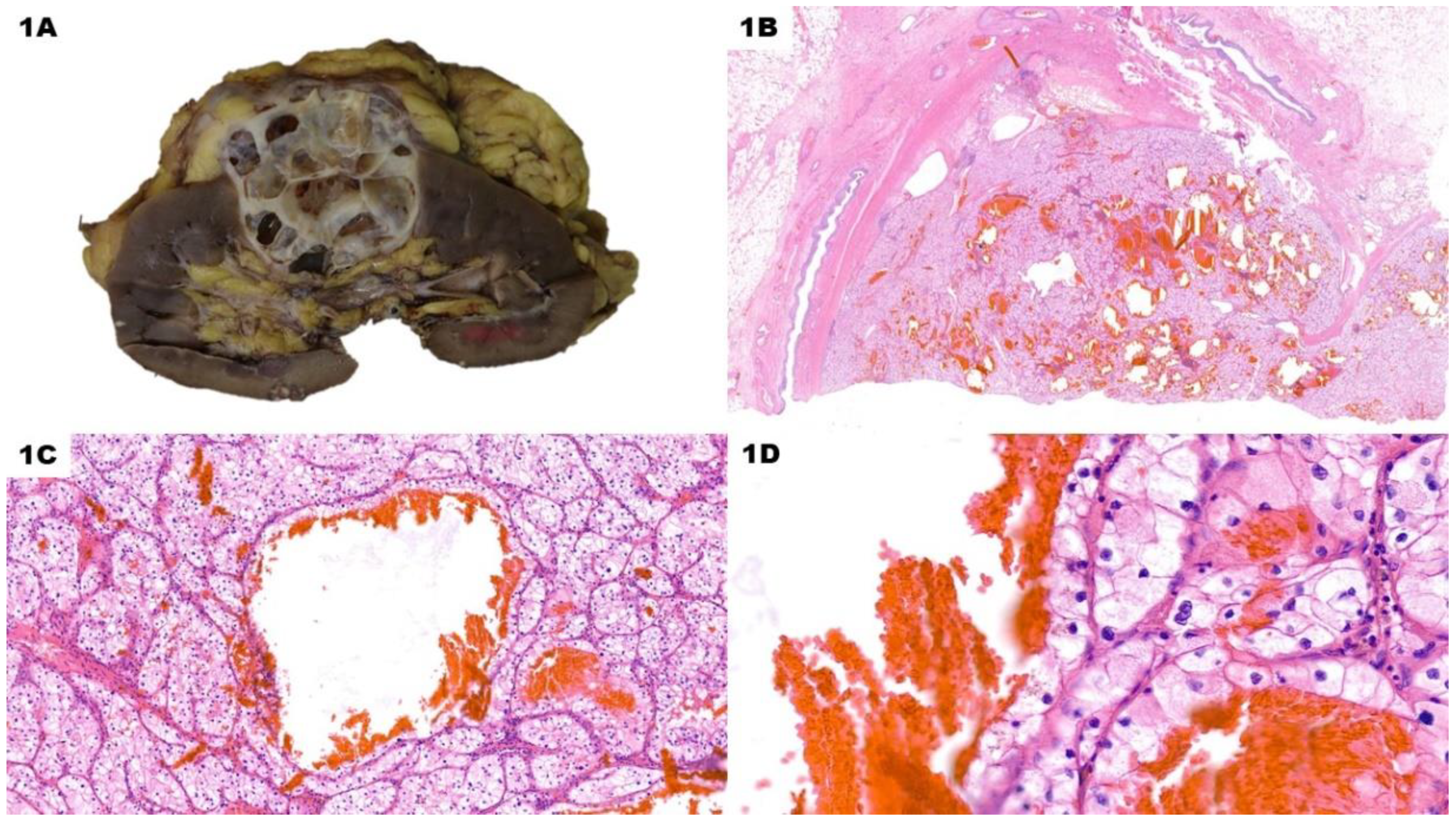
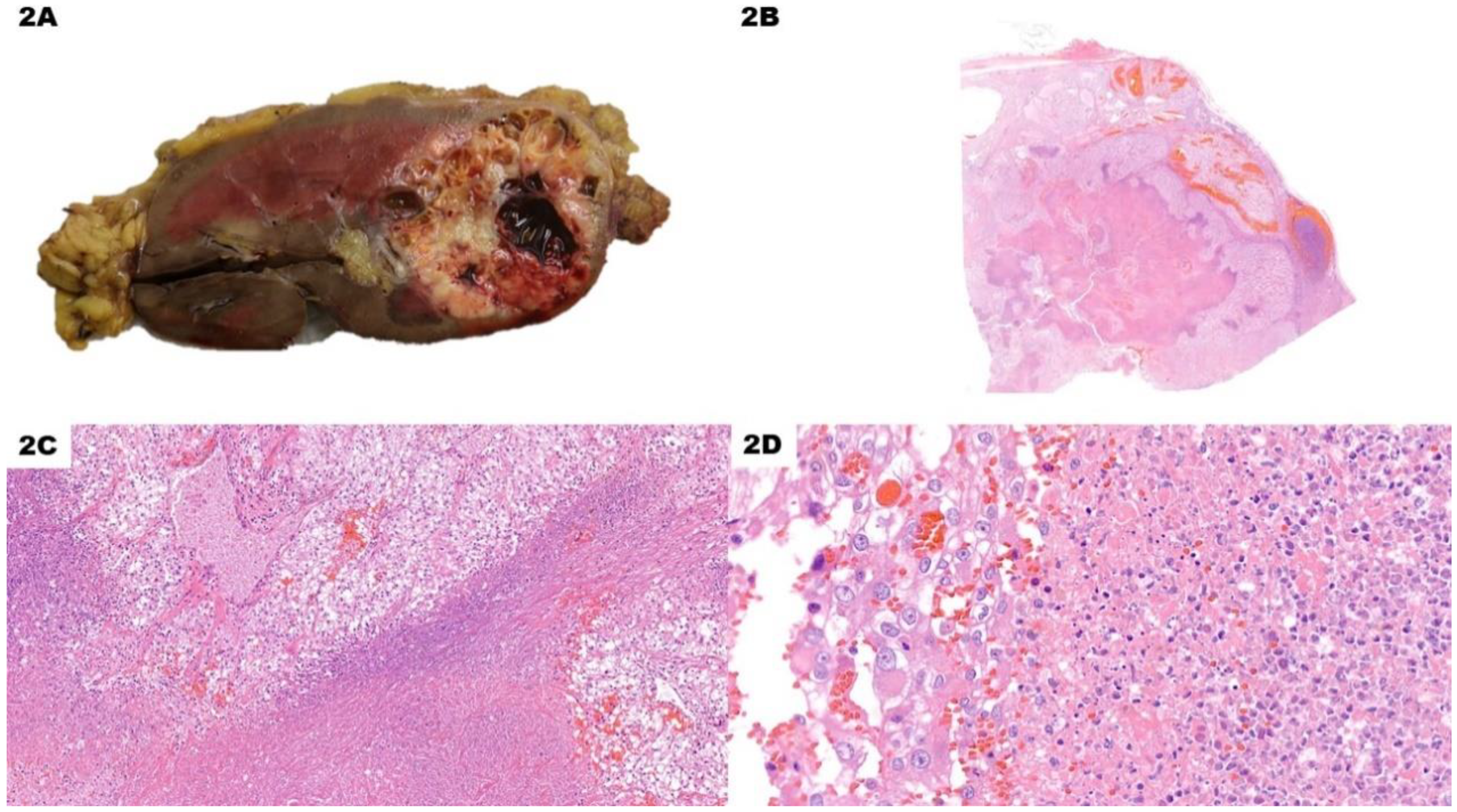
2. Macroscopic and Microscopic Features of Cystic CCRCC
3. Molecular Features of Cystic CCRCC
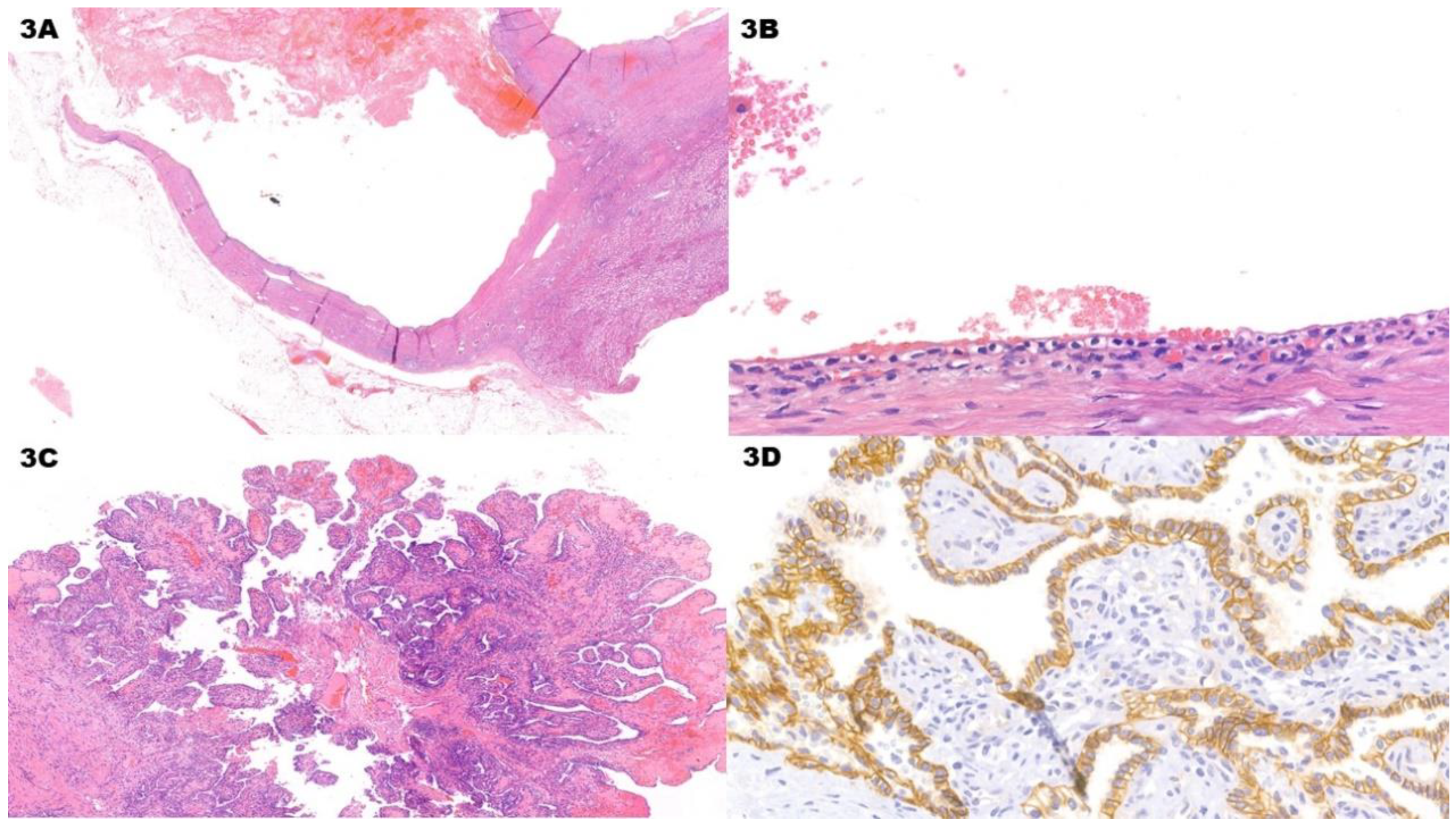
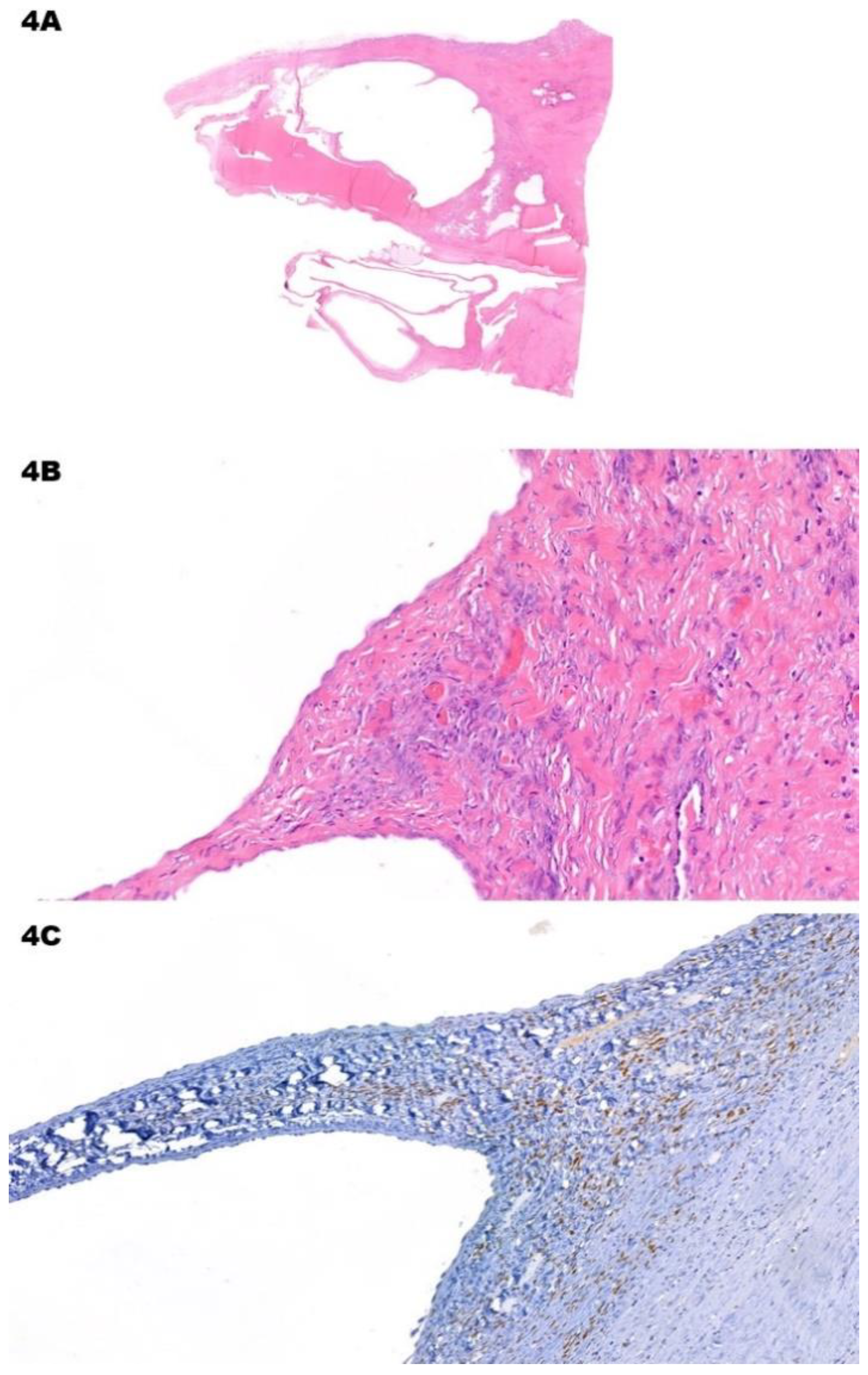
4. Differential Diagnosis of Cystic CCRCC
5. Conclusions and Future Directions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACD-RCD | Acquired Cystic Disease-associated Renal Cell Carcinoma |
| CAN | Adult Cystic Nephroma |
| AMLEC | Angiomyolipoma with Epithelial Cyst |
| BC | Bosniak Classification |
| CAIX | Carbonic Anhydrase IX |
| CCPRCT | Clear Cell Papillary Renal Cell Tumor |
| CCRCC | Clear Cell Renal Cell Carcinoma |
| HIF | Hypoxia-Inducible Factor |
| HMWCK | High Molecular Weight Cytokeratins |
| IHC | Immunohistochemistry |
| MEST | Mixed Epithelial and Stromal Tumor |
| MiTF | Melanocyte Inducing Transcription Factor |
| MCNLMP | Multilocular Cystic Renal Neoplasm of Low Malignant Potential |
| PKD | Polycystic Kidney Disease |
| TcRCC | Tubulo-cystic Renal Cell Carcinoma |
| VEGF | Vascular endothelial growth factor |
| VHL | Von Hippel-Lindau |
| VHLd | Von Hippel-Lindau disease |
References
- Caliò A, Marletta S, Brunelli M, Martignoni G. WHO 2022 Classification of Kidney Tumors: what is relevant? An update and future novelties for the pathologist. Pathologica. 2022 Feb;115(1):23-31. Epub 2023 Jan 16. [CrossRef] [PubMed]
- Alaghehbandan R, Siadat F, Trpkov K. What’s new in the WHO 2022 classification of kidney tumours? Pathologica. 2022 Feb;115(1):8-22. Epub 2023 Jan 16. [CrossRef] [PubMed]
