Submitted:
02 June 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Semen Sample Collection
2.2. Spermatozoa Purification and Analysis
2.2.1. Assessment of Sperm Morphology
2.2.2. Assessment of Sperm Vitality (Eosin Test)
2.2.3. Assessment of the Sperm Membrane Integrity (Hypo-Osmotic Test (HOS))
2.3. RNA Extraction and Synthesis of the cDNA (Reverse Transcription)
2.4. Real-Time Quantitative PCR (qPCR)
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Quantification of mRNA
3.3. Correlation between PGAM5, TYRO3, and PTPRN2 mRNA Expression and Sperm Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A Unique View on Male Infertility around the Globe. Reprod. Biol. Endocrinol. 2015, 13, 1–9. [Google Scholar] [CrossRef]
- Stouffs, K.; Seneca, S.; Lissens, W. Genetic Causes of Male Infertility. Ann. Endocrinol. (Paris). 2014. [Google Scholar] [CrossRef]
- Tognon, M.; Tagliapietra, A.; Magagnoli, F.; Mazziotta, C.; Oton-Gonzalez, L.; Lanzillotti, C.; Vesce, F.; Contini, C.; Rotondo, J.C.; Martini, F. Investigation on Spontaneous Abortion and Human Papillomavirus Infection. Vaccines 2020, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Lanzillotti, C.; Mazziotta, C.; Tognon, M.; Martini, F. Epigenetics of Male Infertility: The Role of DNA Methylation. Front. Cell Dev. Biol. 2021, 9, 689624. [Google Scholar] [CrossRef] [PubMed]
- Portela, A.; Esteller, M. Epigenetic Modifications and Human Disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Kavak, E.; Gregory, M.; Imashimizu, M.; Shutinoski, B.; Kashlev, M.; Oberdoerffer, P.; Sandberg, R.; Oberdoerffer, S. CTCF-Promoted RNA Polymerase II Pausing Links DNA Methylation to Splicing. Nature 2011, 479, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.M.; Bird, A. DNA Methylation Landscapes: Provocative Insights from Epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.K.; Pausova, Z. Cigarette Smoking and DNA Methylation. Front. Genet. 