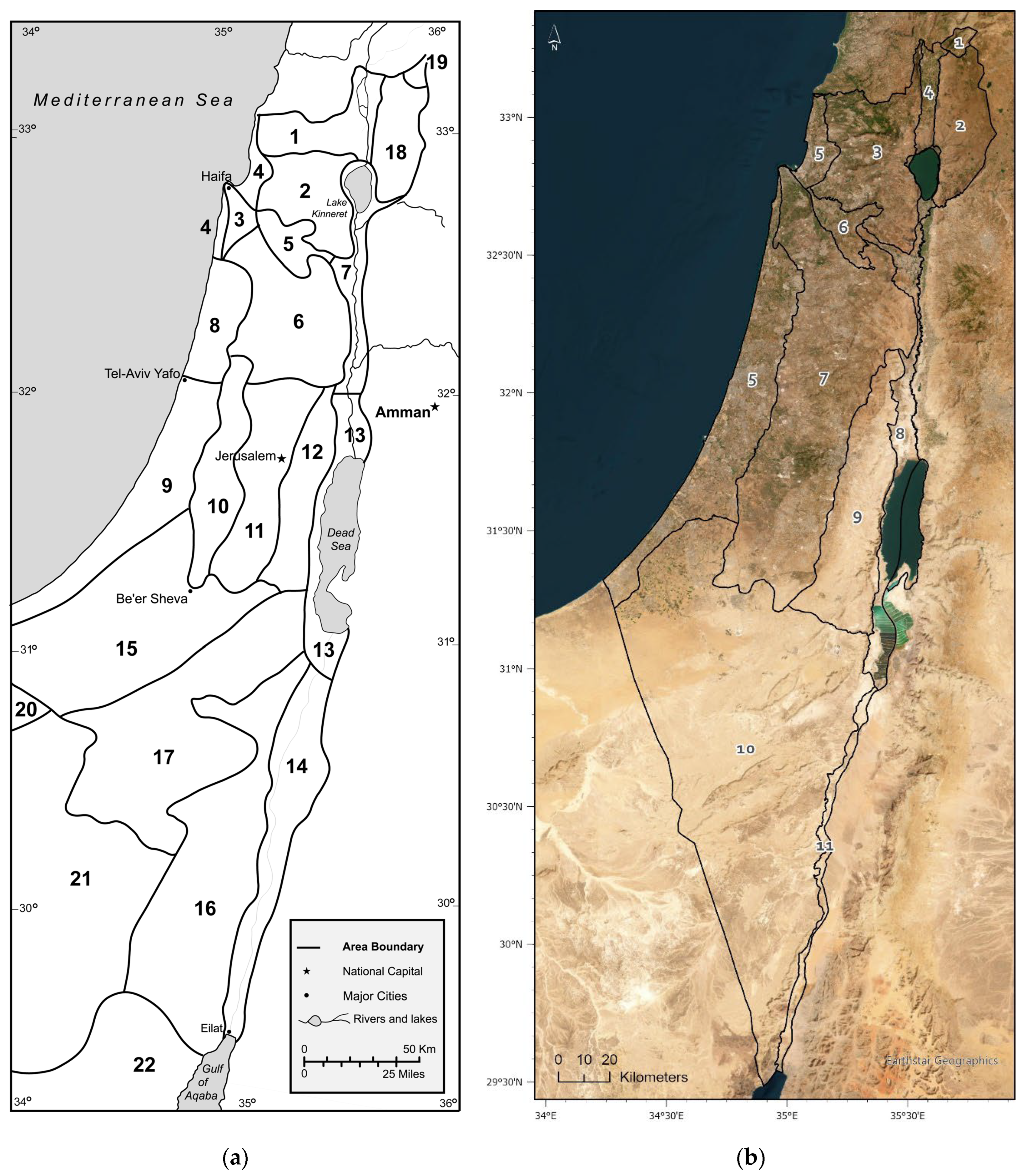
