1. Introduction
Olive germplasm includes more than 2,000 different cultivars, most of them very ancient and restricted to their area of origin [
1], usually in the Mediterranean area. This wide diversity is hosted in many Germplasm Banks, whose evaluation has shown high variability for many agronomic traits [
2,
3]. These repositories are essential to look for genetic variability for fighting against the challenges that threaten olive cultivation, such as diseases [
4] or climate warming [
5].
One of the main effects of climate warming on olive growing could be attributable to the increase in winter temperatures, which may affect flowering [
6]. Many models have been developed to predict how climate change could modify the areas suitable for olive growing [
7,
8,
9]. However, most of these models were based on data taken on the Mediterranean area, where winter temperatures currently fulfil the olive chilling needs for normal flowering [
10,
11]. And normally, those models included data on single or few cultivars. Analyzing the variability for flowering phenology of several cultivars , little genetic variability was observed [
12], even when a large set of cultivars were tested [
10,
13]. However, these works have always been carried out under Mediterranean conditions fulfilling the winter chilling requirements of olive.
To overcome this geographical limitation, some works have been carried out using field observations in conditions different from the current Mediterranean climate by artificially modifying the air temperature [
14], in variable natural conditions in Argentina [
15], and in the Subtropical climate of Tenerife, Canary Islands, with high winter temperature [
16]. Again, in these cases, only one or very few cultivars were tested, limiting the scope of the results obtained.