2013, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Levin, H.L.; Moran, J. V Dynamic Interactions between Transposable Elements and Their Hosts. Nat. Rev. Genet. 2011, 12, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.T.; Pai, A.A.; Pickrell, J.K.; Gaffney, D.J.; Pique-Regi, R.; Degner, J.F.; Gilad, Y.; Pritchard, J.K. DNA Methylation Patterns Associate with Genetic and Gene Expression Variation in HapMap Cell Lines. Genome Biol. 2011, 12, 1–13. [Google Scholar] [CrossRef]
- Breitling, L.P.; Yang, R.; Korn, B.; Burwinkel, B.; Brenner, H. Tobacco-Smoking-Related Differential DNA Methylation: 27K Discovery and Replication. Am. J. Hum. Genet. 2011, 88, 450–457. [Google Scholar] [CrossRef]
- Kovac, J.R.; Khanna, A.; Lipshultz, L.I. The Effects of Cigarette Smoking on Male Fertility. Postgrad. Med. 2015, 127, 338–341. [Google Scholar] [CrossRef]
- Craig, J.R.; Jenkins, T.G.; Carrell, D.T.; Hotaling, J.M. Obesity, Male Infertility, and the Sperm Epigenome. Fertil. Steril. 2017, 107, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Amor, H.; Hammadeh, M.E.; Mohd, I.; Jankowski, P.M. Impact of Heavy Alcohol Consumption and Cigarette Smoking on Sperm DNA Integrity. Andrologia 2022, 54, e14434. [Google Scholar] [CrossRef] [PubMed]
- Amor, H.; Jankowski, P.M.; Dahadhah, F.W.; Al Zoubi, M.S.; Hammadeh, M.E. Impact of Tobacco Smoking in Association with H2BFWT, PRM1 and PRM2 Genes Variants on Male Infertility. Andrologia 2022, 54, e14611. [Google Scholar] [CrossRef] [PubMed]
- Ilacqua, A.; Izzo, G.; Emerenziani, G. Pietro; Baldari, C.; Aversa, A. Lifestyle and Fertility: The Influence of Stress and Quality of Life on Male Fertility. Reprod. Biol. Endocrinol. 2018, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hammadeh, M.E.; Hamad, M.F.; Montenarh, M.; Fischer-Hammadeh, C. Protamine Contents and P1/P2 Ratio in Human Spermatozoa from Smokers and Non-Smokers. Hum. Reprod. 2010, 25, 2708–2720. [Google Scholar] [CrossRef] [PubMed]