- Udager AM, Mehra R. Morphologic, Molecular, and Taxonomic Evolution of Renal Cell Carcinoma: A Conceptual Perspective With Emphasis on Updates to the 2016 World Health Organization Classification. Arch Pathol Lab Med. 2016 Oct;140(10):1026-37. [CrossRef] [PubMed]
- Moch, H. Cystic renal tumors: new entities and novel concepts. Adv Anat Pathol. 2010 May;17(3):209-14. [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Urinary and male genital tumours. Lyon (France): International Agency for Research on Cancer; 2022. (WHO Classification of tumour series; 5th ed.; vol. 8).
- Alrumayyan M, Raveendran L, Lawson KA, Finelli A. Cystic Renal Masses: Old and New Paradigms. Urol Clin North Am. 2023 May;50(2):227-238. Epub 2023 Feb 20. [CrossRef] [PubMed]
- Silverman SG, Pedrosa I, Ellis JH, Hindman NM, Schieda N, Smith AD, Remer EM, Shinagare AB, Curci NE, Raman SS, Wells SA, Kaffenberger SD, Wang ZJ, Chandarana H, Davenport MS. Bosniak Classification of Cystic Renal Masses, Version 2019: An Update Proposal and Needs Assessment. Radiology. 2019 Aug;292(2):475-488. Epub 2019 Jun 18. PMCID: PMC6677285. [CrossRef] [PubMed]
- Krishna S, Schieda N, Pedrosa I, Hindman N, Baroni RH, Silverman SG, Davenport MS. Update on MRI of Cystic Renal Masses Including Bosniak Version 2019. J Magn Reson Imaging. 2021 Aug;54(2):341-356. Epub 2020 Oct 2. PMCID: PMC8017011. [CrossRef] [PubMed]