Therefore, in the present study, we evaluate the genetic variability for flowering phenology of a set of cultivars in the Subtropical climate of Tenerife with high winter temperatures. And we compare them with the phenology of the same cultivars grown in Cordoba, southern Iberian Peninsula, a typical Mediterranean growing area. The interaction between cultivar and contrasting environmental effects is also be evaluated.
3. Results
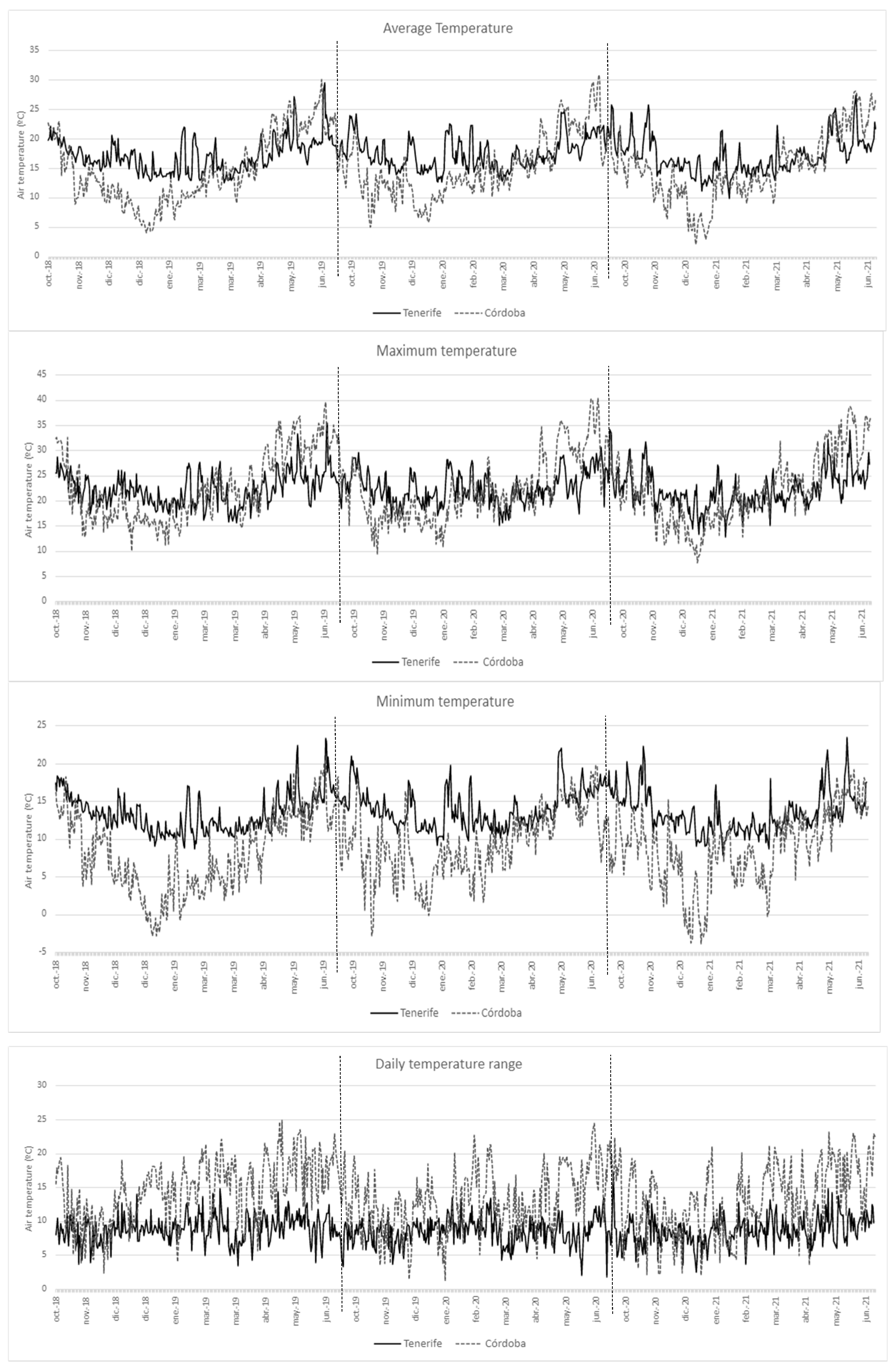
As expected, Tenerife and Cordoba have contrasting climatic characteristics (
Figure 2). Air temperature in Tenerife was in general, milder than in Cordoba. Autumn and winter were colder in Cordoba, with minimum air temperatures below 0ºC in late December and early January. While in Tenerife, the minimum daily winter temperatures were below 10 ºC on only 8 days of the year, while maximum temperatures were above 20 ºC on almost all days of the winter. Besides, spring was hotter in Cordoba, with maximum values reaching 40ºC in May and June. In Tenerife, the highest temperatures recorded during the study were below 35ºC. Differences were also observed in the daily temperature range, which in Cordoba was sometimes close to 25ºC, while in Tenerife rarely exceeded 15ºC.
All these climatic differences between Cordoba and Tenerife promotes significant differences in flowering phenology. Indeed, the analysis of variance for the flowering phenology parameters (
Table 1) showed that environment (defining each year-location combination as a different environment) was the main and significant contributor to the variability of both flowering period (FP) and full bloom date (FBD). For both FP and FBD, the cultivar x environment interaction was also significant. For full bloom period (FBP), only environment was significant, but with little amount of the total variability. However, in the latter case, a high error term of the percentage of the variance was observed, indicating a high variance among olive trees of each cultivar and environment.
