Submitted:
31 May 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Patients and Methods
Results
Characteristics of the patients
Pathological characteristics of tumor lesions
Treatment characteristics and results
| Case | Surgical treatment |
Final surgical margin | Neoadjuvant therapy | Necrosis % post neoadjuvant therapy | Adjuvant therapy | Recurrences | Metastasis | Survival |
|---|---|---|---|---|---|---|---|---|
| 1 | Partial maxillectomy and eye enucleation | + | No | N/A | CTX: ifosfamide-cisplatin + RT: tomotherapy 6MV (5 cycles) Epirubicin (3 cycles) |
No | No | FOD 15 years |
| 2 | Partial maxillectomy | 1st Surgery: - 2nd Surgery: - 3rd Surgery: n/c 4th Surgery: + 5th Surgery: n/c |
CTX: cisplatin-adriamycin (1 cycle) | N/D | After 3º surgery (pregnant in 2º recurrence): CTX: cisplatin-adriamycin/ Methotrexate (5 cycles) |
3 local recurrences | N/D | N/D |
| 3 | Hemimandibulectomy | - | No | N/A | In recurrence (no surgery decided) CTX: ifosfamide |
1 local recurrence | No | Died |
| 4 | Hemimandibulectomy | - | No | N/A | No | No | No | FOD 15 years |
| 5 | Hemimandibulectomy | (<1 mm) | No | N/A | CTX: cisplatin-adriamycin (6 cycles) | No | No | FOD 9 years |
| 6 | Partial maxillectomy | - | No | N/A | CTX: cisplatin-adriamycin (5 cycles) | No | No | FOD 7 years |
| 7 | Partial maxillectomy | - | CTX: methotrexate-adriamicyn-cisplatin | 95% | CTX: adriamicyn (1 cycle) | No | No | FOD 4 years |
| 8 | Hemimandibulectomy | + | CTX: cisplatin-adriamycin (1 cycle) | 15% | CTX: ifosfamide-etoposide (1 cycle) | 1 local recurrence | Yes | Died |
Discussion
Conclusions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Vered M, Wright JM. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Odontogenic and Maxillofacial Bone Tumours. Head and Neck Pathol 2022;16:63-75.
- Clark JL, Unni KK, Dahlin DC, Devine KD. Osteosarcoma of the jaw. Cancer 1983;51:2311-2316. [CrossRef]
- Baumhoer D, MD. Bone-Related Lesions of the Jaws. Surgical Pathology Clinics 2017;10:693-704. [CrossRef]
- WHO Classification of Tumours Editorial Board, editor. Head and neck tumours. Lyon, France: International Agency for Research on Cancer; 2022.
- Chakravarthi PS, Kattimani VS, Prasad LK, Satish PR. Juxtacortical osteosarcoma of the mandible: Challenges in diagnosis and management. Natl J Maxillofac Surg 2015;6:127-131. [CrossRef]
- Lee RJ, Arshi A, Schwartz HC, Christensen RE. Characteristics and Prognostic Factors of Osteosarcoma of the Jaws: A Retrospective Cohort Study. JAMA Otolaryngology-- Head & Neck Surgery 2015;141:470-477. [CrossRef]
- Malik F, Gleysteen JP, Agarwal S. Osteosarcoma of the jaw: report of 3 cases (including the rare epithelioid variant) with review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol 2021;131:e71-e80. [CrossRef]
- Haefliger S, Harder D, Kovac M, Linkeschova K, Eufinger H, Baumhoer D. Osteosarcoma of the Mandible in a Patient with Florid Cemento-Osseous Dysplasia and Li-Fraumeni Syndrome: A Rare Coincidence. Head Neck Pathol 2021;15:704-708. [CrossRef]
- Bertin H, Gomez-Brouchet A, Rédini F. Osteosarcoma of the jaws: An overview of the pathophysiological mechanisms. Crit Rev Oncol Hematol 2020;156:103126. [CrossRef]
- Nissanka E, Amaratunge E, Tilakaratne W. Clinicopathological analysis of osteosarcoma of jaw bones. Oral Diseases 2007;13:82-87. [CrossRef]
- August M, Magennis P, Dewitt D. Osteogenic sarcoma of the jaws: factors influencing prognosis. International Journal of Oral and Maxillofacial Surgery 1997;26:198. [CrossRef]
- Granowski-LeCornu M, Chuang SK, Kaban LB, August M. Osteosarcoma of the jaws: factors influencing prognosis. J Oral Maxillofac Surg 2011;69:2368-2375. [CrossRef]
- Hameed M, Mandelker D. Tumor Syndromes Predisposing to Osteosarcoma. Adv Anat Pathol 2018;25:217-222. [CrossRef]
- Patel SG, Meyers P, Huvos AG, et al. Improved outcomes in patients with osteogenic sarcoma of the head and neck. Cancer 2002;95:1495-1503. [CrossRef]
- Guadagnolo BA, Zagars GK, Raymond AK, Benjamin RS, Sturgis EM. Osteosarcoma of the jaw/craniofacial region. Cancer 2009;115:3262-3270. [CrossRef]
- Piattelli A, Favia GF. Periosteal osteosarcoma of the jaws: report of 2 cases. J Periodontol 2000;71:325-329. [CrossRef]
- Costello L, Toner M, Pierse D, Stassen LFA. Osteosarcoma (osteogenic sarcoma) of the jaws presenting in general dental practice - a series of four cases. Br Dent J 2021;230:583-586. [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Jama 2013;310:2191-2194.