- Westerman ME, Cheville JC, Lohse CM, Sharma V, Boorjian SA, Leibovich BC, Thompson RH. Long-Term Outcomes of Patients With Low Grade Cystic Renal Epithelial Neoplasms. Urology. 2019 Nov;133:145-150. Epub 2019 Jul 26. [CrossRef] [PubMed]
- Tretiakova M, Mehta V, Kocherginsky M, Minor A, Shen SS, Sirintrapun SJ, Yao JL, Alvarado-Cabrero I, Antic T, Eggener SE, Picken MM, Paner GP. Predominantly cystic clear cell renal cell carcinoma and multilocular cystic renal neoplasm of low malignant potential form a low-grade spectrum. Virchows Arch. 2018 Jul;473(1):85-93. Epub 2018 May 17. [CrossRef] [PubMed]
- Pastorekova S, Gillies RJ. The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev. 2019 Jun;38(1-2):65-77. PMCID: PMC6647366. [CrossRef] [PubMed]
- Genega EM, Ghebremichael M, Najarian R, Fu Y, Wang Y, Argani P, Grisanzio C, Signoretti S. Carbonic anhydrase IX expression in renal neoplasms: correlation with tumor type and grade. Am J Clin Pathol. 2010 Dec;134(6):873-9. PMCID: PMC3778911. [CrossRef] [PubMed]
- Jonasch E, Walker CL, Rathmell WK. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat Rev Nephrol. 2021 Apr;17(4):245-261. Epub 2020 Nov 3. PMCID: PMC8172121. [CrossRef] [PubMed]
- Bui TO, Dao VT, Nguyen VT, Feugeas JP, Pamoukdjian F, Bousquet G. Genomics of Clear-cell Renal Cell Carcinoma: A Systematic Review and Meta-analysis. Eur Urol. 2022 Apr;81(4):349-361. Epub 2022 Jan 3. [CrossRef] [PubMed]
- Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol. 2007 May;9(5):588-95. Epub 2007 Apr 22. [CrossRef] [PubMed]
