Submitted:
31 May 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Long-term neurological and cognitive dysfunction of COVID-19
Molecules contribute to COVID-19 penetration into the CNS
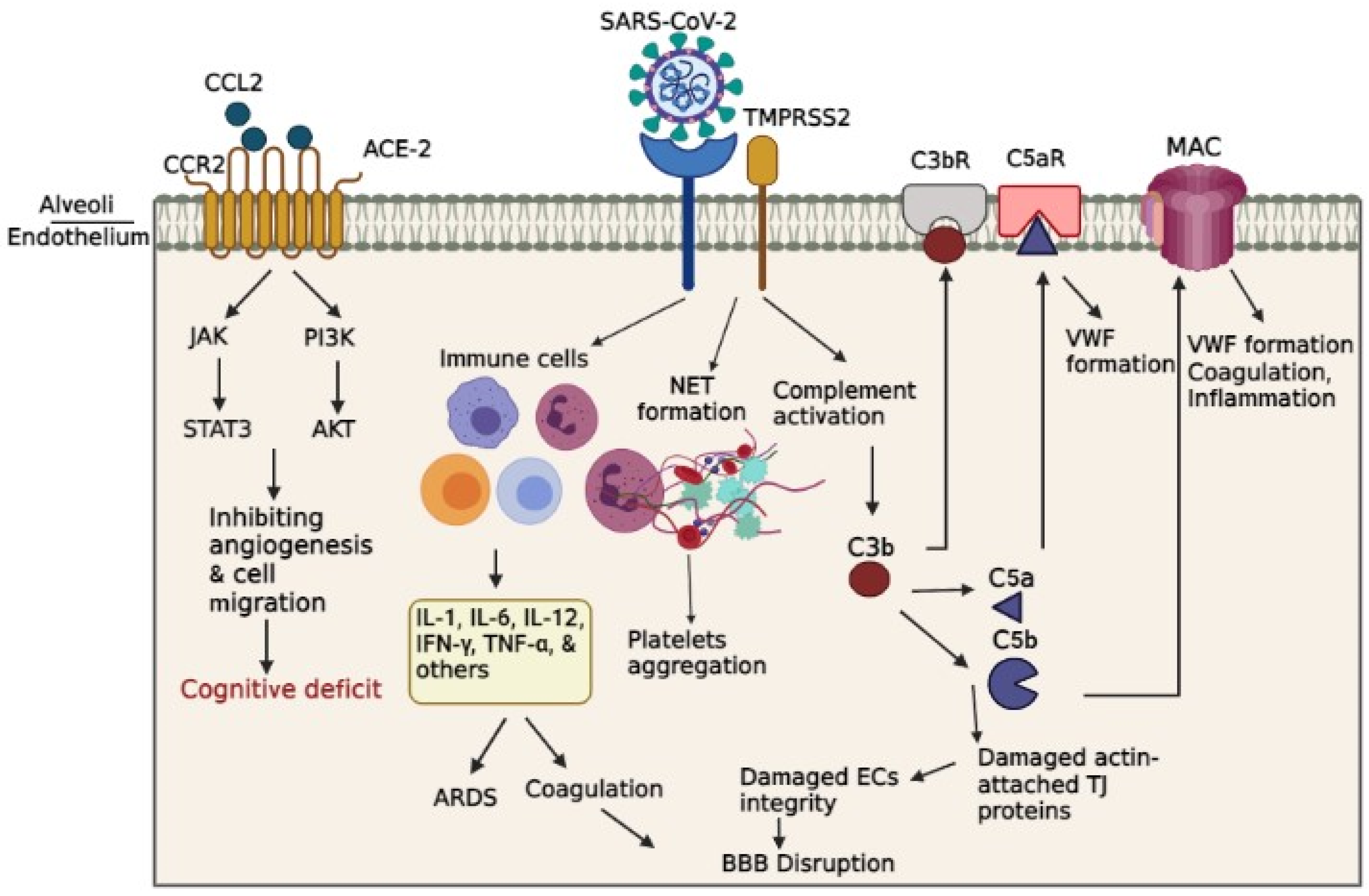
Endothelial cell infection and endotheliitis in COVID-19
Degradation of endothelial glycocalyx makes them vulnerable to SARS-CoV-2 entry.