- Mahat, R.K.; Kumar, S.; Arora, M.; Bhale, D. V; Mehta, R.; Batra, J. Role of Oxidative Stress and Antioxidants in Male Infertility. Int J Heal. Sci Res 2015, 5, 324–333. [Google Scholar]
- Opuwari, C.S.; Henkel, R.R. An Update on Oxidative Damage to Spermatozoa and Oocytes. Biomed Res. Int. 2016. [CrossRef] [PubMed]
- Perrin, J.; Tassistro, V.; Mandon, M.; Grillo, J.M.; Botta, A.; Sari-Minodier, I. Tobacco Consumption and Benzo(a)Pyrene-Diol-Epoxide–DNA Adducts in Spermatozoa: In Smokers, Swim-up Procedure Selects Spermatozoa with Decreased DNA Damage. Fertil. Steril. 2011, 95, 2013–2017. [Google Scholar] [CrossRef]
- Phillips, D.H.; Venitt, S. DNA and Protein Adducts in Human Tissues Resulting from Exposure to Tobacco Smoke. Int. J. Cancer 2012. [Google Scholar] [CrossRef]
- Harlev, A.; Agarwal, A.; Gunes, S.O.; Shetty, A.; du Plessis, S.S. Smoking and Male Infertility: An Evidence-Based Review. World J. Mens. Health 2015, 33, 143–160. [Google Scholar] [CrossRef]
- Beal, M.A.; Yauk, C.L.; Marchetti, F. From Sperm to Offspring: Assessing the Heritable Genetic Consequences of Paternal Smoking and Potential Public Health Impacts. Mutat. Res. - Rev. Mutat. Res. 2017. [Google Scholar]
- Donkin, I.; Barrès, R. Sperm Epigenetics and Influence of Environmental Factors. Mol. Metab. 2018. [CrossRef]
- Alkhaled, Y.; Laqqan, M.; Tierling, S.; Lo Porto, C.; Amor, H.; Hammadeh, M.E. Impact of Cigarette-Smoking on Sperm DNA Methylation and Its Effect on Sperm Parameters. Andrologia 2018, 50. [Google Scholar] [CrossRef]
- World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. Cambridge Cambridge Univ. Press 2010.
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2− ΔΔCT Method. methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Amor, H.; Zeyad, A.; Hammadeh, M.E. Tobacco Smoking and Its Impact on the Expression Level of Sperm Nuclear Protein Genes: H2BFWT, TNP1, TNP2, PRM1 and PRM2. Andrologia 2021, 53, e13964. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.F.; Shelko, N.; Kartarius, S.; Montenarh, M.; Hammadeh, M.E. Impact of Cigarette Smoking on Histone (H2B) to Protamine Ratio in Human Spermatozoa and Its Relation to Sperm Parameters. Andrology 2014. [Google Scholar] [CrossRef] [PubMed]
