Submitted:
01 June 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Characterization methods
2.2. Adsorption with probe gases
3. Results and Discussion
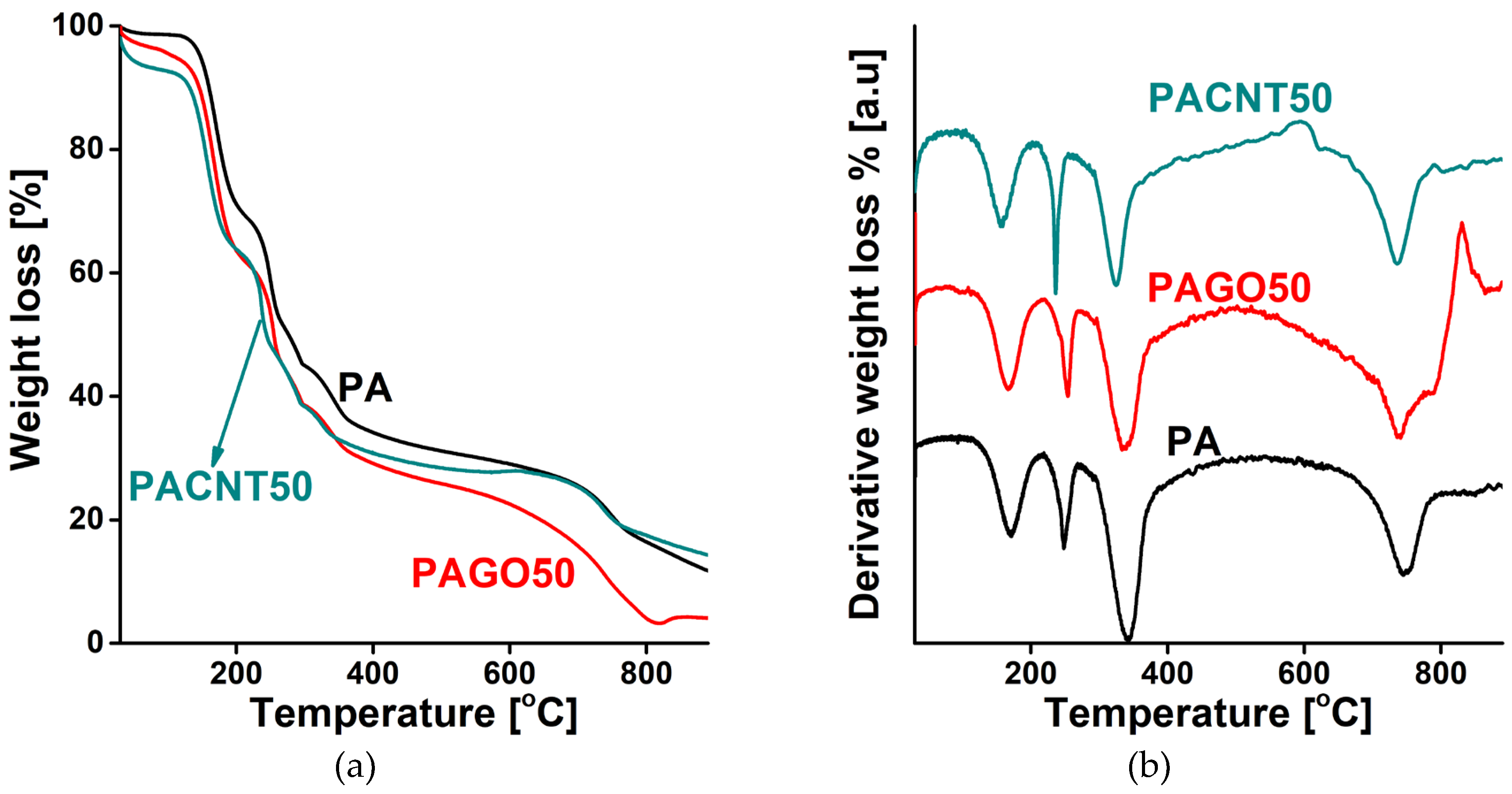
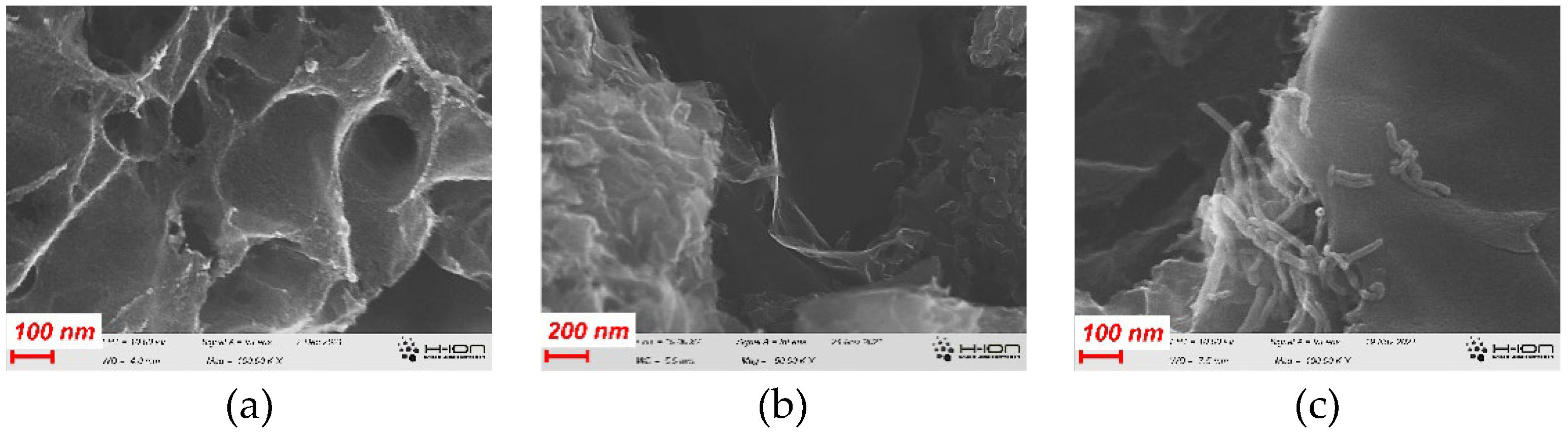
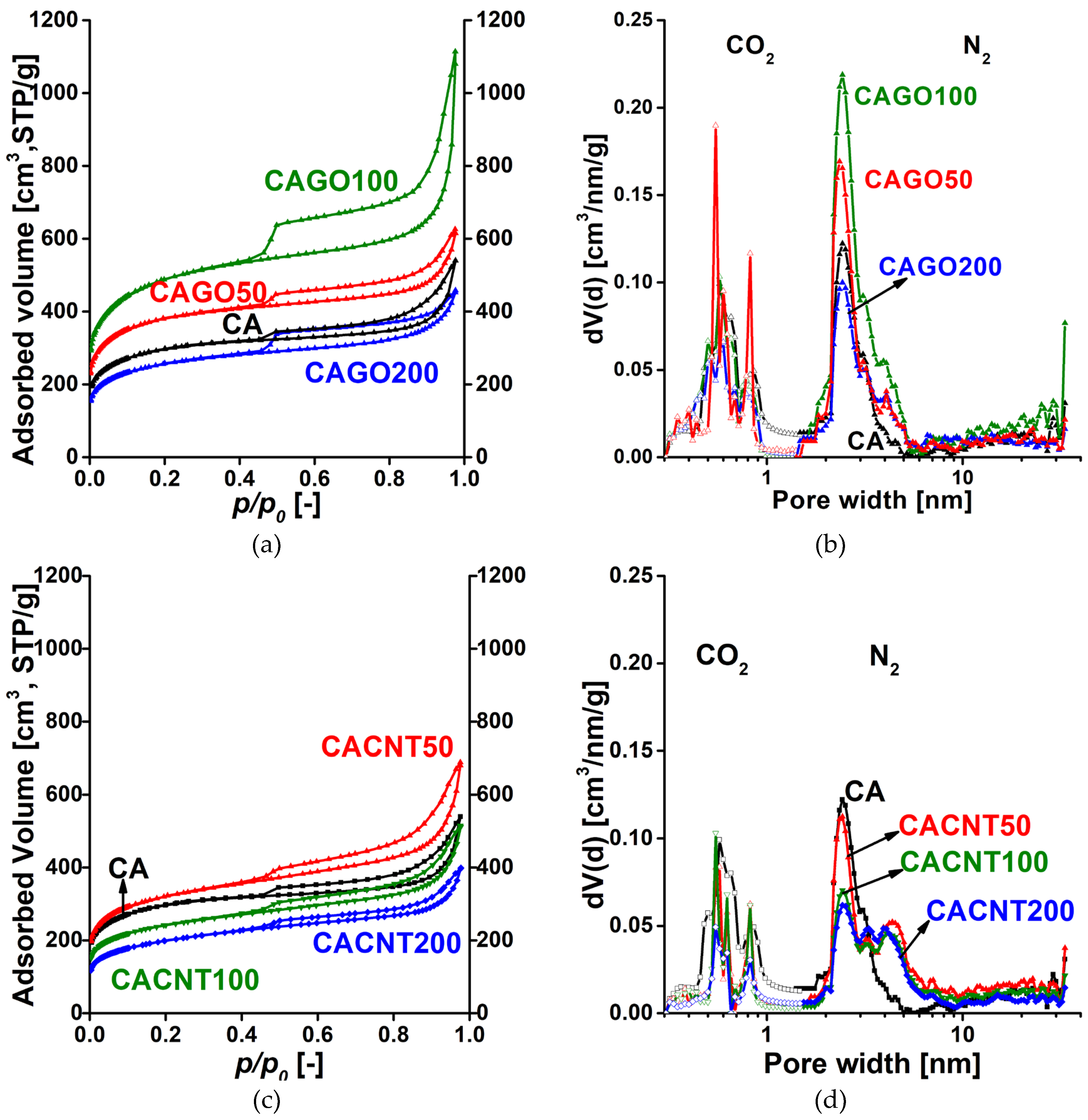
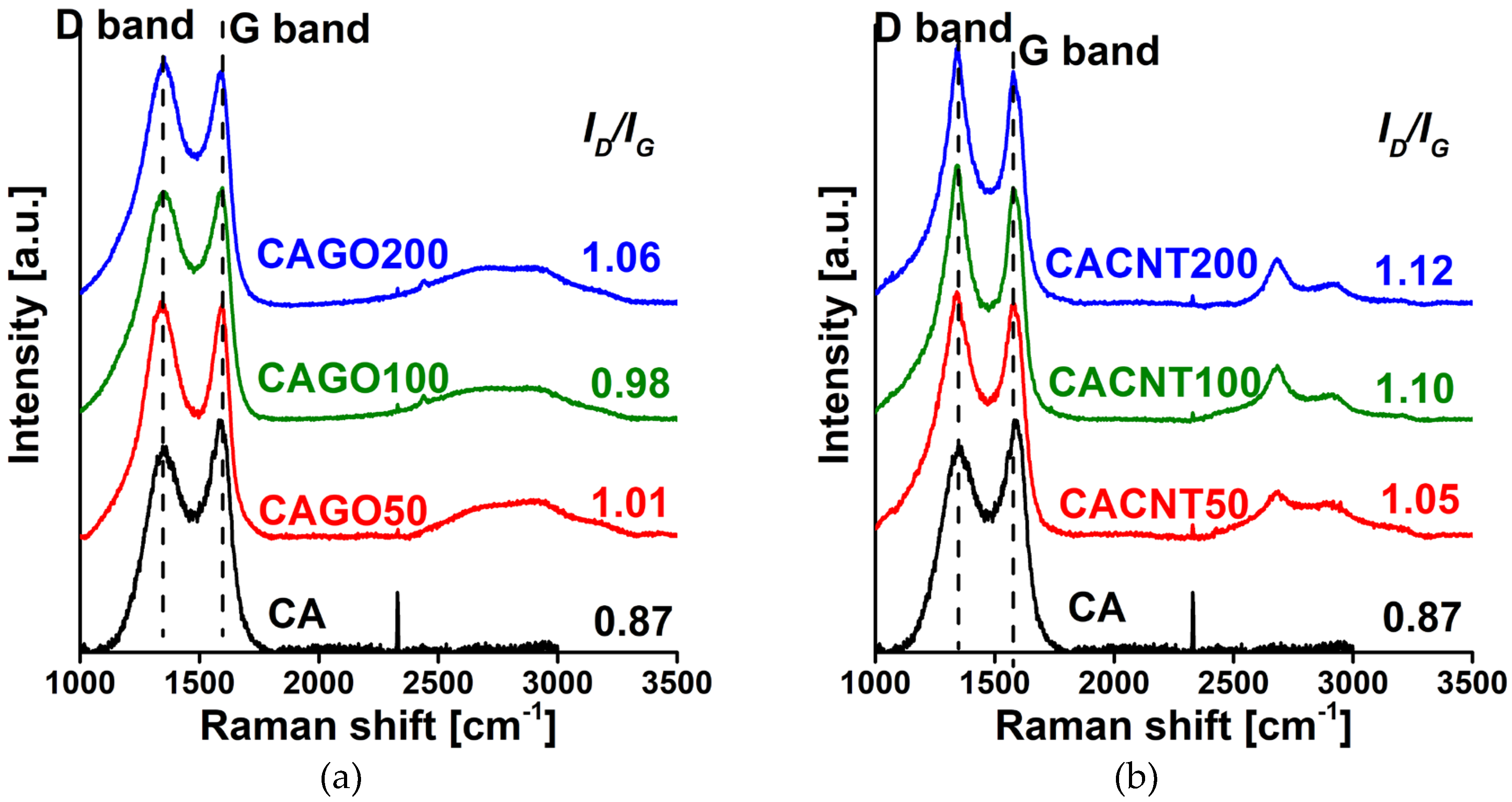
3.1. Effect of the CNPs on the morphology and chemistry of the samples
| from N2 | from CO2 | ||||||
|---|---|---|---|---|---|---|---|
| Sample | SBET | V0.98 | Vmicro | Vmeso | Vu micro DR | Vu micro DFT | |
| m2/g | cm3/g | cm3/g | % | cm3/g | cm3/g | cm3/g | |
| CA | 1070 | 0.83 | 0.40 | 48 | 0.43 | 0.057 | 0.037 |
| CAGO50 | 1408 | 0.95 | 0.56 | 59 | 0.39 | 0.049 | 0.027 |
| CAGO100 | 1779 | 1.72 | 0.64 | 37 | 1.08 | 0.050 | 0.027 |
| CAGO200 | 933 | 0.71 | 0.34 | 48 | 0.37 | 0.040 | 0.022 |
| CACNT50 | 1169 | 1.07 | 0.43 | 40 | 0.64 | 0.032 | 0.018 |
| CACNT100 | 880 | 0.79 | 0.32 | 41 | 0.47 | 0.028 | 0.016 |
| CACNT200 | 727 | 0.62 | 0.26 | 42 | 0.36 | 0.020 | 0.013 |
| Sample | C | O | N | S | O/C | N/C | S/C |
O+N+S C |
S/N |
|---|---|---|---|---|---|---|---|---|---|
| CA | 90.6 | 3.3 | 5.1 | 1.0 | 0.036 | 0.056 | 0.011 | 0.104 | 0.196 |
| CAGO50 | 92.0 | 3.1 | 3.7 | 1.3 | 0.034 | 0.039 | 0.014 | 0.087 | 0.361 |
| CAGO100 | 90.7 | 4.1 | 4.1 | 1.2 | 0.045 | 0.045 | 0.013 | 0.104 | 0.293 |
| CAGO200 | 90.4 | 3.7 | 4.4 | 1.4 | 0.041 | 0.049 | 0.015 | 0.105 | 0.318 |
| GO | 67.4 | 32.1 | - | 0.5 | 0.476 | - | 0.007 | 0.484 | - |
| CACNT50 | 91.6 | 4.0 | 3.1 | 1.2 | 0.044 | 0.034 | 0.013 | 0.091 | 0.387 |
| CACNT100 | 91.8 | 3.7 | 3.4 | 1.1 | 0.040 | 0.037 | 0.012 | 0.089 | 0.323 |
| CACNT200 | 92.2 | 4.6 | 1.9 | 1.2 | 0.050 | 0.021 | 0.013 | 0.084 | 0.632 |
| CNTs | 94.9 | 5.1 | - | - | 0.054 | - | - | 0.054 | - |
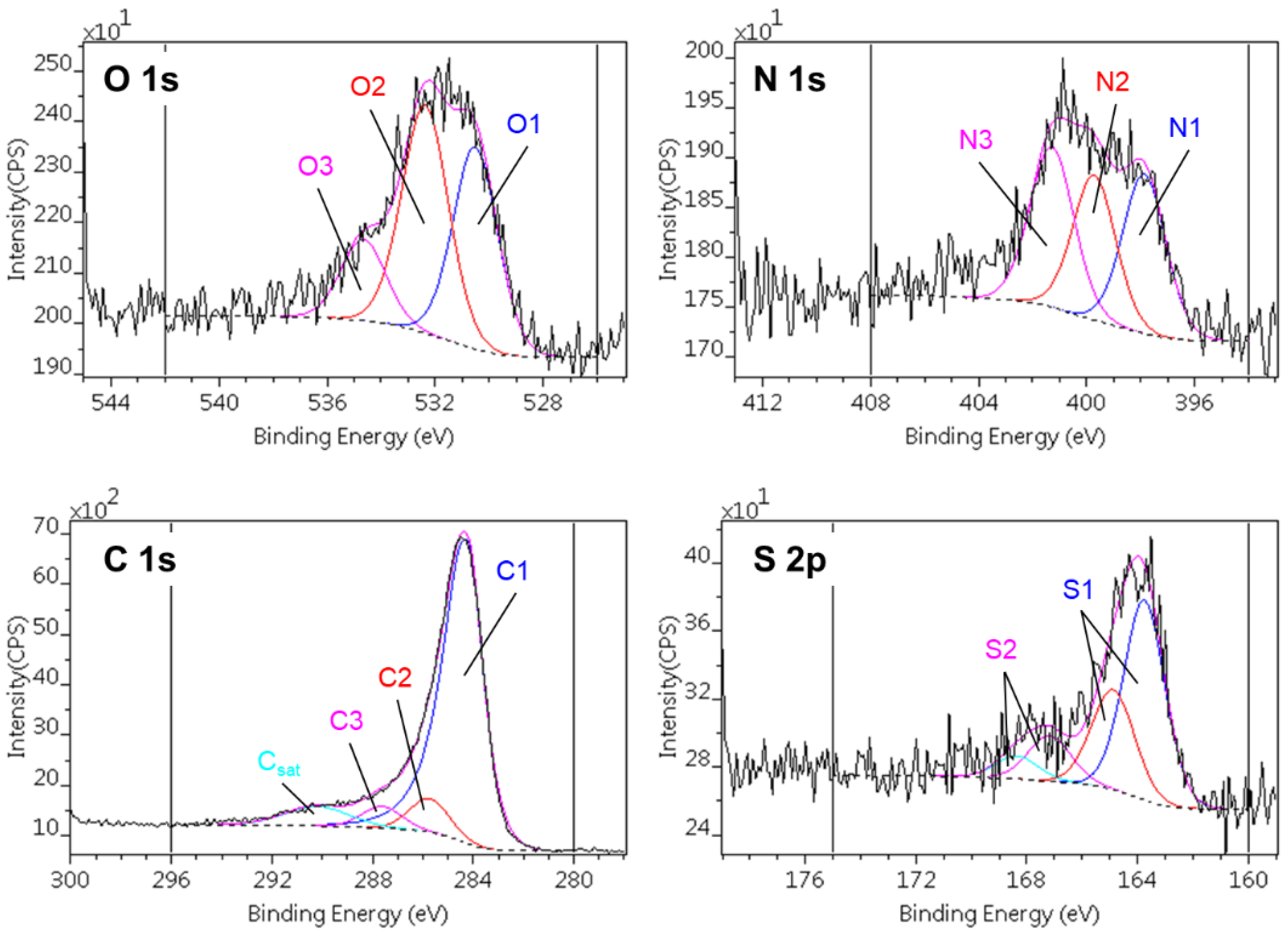