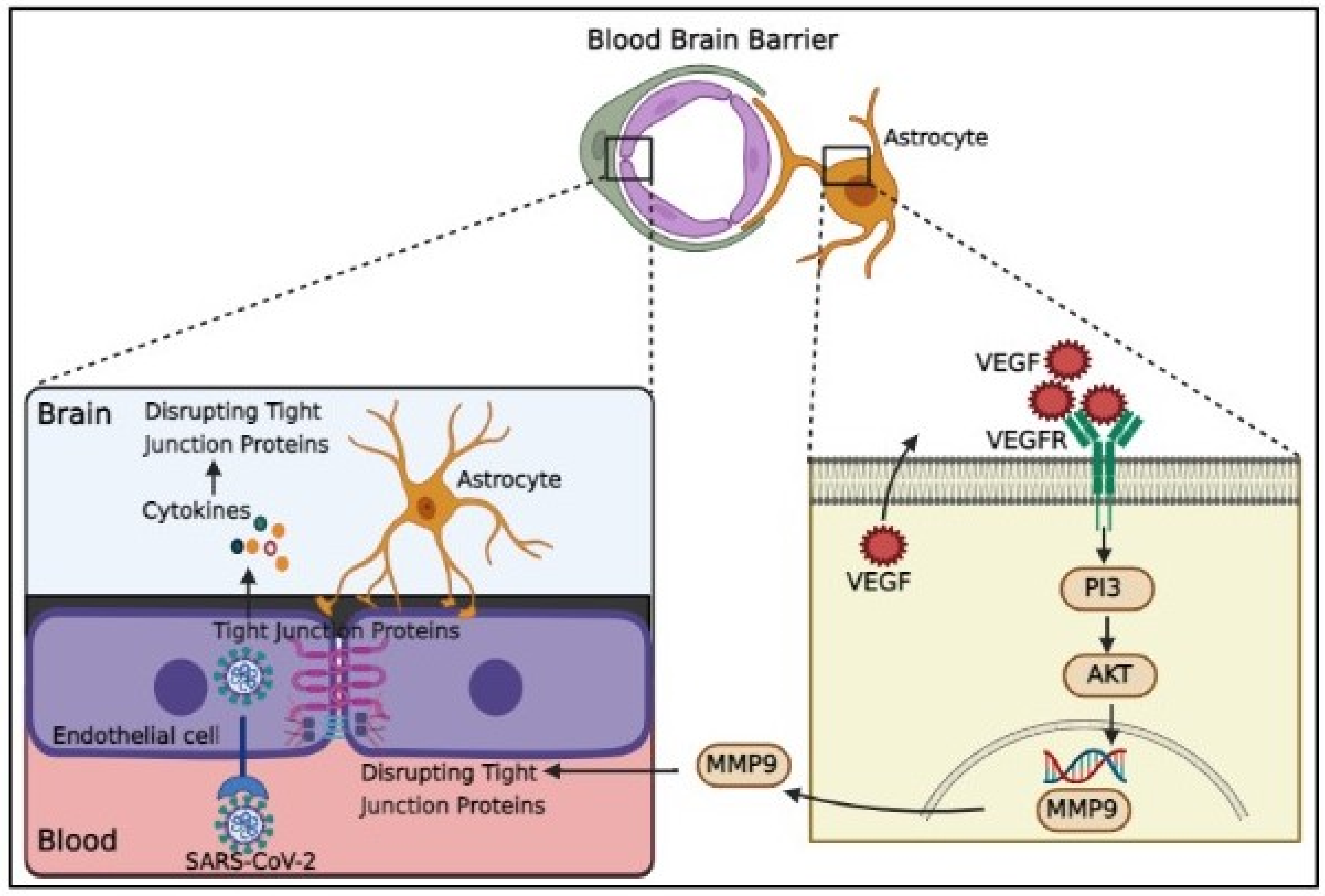
Endothelial cells inflammation and blood brain barrier disruption in COVID-19
Disseminating intravascular coagulation and blood brain barrier disruption in COVID-19
Pneumonia and BBB disruption in COVID-19
Vascular dysfunction, brain inflammation, and cognitive impairment
Conclusion and future directions
Funding
Conflict of Interest
Ethical Approval
References
- Garg, A., et al., A case of COVID-19 with memory impairment and delayed presentation as stroke. Cureus, 2020. 12(8). [CrossRef]
- Wu, Z. and J.M. McGoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. jama, 2020. 323(13): p. 1239-1242. [CrossRef]
- Pilotto, A., et al., Long-term neurological manifestations of COVID-19: prevalence and predictive factors. Neurological Sciences, 2021. 42: p. 4903-4907. [CrossRef]
- Grant, M.C., et al., The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PloS one, 2020. 15(6): p. e0234765. [CrossRef]
- Dubé, M., et al., Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. Journal of virology, 2018. 92(17): p. e00404-18. [CrossRef]
- Goërtz, Y.M., et al., Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ open research, 2020. 6(4). [CrossRef]
- Raja, C.P., et al., Science behind Usefulness of Bacopa monnieri for Memory and Cognition, in Phytopharmaceuticals for Brain Health. 2017, CRC Press. p. 225-250.
