1. Introduction
Age-related macular degeneration (AMD) is the most common cause of severe visual impairment in industrialized countries [
1,
2]. Especially, the late forms of the disease, neovascular AMD (nAMD) and geographic atrophy, are responsible for the most visual loss [
3]. AMD is an increasingly important public health challenge worldwide due increases in aging populations and lifetime expectancy. Accordingly, the prevalence of AMD has been projected to rise from 196 million in 2020 to 288 million by the year 2040 [
4].
AMD risk is influenced by genetic and non-genetic factors [
5,
6,
7,
8]. The previous systematic review and meta-analysis by Chakravarthy et al. has identified four strong and consistent risk factors for the development of late AMD including age, smoking, previous cataract surgery and a family history for AMD. Additional risk factors associated with AMD were obesity, history of cardiovascular disease, hypertension, and plasma fibrinogen concentration [
5]. Association of very rare coding variants in complement factor H (CFH), complement factor I (CFI) and tissue inhibitor of metalloproteinases-3 (TIMP3) has suggested to have causal roles for these genes in AMD pathogenesis [
6].
AMD is divided clinically into early, intermediate, and late forms of the disease [
1,
2]. Early AMD is defined as the presence of medium-sized drusen without retinal hyper- or hypo-pigmentary changes in the macula. The presence of at least one large drusen (> 125 µm) or extensive medium drusen and typical pigmentary changes are typical for intermediate AMD. Early and intermediate stages of AMD are often asymptomatic or associated with only mild central distortion. Both nAMD and atrophic AMD are defined as late AMD causing typical symptoms including reduction of visual acuity, distorted central vision and development of central scotoma [
1]. The symptoms and clinical findings of nAMD may progress rapidly over weeks to months, while atrophic AMD progresses more slowly over years to decades.
The current treatment protocols for AMD are focused on management of nAMD, since there are no commonly available treatment strategies for atrophic AMD at present [
1,
2,
9], although the complement C3 inhibitor pegcetacoplan has recently been reported to reduce the growth of geographic atrophy in patients with dry AMD [
10,
11]. The pathogenesis of nAMD involves development of choroidal neovascularization in macula, vascular leakage leading to macular edema, hemorrhages and occasionally scarring, which may lead to permanent loss of vision [
12,
13]. Several mediators are shown to have a role in this complex pathological process including kinases, cytokines and growth factors [
1,
2,
14,
15]. Vascular endothelial growth factor A (VEGF-A) and its receptors have the notable ability to promote angiogenesis and vascular permeability which explains its significance in the management of nAMD [
15]. Over the past two decades, the use of VEGF-inhibitors, such as ranibizumab, bevacizumab, aflibercept, brolucizumab and faricimab have revolutionized the clinical management of nAMD [
1,
2,
9,
16,
17,
18].
The current population-based study aimed to evaluate visual outcomes of anti-VEGF treatment for nAMD in a real-life setting. In addition, the rates for visual impairment were determined at the onset of nAMD and after the intravitreal anti-VEGF treatment. Our results suggest that a great majority of individuals with nAMD, 90%, benefit from anti-VEGF management in terms of stabilization or improvement of visual acuity.
2. Results
2.1. Characteristics of the Study Population
A total of 827 nAMD patients with 1088 eyes were included in the study. All study eyes had nAMD and were treated with intravitreal anti-VEGF injections. The average age of the nAMD patients at the time of the diagnosis was 78±8 years, and 693 out of 827 patients (64%) were women. The study patients were followed up for 36±25 months (range 3-134 months) on average. The eyes with most ETDRS letters gained were followed-up longer than those with the biggest loss of vision (40±25 vs. 20±20 months, respectively, p < 0.001) (
Table 1).
Bilateral nAMD was diagnosed in 107 (13%) patients already at the onset of nAMD.
During the follow-up period, 154 patients (19%) developed nAMD also in the fellow-eye at the average time-interval of 14±19 months after the initial diagnosis of nAMD. At the end of the follow-up, a total of 261 (32%) patients with nAMD had bilateral disease.