- Rechsteiner MP, von Teichman A, Nowicka A, Sulser T, Schraml P, Moch H. VHL gene mutations and their effects on hypoxia inducible factor HIFα: identification of potential driver and passenger mutations. Cancer Res. 2011 Aug 15;71(16):5500-11. Epub 2011 Jun 29. [CrossRef] [PubMed]
- Cremona M, Espina V, Caccia D, Veneroni S, Colecchia M, Pierobon M, Deng J, Mueller C, Procopio G, Lanzi C, Daidone MG, Cho WC, Petricoin EF, Liotta L, Bongarzone I. Stratification of clear cell renal cell carcinoma by signaling pathway analysis. Expert Rev Proteomics. 2014 Apr;11(2):237-49. Epub 2014 Feb 27. [CrossRef] [PubMed]
- Bakouny Z, Braun DA, et al. Integrative molecular characterization of sarcomatoid and rhabdoid renal cell carcinoma. Nat Commun. 2021 Feb 5;12(1):808. PMCID: PMC7865061. [CrossRef] [PubMed]
- Akgul M, Williamson SR. How New Developments Impact Diagnosis in Existing Renal Neoplasms. Surg Pathol Clin. 2022 Dec;15(4):695-711. Epub 2022 Oct 13. [CrossRef] [PubMed]
- Kapur P, Rajaram S, Brugarolas J. The expanding role of BAP1 in clear cell renal cell carcinoma. Hum Pathol. 2023 Mar;133:22-31. Epub 2022 Aug 4. PMCID: PMC9898467. [CrossRef] [PubMed]
- D.A. Braun, Y. Hou, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma., Nat. Med. 26 (2020) 909–918. [CrossRef]
- Elgendy M, Fusco JP, et al. Identification of mutations associated with acquired resistance to sunitinib in renal cell cancer. Int J Cancer. 2019 Oct 1;145(7):1991-2001. Epub 2019 Mar 30. [CrossRef] [PubMed]