- Stefano, G.B., et al., Selective neuronal mitochondrial targeting in SARS-CoV-2 infection affects cognitive processes to induce ‘brain fog’and results in behavioral changes that favor viral survival. Medical science monitor: international medical journal of experimental and clinical research, 2021. 27: p. e930886-1. [CrossRef]
- Bliddal, S., et al., Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Scientific reports, 2021. 11(1): p. 13153. [CrossRef]
- Iwashyna, T.J., et al., Long-term cognitive impairment and functional disability among survivors of severe sepsis. Jama, 2010. 304(16): p. 1787-1794. [CrossRef]
- Semmler, A., et al., Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. Journal of Neurology, Neurosurgery & Psychiatry, 2013. 84(1): p. 62-69. [CrossRef]
- Rass, V., et al., Neurological outcome and quality of life 3 months after COVID-19: A prospective observational cohort study. European journal of neurology, 2021. 28(10): p. 3348-3359. [CrossRef]
- Carfì, A., R. Bernabei, and F. Landi, Persistent symptoms in patients after acute COVID-19. Jama, 2020. 324(6): p. 603-605. [CrossRef]
- Helms, J., et al., Neurologic features in severe SARS-CoV-2 infection. New England Journal of Medicine, 2020. 382(23): p. 2268-2270. [CrossRef]
- Mao, L., et al., Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA neurology, 2020. 77(6): p. 683-690. [CrossRef]
- Mazza, M.G., et al., Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain, behavior, and immunity, 2021. 94: p. 138-147. [CrossRef]
- Hellmuth, J., et al., Persistent COVID-19-associated neurocognitive symptoms in non-hospitalized patients. Journal of neurovirology, 2021. 27: p. 191-195. [CrossRef]
- Garrigues, E., et al., Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. Journal of Infection, 2020. 81(6): p. e4-e6. [CrossRef]
- Han, Q., et al., Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens, 2022. 11(2): p. 269. [CrossRef]
- Ritchie, K. and D. Chan, The emergence of cognitive COVID. World Psychiatry, 2021. 20(1): p. 52. [CrossRef]
- Zhou, H., et al., The landscape of cognitive function in recovered COVID-19 patients. Journal of psychiatric research, 2020. 129: p. 98-102. [CrossRef]
- Raman, B., et al., Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine, 2021. 31: p. 100683. [CrossRef]
- Woo, M.S., et al., Frequent neurocognitive deficits after recovery from mild COVID-19. Brain communications, 2020. 2(2): p. fcaa205. [CrossRef]
- Kumar, V., et al., The incidence of anosmia in patients with laboratory-confirmed COVID 19 infection in India: An observational study. Journal of Anaesthesiology, Clinical Pharmacology, 2021. 37(1): p. 51. [CrossRef]
- Jaywant, A., et al., Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology, 2021. 46(13): p. 2235-2240. [CrossRef]
- Villani, E.R., et al., Impact of COVID-19-related lockdown on psychosocial, cognitive, and functional well-being in adults with down syndrome. Frontiers in Psychiatry, 2020. 11: p. 578686. [CrossRef]
- Zhou, J., et al., Cognitive disorders associated with hospitalization of COVID-19: Results from an observational cohort study. Brain, Behavior, and Immunity, 2021. 91: p. 383-392. [CrossRef]
- Heesakkers, H., et al., Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. Jama, 2022. 327(6): p. 559-565. [CrossRef]
- Del Brutto, O.H., et al., Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: A longitudinal prospective study nested to a population cohort. European journal of neurology, 2021. 28(10): p. 3245-3253. [CrossRef]