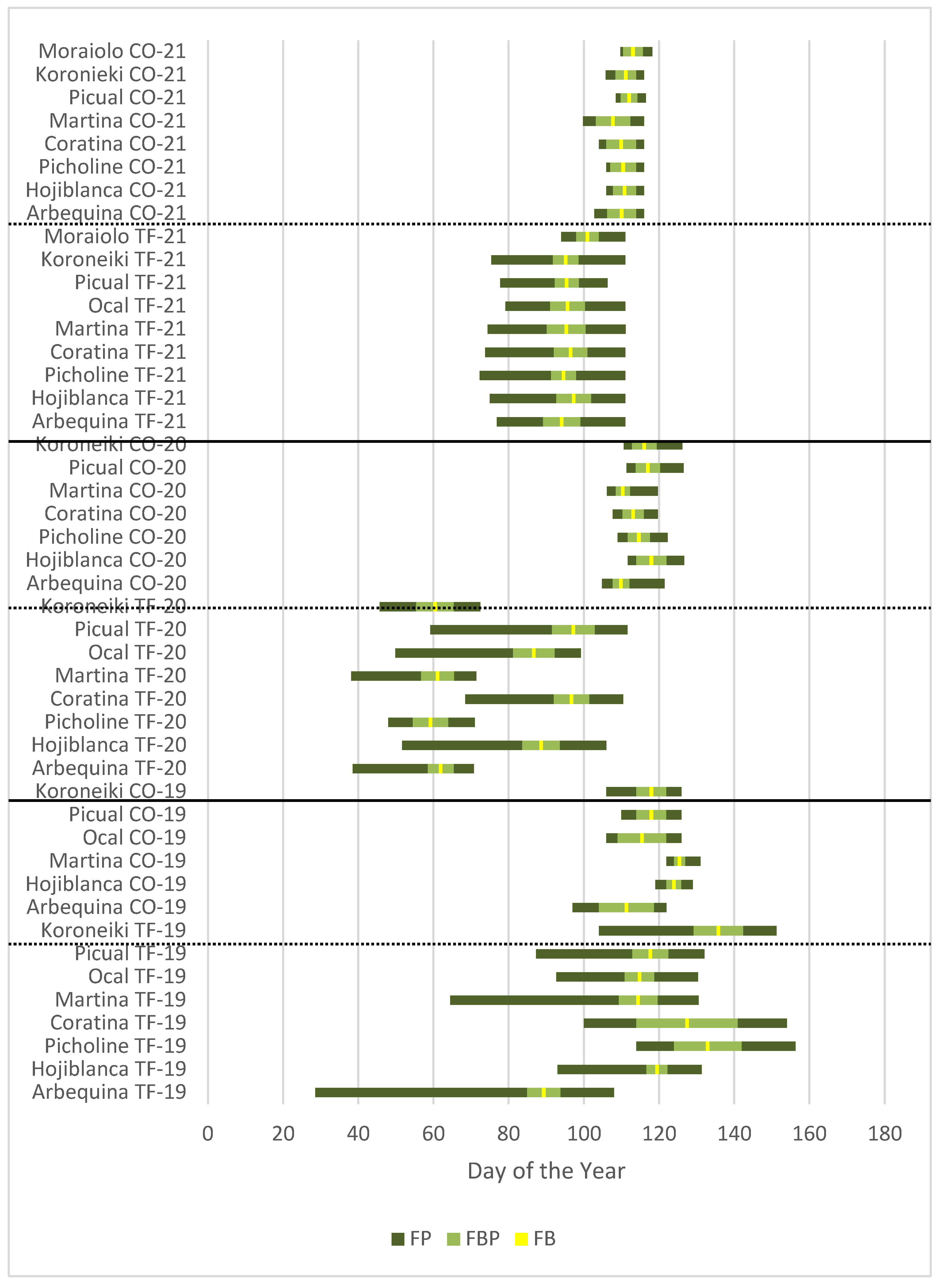
The length of the flowering period (FP) in TF-19 (Tenerife in 2019) was significantly longer than in the other environments, reaching 55 days (
Table 2), five times higher than in CO-21 (Cordoba in 2021). The other two Tenerife environments (TF-20 and TF-21) also had higher FP than those of Cordoba (CO-19, CO-20 and CO-21). Considering the individual FP values per cultivar and environment, TF-19 is the environment with the highest differences between cultivars. Besides, ‘Arbequina’, ‘Koroneiki’ and ‘Martina’ showed significant higher FP values in TF-19 than in TF-20 and TP-21 (
Table 2). In contrast, no significant differences among cultivars were observed in the three Cordoba environments (CO-19, CO-20 and CO-21) and in TF-21. Furthermore, ‘Arbequina’ had by far the longest FP in TF-19, with 79 days, while ‘Picual’ had the shortest in CO-21 with 8 days.
Regarding the length of the full bloom period (FBP), only small differences were found between environments. These differences were mainly due to the low values in CO-20 and CO-21 (
Table 3).