| C1s | O1s | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | O1 | O2 | O3 | |
| Chemical state | sp2 C=C | C–O C–N C–S |
C=O O–C–O N–C–O |
S–O | C–O–C C–OH C=O |
OC–O–CO (H2O) |
| Binding energy [eV] | 284.3 – 284.4 |
285.7 – 285.8 |
287.5 – 287.9 |
530.2 – 530.6 | 532.1 – 532.5 | 533.9 – 534.3 |
| CA | 74.0 | 10.9 | 5.4 | 1.5 | 1.7 | n.d. |
| CAGO50 | 78.8 | 7.4 | 5.5 | 1.9 | 1.3 | n.d. |
| CAGO100 | 74.7 | 11.0 | 4.8 | 1.8 | 1.7 | 0.7 |
| CAGO200 | 75.9 | 9.4 | 4.8 | 1.8 | 1.6 | 0.5 |
| CACNT50 | 78.6 | 7.8 | 5.1 | 1.6 | 1.9 | 0.7 |
| CACNT100 | 80.5 | 7.4 | 4.5 | 1.3 | 2.0 | 0.7 |
| CACNT200 | 75.7 | 10.8 | 5.5 | 1.7 | 2.3 | 0.9 |
| N1s | S2p | ||||
|---|---|---|---|---|---|
| N1 | N2 | N3 | S1 | S2 | |
| Chemical state | C–N | OO–C–N | C–N+ | C–S | C–SO3 |
| Binding energy [eV] | 397.8 – 398.0 |
400.4 – 400.5 | 402.4 – 402.7 | 164.9 – 165.0 | 168.3 – 168.6 |
| CA | 2.3 | 2.3 | 0.8 | 0.9 | 0.2 |
| CAGO50 | 1.6 | 1.7 | 0.6 | 1.2 | n.d. |
| CAGO100 | 1.9 | 1.9 | 0.4 | 1.0 | 0.2 |
| CAGO200 | 2.0 | 2.0 | 0.7 | 1.1 | 0.3 |
| CACNT50 | 1.1 | 1.0 | 1.1 | 1.0 | 0.2 |
| CACNT100 | 0.9 | 0.7 | 0.9 | 1.0 | 0.2 |
| CACNT200 | 0.6 | 0.5 | 0.8 | 0.9 | 0.4 |
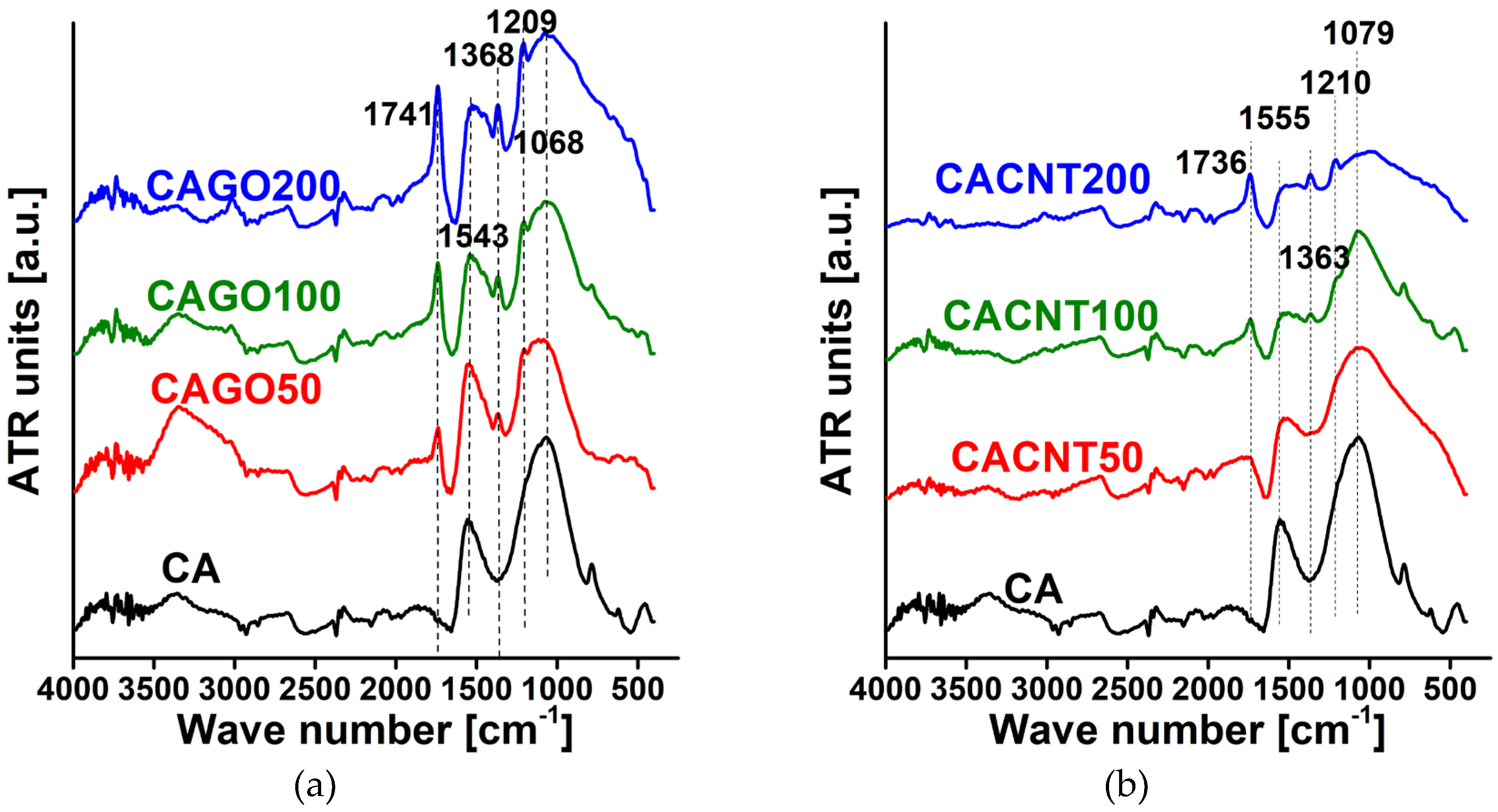
| Wavenumber [cm-1] | Assignation |
|---|---|
| 1750–1705 | aromatic (1730–1705) and aliphatic (1750–1730) C=O stretching |
| 1600–1400 | stretching and contracting of the C=C bonds in the aromatic ring |
| 1350 ± 50 | OH in plane bending of phenol and alcohol groups |
| 1260–1200 | C–O stretching in phenols |
| 1060–1035 | C-O stretching in noncyclic acid anhydrides |
| Sample | C=O/C=C | OH/C=C | C-O(H)/C=C | C-O-C/C=C |
|---|---|---|---|---|
| CA | 0.19 | 0.50 | 1.33 | 1.69 |
| CAGO50 | 0.53 | 0.62 | 1.10 | 1.17 |
| CAGO100 | 0.93 | 0.78 | 1.31 | 1.51 |
| CAGO200 | 1.17 | 1.02 | 1.55 | 1.60 |
| CACNT50 | 0.53 | 0.81 | 1.50 | 1.86 |