- Cristillo, V., et al., Premorbid vulnerability and disease severity impact on Long-COVID cognitive impairment. Aging clinical and experimental research, 2022: p. 1-4. [CrossRef]
- Hoffmann, M., et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. cell, 2020. 181(2): p. 271-280. e8. [CrossRef]
- Sparks, M.A., et al., Classical renin-angiotensin system in kidney physiology. Comprehensive Physiology, 2014. 4(3): p. 1201. [CrossRef]
- Shabani, Z., Demyelination as a result of an immune response in patients with COVID-19. Acta Neurologica Belgica, 2021. 121(4): p. 859-866. [CrossRef]
- Doobay, M.F., et al., Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 2007. 292(1): p. R373-R381. [CrossRef]
- Baig, A.M., et al., Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS chemical neuroscience, 2020. 11(7): p. 995-998. [CrossRef]
- Wang, Q., et al., Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell, 2020. 181(4): p. 894-904. e9. [CrossRef]
- Seyran, M., et al., The structural basis of accelerated host cell entry by SARS-CoV-2. The FEBS journal, 2021. 288(17): p. 5010-5020. [CrossRef]
- Iadecola, C., J. Anrather, and H. Kamel, Effects of COVID-19 on the nervous system. Cell, 2020. 183(1): p. 16-27. e1. [CrossRef]
- Davies, J., et al., Neuropilin-1 as a new potential SARS-CoV-2 infection mediator implicated in the neurologic features and central nervous system involvement of COVID-19. Molecular medicine reports, 2020. 22(5): p. 4221-4226. [CrossRef]
- Ulrich, H. and M.M. Pillat, CD147 as a target for COVID-19 treatment: suggested effects of azithromycin and stem cell engagement. Stem cell reviews and reports, 2020. 16(3): p. 434-440. [CrossRef]
- Istifli, E.S., et al., Interaction of certain monoterpenoid hydrocarbons with the receptor binding domain of 2019 novel coronavirus (2019-nCoV), transmembrane serine protease 2 (TMPRSS2), cathepsin B, and cathepsin L (CatB/L) and their pharmacokinetic properties. Turkish Journal of Biology, 2020. 44(7): p. 242-264. [CrossRef]
- Shang, J., et al., Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences, 2020. 117(21): p. 11727-11734. [CrossRef]
- Shabani, Z., J. Schuerger, and H. Su, Cellular loci involved in the development of brain arteriovenous malformations. Frontiers in Human Neuroscience, 2022. [CrossRef]
- Osburn, W.O., et al., Markers of endothelial cell activation are associated with the severity of pulmonary disease in COVID-19. Plos one, 2022. 17(5): p. e0268296. [CrossRef]
- Rotoli, B.M., et al., Endothelial cell activation by sars-cov-2 spike s1 protein: A crosstalk between endothelium and innate immune cells. Biomedicines, 2021. 9(9): p. 1220. [CrossRef]
- Szekely, L., et al., Pulmonary stromal expansion and intra-alveolar coagulation are primary causes of COVID-19 death. Heliyon, 2021. 7(5): p. e07134. [CrossRef]
- Bhatnagar, J., et al., Evidence of severe acute respiratory syndrome coronavirus 2 replication and tropism in the lungs, airways, and vascular endothelium of patients with fatal coronavirus disease 2019: an autopsy case series. The Journal of infectious diseases, 2021. 223(5): p. 752-764. [CrossRef]
- Hamming, I., et al., Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland, 2004. 203(2): p. 631-637. [CrossRef]
- Chen, L. and G. Hao, The role of angiotensin-converting enzyme 2 in coronaviruses/influenza viruses and cardiovascular disease. Cardiovascular research, 2020. 116(12): p. 1932-1936. [CrossRef]