- Broders AC. The Grading of Carcinoma. Minn Med 1925;8:726-730.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [CrossRef]
- Doval DC, Kumar RV, Kannan V, et al. Osteosarcoma of the jaw bones. Br J Oral Maxillofac Surg 1997;35:357-362.
- Bertoni F, Dallera P, Bacchini P, Marchetti C, Campobassi A. The Istituto Rizzoli-Beretta experience with osteosarcoma of the jaw. Cancer 1991;68:1555-1563. [CrossRef]
- Delgado R, Maafs E, Alfeiran A, et al. Osteosarcoma of the jaw. Head Neck 1994;16:246-252.
- Fernandes R, Nikitakis NG, Pazoki A, Ord RA. Osteogenic sarcoma of the jaw: a 10-year experience. J Oral Maxillofac Surg 2007;65:1286-1291. [CrossRef]
- Ha PK, Eisele DW, Frassica FJ, Zahurak ML, McCarthy EF. Osteosarcoma of the head and neck: a review of the Johns Hopkins experience. Laryngoscope 1999;109:964-969. [CrossRef]
- Mardinger O, Givol N, Talmi YP, Taicher S. Osteosarcoma of the jaw. The Chaim Sheba Medical Center experience. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:445-451. [CrossRef]
- Ogunlewe MO, Ajayi OF, Adeyemo WL, Ladeinde AL, James O. Osteogenic sarcoma of the jaw bones: a single institution experience over a 21-year period. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006;101:76-81. [CrossRef]
- Junior AT, de Abreu Alves F, Pinto CAL, Carvalho AL, Kowalski LP, Lopes MA. Clinicopathological and immunohistochemical analysis of twenty-five head and neck osteosarcomas. Oral Oncol 2003;39:521-530. [CrossRef]
- Paparella ML, Olvi LG, Brandizzi D, Keszler A, Santini-Araujo E, Cabrini RL. Osteosarcoma of the jaw: an analysis of a series of 74 cases. Histopathology 2013;63:551-557. [CrossRef]
- Tanzawa H, Uchiyama S, Sato K. Statistical observation of osteosarcoma of the maxillofacial region in Japan. Analysis of 114 Japanese cases reported between 1930 and 1989. Oral Surg Oral Med Oral Pathol 1991;72:444-448. [CrossRef]
- Brown JM, Steffensen A, Trump B. Clinical features and overall survival of osteosarcoma of the mandible. Int J Oral Maxillofac Surg 2022; 52:524-530. [CrossRef]
- Garrington GE, Scofield HH, Cornyn J, Hooker SP. Osteosarcoma of the jaws. Analysis of 56 cases. Cancer 1967;20:377-391. [CrossRef]
- Ajura AJ, Lau SH. A retrospective clinicopathological study of 59 osteogenic sarcoma of jaw bone archived in a stomatology unit. Malays J Pathol 2010;32:27-34.