The duration of typical visual symptoms of nAMD before diagnosis was less than two months in 36% of the patients and over two months in 64% of the patients. Improvement in visual acuity >5 ETDRS letters was noted in 69% of the patients with the duration of ocular symptoms of nAMD for less than two months and in 62% with symptoms longer than two months (p=0.211). In both groups, 9% of the patients lost >5 ETDRS letters. The symptoms complained were decrease in visual acuity (76%), distortion of the straight lines (34%), blurring of the central vision (19%) and other discomfort of the eyes (2%). Several subsequential symptoms occurred commonly in study participants with nAMD.
2.2. Visual Outcomes
An average baseline visual acuity was 56±20 ETDRS letters. During the follow-up period, visual acuity was stable or improved in 984 (90%) eyes. The gain of >5 ETDRS letters was achieved in 686 (63%) of the total of 1088 study eyes with nAMD (
Figure 1). In this visual outcome group, ≥15 ETDRS letters gain was noted in 377 (35%) eyes. Visual acuity remained stable (±5 ETDRS letters) in 298 (27%) eyes (
Figure 1). The loss of >5 ETDRS letters was noted in 104 (10%), which includes 60 (6%) eyes with a loss of ≥15 ETDRS letters (
Figure 1). The changes in visual acuity in the study eyes are presented in
Figure 1. The average age of the patients in each visual outcome group at the onset of nAMD ranged from 77 to 80, and the difference was non-significant (p>0.02). In addition, female gender was represented in 63-64% of the patients in all visual outcome groups (p>0.9).
2.3. Intravitreal Anti-VEGF Injections
The number of anti-VEGF injections varied in different visual outcome groups. An average number of anti-VEGF injections were 8±10, 15±14, and 19±15 in the groups of loss > 5 ETDRS letters, no change in vision, or gain of > 5 ETDRS letters, respectively (p<0.001)(
Table 1). 45% of the eyes received less than ten injections, and 81% received less than 30 injections during the follow-up period. Only single eyes were treated with over 50 injections and an eye with the most injections received 80 injections during the study period. An average number of injections per eye was 17±15 in the whole study population during the follow-up.
The time interval between anti-VEGF injections ranged from 28 to 2117 days. 695 (64%) eyes had chronic nAMD and need for continuous intravitreal treatment during the follow-up period. Intravitreal anti-VEGF treatment breaks varying from 4 to 71 months were noted in 393 (36%) study eyes (p<0.001)(
Table 1). There were several reasons for the discontinuation of anti-VEGF injections during the follow-up: no need (dry macula) in 52%, no fulfillment of the treatment criteria (visual acuity >0.05 in Snellen E chart and sufficient compliance for treatment) in 20%, or no response to anti-VEGF treatment in 10% of the eyes. The patient asked for discontinuation of the injections in 9% of the cases and in 9% the reason for stopping the treatment was the death of the patient. No information about the reason for the discontinuation of the treatment was available in 1% of the eyes.
Remission of active nAMD (>4 month break with no need for anti-VEGF treatment) was noted in 42% of the eyes gaining most vision and in 31% of those with stable visual activity (
Table 1). In contrast, only 14% of the eyes with a loss of vision emerged without continuous treatment.
Bevacitzumab was the first-line drug in 1083 (99.5%) eyes, whereas 5 eyes (0.5%) were initially treated by aflibercept. Endophthalmitis occurred in 11 out of a total of 18359 injections (0.06%) during the study period.