| CACNT100 | 0.91 | 1.00 | 1.73 | 2.68 |
| CACNT200 | 1.33 | 1.33 | 1.72 | 1.89 |
3.2. Gas storage and separation results
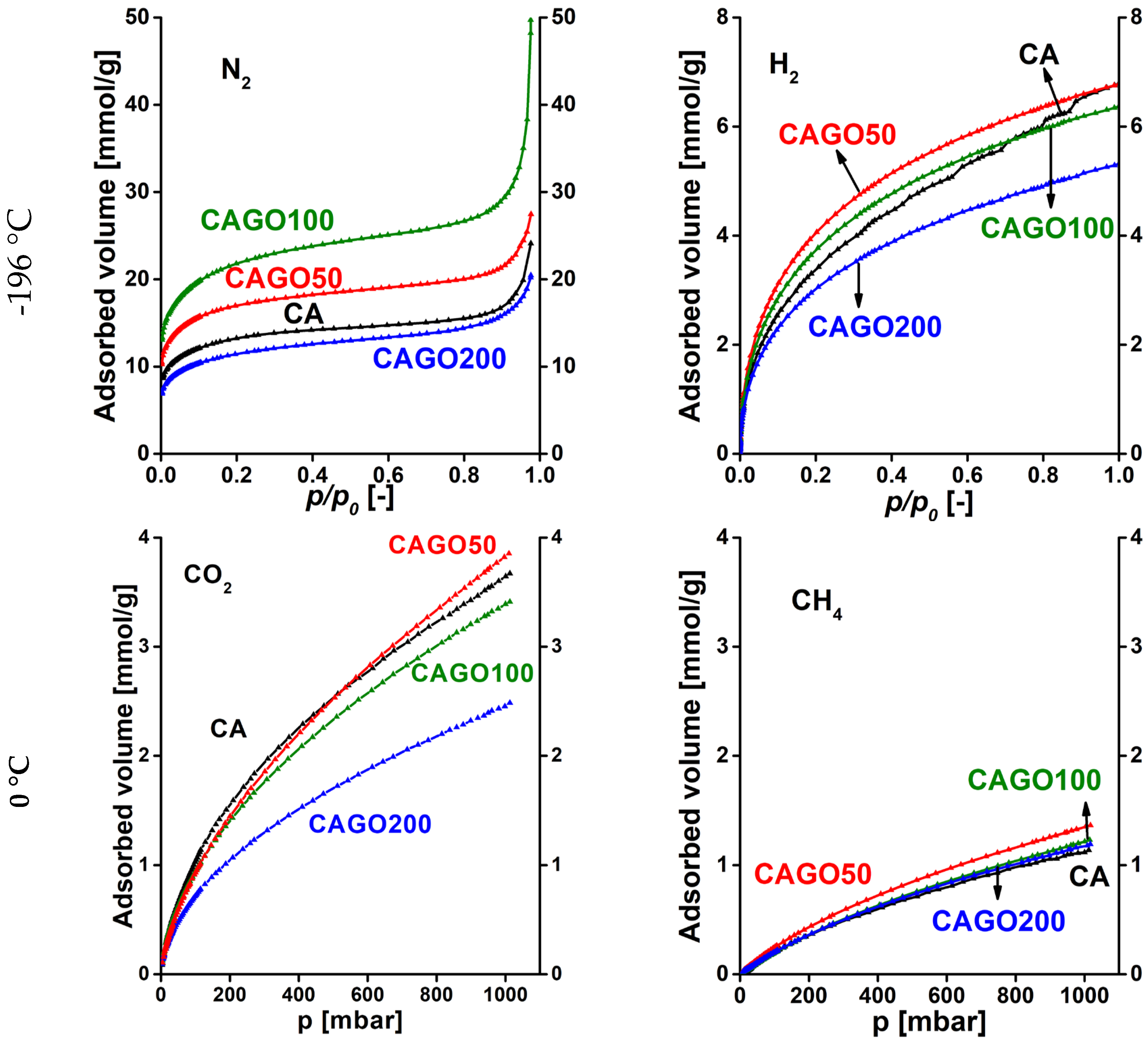
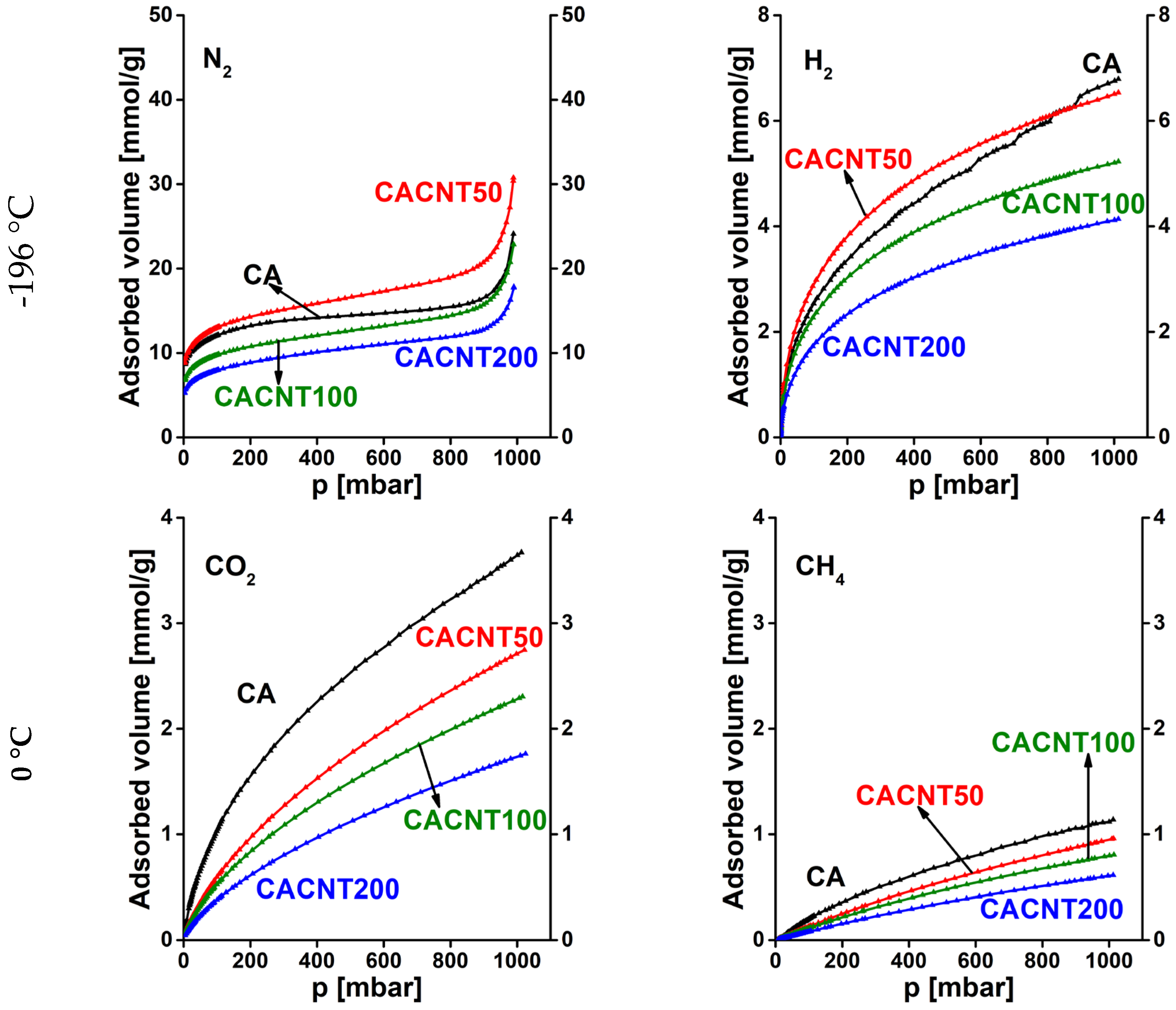
| CA | CAGO50 | CAGO100 | CAGO200 | CACNT50 | CACNT100 | CACNT200 | ||
|---|---|---|---|---|---|---|---|---|
| N2, -196 °C | 0.0236 | 0.0254 | 0.0271 | 0.0278 | 0.0297 | 0.0292 | 0.0298 | |
| 0.154 | 0.159 | 0.165 | 0.167 | 0.172 | 0.171 | 0.173 | ||
| KH* | 0.151 | 0.284 | 0.338 | 0.156 | 0.213 | 0.152 | 0.140 | |
| H2, -196 °C | 0.0978 | 0.106 | 0.108 | 0.0988 | 0.0842 | 0.116 | 0.0870 | |
| 0.313 | 0.325 | 0.329 | 0.314 | 0.290 | 0.341 | 0.295 | ||
| KH | 0.543 | 0.661 | 0.541 | 0.437 | 0.430 | 0.329 | 0.241 | |
| N2/H2, -196 °C |
ratio |
0.241 | 0.240 | 0.251 | 0.281 | 0.353 | 0.252 | 0.343 |
|
ratio |
0.491 | 0.490 | 0.501 | 0.530 | 0.594 | 0.502 | 0.586 | |
| KH ratio | 0.277 | 0.430 | 0.625 | 0.357 | 0.495 | 0.461 | 0.582 | |
| CO2, 0 °C | 0.212 | 0.196 | 0.182 | 0.163 | 0.197 | 0.198 | 0.201 | |
| 0.461 | 0.442 | 0.427 | 0.403 | 0.444 | 0.444 | 0.448 | ||
| KH | 0.0186 | 0.0166 | 0.0190 | 0.0148 | 0.00950 | 0.00890 | 0.00620 | |
| CH4, 0 °C | 0.551 | 0.239 | 0.195 | 0.251 | 0.236 | 0.239 | 0.220 | |
| 0.742 | 0.488 | 0.442 | 0.501 | 0.486 | 0.488 | 0.469 | ||
| KH | 0.00210 | 0.00300 | 0.00210 | 0.00240 | 0.00140 | 0.00120 | 0.000900 | |
| CO2/CH4, 0 °C |
ratio |
0.385 | 0.821 | 0.932 | 0.649 | 0.835 | 0.828 | 0.913 |
|
ratio |
0.620 | 0.906 | 0.966 | 0.805 | 0.914 | 0.910 | 0.956 | |
| KH ratio | 8.86 | 5.53 | 9.048 | 6.17 | 6.79 | 7.42 | 6.89 |
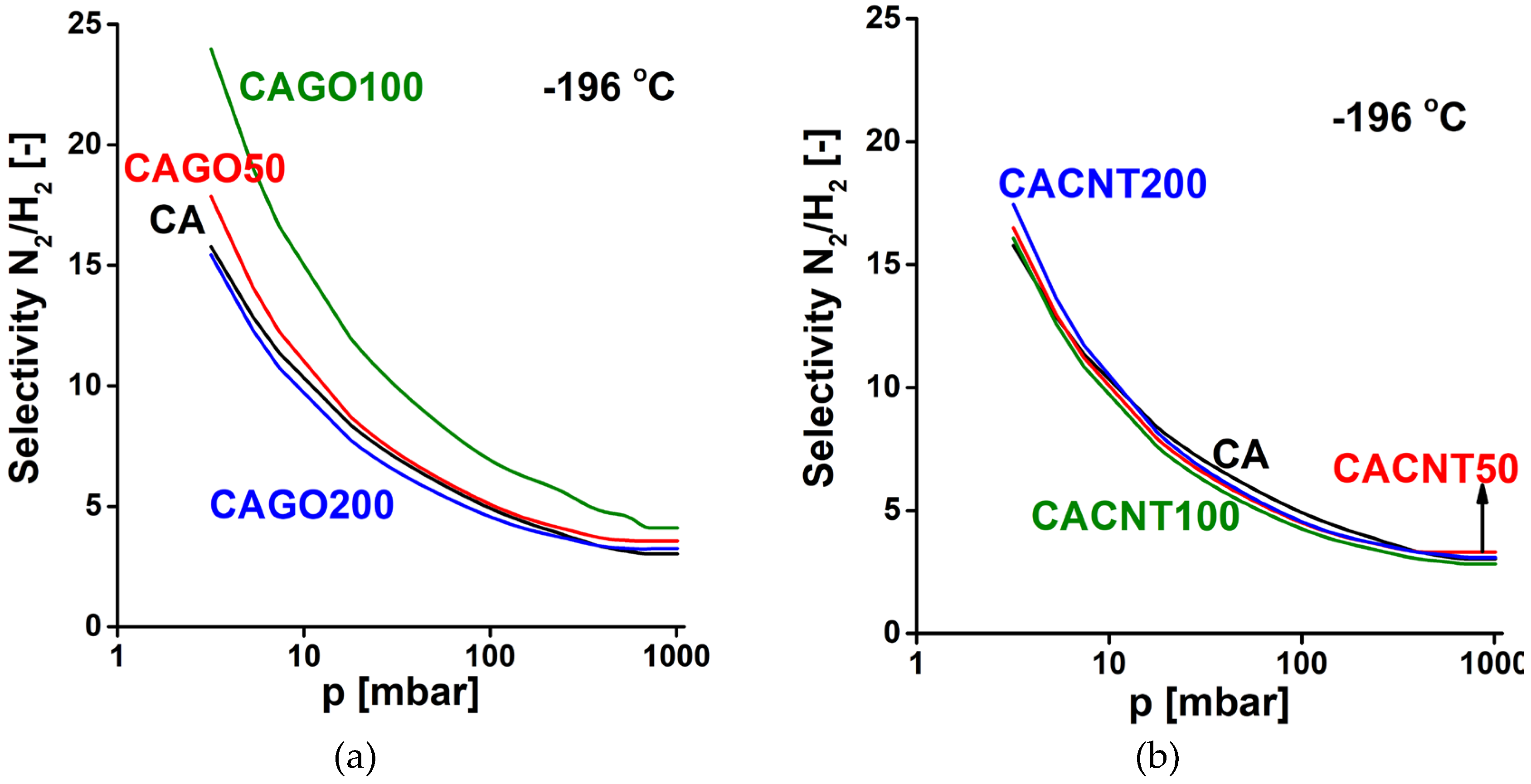
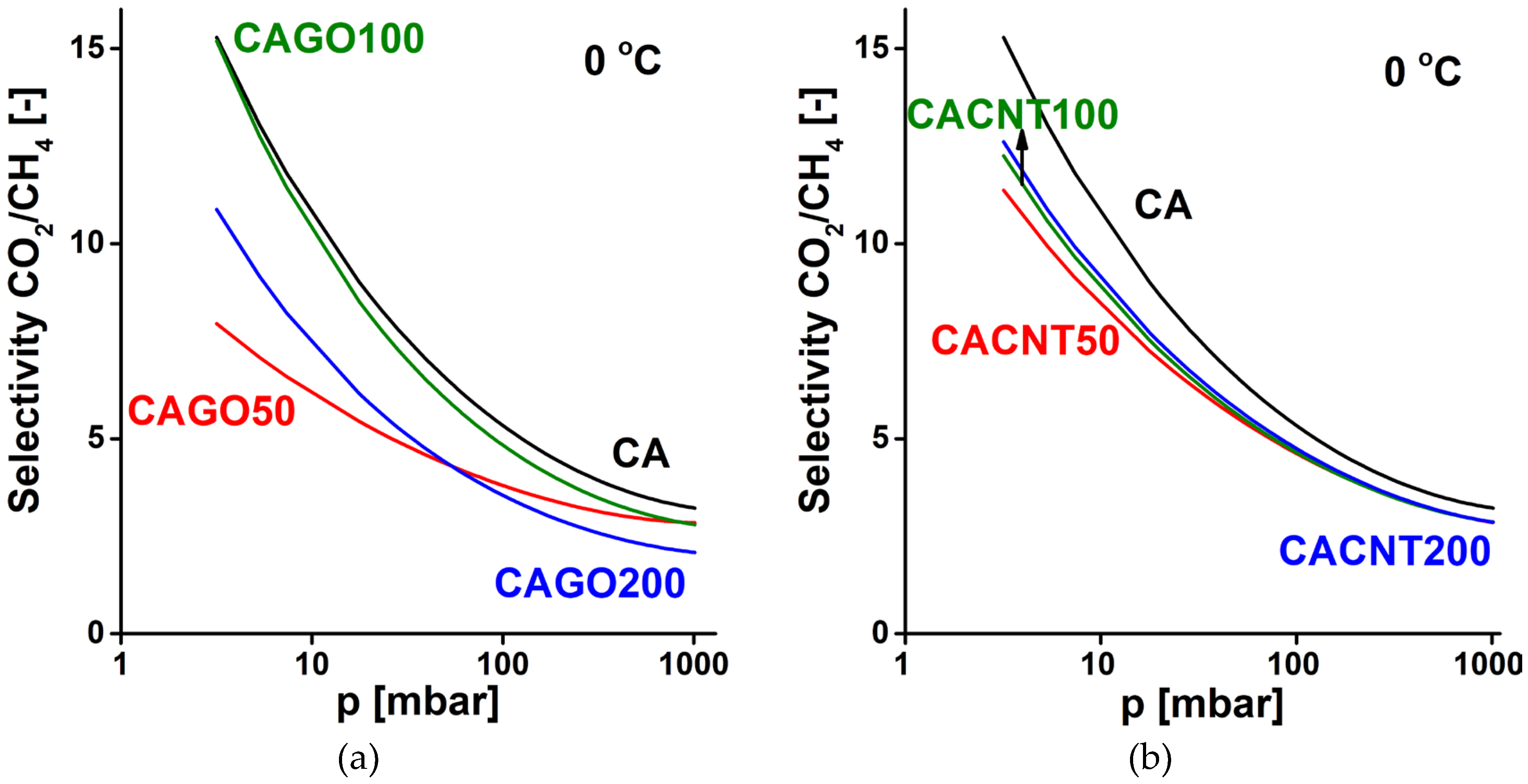
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- M.M. Titirici, R.J. White, C. Falco, M. Sevilla. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ Sci. 2012, 5, 6796-6822. DOI: 10.1039/C2EE21166A. [CrossRef]