- Bennett JH, Thomas G, Evans AW, Speight PM. Osteosarcoma of the jaws: A 30-year retrospective review. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 2000;90:323-333. [CrossRef]
- Givol N, Buchner A, Taicher S, Kaffe I. Radiological features of osteogenic sarcoma of the jaws. A comparative study of different radiographic modalities. Dentomaxillofac Radiol 1998;27:313-320. [CrossRef]
- Lindqvist C, Teppo L, Sane J, Holmström T, Wolf J. Osteosarcoma of the mandible: analysis of nine cases. J Oral Maxillofac Surg 1986;44:759-764. [CrossRef]
- Chaudhary M, Chaudhary SD. Osteosarcoma of jaws. J Oral Maxillofac Pathol 2012;16:233-238.
- ALQahtani D, AlSheddi M, Al-Sadhan R. Epithelioid Osteosarcoma of the Maxilla: A Case Report and Review of the Literature. Int J Surg Pathol 2015;23:495-499.
- Okada K, Hasegawa T, Yokoyama R. Rosette-forming epithelioid osteosarcoma: a histologic subtype with highly aggressive clinical behavior. Hum Pathol 2001;32:726-733. [CrossRef]
- Unni KK, Dahlin DC. Osteosarcoma: pathology and classification. Semin Roentgenol 1989;24:143-152. [CrossRef]
- Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 1990;250:1233-1238. [CrossRef]
- Tilden W, Saifuddin A. An update on imaging of Paget's sarcoma. Skeletal Radiol 2021;50:1275-1290. [CrossRef]
- Scranton PE,Jr, DeCicco FA, Totten RS, Yunis EJ. Prognostic factors in osteosarcoma. A review of 20 year's experience at the University of Pittsburgh Health Center Hospitals. Cancer 1975;36:2179-2191. [CrossRef]
- Lucas DR, Unni KK, McLeod RA, O'Connor MI, Sim FH. Osteoblastoma: clinicopathologic study of 306 cases. Hum Pathol 1994;25:117-134. [CrossRef]
- Kimura Y, Tomihara K, Tachinami H, et al. Conventional osteosarcoma of the mandible successfully treated with radical surgery and adjuvant chemotherapy after responding poorly to neoadjuvant chemotherapy: a case report. J Med Case Rep 2017;11:210-0. [CrossRef]
- Canadian Society of Otolaryngology-Head and Neck Surgery Oncology Study Group. Osteogenic sarcoma of the mandible and maxilla: a Canadian review (1980-2000). J Otolaryngol 2004;33:139-144.
- Thiele OC, Freier K, Bacon C, Egerer G, Hofele CM. Interdisciplinary combined treatment of craniofacial osteosarcoma with neoadjuvant and adjuvant chemotherapy and excision of the tumour: a retrospective study. Br J Oral Maxillofac Surg 2008;46:533-536. [CrossRef]
- Thariat J, Julieron M, Brouchet A, et al. Osteosarcomas of the mandible: are they different from other tumor sites? Crit Rev Oncol Hematol 2012;82:280-295. [CrossRef]
- Friebele JC, Peck J, Pan X, Abdel-Rasoul M, Mayerson JL. Osteosarcoma: A Meta-Analysis and Review of the Literature. Am J Orthop (Belle Mead NJ) 2015;44:547-553.
- Khadembaschi D, Jafri M, Praveen P, Parmar S, Breik O. Does neoadjuvant chemotherapy provide a survival benefit in maxillofacial osteosarcoma: A systematic review and pooled analysis. Oral Oncol 2022;135:106133. [CrossRef]
- Bouaoud J, Beinse G, Epaillard N, et al. Lack of efficacy of neoadjuvant chemotherapy in adult patients with maxillo-facial high-grade osteosarcomas: A French experience in two reference centers. Oral Oncol 2019;95:79-86. [CrossRef]
- Grimer R, Athanasou N, Gerrand C, et al. UK Guidelines for the Management of Bone Sarcomas. Sarcoma 2010;2010:317462. [CrossRef]
- Jasnau S, Meyer U, Potratz J, et al. Craniofacial osteosarcoma Experience of the cooperative German-Austrian-Swiss osteosarcoma study group. Oral Oncol 2008;44:286-294. [CrossRef]
- Yamaguchi DT. "Ins" and "Outs" of mesenchymal stem cell osteogenesis in regenerative medicine. World J Stem Cells 2014;6:94-110.