2.4. The Impact of Cataract
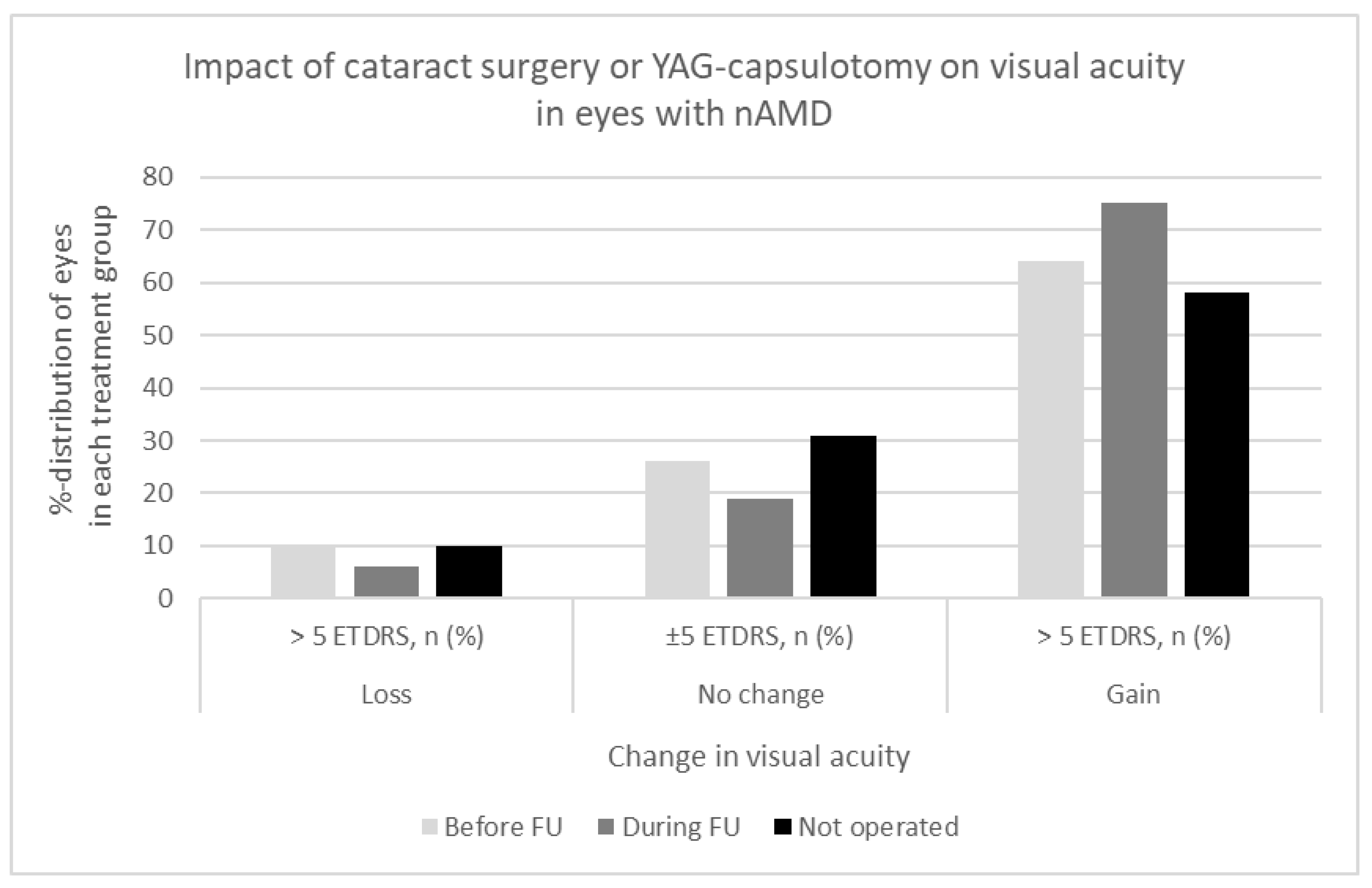
The impact of the lens status (pseudophakic, phakic), cataract, secondary cataract, cataract surgery and YAG-capsulotomy on visual acuity was evaluated according to the relatively old age of the study patients and long follow-up period. Cataract surgery or YAG-capsulotomy had been completed in 428 (39%) eyes before the diagnosis of nAMD. In 162 (15%) eyes either cataract or secondary cataract were operated during the follow-up of nAMD, and 498 (46%) eyes remained phakic until the end of follow-up period. The effect of the cataract surgery, YAG-capsulotomy, or developing cataract on visual acuity in the eyes with nAMD is shown in detail in
Figure 2. Visual acuity improved > 5 ETDRS letters in 64% of the eyes in the pseudophakic group, in 75% in the group of cataract surgery/YAG-capsulotomy during the follow-up, and in 58% in the group without any intervention for cataract (p=0.003).
2.5. Visual Impairment
At the time of the diagnosis of nAMD, 110 out of 827 patients (13%) fulfilled the criteria of visual impairment, whereas a total of 179 patients (22%) were visually impaired after the follow-up. However, 28 (25%) patients with initial vision impairment were not visually impaired at the end of follow-up, and 97 out of 717 (14%) patients not visually impaired at the diagnosis of nAMD fulfilled the criteria at the end of follow-up period.