- Guinot A, Lehmann H, Wild PJ, Frew IJ. Combined deletion of Vhl, Trp53 and Kif3a causes cystic and neoplastic renal lesions. J Pathol. 2016 Jul;239(3):365-73. Epub 2016 May 30. [CrossRef] [PubMed]
- Kuehn EW, Walz G, Benzing T. Von hippel-lindau: a tumor suppressor links microtubules to ciliogenesis and cancer development. Cancer Res. 2007 May 15;67(10):4537-40. [CrossRef] [PubMed]
- Seeger-Nukpezah T, Geynisman DM, Nikonova AS, Benzing T, Golemis EA. The hallmarks of cancer: relevance to the pathogenesis of polycystic kidney disease. Nat Rev Nephrol. 2015 Sep;11(9):515-34. Epub 2015 Apr 14. PMCID: PMC5902186. [CrossRef] [PubMed]
- Santoni M, Piva F, Cimadamore A, Giulietti M, Battelli N, Montironi R, Cosmai L, Porta C. Exploring the Spectrum of Kidney Ciliopathies. Diagnostics (Basel). 2020 Dec 16;10(12):1099. PMCID: PMC7766105. [CrossRef] [PubMed]
- Louise M Binderup M, Smerdel M, et al. von Hippel-Lindau disease: Updated guideline for diagnosis and surveillance. Eur J Med Genet. 2022 Aug;65(8):104538. Epub 2022 Jun 13. [CrossRef] [PubMed]
- Chahoud J, McGettigan M, Parikh N, Boris RS, Iliopoulos O, Rathmell WK, Daniels AB, Jonasch E, Spiess PE; International VHL Surveillance Guidelines Consortium-Renal Committee. Evaluation, diagnosis and surveillance of renal masses in the setting of VHL disease. World J Urol. 2021 Jul;39(7):2409-2415. Epub 2020 Sep 16. PMCID: PMC8101019. [CrossRef] [PubMed]