- Wang, K., et al., SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. biorxiv, 2020: p. 2020.03. 14.988345. [CrossRef]
- Maccio, U., et al., SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine, 2021. 63: p. 103182. [CrossRef]
- Choudhary, S., et al., Modeling SARS-CoV-2: comparative pathology in rhesus macaque and Golden Syrian hamster models. Toxicologic Pathology, 2022. 50(3): p. 280-293. [CrossRef]
- Adesse, D., et al., Role of aging in Blood–Brain Barrier dysfunction and susceptibility to SARS-CoV-2 infection: impacts on neurological symptoms of COVID-19. Fluids and Barriers of the CNS, 2022. 19(1): p. 63. [CrossRef]
- Motta, C.S., et al., Human Brain Microvascular Endothelial Cells Exposure to SARS-CoV-2 Leads to Inflammatory Activation through NF-κB Non-Canonical Pathway and Mitochondrial Remodeling. Viruses, 2023. 15(3): p. 745. [CrossRef]
- Qin, Z., et al., Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics, 2021. 11(16): p. 8076. [CrossRef]
- Werlein, C., et al., Inflammation and vascular remodeling in COVID-19 hearts. Angiogenesis, 2022: p. 1-16. [CrossRef]
- Potje, S.R., et al., Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life sciences, 2021. 276: p. 119376. [CrossRef]
- du Preez, H.N., et al., Pathogenesis of COVID-19 described through the lens of an undersulfated and degraded epithelial and endothelial glycocalyx. The FASEB Journal, 2022. 36(1): p. e22052. [CrossRef]
- Targosz-Korecka, M., et al., Endothelial glycocalyx shields the interaction of SARS-CoV-2 spike protein with ACE2 receptors. Scientific reports, 2021. 11(1): p. 12157. [CrossRef]
- Vollenberg, R., et al., Indications of persistent glycocalyx damage in convalescent COVID-19 patients: a prospective multicenter study and hypothesis. Viruses, 2021. 13(11): p. 2324. [CrossRef]
- Langen, U.H., S. Ayloo, and C. Gu, Development and cell biology of the blood-brain barrier. Annual review of cell and developmental biology, 2019. 35: p. 591-613. [CrossRef]
- Greene, C. and M. Campbell, Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue barriers, 2016. 4(1): p. e1138017. [CrossRef]
- Bleau, C., et al., Brain invasion by mouse hepatitis virus depends on impairment of tight junctions and beta interferon production in brain microvascular endothelial cells. Journal of virology, 2015. 89(19): p. 9896-9908. [CrossRef]
- Alquisiras-Burgos, I., et al., Neurological complications associated with the blood-brain barrier damage induced by the inflammatory response during SARS-CoV-2 infection. Molecular neurobiology, 2021. 58(2): p. 520-535. [CrossRef]
- Yang, R.-C., et al., SARS-CoV-2 productively infects human brain microvascular endothelial cells. Journal of Neuroinflammation, 2022. 19(1): p. 149. [CrossRef]
- Almutairi, M.M., et al., Factors controlling permeability of the blood–brain barrier. Cellular and molecular life sciences, 2016. 73: p. 57-77. [CrossRef]
- Ranaivo, H.R., et al., Mild stretch-induced injury increases susceptibility to interleukin-1β-induced release of matrix metalloproteinase-9 from astrocytes. Journal of neurotrauma, 2011. 28(9): p. 1757-1766. [CrossRef]
- Erickson, M.A., et al., Interactions of SARS-CoV-2 with the blood–brain barrier. International Journal of Molecular Sciences, 2021. 22(5): p. 2681. [CrossRef]
- Rauti, R., et al., Effect of SARS-CoV-2 proteins on vascular permeability. Elife, 2021. 10: p. e69314. [CrossRef]