- Hammadeh, M.E.; Hamad, M.F.; Montenarh, M.; Fischer-Hammadeh, C. Protamine Contents and P1/P2 Ratio in Human Spermatozoa from Smokers and Non-Smokers. Hum. Reprod. 2010, 25, 2708–2720. [Google Scholar] [CrossRef]
- Amor, H.; Nyaz, S.; Hammadeh, M.E. Paternal Smoking in Relation to Sperm Quality and Intracytoplasmic Sperm Injection Outcomes. Int. J. Women’s Heal. Reprod. Sci. 2019, 7, 451–460. [Google Scholar] [CrossRef]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette Smoking and Semen Quality: A New Meta-Analysis Examining the Effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Sepaniak, S.; Forges, T.; Gerard, H.; Foliguet, B.; Bene, M.C.; Monnier-Barbarino, P. The Influence of Cigarette Smoking on Human Sperm Quality and DNA Fragmentation. Toxicology 2006, 223, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, A.; Zafari, M. The Impact of Cigarette Smoking on Sperm Parameters: A Cross-Sectional Study. In Proceedings of the International Conference on Environmental, Biomedical and Biotechnology Web site. http://www. ipcbee. com/vol16/17-E20004. pdf; 2011. [Google Scholar]
- Cinar, O.; Dilbaz, S.; Terzioglu, F.; Karahalil, B.; Yücel, C.; Turk, R.; Taskin, L.; Kose, S.K. Does Cigarette Smoking Really Have Detrimental Effects on Outcomes of IVF? Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 174, 106–110. [Google Scholar] [CrossRef]
- Hamad, M.; Shelko, N.; Montenarh, M.; Hammadeh, M.E. The Impact of Cigarette Smoking on Protamines 1 and 2 Transcripts in Human Spermatozoa. Hum. Fertil. 2019. [Google Scholar] [CrossRef]