3. Discussion
Intravitreal anti-VEGF therapy is the standard of care for the treatment of nAMD [
17]. Currently available therapies include aflibercept, bevacizumab, brolucizumab, faricimab and ranibizumab [
16,
19,
20,
21,
22]. Landmark trials for anti-VEGF agents have proven major advancement in the management and prognosis of nAMD, which is known to lead to permanently impaired vision over time if not treated [
23,
24,
25,
26,
27]. However, clinical trial treatment regimens may not reflect real-world practice and high frequency of intravitreal anti-VEGF injections in registration trial design may lead to a high treatment burden and affect the outcomes. The present study was designed to expose the real-life treatment outcomes of nAMD in a population-based cohort during the long follow-up.
The study nAMD cohort is comparable to the recent study of 67 666 eyes treated with intravitreal anti-VEGF agents for nAMD according to age of the patients, sex distribution, and baseline visual acuity [
28]. Ciulla et al. reported an improvement in visual acuity of 3 ETDRS letters during the first year of intravitreal treatment for nAMD followed by the gradual loss of vision back to baseline level over the next 4 years [
28]. Similarly, several clinical extension studies of randomized nAMD trials have reported that visual acuity commonly declines over the years after an increase at the start of the anti-VEGF treatment [
29,
30,
31]. These studies revealed mean visual gain of only 2 ETDRS letters by treatment year 4, and a loss of 3 and 9 letters by 5.5 and 7 years, respectively. Our results show stable or improved visual acuity in 90% of the eyes during the follow-up for 3 years on average. This is in line with the recent study that revealed stable or >10 ETDRS letter improvement in visual acuity by brolucizumab in 84% of treatment-naïve and 86% of previously treated eyes with nAMD [
32]. In the current study, the losses of >5 ETDRS letters were noted in 10% of all eyes. Chandra et al. reported 15 letters decline in visual acuity in 24% of eyes with nAMD, mostly caused by macular atrophy [
33]. These differences in visual outcomes can possibly be explained by differences in the rates of macular atrophy in study cohorts as well as continuance and frequency of the anti-VEGF injections.
A recent register study documented that over one third of the patients with nAMD had discontinued intravitreal anti-VEGF treatment by the end of first year [
34]. Continuous and frequent treatment triggers a remarkable load not only for healthcare providers but also on the patients, although the injection frequency may decline over the years [
35]. In our cohort, intravitreal anti-VEGF-treatment was discontinued in cases of dry macula with no intra- or subretinal fluid observed, treatment resistance, or if the patients requested for discontinuation. Anti-VEGF injections were restarted in a case of renewal of active disease. Despite almost 6-year-breaks in anti-VEGF treatment in the inactive phase of the disease in some individuals, no reactivation of nAMD nor reduced visual acuity was noted after the follow-up. One may assume that marked cost savings can be achieved by active follow-up of discontinued patient cohort when compared to continuous and frequent injections in all patients. In this cohort of 1088 eyes, only a part, 64%, needed continuous anti-VEGF treatment for active nAMD. Some previous studies have shown that interruption of the regular treatment may have caused at least transient worsening of visual acuity mostly due to development of macular hemorrhages [
36,
37,
38,
39]. It seems possible that progression of nAMD may have occurred if the interruptions of injections were implemented while the disease was still active with choroidal neovascularization. Nassisi et al. reported no significant differences in visual outcomes in patients with a delay in anti-VEGF injections for a few months due to COVID-19 lockdown, and no development of atrophy or fibrosis at 6 months [
40].
Bevacitzumab was the first-line drug in almost all (99.5%) of the study eyes, which is in accordance with the Finnish Current Care Guidelines [
9]. Similarly, in a recent study by Nassisi et al. over 95% of the individuals with nAMD were treated using bevacizumab [
40]. Ross et al. compared the cost-effectiveness of aflibercept, bevacizumab and ranibizumab for treatment of diabetic macular edema. Aflibercept and ranibizumab were found to be 31 and 20 times more expensive than bevacizumab, respectively [
41]. Off-label use of bevacizumab in the management of both nAMD and diabetic macular edema has become commonly available throughout Europe and the US, because its efficacy and safety compares to that of other anti-VEGF agents with lower costs [
30,
42,
43]. Recurrent intravitreal injections typically at 4-8 week-intervals for increasing aging population with nAMD impose a high financial burden on health care. The eyes of the present study received 17 anti-VEGF injections on average during the follow-up, and the number of injections increased alongside with the number of letters gained. Recently, bispecific antibody faricimab that acts via dual inhibition of both VEGF-A and angiopoietin-2, has shown a possibility to extend the injection intervals up to 16 weeks with sustained efficacy for nAMD [
27]. The less intravitreal treatments with longer intervals between treatments would be helpful for reducing the burden and costs for both patients and health care providers.