- Erickson, M.A. and W.A. Banks, Neuroimmune axes of the blood–brain barriers and blood–brain interfaces: bases for physiological regulation, disease states, and pharmacological interventions. Pharmacological reviews, 2018. 70(2): p. 278-314. [CrossRef]
- Kilic, E., et al., The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF's neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB journal, 2006. 20(8): p. 1185. [CrossRef]
- Ahmad, S.J., et al., Neurological sequelae of COVID-19. Journal of Integrative Neuroscience, 2022. 21(3): p. 77. [CrossRef]
- Staekenborg, S.S., et al., Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke, 2008. 39(2): p. 317-322. [CrossRef]
- Miners, S., P.G. Kehoe, and S. Love, Cognitive impact of COVID-19: looking beyond the short term. Alzheimer's research & therapy, 2020. 12(1): p. 1-16. [CrossRef]
- Loo, J., D.A. Spittle, and M. Newnham, COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax, 2021. 76(4): p. 412-420. [CrossRef]
- Magro, C., et al., Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translational Research, 2020. 220: p. 1-13. [CrossRef]
- Zuo, Y., et al., Neutrophil extracellular traps in COVID-19. JCI insight, 2020. 5(11). [CrossRef]
- Harris, A.G. and T.C. Skalak, Leukocyte cytoskeletal structure determines capillary plugging and network resistance. American Journal of Physiology-Heart and Circulatory Physiology, 1993. 265(5): p. H1670-H1675. [CrossRef]
- Cruz Hernández, J.C., et al., Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nature neuroscience, 2019. 22(3): p. 413-420. [CrossRef]
- Wang, S., et al., Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. 2012. [CrossRef]
- Paul, R., et al., Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nature medicine, 2001. 7(2): p. 222-227. [CrossRef]
- Liu, D.Z., et al., Blood–brain barrier breakdown and repair by Src after thrombin-induced injury. Annals of neurology, 2010. 67(4): p. 526-533. [CrossRef]
- Tyagi, N., et al., Fibrinogen induces endothelial cell permeability. Molecular and cellular biochemistry, 2008. 307: p. 13-22. [CrossRef]
- Trepat, X., et al., Thrombin and histamine induce stiffening of alveolar epithelial cells. Journal of Applied Physiology, 2005. 98(4): p. 1567-1574. [CrossRef]
- Yepes, M., et al., Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor–related protein. The Journal of clinical investigation, 2003. 112(10): p. 1533-1540. [CrossRef]
- Grammas, P., P.G. Samany, and L. Thirumangalakudi, Thrombin and inflammatory proteins are elevated in Alzheimer's disease microvessels: implications for disease pathogenesis. Journal of Alzheimer's Disease, 2006. 9(1): p. 51-58. [CrossRef]
- Shah, F.A., et al., Bidirectional relationship between cognitive function and pneumonia. American journal of respiratory and critical care medicine, 2013. 188(5): p. 586-592. [CrossRef]
- Salive, M.E., et al., Disability and cognitive impairment are risk factors for pneumonia-related mortality in older adults. Public Health Reports, 1993. 108(3): p. 314.
- Herridge, M.S., et al., Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive care medicine, 2016. 42: p. 725-738. [CrossRef]
- Tate, J.A., et al., Infection hospitalization increases risk of dementia in the elderly. Critical care medicine, 2014. 42(5): p. 1037. [CrossRef]
- Wilcox, M.E., et al., Cognitive dysfunction in ICU patients: risk factors, predictors, and rehabilitation interventions. Critical care medicine, 2013. 41(9): p. S81-S98. [CrossRef]
- Cervos-Navarro, J. and N. Diemer, Selective vulnerability in brain hypoxia. Critical reviews in neurobiology, 1991. 6(3): p. 149-182.
