Submitted:
01 June 2023
Posted:
01 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Fish and Rearing Conditions
2.2. Experimental Design

2.3. Hypoxic Shock and Sampling Procedure
2.4. Hematological Analysis
2.6. Physiological Analysis
2.7. Statistical Analysis
3. Results
3.1. Treatment Effects
3.2. Time Effects
3.3. Interaction Effects
4. Discussion
4.3. Physiological Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
References
- FAO. 2022. The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome.
- Petitjean, Q., Jean, S., Gandar, A., Côte, J., Laffaille, P., & Jacquin, L. (2019). Stress responses in fishes: From molecular to evolutionary processes. Science of The Total Environment. [CrossRef]
- Bera, A., Sawant, P. B., Dasgupta, S., Chadha, N. K., Sawant, B. T., & Pal, A. K. (2017). Diel cyclic hypoxia alters plasma lipid dynamics and impairs reproduction in goldfish (Carassius auratus). Fish physiology and biochemistry, 43, 1677-1688. [CrossRef]
- Abdel-Tawwab, M., Monier, M. N., Hoseinifar, S. H., & Faggio, C. (2019). Fish response to hypoxia stress: growth, physiological, and immunological biomarkers. Fish Physiology and Biochemistry, 45(3), 997–1013. [CrossRef]
- Xiao, W. (2015). The hypoxia signaling pathway and hypoxic adaptation in fishes. Science China. Life Sciences, 58(2), 148–155. [CrossRef]
- Magnoni, L. J., Novais, S. C., Eding, E., Leguen, I., Lemos, M. F. L., Ozório, R. O. A., Geurden, I., Prunet, P., & Schrama, J. W. (2019). Acute stress and an electrolyte- imbalanced diet, but not chronic hypoxia, increase oxidative stress and hamper innate immune status in a rainbow trout (Oncorhynchus mykiss) isogenic line. Frontiers in Physiology, 10, 453. [CrossRef]
- Barton, B. A. (2002). Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integrative and Comparative Biology, 42(3), 517–525. [CrossRef]
- Balasch, J. C., & Tort, L. (2019). Netting the stress responses in fish. Frontiers in Endocrinology, 10, 62. [CrossRef]
- Schreck, C. B., & Tort, L. (2016). The concept of stress in fish. In C. B. Schreck, L. Tort, A. P. Farrell, & C. J. Brauner (Eds.), Fish Physiology (Vol. 35, pp. 1–34). Elsevier.
- Conde-Sieira, M., Valente, L. M. P., Hernández-Pérez, J., Soengas, J. L., Míguez, J. M., & Gesto, M. (2018). Short-term exposure to repeated chasing stress does not induce habituation in Senegalese sole, Solea senegalensis. Aquaculture (Amsterdam, Netherlands), 487, 32–40. [CrossRef]
- Nilsson, J., Stien, L. H., Fosseidengen, J. E., Olsen, R. E., & Kristiansen, T. S. (2012). From fright to anticipation: Reward conditioning versus habituation to a moving dip net in farmed Atlantic cod (Gadus morhua). Applied Animal Behaviour Science, 138(1–2), 118–124. [CrossRef]
- Koakoski, G., Kreutz, L. C., Fagundes, M., Oliveira, T. A., Ferreira, D., Rosa, J. G. S. da, & Barcellos, L. J. G. (2013). Repeated stressors do not provoke habituation or accumulation of the stress response in the catfish Rhamdia quelen. Neotropical ichthyology, 11(2), 453–457. [CrossRef]
- Fazio, F. (2019). Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture, 500, 237-242. [CrossRef]
- Pavlidis, M., Futter, W. C., Katharios, P., & Divanach, P. (2007). Blood cell profile of six Mediterranean mariculture fish species. Journal of Applied Ichthyology, 23(1), 70-73. [CrossRef]
- Fish, E. J., Hansen, S. C., Spangler, E. A., Gaillard, P. R., Fan, S., & Bacek, L. M. (2019). Retrospective evaluation of serum/plasma iron, red blood cell distribution width, and nucleated red blood cells in dogs with acute trauma (2009–2015): 129 cases. Journal of Veterinary Emergency and Critical Care, 29(5), 521-527.