Age-related cataract often coexists with AMD. The impact of cataract or secondary cataract was considered as the visual outcomes were analyzed. Pseudophakia has been found to be associated with better visual outcomes compared to phakic eyes [
44,
45]. In accordance, our results showed that approximately 60% of the patients with the most ≥15 ETDRS letter increase in visual acuity had undergone cataract surgery before or during the follow-up. In addition, after cataract surgery completed before or during the follow-up 64% and 75% of the eyes, respectively, had a gain of >5 ETDRS letters compared to 58% in the phakic eyes. However, marked increase in visual acuity was also noted in phakic eyes, which may suggest that not all individuals develop visually significant cataract and progressed cataract is likely to be treated timely in Finland according to the Finnish Current Care Guidelines for Cataract [
46]. Cataract surgery has been shown not to have clinically significant impact on the activity of pre-existent choroidal neovascularization secondary to nAMD and may be recommended to patients with nAMD and cataract that limits vision [
47].
Anti-VEGF agents have reduced visual impairment due to nAMD, and improved patients’ quality of life [
2,
48,
49]. In our study the number of patients with visual impairment increased during the 10-year-period, which reflects the natural course of the disease. It is, however, notable that one fourth of the patients with initial visual impairment benefit from anti-VEGF treatment and were no longer visually impaired at the end of the follow-up. Despite treatment, 14% of the study patients had declined visual acuity to the level of visual impairment by the end of the study period. Poor visual gain among patients with longstanding symptoms suggests that timely diagnosis and treatment of nAMD is prudent, and the delay in the initiation of treatment may have negative impact on visual outcomes.
The study has some limitations. There are variations in the duration of follow-up, amount of anti-VEGF injections and implementation of treatment due to real-life setting and retrospective nature of the study. The administration of anti-VEGF agents in various treatment regimens may vary and thus affect the treatment outcomes and the number of injections. In most cases, treatment started with monthly injections with anti-VEGF agents and continued according to the treatment response of the individual patient. The real-life setting might be considered as a strength of the present study, as well as a population-based cohort of 827 patients with 1088 eyes with nAMD in a long-term follow-up period. The implementation of the treatment and the results in the randomized, controlled studies may differ from those obtained from the real-world setting. In addition, the present study cohort includes only patients with nAMD in contrast to numerous studies including patients with any AMD.
4. Materials and methods
4.1. Characterization of the Study Population and Environment
The population-based cohort consisted of the patients with neovascular age-related macular degeneration (nAMD) diagnosed in Northern Ostrobothnia Hospital District in 2010-2016. The data from patient records was collected until 2019 to ensure sufficient duration of follow-up for all patients. The treatment of nAMD was completed at Oulu University Hospital responsible for tertiary care for a population of approximately 410 000 inhabitants. According to the data from Statistics Finland over 98% of the population aged over 75 years in Finland are of Finnish background (white Caucasian by ethnicity). In Finland, municipalities are responsible for organizing and financing health care, and every citizen is entitled to adequate health services. Specialized healthcare refers to examinations and treatments arranged in hospitals in specialized fields, such as ophthalmological examinations and treatment of eye diseases, including nAMD. Access to ophthalmic treatment requires a referral.
4.2. Diagnostic Evaluations for nAMD and Exclusion Criteria
The hospital’s electronic patient database was used to search for the nAMD patients treated with intravitreal anti-VEGF agents by using the ICD-10 (International Classification of Diseases) diagnosis code for nAMD (H35.31). All patients with a nAMD diagnosis had undergone a comprehensive ophthalmic examination, evaluation of best-corrected visual acuity and fundus imaging ((fundus photography, fluorescein angiogram, optical coherence tomography (OCT), optical coherence tomography angiography (OCT-A)) based on discretion of the treating physician and the availability of the imaging techniques during the follow-up period. The cases of dry AMD, polypoidal choroidal vasculopathy (PCV), myopic degeneration or other retinal disorders, such as diabetic macular edema, treated with anti-VEGF injections were excluded from the study. The patients with less than three consecutive anti-VEGF injections, follow-up time less than 3 months, or diagnosis of dry AMD were excluded from the study.