- E. Azwar, W.A.W. Mahari, J. Huang Chuah, D.V.N. Vo, N.L. Ma, W.H. Lam, S.S. Lam. Transformation of biomass into carbon nanofiber for supercapacitor application—a review. Int J Hydrog Energy. 2018, 43, 20811–20821. DOI: 10.1016/j.ijhydene.2018.09.111. [CrossRef]
- D. Praveen Kumar, D. Ramesh, V. Karuppasamy Vikraman, P. Subramanian. Synthesis of carbon molecular sieves from agricultural residues: status, challenges and prospects. Environ. Res., 2022, 214, 114022. DOI: 10.1016/j.envres.2022.114022. [CrossRef]
- Rehman, G. Nazir, K.Y. Rhee, S.J. Park. A rational design of cellulose-based heteroatom-doped porous carbons: Promising contenders for CO2 adsorption and separation. J. Chem. Eng., 2021, 420, 130421. DOI: 10.1016/j.cej.2021.130421. [CrossRef]
- Y. Tan, X. Wang, S. Song, M. Sun, Y. Xue, G. Yang. Preparation of nitrogen-doped cellulose-based porous carbon and its carbon dioxide adsorption properties. ACS Omega, 2021, 6, 24814-24825. DOI: 10.1021/acsomega.1c03664. [CrossRef]
- G. Onyestyák, K. László, L.V.C. Rees. Molecular sieve honeycomb for air separation from Picea abies. Helv. Chim. Acta, 2004, 87 (7), 1888-1893. DOI: 10.1002/hlca.200490167. [CrossRef]
- A.A. Alhwaige, T. Agag, H, Ishida, S. Qutubuddin. Biobased chitosan hybrid aerogels with superior adsorption: role of graphene oxide in CO2 capture. RSC Adv., 2013, 3 (36), 16011-16044. DOI: 10.1039/C3RA42022A. [CrossRef]
- S. Shi, F.O. Ochedi, S. Cui, Y. Liu. Removal of CO2 from flue gas using seaweed porous carbons prepared by urea doping and KOH activation. Energy Fuels, 2020, 34 (12), 16411–16422. DOI: 10.1021/acs.energyfuels.0c03200. [CrossRef]
- M. Yang, C. Ma, M. Xu, S. Wang, L. Xu. Recent advances in CO2 adsorption from air: a review. Current Pollution Reports, 2019, 5, 272–293. DOI: 10.1007/s40726-019-00128-1. [CrossRef]