Average FBD was earliest in TF-20, followed by TF-21 (
Table 4), while no differences in FBD were observed in the other environments. In TF-19 and TF-20, significant differences in FBD were observed between cultivars. ‘Arbequina’ in TF-19 showed an earlier FBD compared to the rest of cultivars in this environment. Very early FBD was observed in TF-20 for ‘Arbequina’, ‘Koroneiki’, ‘Martina’ and ‘Picholine Marocaine’ around day 60 (1
st March). In the other environments, the behavior of all cultivars was very homogeneous, as in the case of FP. Therefore, only slight significant differences of cultivars across environments were found.
In general, flowering in Cordoba environments started and ended 60 and 30 days later than in TF-20 and TF-21, respectively (
Figure 3). While in TF-19, flowering duration was very variable between cultivars but, in generally occurred at a similar date to that in Cordoba environments.
It is remarkable that the differences between the most advanced and the most delayed phenology stages for a given date were much greater in Tenerife than in Cordoba for all cultivars (
Figure 4). Consequently, two very distant phenological stages were observed simultaneously in a tree in all cultivars in Tenerife environments compared to those in Cordoba. Moreover, in Tenerife, stage 53 (Inflorescence buds open, flower cluster development starts) was the most delayed stage during a long time for all the cultivars tested; and the variation of the phenological stages with time was not always ascending, as happened in Cordoba. All this is due to the asynchronous bud blooming in the Tenerife environments, where new flowers appear on the trees over a very long period.
4. Discussion
In this study, we evaluate the relative influence of genotype, environment and their interaction on the olive flowering phenology. The genotype consisted of seven cultivars cultivated traditionally in growing areas with very different climatic conditions. Environment included two locations, one with a Mediterranean climate (Cordoba), typical of olive growing, and the other with a Subtropical climate (Tenerife), with winter temperatures higher than those considered suitable for olive growing [
11]. These warm winter temperatures have been reported as a good natural scenario to simulate the effect of climate warming in the Mediterranean [
16]. The environment factor was the combination of these two locations and three years (2019 to 2021) in which climatic conditions were variable.
Flowering was observed in all the Tenerife environments and in all the cultivars tested. This despite the fact that the optimal chilling accumulation temperature previously established at 7-12 ºC for olive crop [15,20,21] was rarely reached in winter in the three Tenerife environments.
The analysis of variance of the flowering phenology parameters showed that the environment was the main factor responsible for the variability in the length of the flowering period, and the date of full bloom. This factor was also significant for the full flowering periods. A high environmental influence has been reported for flowering phenology in Mediterranean conditions [
12] and in diverse climatic conditions, such as in Argentina [22]. In this work, the high environmental influence on flowering phenology is mainly due to the lack of winter chilling in the three Tenerife environments (TF-19, TF-20 and TF-21) above mentioned. The most remarkable effect of this lack of winter chilling is an asynchronous flower bud burst that was also previously observed for ‘Arbequina’ and ‘Picual’ in the Subtropical climate [
16]. This asynchrony in the flowering could be the cause of the high error variance for the full flowering period observed in Tenerife. It also caused a much greater difference between the most delayed and the most advanced phenological stage, for a given date, in Tenerife than in Cordoba; and consequently, the flowering period was much longer in the former location. A longer flowering period has also been observed in experiments with artificial temperature increases in Mediterranean conditions [
14] and with warmer winters [23].
Both the asynchrony and the increase in the length of the flowering period might have a negative impact on the profitability of olive cultivation in warm areas. It also causes an asynchronous olive ripening, with important consequences for the quality of the olive oil obtained. In the case of Tenerife, this long flowering period is exposed to a large number of extreme climatic phenomena, such as hot sub-Saharan air masses, which could cause a massive drop in flowers, a deficit in fruit set or pistil abortion [24]. In addition, flowering period occurred earlier in Tenerife than in Cordoba. This fact is consistent with the observations registered under natural conditions [
13], using artificial increase of air temperature during winter [
14] and in flowering phenology models [10,25].
The significant differences observed among the three Tenerife environments are difficult to explain, as the air temperatures in the three years under study were relatively similar. Maybe other factors apart from air temperature are involved.