4.3. Treatment Regimens for nAMD
The patients with nAMD were treated following the PRN or treat -and-extend-regimens of nAMD following the Finnish Current Care Guidelines for Age-related Macular Degeneration [
9]. Generally, the first 3-6 injections were scheduled in 4-6 week-intervals and the response to treatment was assessed by evaluation of visual acuity, optical coherence tomography (OCT) and fundus photography. Intra- and subretinal fluid and macular hemorrhages were indications to continue anti-VEGF-treatment. In cases of dry macula, the patients were followed-up in 1-2-month-interval to diagnose the possible renewal of macular edema. Pro re nata (PRN) or treat and extend (T&E) regimens were followed. In Finland, bevacizumab is commonly the first-line choice for the treatment of nAMD.
4.4. Clinical Outcome Measures
Demographic and clinical data of the patients was collected and included parameters for age, gender, age at the diagnosis of nAMD, duration of ocular symptoms, time of follow-up, laterality, chronicity, best-corrected visual acuity, and the rate for visual impairment at the baseline and during the follow-up, the number of intravitreal injections, lens status (phakic or pseudophakic) and rates for cataract surgery or YAG-capsulotomy. The intravitreal anti-VEGF treatment was considered as continuous if the longest interval between the two injections was less than 4 months during the follow-up. The impact of the lens status on visual acuity was observed according to the relatively old age of the cohort patients and long follow-up period. The comprehensive ophthalmic evaluations, macular optical coherence tomography (OCT) and fundus photography were completed at the onset and repeatedly during the follow-up period. Visual acuity was evaluated routinely by Snellen E-chart, and the decimals were converted to ETDRS letters for the evaluation of changes in visual acuity. The WHO criteria of distance vision impairment (Snellen visual acuity worse than 0.3 or ETDRS less than 59 letters in a better eye) was used.
4.5. Ethical Aspects of the Study
The study followed the tenets of the Declaration of Helsinki and it was conducted with the approval of the Oulu University Hospital Research Committee (221/2016). A written informed consent was obtained from the participants at the time of the clinical ophthalmic evaluation.
4.6. Statistical Analysis
SPSS for Windows (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp) was used for data analysis. Between group comparisons for continuous variables were performed using analysis of variance or Brown-Forsythe test, the latter if homogeneity of variances test failed. Categorical data was analyzed using Pearson Chi-squared test.
5. Conclusions
These real-life results show that a great majority of patients with nAMD benefit from intravitreal anti-VEGF treatment in terms of stability or improvement in visual acuity.
Bevacizumab seems to be an effective and safe treatment for nAMD with relatively low costs.
The results of this study suggest that continuous anti-VEGF injections are not necessary in cases of dry macula after treatment, because several nAMD patients had long, even several-year intervals without frequent injections and still no decline in long term visual outcomes. This should be considered when the burden for nAMD treatment increases alongside with an increase in the number of aging populations.
Author Contributions
Conceptualization, NH and AMK; formal analysis and interpretation of data for the work, IK-G, AMK, PO, NH.; writing—original draft preparation, NH, AMK; writing—review and editing, IK-G, AMK, PO, NH; visualization, IK, AMK, PO and NH; supervision, NH. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a PPSHP VTR grant (K71769) and Finnish Eye Foundation (grant number 20220008).
Acknowledgments
The investigation was conducted at the Oulu University Hospital, Finland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Apte RS. Age-Related Macular Degeneration. N Engl J Med. 2021;385:539–47.
- Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. 2018;392:1147–59.
- Fernandes AR, Zielińska A, Sanchez-Lopez E, Dos Santos T, Garcia ML, Silva AM, et al. Exudative versus Nonexudative Age-Related Macular Degeneration: Physiopathology and Treatment Options. Int J Mol Sci [Internet]. 2022;23. [CrossRef]
- Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng C-Y, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16.
- Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31. [CrossRef]
- Fritsche LG, Igl W, Bailey JNC, Grassmann F, Sengupta S, Bragg-Gresham JL, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–43. [CrossRef]
- Heesterbeek TJ, Lorés-Motta L, Hoyng CB, Lechanteur YTE, den Hollander AI. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol Opt. 2020;40:140–70. [CrossRef]
- Rossi S, D’Amico M, Bucolo C, Sanderson J. Chronic Inflammation and Neurodegeneration in Retinal Disease. Frontiers Media SA; 2021. [CrossRef]
- Tuuminen R, Uusitalo-Järvinen H, Aaltonen V, Hautala N, Kaipiainen S, Laitamäki N, et al. The Finnish national guideline for diagnosis, treatment and follow-up of patients with wet age-related macular degeneration. Acta Ophthalmol. 2017;95:1–9.
- Liao DS, Grossi FV, El Mehdi D, Gerber MR, Brown DM, Heier JS, et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology. 2020;127:186–95.
- Wykoff CC, Rosenfeld PJ, Waheed NK, Singh RP, Ronca N, Slakter JS, et al. Characterizing New-Onset Exudation in the Randomized Phase 2 FILLY Trial of Complement Inhibitor Pegcetacoplan for Geographic Atrophy. Ophthalmology. 2021;128:1325–36. [CrossRef]
- Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120:106–14.
- Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology. 2020;127:616–36.
- Kyosseva SV. Targeting MAPK Signaling in Age-Related Macular Degeneration. Ophthalmol Eye Dis. 2016;8:23–30. [CrossRef]
- Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell. 2019;176:1248–64.
- Sarwar S, Clearfield E, Soliman MK, Sadiq MA, Baldwin AJ, Hanout M, et al. Aflibercept for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2016;2:CD011346.
- Li E, Donati S, Lindsley KB, Krzystolik MG, Virgili G. Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2020;5:CD012208. [CrossRef]
- Bucolo C, Eandi CM, Toro MD. Ocular Pharmacology: Recent Breakthroughs and Unmet Needs. Frontiers Media SA; 2023. [CrossRef]
- Khanani AM, Guymer RH, Basu K, Boston H, Heier JS, Korobelnik J-F, et al. TENAYA and LUCERNE: Rationale and Design for the Phase 3 Clinical Trials of Faricimab for Neovascular Age-Related Macular Degeneration. Ophthalmol Sci. 2021;1:100076.
- ElSheikh RH, Chauhan MZ, Sallam AB. Current and Novel Therapeutic Approaches for Treatment of Neovascular Age-Related Macular Degeneration. Biomolecules [Internet]. 2022;12. [CrossRef]
- Jern I, Forsell S, Norberg H. Eligibility for faricimab in a real-world neovascular age-related macular degeneration population: a cross-sectional study. BMJ Open. 2022;12:e065001. [CrossRef]
- Stanga PE, Valentín-Bravo FJ, Stanga SEF, Reinstein UI, Pastor-Idoate S, Downes SM. Faricimab in neovascular AMD: first report of real-world outcomes in an independent retina clinic. Eye [Internet]. 2023; http://dx.doi.org/10.1038/s41433-023-02505-z.
- Folk JC, Stone EM. Ranibizumab therapy for neovascular age-related macular degeneration. N Engl J Med. 2010;363:1648–55. [CrossRef]
- Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695.e1–15. [CrossRef]
- Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2020;127:72–84. [CrossRef]
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31.
- Heier JS, Khanani AM, Quezada Ruiz C, Basu K, Ferrone PJ, Brittain C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet. 2022;399:729–40. [CrossRef]
- Ciulla TA, Hussain RM, Taraborelli D, Pollack JS, Williams DF. Longer-Term Anti-VEGF Therapy Outcomes in Neovascular Age-Related Macular Degeneration, Diabetic Macular Edema, and Vein Occlusion-Related Macular Edema: Clinical Outcomes in 130 247 Eyes. Ophthalmol Retina. 2022;6:796–806. [CrossRef]
- Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119:1175–83.
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Maguire MG, Martin DF, Ying G-S, Jaffe GJ, Daniel E, et al. Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology. 2016;123:1751–61.
- Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120:2292–9. [CrossRef]
- MacCumber MW, Wykoff CC, Karcher H, Adiguzel E, Buxy Sinha S, Vishwakarma S, et al. One-Year Real-World Brolucizumab Outcomes in Neovascular Age-Related Macular Degeneration from a Large US Cohort in the IRIS® Registry. Ophthalmology [Internet]. 2023; http://dx.doi.org/10.1016/j.ophtha.2023.04.012.