- T.S. Lee, J.H. Cho, S.H. Chi. Carbon dioxide removal using carbon monolith as electric swing adsorption to improve indoor air quality. Build Environ., 2015, 92, 209–21. DOI: 10.1016/j.buildenv.2015.04.028. [CrossRef]
- Z. Zhang, J. Zhou, W. Xing, et al. Critical role of small micropores in high CO2 uptake. Phys Chem Chem Phys., 2013, 15 (7), 2523–2529. DOI: 10.1039/c2cp44436d. [CrossRef]
- L.F. Villalobos, D.J. Babu, K-J. Hsu, C. Van Goethem, K.V. Agrawal. Gas separation membranes with atom-thick nanopores: The potential of nanoporous single-layer graphene. Acc. Mater. Res., 2022, 3, 1073-1087. DOI: 10.1021/accountsmr.2c00143. [CrossRef]
- L. Zhu, D. Shen, K.H. Luo. A critical review on VOCs adsorption by different porous materials: species, mechanisms and modification methods. J. Hazard Mater., 2020, 389, 122102. DOI: 10.1016/j.jhazmat.2020.122102. [CrossRef]
- X. Jia, S. Xu, Y. Cong. Kinetics of spontaneous liquid-gas imbibition in carbon molecular sieves used for O2/N2 separation. Microporous Mesoporous Mater., 2017, 241, 185–191. DOI: 10.1016/j.micromeso.2016.12.040. [CrossRef]
- A.R. Mohamed, M. Mohammadi, G.N. Darzi. Preparation of carbon molecular sieve from lignocellulosic biomass: a review. Renew. Sustain. Energy Rev., 2010, 14, 1591–1599. DOI: 10.1016/j.rser.2010.01.024. [CrossRef]
- S. Villar-Rodil, R. Navarrete, R. Denoyel, A. Albiniak, J.I. Paredes, A. Martínez-Alonso, J.M.D. Tascón. Carbon molecular sieve cloths prepared by chemical vapour deposition of methane for separation of gas mixtures. Microporous Mesoporous Mater., 2005, 77, 109–118. DOI: 10.1016/j.micromeso.2004.08.017. [CrossRef]
- H. Marsh, F. Rodríguez Reinoso: Activated Carbon. Elsevier, 2006. DOI: 10.1016/B978-0-08-044463-5.X5013-4. [CrossRef]