In contrast to the strong environmental effect, the evaluation here performed seems to suggest that genotype has little influence on the flowering phenology. This is despite the fact that the cultivars studied come from distant areas and have a high genetic distance between them [
1]. Only some differences were observed in the day of full flowering, which was earlier in ‘Arbequina’, curiously the only cultivar evaluated from the northern part of the Mediterranean olive-growing area.
The interaction between genotype and environment was significant for the length of the flowering period (FP) and for the day of full flowering (FBD). In particular, no significant differences between cultivars were found in the Mediterranean climate of Cordoba, where olive winter temperatures are low enough to fulfill the chilling requirements of all cultivars [
12]. However, in Tenerife significant differences among cultivars were observed. And it is noteworthy that there is no specific pattern of variation for cultivars for both FP and FBD along the three Tenerife environments tested. For example, ‘Koroneiki’ had the longest FP in TF-19, and one of the lowest in TF-20. In other words, the flowering behavior of the cultivars in the warmer winters of Tenerife seems to be erratic and with little consistent genetic influence. Previous work has reported that different cultivars have different winter chilling requirements or chilling portions for flowering, including some of those used in the present study such as ‘Arbequina’, ‘Hojiblanca’ and ‘Picual’ [
6,
11]. However, in this work, where cultivars were placed in a natural environment with a warm winter as Tenerife, no consistent significant differences were observed among these cultivars for flowering phenology or winter chilling needs. Previously, differences in terms of flowering intensity due to the lack of sufficient winter chilling were reported among cultivars in Argentina [
15], but the length of the flowering period was not reported. In the only previous report of cultivar evaluation for the length of flowering period in multiple Mediterranean environments, no significant differences among cultivars were observed [
12].
Therefore, more cultivars need to be tested to identify genetic variability for adaptation to the warmer winters, with low chilling accumulation, predicted by climate models [
5]. Unfortunately, few genetic influence on the full bloom date has been observed when evaluated in typical Mediterranean climates such as Cordoba [