- Schönenberger D, Harlander S, Rajski M, Jacobs RA, Lundby AK, Adlesic M, Hejhal T, Wild PJ, Lundby C, Frew IJ. Formation of Renal Cysts and Tumors in Vhl/Trp53-Deficient Mice Requires HIF1α and HIF2α. Cancer Res. 2016 Apr 1;76(7):2025-36. Epub 2016 Jan 12. [CrossRef] [PubMed]
- Tao S, Kakade VR, Woodgett JR, Pandey P, Suderman ED, Rajagopal M, Rao R. Glycogen synthase kinase-3β promotes cyst expansion in polycystic kidney disease. Kidney Int. 2015 Jun;87(6):1164-75. Epub 2015 Jan 28. PMCID: PMC4449797. [CrossRef] [PubMed]
- Bilim V, Ougolkov A, Yuuki K, Naito S, Kawazoe H, Muto A, Oya M, Billadeau D, Motoyama T, Tomita Y. Glycogen synthase kinase-3: a new therapeutic target in renal cell carcinoma. Br J Cancer. 2009 Dec 15;101(12):2005-14. Epub 2009 Nov 17. PMCID: PMC2795437. [CrossRef] [PubMed]
- Favazza L, Chitale DA, Barod R, Rogers CG, Kalyana-Sundaram S, Palanisamy N, Gupta NS, Williamson SR. Renal cell tumors with clear cell histology and intact VHL and chromosome 3p: a histological review of tumors from the Cancer Genome Atlas database. Mod Pathol. 2017 Nov;30(11):1603-1612. Epub 2017 Jul 21. [CrossRef] [PubMed]
- Halat S, Eble JN, Grignon DJ, Lopez-Beltran A, Montironi R, Tan PH, Wang M, Zhang S, MacLennan GT, Cheng L. Multilocular cystic renal cell carcinoma is a subtype of clear cell renal cell carcinoma. Mod Pathol. 2010 Jul;23(7):931-6. Epub 2010 Mar 26. [CrossRef] [PubMed]
- Gong K, Zhang N, He Z, Zhou L, Lin G, Na Y. Multilocular cystic renal cell carcinoma: an experience of clinical management for 31 cases. J Cancer Res Clin Oncol. 2008 Apr;134(4):433-7. Epub 2007 Sep 1. [CrossRef] [PubMed]