- Weber M, Söder S, Sander J, et al. Craniofacial Osteosarcoma-Pilot Study on the Expression of Osteobiologic Characteristics and Hypothesis on Metastasis. Front Oncol 2020;10:745. [CrossRef]
- Saito K, Unni KK, Wollan PC, Lund BA. Chondrosarcoma of the jaw and facial bones. Cancer 1995;76:1550-1558. [CrossRef]
- Clark JL, Unni KK, Dahlin DC, Devine KD. Osteosarcoma of the jaw. Cancer 1983;51:2311-2316.
- van den Berg H, Schreuder WH, de Lange J. Osteosarcoma: A Comparison of Jaw versus Nonjaw Localizations and Review of the Literature. Sarcoma 2013;2013:316123. [CrossRef]
- van Es RJ, Keus RB, van der Waal I, Koole R, Vermey A. Osteosarcoma of the jaw bones. Long-term follow up of 48 cases. Int J Oral Maxillofac Surg 1997;26:191-197.
- Mark RJ, Sercarz JA, Tran L, Dodd LG, Selch M, Calcaterra TC. Osteogenic sarcoma of the head and neck. The UCLA experience. Arch Otolaryngol Head Neck Surg 1991;117:761-766.
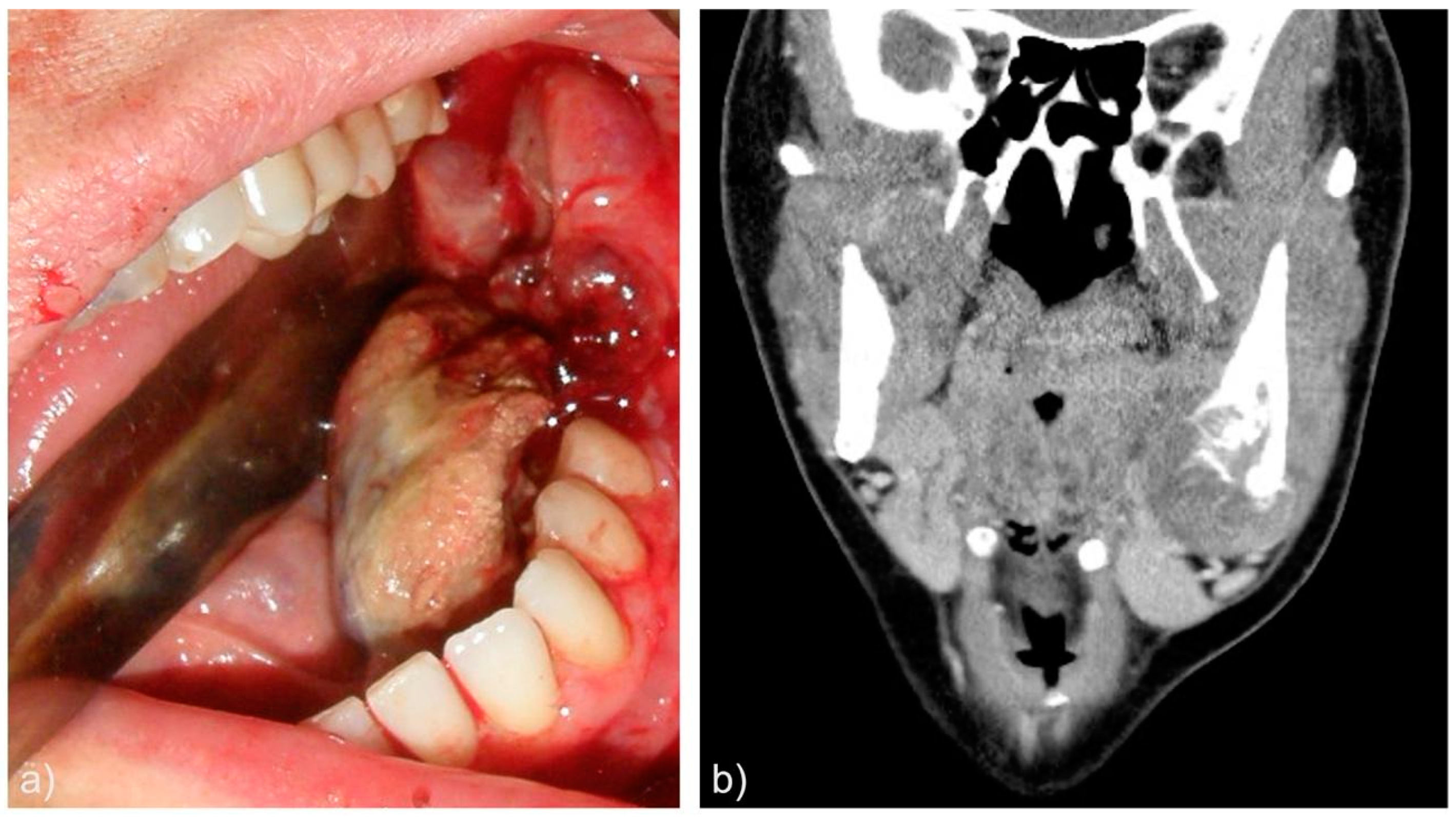
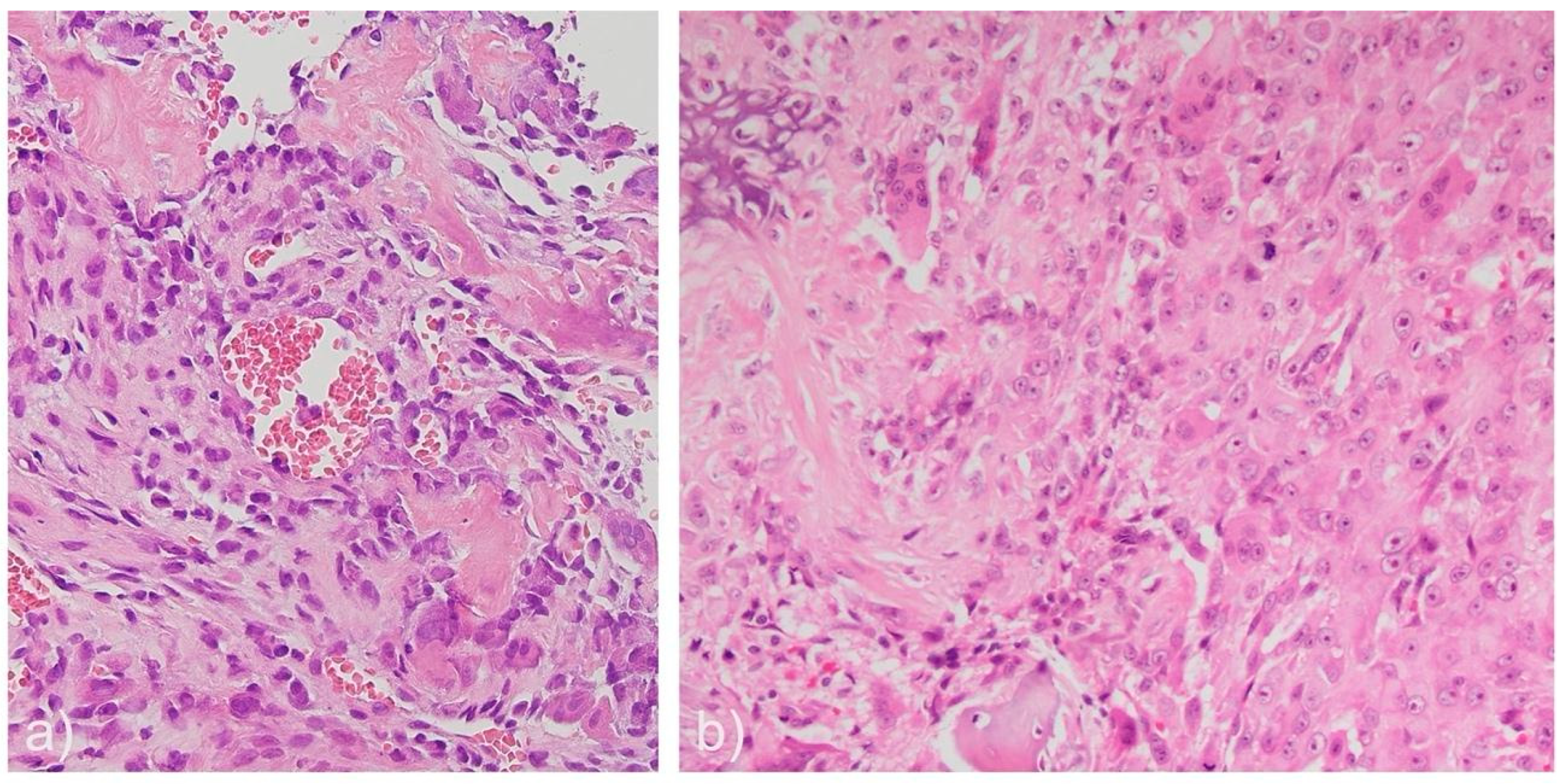
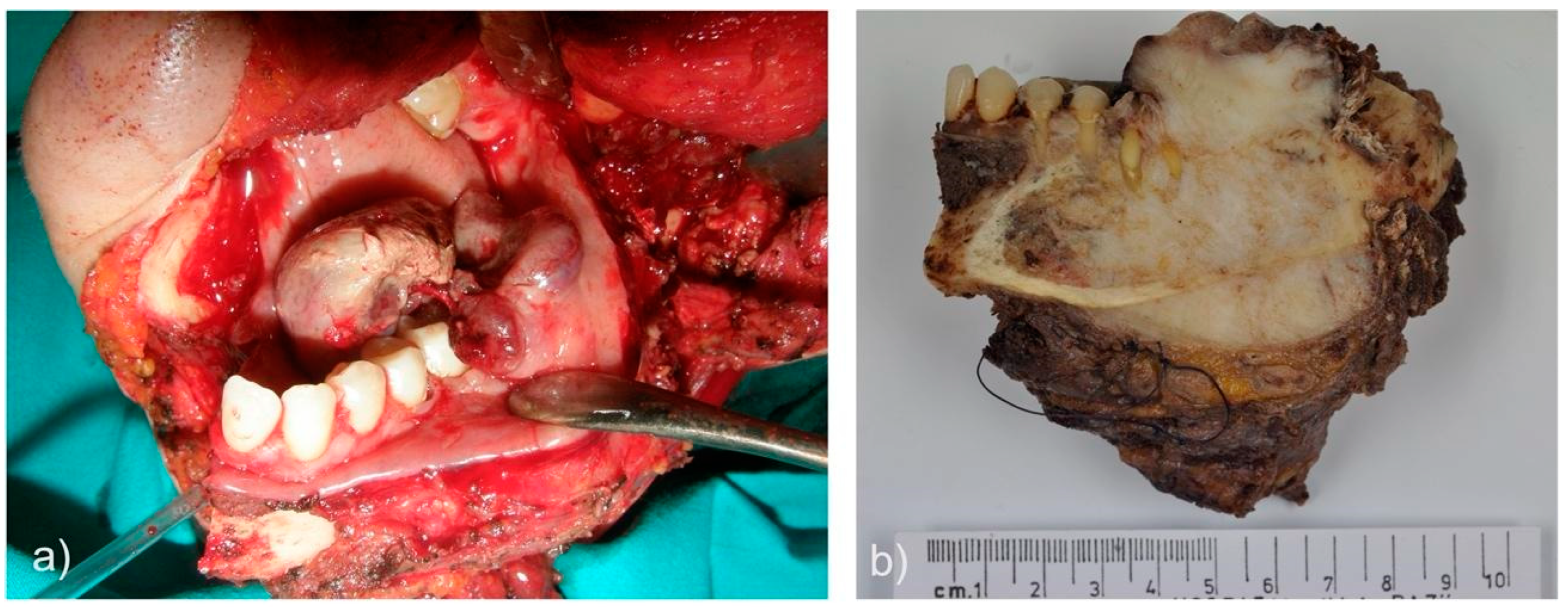
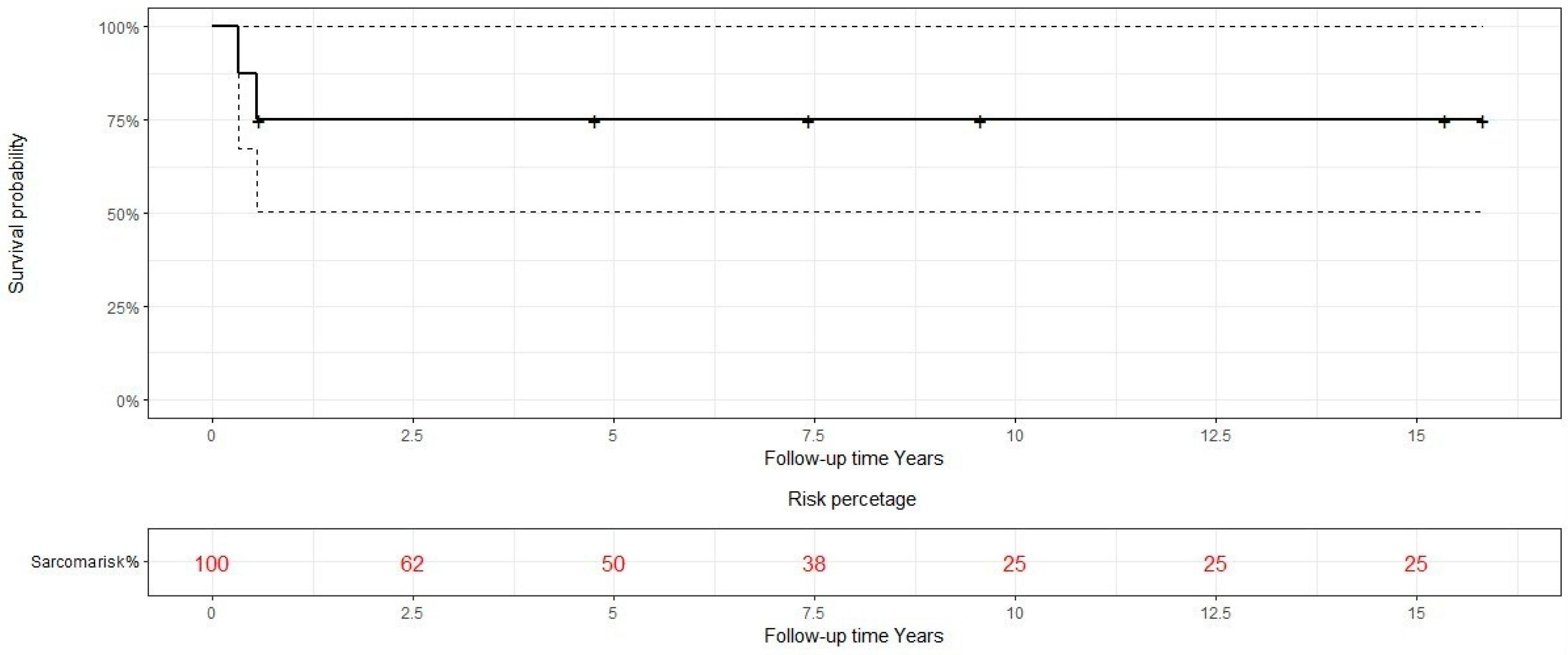
| Case | Age | Sex | Medical History | Signs and symptoms | Bone | Side |
|---|---|---|---|---|---|---|
| 1 | 51 | M | Pneumothorax at age 26 | -Sensation of nasal obstruction -Progressive proptosis -Diplopia |
Maxilla | Left |
| 2 | 35 | F | None | Painful swelling | Maxilla | Left |