- Yu, B.; Ding, Q.; Zheng, T.; Jiang, L.; Li, Q.; Sun, X.; Bai, C.; Huang, Z. Smoking Attenuated the Association between IκBα Rs696 Polymorphism and Defective Spermatogenesis in Humans. Andrologia 2015, 47, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Laqqan, M.M.; Yassin, M.M. Cigarette Heavy Smoking Alters DNA Methylation Patterns and Gene Transcription Levels in Humans Spermatozoa. Environ. Sci. Pollut. Res. 2022, 29, 26835–26849. [Google Scholar] [CrossRef]
- Consortium, E.P. An Integrated Encyclopedia of DNA Elements in the Human Genome. Nature 2012, 489, 57. [Google Scholar] [CrossRef]
- Chen, G.; Han, Z.; Feng, D.; Chen, Y.; Chen, L.; Wu, H.; Huang, L.; Zhou, C.; Cai, X.; Fu, C.; et al. A Regulatory Signaling Loop Comprising the PGAM5 Phosphatase and CK2 Controls Receptor-Mediated Mitophagy. Mol. Cell 2014, 54, 362–377. [Google Scholar] [CrossRef]
- Wu, H.; Xue, D.; Chen, G.; Han, Z.; Huang, L.; Zhu, C.; Wang, X.; Jin, H.; Wang, J.; Zhu, Y. The BCL2L1 and PGAM5 Axis Defines Hypoxia-Induced Receptor-Mediated Mitophagy. Autophagy 2014, 10, 1712–1725. [Google Scholar] [CrossRef]
- Chen, Y.; Gong, K.; Xu, Q.; Meng, J.; Long, T.; Chang, C.; Wang, Z.; Liu, W. Phosphoglycerate Mutase 5 Knockdown Alleviates Neuronal Injury after Traumatic Brain Injury through Drp1-Mediated Mitochondrial Dysfunction. Antioxid. Redox Signal. 2021, 34, 154–170. [Google Scholar] [CrossRef]
- Villavicencio Tejo, F.; Quintanilla, R.A. Contribution of the Nrf2 Pathway on Oxidative Damage and Mitochondrial Failure in Parkinson and Alzheimer’s Disease. Antioxidants 2021, 10, 1069. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Balercia, G.; Vicari, E.; Calogero, A.E. Does Alcohol Have Any Effect on Male Reproductive Function? A Review of Literature. Asian J. Androl. 2013, 15, 221. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Aston, K.I.; Meyer, T.D.; Hotaling, J.M.; Shamsi, M.B.; Johnstone, E.B.; Cox, K.J.; Stanford, J.B.; Porucznik, C.A.; Carrell, D.T. Decreased Fecundity and Sperm DNA Methylation Patterns. Fertil. Steril. 2016, 105, 51–57. [Google Scholar] [CrossRef]