- Groff, J. M., & Zinkl, J. G. (1999). Hematology and clinical chemistry of cyprinid fish: common carp and goldfish. Veterinary Clinics of North America: Exotic Animal Practice, 2(3), 741-776. [CrossRef]
- Pollock, M. S., Clarke, L. M. J., & Dubé, M. G. (2007). The effects of hypoxia on fishes: from ecological relevance to physiological effects. Environmental Reviews, 15(NA), 1-14. [CrossRef]
- Pilgaard, L., Malte, H., & Jensen, F. B. (1994). Physiological effects and tissue accumulation of copper in freshwater rainbow trout (Oncorhynchus mykiss) under normoxic and hypoxic conditions. Aquatic toxicology, 29(3-4), 197-212. [CrossRef]
- Sappal, R., Fast, M., Purcell, S., MacDonald, N., Stevens, D., Kibenge, F., ... & Kamunde, C. (2016). Copper and hypoxia modulate transcriptional and mitochondrial functional-biochemical responses in warm acclimated rainbow trout (Oncorhynchus mykiss). Environmental Pollution, 211, 291-306. [CrossRef]
- Zhang, Y., Healy, T. M., Vandersteen, W., Schulte, P. M., & Farrell, A. P. (2018). A rainbow trout Oncorhynchus mykiss strain with higher aerobic scope in normoxia also has superior tolerance of hypoxia. Journal of Fish Biology, 92(2), 487-503.
- Kolosov, D., & Kelly, S. P. (2019). The mineralocorticoid receptor contributes to barrier function of a model fish gill epithelium. The Journal of Experimental Biology, 222(Pt 11), jeb192096. [CrossRef]
- Carbajal, A., Soler, P., Tallo-Parra, O., Isasa, M., Echevarria, C., Lopez-Bejar, M., & Vinyoles, D. (2019). Towards non-invasive methods in measuring fish welfare: the measurement of cortisol concentrations in fish skin mucus as a biomarker of habitat quality. Animals, 9(11), 939. [CrossRef]
- Acerete, L., Balasch, J. C., Espinosa, E., Josa, A., & Tort, L. (2004). Physiological responses in Eurasian perch (Perca fluviatilis, L.) subjected to stress by transport and handling. Aquaculture, 237(1-4), 167-178. [CrossRef]
- Pichavant, K., Maxime, V., Thebault, M. T., Ollivier, H., Garnier, J. P., Bousquet, B., ... & Nonnotte, G. (2002). Effects of hypoxia and subsequent recovery on turbot Scophthalmus maximus: hormonal changes and anaerobic metabolism. Marine Ecology Progress Series, 225, 275-285. [CrossRef]
- Aboagye, D. L., & Allen, P. J. (2018). Effects of acute and chronic hypoxia on acid-base regulation, hematology, ion, and osmoregulation of juvenile American paddlefish. Journal of Comparative Physiology. B, Biochemical, Systemic, and Environmental Physiology, 188(1), 77–88. [CrossRef]
- Muusze, B., Marcon, J., van den Thillart, G., & Almeida-Val, V. (1998). Hypoxia tolerance of Amazon fish: respirometry and energy metabolism of the cichlid Astronotus ocellatus. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 120(1), 151-156.
- Petersen, L. H., & Gamperl, A. K. (2011). Cod (Gadus morhua) cardiorespiratory physiology and hypoxia tolerance following acclimation to low-oxygen conditions. Physiological and Biochemical Zoology, 84(1), 18-31. [CrossRef]
- Remen, M., Oppedal, F., Torgersen, T., Imsland, A. K., & Olsen, R. E. (2012). Effects of cyclic environmental hypoxia on physiology and feed intake of post-smolt Atlantic salmon: initial responses and acclimation. Aquaculture, 326, 148-155. [CrossRef]
- Caldwell, C. A., & Hinshaw, J. (1994). Physiological and haematological responses in rainbow trout subjected to supplemental dissolved oxygen in fish culture. Aquaculture, 126(1-2), 183-193. [CrossRef]
- Swift, D. J., & Lloyd, R. (1974). Changes in urine flow rate and haematocrit value of rainbow trout Salmo gairdneri (Richardson) exposed to hypoxia. Journal of Fish Biology, 6(4), 379-387. [CrossRef]
- Khan, S., Ahmed, S., & Saifullah, M. K. (2017). Haematological studies on Trichogaster fasciatus fish infected with Clinostomum complanatum metacercariae. Journal of Biological Sciences and Medicine, 3(4), 35-39.
- Goran, S. M. A., Omar, S. S., & Anwer, A. Y. (2017, September). Assessment of yeast as a dietary additive on haematology and water quality of common carp in a recirculating aquaculture system. In AIP conference proceedings (Vol. 1888, No. 1, p. 020023). AIP Publishing LLC.