- Chandra S, Arpa C, Menon D, Khalid H, Hamilton R, Nicholson L, et al. Ten-year outcomes of antivascular endothelial growth factor therapy in neovascular age-related macular degeneration. Eye . 2020;34:1888–96. [CrossRef]
- MacCumber MW, Yu JS, Sagkriotis A, B G, Burugapalli B, Bi X, et al. Antivascular endothelial growth factor agents for wet age-related macular degeneration: an IRIS registry analysis. Can J Ophthalmol [Internet]. 2021; http://dx.doi.org/10.1016/j.jcjo.2021.10.008.
- Leys AM, Ramboer E, Favreau M, Denhaerynck K, MacDonald K, Abraham I, et al. Long-Term Ranibizumab Treatment in Neovascular Age-Related Macular Degeneration: A Belgian Subanalysis from the Global Real-World LUMINOUS Study. Clin Ophthalmol. 2020;14:1473–81.
- Invernizzi A, Torre A, Parrulli S, Zicarelli F, Schiuma M, Colombo V, et al. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine. 2020;27:100550. [CrossRef]
- Romano F, Monteduro D, Airaldi M, Zicarelli F, Parrulli S, Cozzi M, et al. Increased Number of Submacular Hemorrhages as a Consequence of Coronavirus Disease 2019 Lockdown. Ophthalmol Retina. 2020;4:1209–10. [CrossRef]
- Borrelli E, Grosso D, Vella G, Sacconi R, Battista M, Querques L, et al. Short-term outcomes of patients with neovascular exudative AMD: the effect of COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258:2621–8. [CrossRef]
- Chong Teo KY, Saxena N, Gan A, Wong TY, Gillies MC, Chakravarthy U, et al. Detrimental Effect of Delayed Re-treatment of Active Disease on Outcomes in Neovascular Age-Related Macular Degeneration: The RAMPS Study. Ophthalmol Retina. 2020;4:871–80.
- Nassisi M, Pozzo Giuffrida F, Milella P, Ganci S, Aretti A, Mainetti C, et al. Delaying anti-VEGF therapy during the COVID-19 pandemic: long-term impact on visual outcomes in patients with neovascular age-related macular degeneration. BMC Ophthalmol. 2023;23:156. [CrossRef]
- Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR, et al. Cost-effectiveness of Aflibercept, Bevacizumab, and Ranibizumab for Diabetic Macular Edema Treatment: Analysis From the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial. JAMA Ophthalmol. 2016;134:888–96.
- Bro T, Derebecka M, Jørstad ØK, Grzybowski A. Off-label use of bevacizumab for wet age-related macular degeneration in Europe. Graefes Arch Clin Exp Ophthalmol. 2020;258:503–11. [CrossRef]
- Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36:331–5. [CrossRef]
- Hanhart J, Wiener R, Totah H, Brosh K, Zadok D. Pseudophakia as a surprising protective factor in neovascular age-related macular degeneration. J Fr Ophtalmol [Internet]. 2023; http://dx.doi.org/10.1016/j.jfo.2022.11.015.
- Kessel L, Koefoed Theil P, Lykke Sørensen T, Munch IC. Cataract surgery in patients with neovascular age-related macular degeneration. Acta Ophthalmol. 2016;94:755–60.
- Suomalaisen Lääkäriseuran Duodecimin, Suomen Silmälääkäriyhdistyksen Ja Suomen Silmäkirurgiyhdistyksen Asettama Työryhmä. [Update on current care guidelines: cataract in adults]. Duodecim. 2010;126:2541–2.
- Hogg HDJ, Chung N, Reed J, Berrett G, Pearce M, Di Simplicio S. An observational clinical study of the influence of phacoemulsification on choroidal neovascular membrane activity in age related macular degeneration. Eye . 2022;36:1379–83. [CrossRef]
- Purola P, Kaarniranta K, Ojamo M, Gissler M, Uusitalo H. Visual impairment due to age-related macular degeneration during 40 years in Finland and the impact of novel therapies. Acta Ophthalmol. 2023;101:57–64. [CrossRef]
- Finger RP, Guymer RH, Gillies MC, Keeffe JE. The impact of anti-vascular endothelial growth factor treatment on quality of life in neovascular age-related macular degeneration. Ophthalmology. 2014;121:1246–51. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).