- Li, Z.; Zhuang, X.; Zeng, J.; Tzeng, C.-M. Integrated Analysis of DNA Methylation and MRNA Expression Profiles to Identify Key Genes in Severe Oligozoospermia. Front. Physiol. 2017, 8, 261. [Google Scholar] [CrossRef]
- Sarkar, S.; Sujit, K.M.; Singh, V.; Pandey, R.; Trivedi, S.; Singh, K.; Gupta, G.; Rajender, S. Array-Based DNA Methylation Profiling Reveals Peripheral Blood Differential Methylation in Male Infertility. Fertil. Steril. 2019, 112, 61–72. [Google Scholar] [CrossRef]
- Godowski, P.J.; Mark, M.R.; Chen, J.; Sadick, M.D.; Raab, H.; Hammonds, R.G. Reevaluation of the Roles of Protein S and Gas6 as Ligands for the Receptor Tyrosine Kinase Rse/Tyro 3. Cell 1995, 82, 355–358. [Google Scholar] [CrossRef]
- Joseph, D.R. Sequence and Functional Relationships between Androgen-Binding Protein/Sex Hormone-Binding Globulin and Its Homologs Protein S, Gas6, Laminin, and Agrin. Steroids 1997, 62, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Richard, I.; Broux, O.; Chiannilkulchai, N.; Fougerousse, F.; Allamand, V.; Bourg, N.; Brenguier, L.; Devaud, C.; Pasturaud, P.; Roudaut, C. Regional Localization of Human Chromosome 15 Loci. Genomics 1994, 23, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative Stress and Male Infertility. Nat. Rev. Urol. 2017. [CrossRef] [PubMed]
- Tawadrous, G.A.; Aziz, A.A.; Mostafa, T. Effect of Smoking Status on Seminal Parameters and Apoptotic Markers in Infertile Men. J. Urol. 2011, 186, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- La Maestra, S.; De Flora, S.; Micale, R.T. Effect of Cigarette Smoke on DNA Damage, Oxidative Stress, and Morphological Alterations in Mouse Testis and Spermatozoa. Int. J. Hyg. Environ. Health 2015. [Google Scholar] [CrossRef] [PubMed]
- Hamad, M.F.; Dayyih, W.A.A.; Laqqan, M.; AlKhaled, Y.; Montenarh, M.; Hammadeh, M.E. The Status of Global DNA Methylation in the Spermatozoa of Smokers and Non-Smokers. Reprod. Biomed. Online 2018, 37, 581–589. [Google Scholar] [CrossRef] [PubMed]