- R. Muhammad, Y-C. Nah, H. Oh. Spider silk-derived nanoporous activated carbon fiber for CO2 capture and CH4 and H2 storage. J. CO2 Util., 2023, 69, 102401. DOI: 10.1016/j.jcou.2023.102401. [CrossRef]
- M. Sevilla, A.S.M. Al-Jumialy, A.B. Fuertes, R. Mokaya. Optimization of the pore structure of biomass-based carbons in relation to their use for CO2 capture under low- and high-pressure regimes. ACS Appl. Mater. Interfaces, 2018, 10, 1623-1633. DOI: 10.1021/acsami.7b10433. [CrossRef]
- T.S. Blankenship, N. Balahmar, R. Mokaya. Oxygen-rich microporous carbons with exceptional hydrogen storage capacity. Nat. Commun., 2017, 8, 1545. DOI: 10.1038/s41467-017-01633-x. [CrossRef]
- J. Park, N.F. Attia, M. Jung, M. Lee, K. Lee, J. Chung, H. Oh. Sustainable nanoporous carbon for CO2, CH4, N2, H2 adsorption and CO2/CH4 and CO2/N2 separation. Energy, 2018, 158, 9-16. DOI: 10.1016/j.energy.2018.06.010. [CrossRef]
- Ashourirad, P. Arab, A. Verlander, H.M. El-Kaderi. From Azo-linked polymers to microporous heteroatom-doped carbons: tailored chemical and textural properties for gas separation. ACS Appl. Mater. Interfaces, 2016, 8, 8491-8501. DOI: 10.1021/acsami.6b00567. [CrossRef]
- X. Wang, G. Sun, P. Routh, D-H. Kim, W. Huang, P. Chen. Heteroatom-doped graphene materials: synthesis, properties and applications. Chem. Soc. Rev., 2014, 43, 7067. DOI: 10.1039/c4cs00141a. [CrossRef]
- M. Sevilla, C. Falco, M.M. Titirici, A.B. Fuertes. High-performance CO2 sorbents from algae. RSC Adv., 2012, 2 (33), 12792-12797. DOI: 10.1039/C2RA22552B. [CrossRef]
- J. Liang, Y. Jiao, M. Jaroniec, S.Z. Qiao. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed., 2012, 51 (46), 11496-11500. DOI: 10.1002/anie.201206720. [CrossRef]
- B.J. Bucior, D-L. Chen, J. Liu, J.K. Johnson. Porous carbon nanotube membranes for separation of H2/CH4 and CO2/CH4 mixtures. J. Phys. Chem. C., 2012, 116 (49), 25904-25910. DOI:10.1021/jp3098022. [CrossRef]