13] and Morocco [
10], suggesting that differences in winter chilling requirements may also be difficult to find. Also, current breeding programs has been focused in other characters as disease resistance [26] or adaptation to new growing systems [27] but low chilling requirements has not yet been reported as a selection trait. Perhaps the use of wild olives from the Canary Islands as
Olea europaea subsp.
guanchica [6,28] could be a long-term strategy to introduce warm winter adaptation genes into cultivated material. In other fruit crops, breeding programs have identified new genotypes adapted to climates with warm winter temperatures [29].
Future work should also consider the effect of lack of winter chilling on flower quality. Indeed, lack of winter chilling seems to reduce the number of inflorescences and increase flower abortion [15,30], and to deform floral buds [31].
All these studies, carried out under non-Mediterranean weather conditions, will contribute to reducing the uncertainty in phenology assessment [32,33] in the context of climate change, complementing previous studies carried out under colder winter weather conditions [
6,
11]
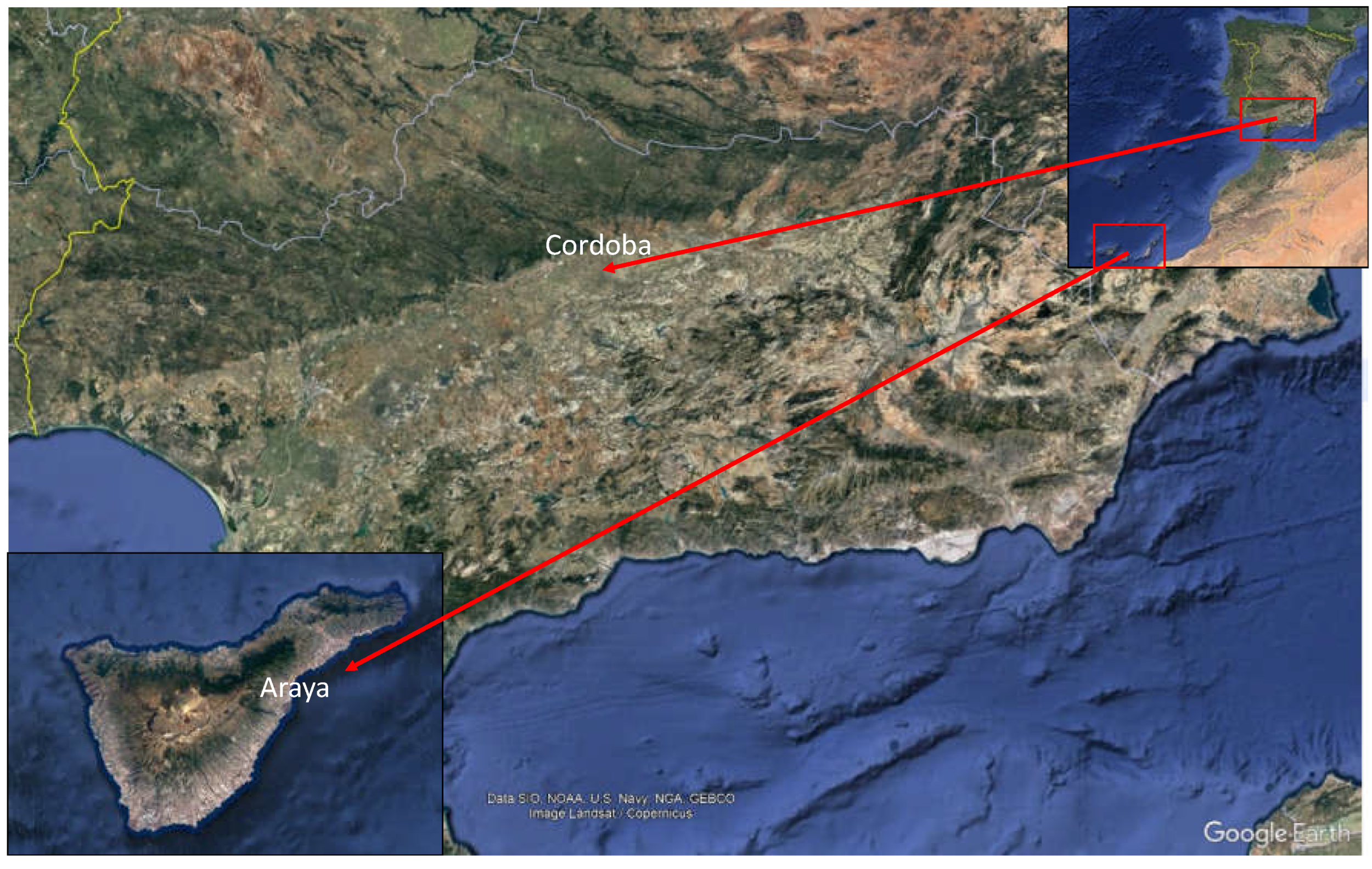
Figure 1.
Location of the two olive trials under evaluation.
Figure 1.
Location of the two olive trials under evaluation.
Figure 2.
Weather data of Tenerife and Andalucía locations from October to June in the three seasons considered (2018-2019, 2019-2020 and 2020-2021). Daily mean temperature (mean, maximum, average and range) are included.
Figure 2.
Weather data of Tenerife and Andalucía locations from October to June in the three seasons considered (2018-2019, 2019-2020 and 2020-2021). Daily mean temperature (mean, maximum, average and range) are included.
Figure 3.
Means of flowering period in days (FP in dark green), full bloom period in days (FBP, in light green) and full bloom date in Day of the Year (FBD in yellow) in seven cultivars in the Tenerife and Andalucía locations in the three seasons considered (2018-2019, 2019-2020 and 2020-2021).
Figure 3.
Means of flowering period in days (FP in dark green), full bloom period in days (FBP, in light green) and full bloom date in Day of the Year (FBD in yellow) in seven cultivars in the Tenerife and Andalucía locations in the three seasons considered (2018-2019, 2019-2020 and 2020-2021).
Figure 4.