- Maiolo, A. T., Gidiuli, R., Damilano, I., Massaro, P., & Polli, e. e. (1991). Relevance of red cell distribution width (RDW) in the differential diagnosis of microcytic anaemias. Clinical & Laboratory Haematology, 13(2), 141-151. [CrossRef]
- Sheng, Y., Hua, Z. Y., Yang, Z., Wei, X. L., Sheng, Y. J., Jia, H. L., & Jun, Q. (2019). Effects of acute hypoxic stress on biochemical parameters, immune regulation and metabolic capacity of the blood in genetically improved farmed tilapia (GIFT, Oreochromis niloticus). Journal of Applied Ichthyology. [CrossRef]
- Dagoudo, M., Qiang, J., Bao, J.-W., Tao, Y.-F., Zhu, H.-J., Tumukunde, E. M., Ngoepe, T. K., & Xu, P. (2021). Effects of acute hypoxia stress on hemato-biochemical parameters, oxidative resistance ability, and immune responses of hybrid yellow catfish (Pelteobagrus fulvidraco × P. vachelli) juveniles.Aquaculture International: Journal of the European Aquaculture Society, 29(5), 2181–2196. [CrossRef]
- McLeay, D. J. (1973). Effects of cortisol and dexamethasone on the pituitary-interrenal axis and abundance of white blood cell types in juvenile coho salmon, Oncorhynchus kisutch. General and Comparative Endocrinology, 21(3), 441-450. [CrossRef]
- Wojtaszek, J., Dziewulska-Szwajkowska, D., Łozińska-Gabska, M., Adamowicz, A., & Dżugaj, A. (2002). Hematological effects of high dose of cortisol on the carp (Cyprinus carpio L.): cortisol effect on the carp blood. General and comparative endocrinology, 125(2), 176-183. [CrossRef]
- Mazeaud, M. M., Mazeaud, F., & Donaldson, E. M. (1977). Primary and secondary effects of stress in fish: some new data with a general review. Transactions of the American Fisheries Society, 106(3), 201-212. [CrossRef]
- O’Connor, E. A., Pottinger, T. G., & Sneddon, L. U. (2011). The effects of acute and chronic hypoxia on cortisol, glucose and lactate concentrations in different populations of three-spined stickleback. Fish Physiology and Biochemistry, 37(3), 461–469. [CrossRef]
- 40. Pages. (1995). Effects of daily management stress on haematology and blood rheology of the gilthead seabream. Journal of fish biology, 46(5), 775–786. [CrossRef]
- Pottinger, T. G., & Carrick, T. R. (1999). Modification of the plasma cortisol response to stress in rainbow trout by selective breeding. General and comparative endocrinology, 116(1), 122-132. [CrossRef]
- Barcellos, L. J. G., Kreutz, L. C., de Souza, C., Rodrigues, L. B., Fioreze, I., Quevedo, R. M., ... & Terra, S. (2004). Hematological changes in jundiá (Rhamdia quelen Quoy and Gaimard Pimelodidae) after acute and chronic stress caused by usual aquacultural management, with emphasis on immunosuppressive effects. Aquaculture, 237(1-4), 229-236.
- Franco-Martinez, L., Brandts, I., Reyes-López, F., Tort, L., Tvarijonaviciute, A., & Teles, M. (2022). Skin mucus as a relevant low-invasive biological matrix for the measurement of an acute stress response in rainbow trout (Oncorhynchus mykiss). Water, 14(11), 1754.