- Williamson SR, Halat S, et al. Multilocular cystic renal cell carcinoma: similarities and differences in immunoprofile compared with clear cell renal cell carcinoma. Am J Surg Pathol. 2012 Oct;36(10):1425-33. [CrossRef] [PubMed]
- Kim SH, Park WS, Chung J. SETD2, GIGYF2, FGFR3, BCR, KMT2C, and TSC2 as candidate genes for differentiating multilocular cystic renal neoplasm of low malignant potential from clear cell renal cell carcinoma with cystic change. Investig Clin Urol. 2019 May;60(3):148-155. Epub 2019 Apr 1. PMCID: PMC6495037. [CrossRef] [PubMed]
- Diolombi ML, Cheng L, Argani P, Epstein JI. Do Clear Cell Papillary Renal Cell Carcinomas Have Malignant Potential? Am J Surg Pathol. 2015 Dec;39(12):1621-34. [CrossRef] [PubMed]
- Williamson, SR. Clear cell papillary renal cell carcinoma: an update after 15 years. Pathology. 2021 Jan;53(1):109-119. Epub 2020 Nov 19. [CrossRef] [PubMed]
- Munari E, Marchionni L, Chitre A, Hayashi M, Martignoni G, Brunelli M, Gobbo S, Argani P, Allaf M, Hoque MO, Netto GJ. Clear cell papillary renal cell carcinoma: micro-RNA expression profiling and comparison with clear cell renal cell carcinoma and papillary renal cell carcinoma. Hum Pathol. 2014 Jun;45(6):1130-8. Epub 2014 Jan 31. PMCID: PMC4332813. [CrossRef] [PubMed]
- Mohanty SK, Parwani AV. Mixed epithelial and stromal tumors of the kidney: an overview. Arch Pathol Lab Med. 2009 Sep;133(9):1483-6. [CrossRef] [PubMed]