Variation of the average most delayed, common and advanced flowering stage (BBCH scale) in the seven studied cultivars along the flowering period (Julian day) in Tenerife and Cordoba in 2020.
Figure 4.
Variation of the average most delayed, common and advanced flowering stage (BBCH scale) in the seven studied cultivars along the flowering period (Julian day) in Tenerife and Cordoba in 2020.
Table 1.
Percentage of sums of squares of cultivar, environment and their interaction for the flowering period (FP in days), full flowering period (FBP in days) and full bloom time (FBD in Day of the Year). Values in bold indicate significant influence of the factor at p<0.01.
Table 1.
Percentage of sums of squares of cultivar, environment and their interaction for the flowering period (FP in days), full flowering period (FBP in days) and full bloom time (FBD in Day of the Year). Values in bold indicate significant influence of the factor at p<0.01.
| |
FP |
FBP |
FBD |
| Cultivar |
2,5 |
1,1 |
8,0 |
| Environment |
52,5 |
8,3 |
54,4 |
| Cultivar*Environment |
11,8 |
12,1 |
16,1 |
| Error |
33,2 |
78,6 |
21,6 |
Table 2.
Comparison of means of length of flowering period (FP, in days) by cultivar, environment and their interaction. Each environment was considered as a combination of a year (2019, 2020 and 2021) and location (Cordoba-CO and Tenerife-TF). Different letters indicate significant differences (p<0.01) among means within each source of variation.
Table 2.
Comparison of means of length of flowering period (FP, in days) by cultivar, environment and their interaction. Each environment was considered as a combination of a year (2019, 2020 and 2021) and location (Cordoba-CO and Tenerife-TF). Different letters indicate significant differences (p<0.01) among means within each source of variation.
| |
CO-19 |
CO-20 |
CO-21 |
TF-19 |
TF-20 |
TF-21 |
Average |
| Arbequina |
25,0 |
fgh |
16,7 |
gh |
13,2 |
gh |
79,4 |
a |
32,3 |
efg |
34,2 |
efg |
33,5 |
n.s. |
| Coratina |
|
|
12,0 |
gh |
12,0 |
gh |
54,0 |
bcde |
42,0 |
cdefg |
37,3 |
defg |
31,5 |
n.s. |
| Hojiblanca |
|
|
15,0 |
gh |
10,0 |
gh |
38,3 |
defg |
54,3 |
bcd |
36,0 |
defg |
30,7 |
n.s. |
| Koroneiki |
20,0 |
gh |
15,6 |
gh |
10,2 |
gh |
62,6 |
bc |
26,7 |
fg |
35,6 |
defg |
28,5 |
n.s. |
| Martina |
9,0 |
gh |
13,5 |
gh |
16,2 |
gh |
66,1 |
b |
33,3 |
efg |
36,7 |
defg |
29,1 |
n.s. |
| Picholine |
|
|
13,3 |
gh |
10,0 |
gh |
42,3 |
cdefg |
23,0 |
fgh |
38,7 |
defg |
25,5 |
n.s. |
| Picual |
16,0 |
gh |
15,2 |
gh |
8,0 |
h |
44,8 |
cdef |
52,5 |
cde |
28,5 |
fg |
27,5 |
n.s. |
| Average |
17,5 |
c |
14,5 |
c |
11,4 |
c |
55,4 |
a |
37,7 |
b |
35,3 |
b |
|
|
Table 3.
Comparison of means of length of full flowering period (FBP in days) by cultivar, environment and their interaction. Each environment was considered as a combination of a year (2019, 2020 and 2021) and location (Cordoba-CO and Tenerife-TF). Different letters indicate significant differences (p<0.01) among means within each source of variation.
Table 3.