| 3 | 56 | F | Cavum cancer (T2N2M0) at age 45. Treated with CT and RT | Painful swelling | Mandible (angle) | Right |
| 4 | 56 | F | None | Asymptomatic mass | Mandible (body and angle) | Right |
| 5 | 49 | F | Uterine myoma. Breast fibroadenoma | -Inferior dental nerve hypoesthesia -Painful swelling -Dental mobility of involved teeth |
Mandible (body and angle) | Left |
| 6 | 47 | F | Maxilar ossifying fibroma at age 42 | Asymptomatic mass | Maxilla | Right |
| 7 | 10 | F | Retinoblastoma at age 3. Treated with CT and RT. Right eye enucleation |
Painful swelling | Maxilla | Right |
| 8 | 31 | F | Appendectomy at age 21 | Painful swelling Oral cavity bleeding |
Mandible (body and angle) | Left |
| Case | Histological morphology | Grade | Broders Classification | Soft tissue involvement | Lymphovascular invasion |
|---|---|---|---|---|---|
| 1 | Fibroblastic | High | Grade 3-4 | No | No |
| 2 | Chondroblastic | High | Grade 3 | No Yes (after recurrence) |
No Yes (after 3rd recurrence) |
| 3 | Epithelioid | High | Grade 3 | Yes | No |
| 4 | Osteoblastic | High | Grade 4 | No | No |
| 5 | Osteoblastic | High | Grade 3 | Yes | No |
| 6 | Chondroblastic | High | Grade 2-3 | Yes | No |
| 7 | Osteoblastic | High | Grade 4 | No | No |
| 8 | Epithelioid | High | Grade 4 | No | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