- Gesto, M., López-Patiño, M. A., Hernández, J., Soengas, J. L., & Míguez, J. M. (2015). Gradation of the stress response in rainbow trout exposed to stressors of different severity: the role of brain serotonergic and dopaminergic systems. Journal of Neuroendocrinology, 27(2), 131–141. [CrossRef]
- 45. Gesto, Manuel, López-Patiño, M. A., Hernández, J., Soengas, J. L., & Míguez, J. M. (2013). The response of brain serotonergic and dopaminergic systems to an acute stressor in rainbow trout: a time course study. The Journal of Experimental Biology, 216(Pt 23), 4435–4442. [CrossRef]
- Dunn, J. F., & Hochachka, P. W. (1986). Metabolic responses of trout (Salmo gairdneri) to acute environmental hypoxia. Journal of Experimental Biology, 123(1), 229-242. [CrossRef]
- Richards, J. G. (2011). Physiological, behavioral and biochemical adaptations of intertidal fishes to hypoxia. Journal of Experimental Biology, 214(2), 191-199. [CrossRef]
- Tort, L., Montero, D., Robaina, L., Fernandez-Palacios, H., & Izquierdo, M. S. (2001). Consistency of stress response to repeated handling in the gilthead sea bream Sparus aurata Linnaeus, 1758. Aquaculture Research,32(7), 593–598. [CrossRef]
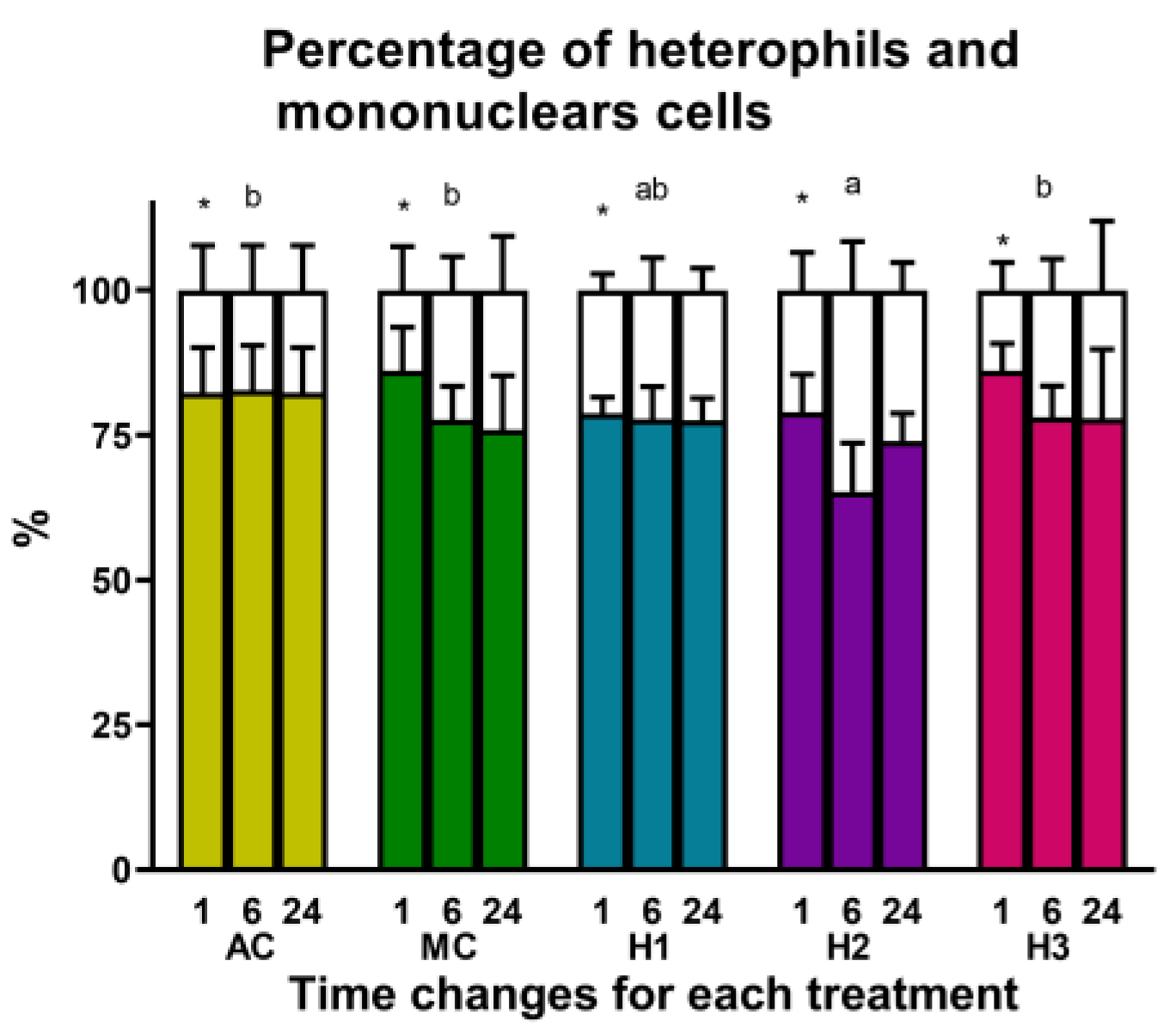
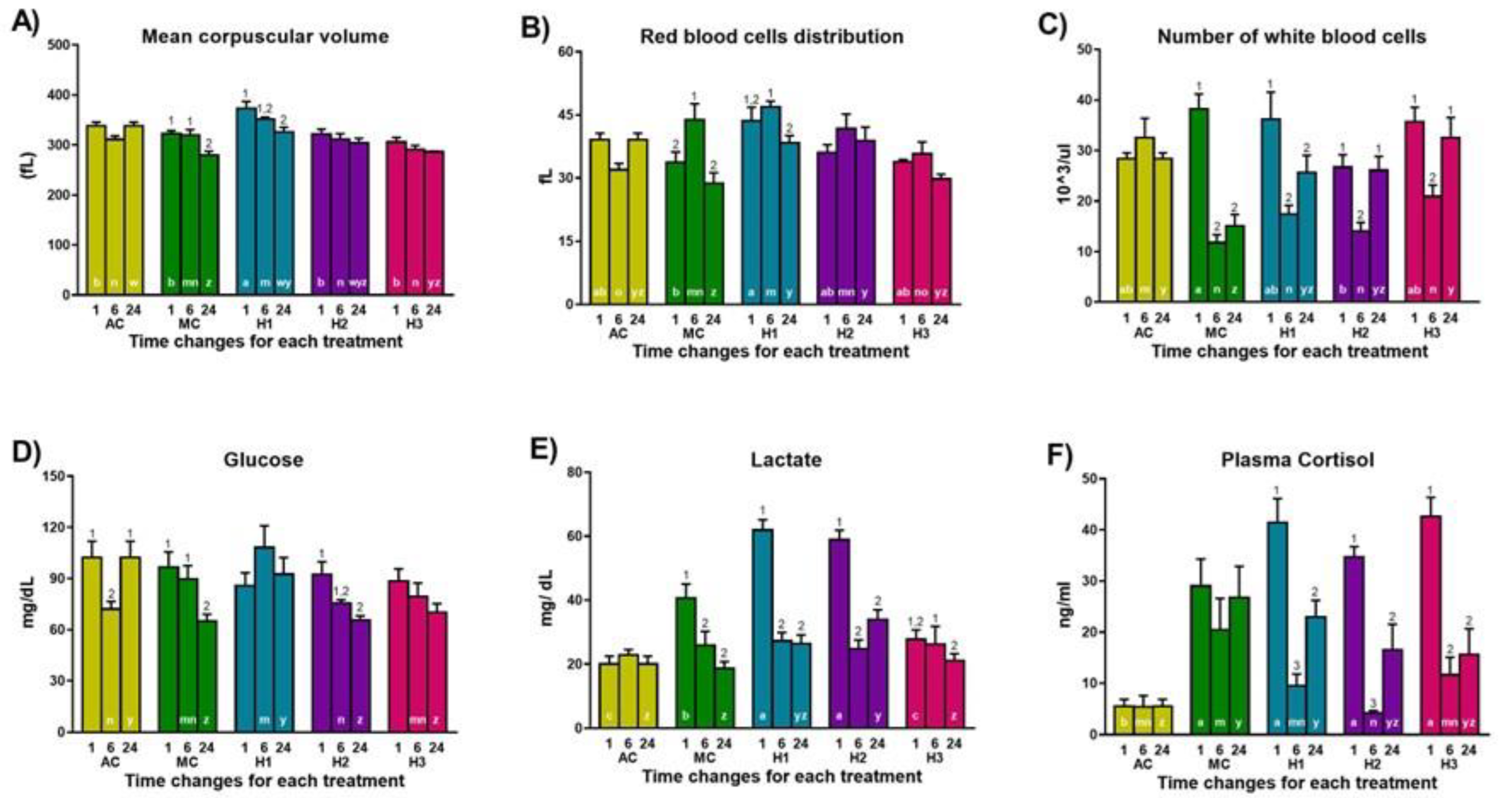
| Group | RBC (103/µL) | HGB (g/dL) | PLT (103/µL) | MCH (pg) |
|---|---|---|---|---|
| AC | 0.961 ± 0.0224 b | 5.57 ± 0.108 ab | 2.34 ± 0.140 | 50.8 ± 0.770 ab |
| MC | 1.058 ± 0.0229 a | 5.78 ± 0.110 a | 2.34 ± 0.162 | 48.3 ± 0.770 bc |
| H1 | 0.939 ± 0.0224 b | 5.61 ± 0.108ab | 2.38 ± 0.149 | 52.8 ± 0.770 a |
| H2 | 1.031 ± 0.0229 a | 5.77 ± 0.123 ab | 2.28 ± 0.154 | 49.5 ± 0.846 bc |
| H3 | 0.975 ± 0.0224 ab | 5.35 ± 0.108 b | 2.61 ± 0.154 | 47.6 ± 0.770 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