Comparison of means of length of full flowering period (FBP in days) by cultivar, environment and their interaction. Each environment was considered as a combination of a year (2019, 2020 and 2021) and location (Cordoba-CO and Tenerife-TF). Different letters indicate significant differences (p<0.01) among means within each source of variation.
| |
CO-19 |
CO-20 |
CO-21 |
TF-19 |
TF-20 |
TF-21 |
Average |
| Arbequina |
14,8 |
n.s. |
4,5 |
n.s. |
7,8 |
n.s. |
8,9 |
n.s. |
6,9 |
n.s. |
10,0 |
n.s. |
8,8 |
n.s. |
| Coratina |
|
|
5,7 |
n.s. |
8,0 |
n.s. |
27,0 |
n.s. |
9,5 |
n.s. |
9,0 |
n.s. |
11,8 |
n.s. |
| Hojiblanca |
|
|
8,0 |
n.s. |
6,2 |
n.s. |
5,7 |
n.s. |
10,0 |
n.s. |
9,3 |
n.s. |
7,8 |
n.s. |
| Koroneiki |
8,0 |
n.s. |
6,6 |
n.s. |
5,6 |
n.s. |
13,2 |
n.s. |
10,0 |
n.s. |
6,8 |
n.s. |
8,4 |
n.s. |
| Martina |
3,0 |
n.s. |
3,8 |
n.s. |
9,2 |
n.s. |
10,3 |
n.s. |
8,8 |
n.s. |
10,3 |
n.s. |
7,6 |
n.s. |
| Picholine |
|
|
6,0 |
n.s. |
7,0 |
n.s. |
18,0 |
n.s. |
9,5 |
n.s. |
6,7 |
n.s. |
9,4 |
n.s. |
| Picual |
8,0 |
n.s. |
6,5 |
n.s. |
4,5 |
n.s. |
9,7 |
n.s. |
11,5 |
n.s. |
6,5 |
n.s. |
7,8 |
n.s. |
| Average |
8,4 |
ab |
5,9 |
c |
6,9 |
bc |
13,3 |
a |
9,5 |
ab |
8,4 |
ab |
|
|
Table 4.
Comparison of means of length full bloom date (FBD in day of the year) by cultivar and environment and their interaction. Each environment was considered as a combination of a year (2019, 2020 and 2021) and location (Cordoba-CO and Tenerife-TF). Different letters indicate significant differences (p<0.01) among means within each source of variation.
Table 4.
Comparison of means of length full bloom date (FBD in day of the year) by cultivar and environment and their interaction. Each environment was considered as a combination of a year (2019, 2020 and 2021) and location (Cordoba-CO and Tenerife-TF). Different letters indicate significant differences (p<0.01) among means within each source of variation.
| |
CO-19 |
CO-20 |
CO-21 |
TF-19 |
TF-20 |
TF-21 |
Average |
| Arbequina |
111,4 |
cdef |
109,9 |
cdefg |
110,1 |
cdefg |
89,4 |
h |
62,0 |
i |
94,5 |
gh |
96,2 |
c |
| Coratina |
|
|
113,2 |
cdef |
110,0 |
cdefg |
127,5 |
abc |
96,8 |
efgh |
96,5 |
fgh |
108,8 |
a |
| Hojiblanca |
|
|
115,7 |
bcde |
110,9 |
cdef |
119,5 |
bcd |
88,7 |
h |
97,3 |
efgh |
106,4 |
a |
| Koroneiki |
118,0 |
bcde |
116,1 |
bcde |
111,2 |
cdef |
135,8 |
a |
60,5 |
i |
95,2 |
fgh |
106,1 |
ab |
| Martina |
125,5 |
abcd |
110,4 |
cdefg |
107,8 |
defg |
114,5 |
cde |
61,1 |
i |
95,4 |
fgh |
102,4 |
bc |
| Picholine |
|
|
114,7 |
bcde |
110,5 |
cdefg |
133,0 |
ab |
59,3 |
i |
94,7 |
fgh |
102,4 |
bc |
| Picual |
118,0 |
bcde |
117,1 |
bcde |
112,1 |
cdef |
117,7 |
bcde |
97,3 |
efgh |
95,5 |
fgh |
109,6 |
a |
| Average |
118,2 |
c |
113,9 |
c |
110,4 |
c |
119,6 |
c |
75,1 |
a |
95,6 |
b |
|
|