| Variables | Heavy Smokers (Mean ± SD) |
Non-smokers (Mean ± SD) |
P-value |
|---|---|---|---|
| Age | 37.42 ± 5.24 | 36.33 ± 6.18 | ≤ 0.4* |
| Count [mill/ml] | 52.38 ± 34.52 | 64.42 ± 39.18 | ≤ 0.01* |
| Total motility (%) | 44.61 ± 22.47 | 53.67 ± 20.51 | ≤ 0.003* |
| Progressive motility (%) | 33.17 ± 21.87 | 40.96 ± 20.78 | ≤ 0.01* |
| Immotile (%) | 51.13 ± 24.23 | 42.21 ± 18.24 | ≤0.002* |
| Normal form (%) | 17.22 ± 8 .26 | 22.98 ± 12.62 | ≤ 0.05* |
| Vitality (%) | 59.95 ± 13.08 | 64.87 ± 15.18 | ≤ 0.001* |
| Membrane integrity (HOS) (%) | 72.19 ± 10.03 | 78.73± 11.21 | ≤ 0.02* |
| SD, stander deviation. *Mann-Whitney test. P> 0.05: not significant. P ≤0.05: significant. ≤0.01 highly significant | |||
|
Target |
ΔCT Heavy smokers |
ΔCT Non-smokers |
ΔΔCT |
Fold Change 2- ΔΔCT |
Regulation |
P-value |
|---|---|---|---|---|---|---|
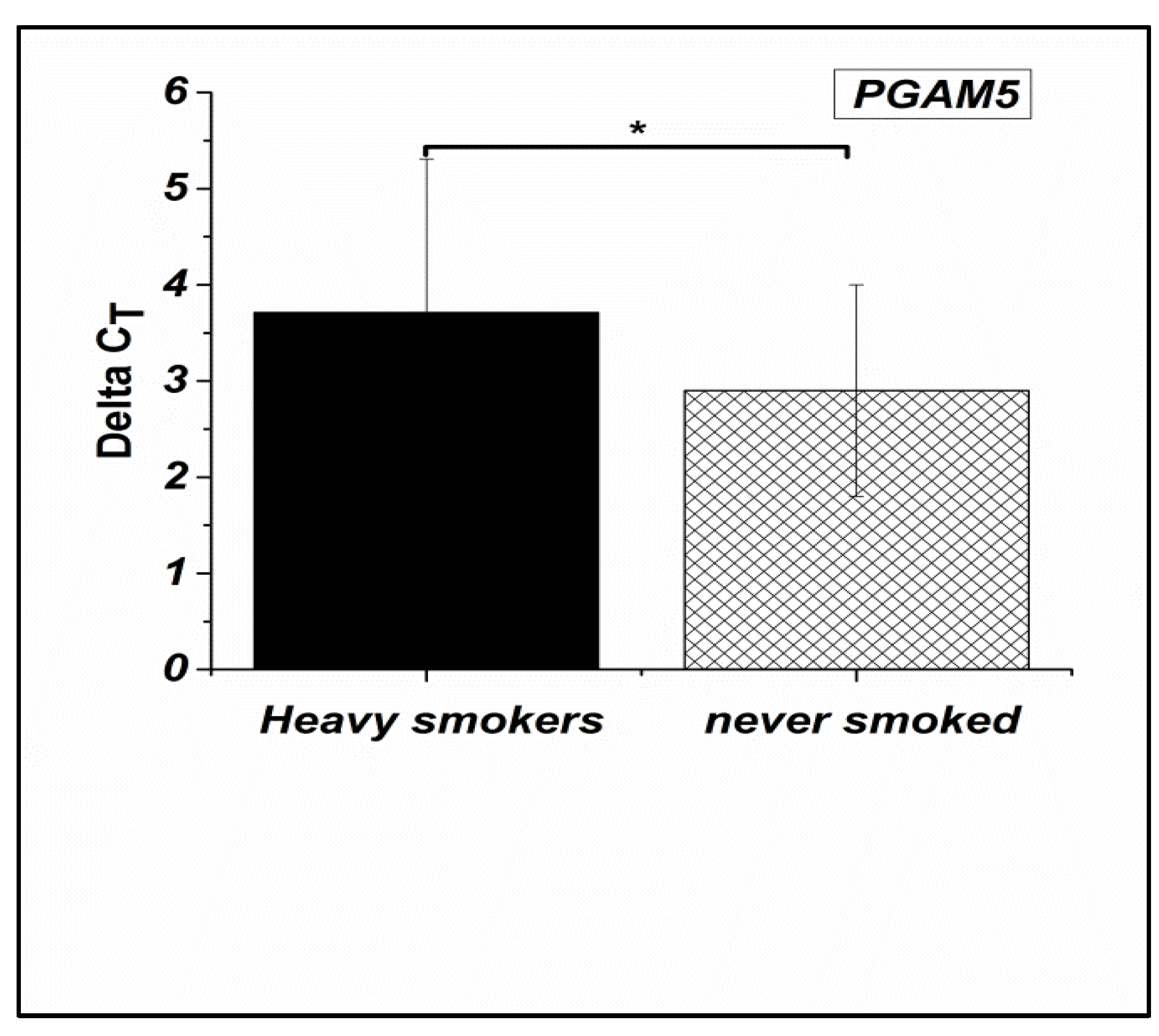
| PGAM5 | 3.7 ± 1.6 |
2.9 ± 1.1 |
0.804 |
0.573 |
Down |
≤0.03* |
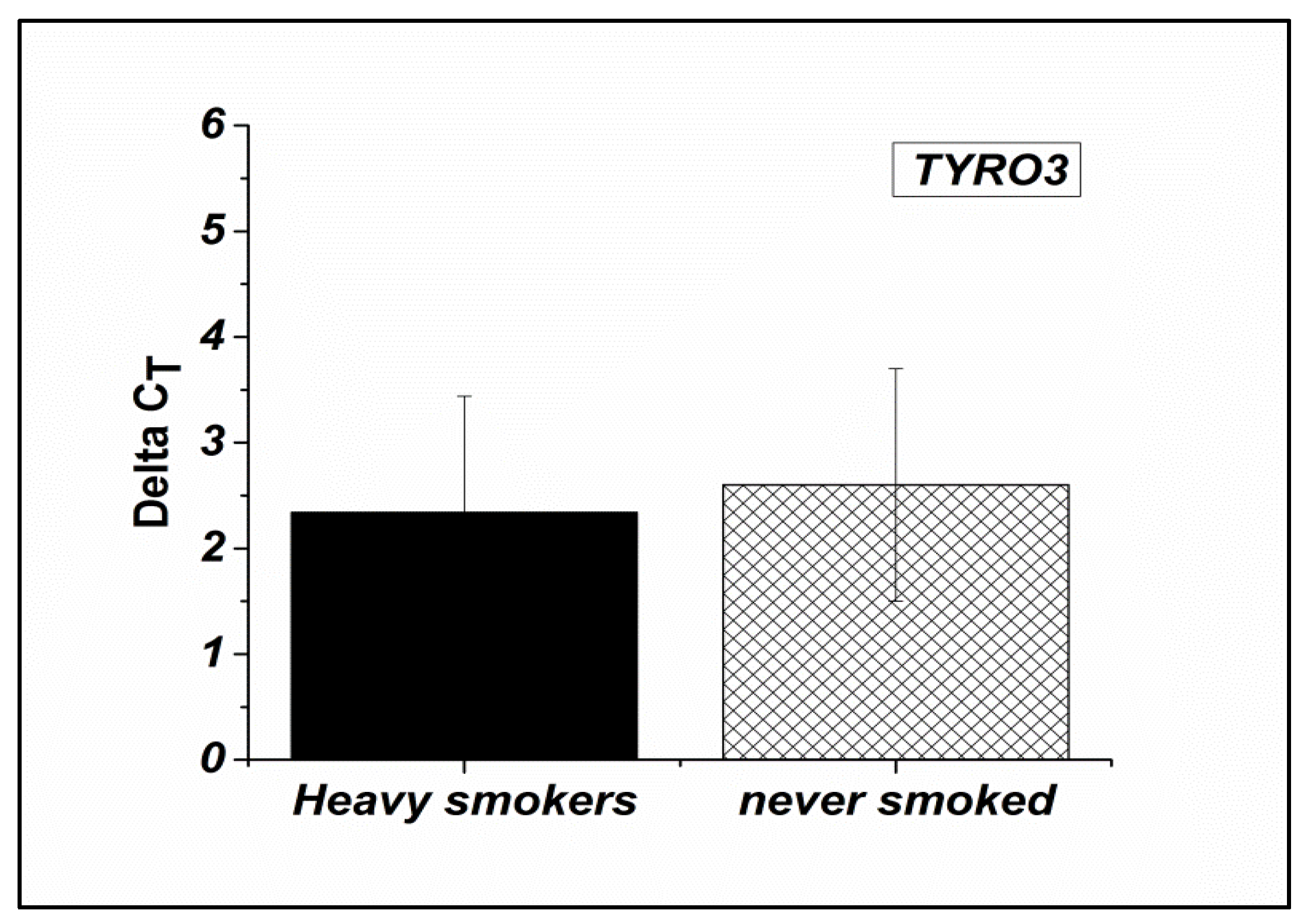
| TYRO3 |
2.3 ± 1.1 |
2.4 ± 1.0 |
-0.275 |
1.209 |
Up |
≤0.3* |
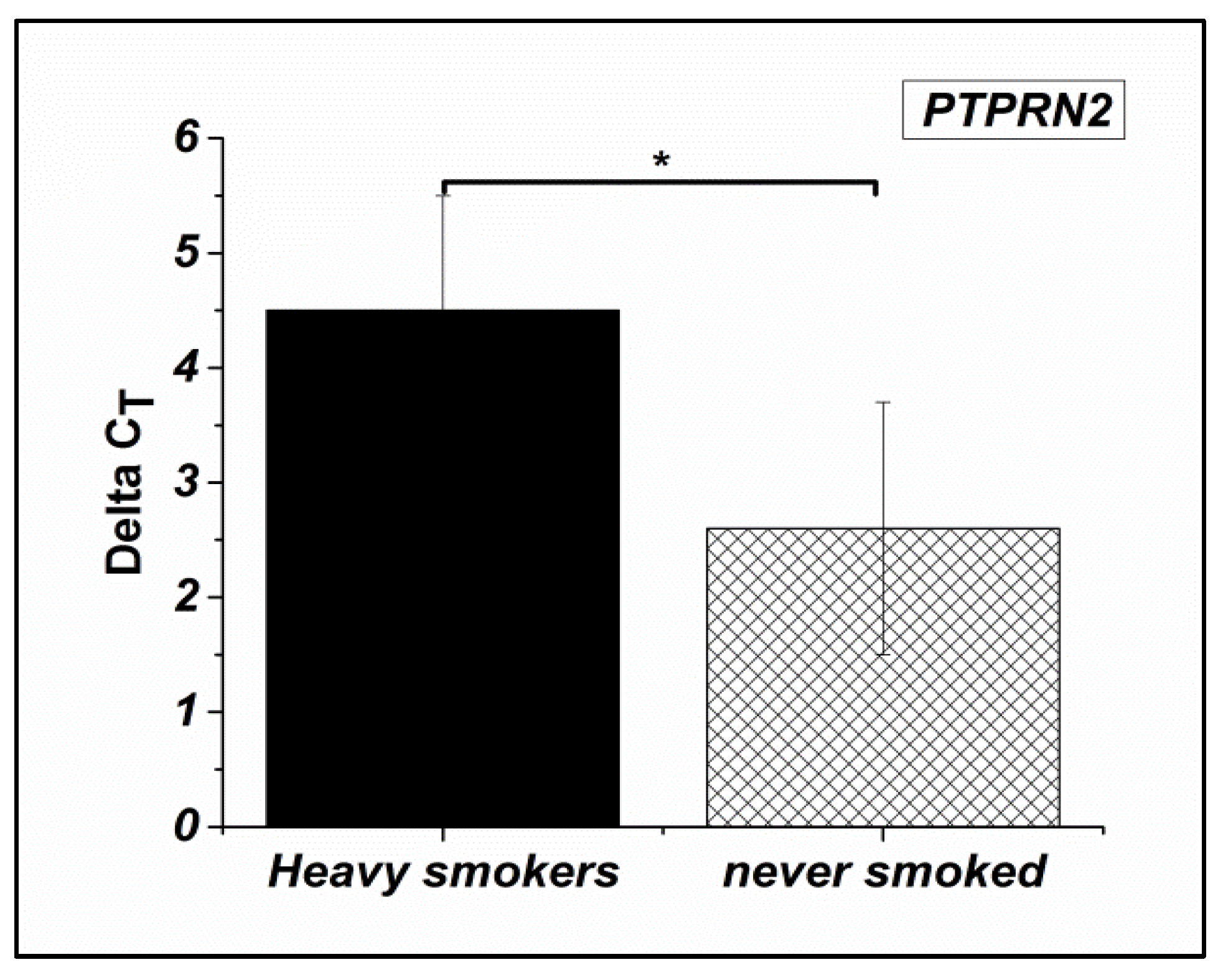
| PTPRN2 |
4.5 ± 1.0 |
3.9 ± 0.9 |
0.587 |
0.666 |
Down |
≤0.01* |
| Total sperm count (mill/ml) |
Motility (%) | Progressive (%) | Immotile (%) |
Normal form (%) | Vitality (%) | Membrane integrity (HOS) (%) |
||
|---|---|---|---|---|---|---|---|---|
|
PGAM5 |
R | 0.078 | -0.336** | -0.274* | -0.001 | -0.017 | 0.253* | 0.020 |
| P | 0.668 | 0.005 | 0.015 | 0.994 | 0.925 | 0.026 | 0.913 | |
|
TYRO3 |
R | -0.331** | 0.239** | 0.308* | -0.218 | 0.259* | -0.230* | 0.269* |
| P | 0.009 | 0.008 | 0.016 | 0.057 | 0.045 | 0.044 | 0.018 | |
|
PTPRN5 |
R | -0.273* | -0.276* | -0.293** | 0.268* | -0.228* | -0.344* | -0.194 |
| P | 0.016 | 0.015 | 0.009 | 0.018 | 0.046 | 0.021 | 0.202 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