- Li, Y. Jia, G. Chang, J. Chen, H. Liu, J. Wang, Y. Hu, Y. Xia, D. Yang, X. Yao. A defect-driven metal-free electrocatalyst for oxygen reduction in acidic electrolyte. Chem, 2018, 4, 2345-2356. DOI: 10.1016/j.chempr.2018.07.005. [CrossRef]
- S.K. Samaniego Andrade, I. Bakos, G. Dobos, A. Farkas, G. Kiss, S. Klébert, J. Madarász, K. László. Biomass related highly porous metal free carbon for gas storage and electrocatalytic applications. Materials, 2021, 14 (13), 3488. DOI: 10.3390/ma14133488. [CrossRef]
- A.L. Myers, J.M. Prausnitz. Thermodynamics of mixed-gas adsorption. AIChE J, 1965, 11, 121-127. DOI: 10.1002/aic.690110125. [CrossRef]
- W.S. Hummers, R.E. Offeman. Preparation of graphitic oxide. J. Am. Chem. Soc., 1958, 80, 1339–1339. DOI: 10.1021/ja01539a017. [CrossRef]
- D.C. Marcano, D.V. Kosynkin, J.M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, et al.: Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–14. DOI: 10.1021/nn1006368. [CrossRef]
- A. Tóth, K. V. Voitko, O. Bakalinska, G. P. Prykhod’ko, I. Bertóti, V. M. Gun’ko, K. László: Morphology and adsorption properties of chemically modified MWCNT probed by nitrogen, n-propane and water vapor. Carbon, 2012, 50 (2), 577-585. DOI: 10.1016/j.carbon.2011.09.016. [CrossRef]
- S. Brunauer, P.H. Emmett, E. Teller. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. DOI: 10.1021/ja01269a023. [CrossRef]
- M.M. Dubinin, L.V. Radushkevich. The Equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR., 1947, 55, 327-329.
- J. Landers, G.Y. Gor, A.V. Neimark. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng., 2013, 437, 3-32. DOI: 10.1016/j.colsurfa.2013.01.007. [CrossRef]