- Moskowitz, M.A., E.H. Lo, and C. Iadecola, The science of stroke: mechanisms in search of treatments. Neuron, 2010. 67(2): p. 181-198. [CrossRef]
- Fernando, M.S., et al., White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke, 2006. 37(6): p. 1391-1398. [CrossRef]
- Yang, Y. and G.A. Rosenberg, Blood–brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke, 2011. 42(11): p. 3323-3328. [CrossRef]
- Esch, T., et al., Emerging roles of blood-borne intact and respiring mitochondria as bidirectional mediators of pro-and anti-inflammatory processes. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 2020. 26: p. e924337-1. [CrossRef]
- Shenoy, S., Coronavirus (Covid-19) sepsis: revisiting mitochondrial dysfunction in pathogenesis, aging, inflammation, and mortality. Inflammation research, 2020. 69: p. 1077-1085. [CrossRef]
- Singh, K.K., et al., Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. American Journal of Physiology-Cell Physiology, 2020. [CrossRef]
- Ptacek, R., et al., Psychiatric manifestations of COVID-19 and their social significance. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 2020. 26: p. e930340-1. [CrossRef]
- Bostancıklıoğlu, M., SARS-CoV2 entry and spread in the lymphatic drainage system of the brain. Brain, behavior, and immunity, 2020. 87: p. 122. [CrossRef]
- Steardo, L., et al., Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiologica (Oxford, England), 2020. 229(3). [CrossRef]
- Bellon, M., et al., Cerebrospinal fluid features in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcription polymerase chain reaction (RT-PCR) positive patients. Clinical Infectious Diseases, 2021. 73(9): p. e3102-e3105. [CrossRef]
- Geng, J., et al., Blood-brain barrier disruption induced cognitive impairment is associated with increase of inflammatory cytokine. Frontiers in aging neuroscience, 2018. 10: p. 129. [CrossRef]
- Montagne, A., et al., APOE4 leads to blood–brain barrier dysfunction predicting cognitive decline. Nature, 2020. 581(7806): p. 71-76. [CrossRef]
- Wu, Y., et al., Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain, behavior, and immunity, 2020. 87: p. 18-22. [CrossRef]
- Anwar, M.M., Immunotherapies and COVID-19 related neurological manifestations: A comprehensive review article. Journal of Immunoassay and Immunochemistry, 2020. 41(6): p. 960-975. [CrossRef]
- Alnefeesi, Y., et al., Impact of SARS-CoV-2 infection on cognitive function: a systematic review. Frontiers in Psychiatry, 2021: p. 1629. [CrossRef]
- Gao, W., et al., Systematic analysis of chemokines reveals CCL18 is a prognostic biomarker in glioblastoma. Journal of Inflammation Research, 2022: p. 2731-2743. [CrossRef]
- Kolodziej, A., et al., Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. Journal of Neuroscience, 2008. 28(17): p. 4488-4500. [CrossRef]
- Senf, K., et al., Chemokine signaling is required for homeostatic and injury-induced neurogenesis in the olfactory epithelium. Stem Cells, 2021. 39(5): p. 617-635. [CrossRef]
- Karimabad, M.N., et al., The Chemokines CXC, CC and C in the Pathogenesis of COVID-19 Disease and as Surrogates of Vaccine-Induced Innate and Adaptive Protective Responses. Vaccines, 2022. 10(8): p. 1299. [CrossRef]
- Yasui, H., et al., CCL2 secreted from cancer-associated mesothelial cells promotes peritoneal metastasis of ovarian cancer cells through the P38-MAPK pathway. Clinical & Experimental Metastasis, 2020. 37: p. 145-158. [CrossRef]
- Villeda, S.A., et al., The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature, 2011. 477(7362): p. 90-94. [CrossRef]
- Minton, K., Mechanistic insights into Long COVID in hamsters. Nature Reviews Immunology, 2022. 22(8): p. 463-463. [CrossRef]
- Hong, S., et al., Abnormalities in chemokine levels in schizophrenia and their clinical correlates. Schizophrenia research, 2017. 181: p. 63-69. [CrossRef]
- Fernández-Castañeda, A., et al., Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. BioRxiv, 2022. [CrossRef]
- Xu, J., et al., Astrocyte-derived CCL2 participates in surgery-induced cognitive dysfunction and neuroinflammation via evoking microglia activation. Behavioural Brain Research, 2017. 332: p. 145-153. [CrossRef]
- Thirumangalakudi, L., et al., Angiogenic proteins are expressed by brain blood vessels in Alzheimer's disease. Journal of Alzheimer's Disease, 2006. 10(1): p. 111-118. [CrossRef]
- Teuwen, L.-A., et al., COVID-19: the vasculature unleashed. Nature Reviews Immunology, 2020. 20(7): p. 389-391. [CrossRef]
- Varga, Z., et al., Endothelial cell infection and endotheliitis in COVID-19. The Lancet, 2020. 395(10234): p. 1417-1418. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