- Turbiner J, Amin MB, Humphrey PA, Srigley JR, De Leval L, Radhakrishnan A, Oliva E. Cystic nephroma and mixed epithelial and stromal tumor of kidney: a detailed clinicopathologic analysis of 34 cases and proposal for renal epithelial and stromal tumor (REST) as a unifying term. Am J Surg Pathol. 2007 Apr;31(4):489-500. [CrossRef] [PubMed]
- Zhou M, Kort E, Hoekstra P, Westphal M, Magi-Galluzzi C, Sercia L, Lane B, Rini B, Bukowski R, Teh BT. Adult cystic nephroma and mixed epithelial and stromal tumor of the kidney are the same disease entity: molecular and histologic evidence. Am J Surg Pathol. 2009 Jan;33(1):72-80. [CrossRef] [PubMed]
- Vanecek T, Pivovarcikova K, Pitra T, Peckova K, Rotterova P, Daum O, Davidson W, Montiel DP, Kalusova K, Hora M, Ondic O, Dubova M, Michal M, Hes O. Mixed Epithelial and Stromal Tumor of the Kidney: Mutation Analysis of the DICER 1 Gene in 29 Cases. Appl Immunohistochem Mol Morphol. 2017 Feb;25(2):117-121. [CrossRef] [PubMed]
- Argani P, Beckwith JB. Metanephric stromal tumor: report of 31 cases of a distinctive pediatric renal neoplasm. Am J Surg Pathol. 2000 Jul;24(7):917-26. [CrossRef] [PubMed]
- Kacar A, Azili MN, Cihan BS, Demir HA, Tiryaki HT, Argani P. Metanephric stromal tumor: a challenging diagnostic entity in children. J Pediatr Surg. 2011 Dec;46(12):e7-e10. [CrossRef] [PubMed]
- Idrissi-Serhrouchni K, El-Fatemi H, El madi A, Benhayoun K, Chbani L, Harmouch T, Bouabdellah Y, Amarti A. Primary renal teratoma: a rare entity. Diagn Pathol. 2013 Jun 25;8:107. PMCID: PMC3751105. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