- Bertóti, M. Mohai, K. László. Surface modification of graphene and graphite by nitrogen plasma: Determination of chemical state alterations and assignments by quantitative X-ray photoelectron spectroscopy. Carbon, 2015, 84, 185-196. DOI: 10.1016/j.carbon.2014.11.056. [CrossRef]
- M. Mohai. XPS MultiQuant: multimodel XPS quantification software. Surf. Interface Anal., 2004, 36 (8), 828-832. DOI: 10.1002/sia.1775. [CrossRef]
- S. Evans, R.G. Pritchard, J.M. Thomas. Relative differential subshell photoionization cross-sections (MgKα) from lithium to uranium. J. Electron Spectrosc. Relat. Phenom., 1978, 14 (5), 341-358. DOI: 10.1016/0368-2048(78)80008-5. [CrossRef]
- R.F. Reilman, A. Msezane, S.T. Manson. Relative intensities in photoelectron spectroscopy of atoms and molecules. J. Electron Spectrosc. Relat. Phenom., 1976, 8 (5), 389-394. DOI: 10.1016/0368-2048(76)80025-4. [CrossRef]
- M. Thommes, K. Kaneko, A.V. Neimark, J.P. Oliver, F. Rodriguez-Reinoso, J. Rouquerol, K.S.W. Sing. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem., 2015, 87 (9-10), 1051-1069. DOI: 10.1515/pac-2014-1117. [CrossRef]
- Y. Wang, D.C. Alsmeyer, R.L. McCreery. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater., 1990, 2 (5), 557-563. DOI: 10.1021/cm00011a018. [CrossRef]
- B.C. Smith: Infrared Spectral Interpretation. CRC Press, 2018. DOI: 10.1201/9780203750841. [CrossRef]
- U. Kamran, S.J. Park. Acetic acid-mediated cellulose-based carbons: Influence of activation conditions on textural features and carbon dioxide uptakes. J. Colloid Interface Sci., 2021, 594, 745–758. DOI: 10.1016/j.jcis.2021.03.069. [CrossRef]
- R. Sips. On the structure of a catalyst surface. J. Chem. Phys., 1948, 16, 490-495. DOI: 10.1063/1.1746922. [CrossRef]
- R. Sips. On the structure of a catalyst surface. II. J. Chem. Phys., 1950, 18, 1024-1026. DOI: 10.1063/1.1747848. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