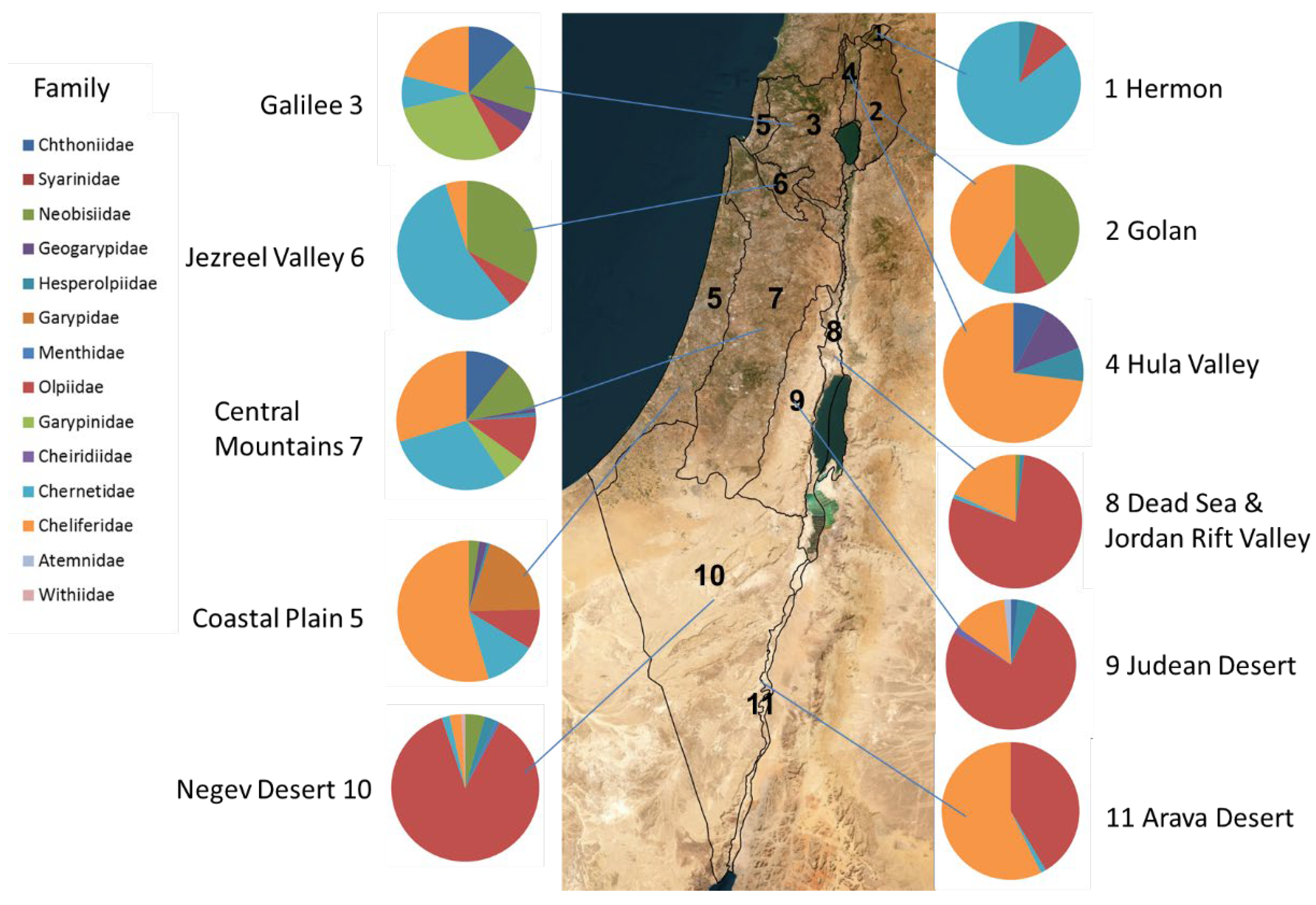
3.1.2. The pseudoscorpion families
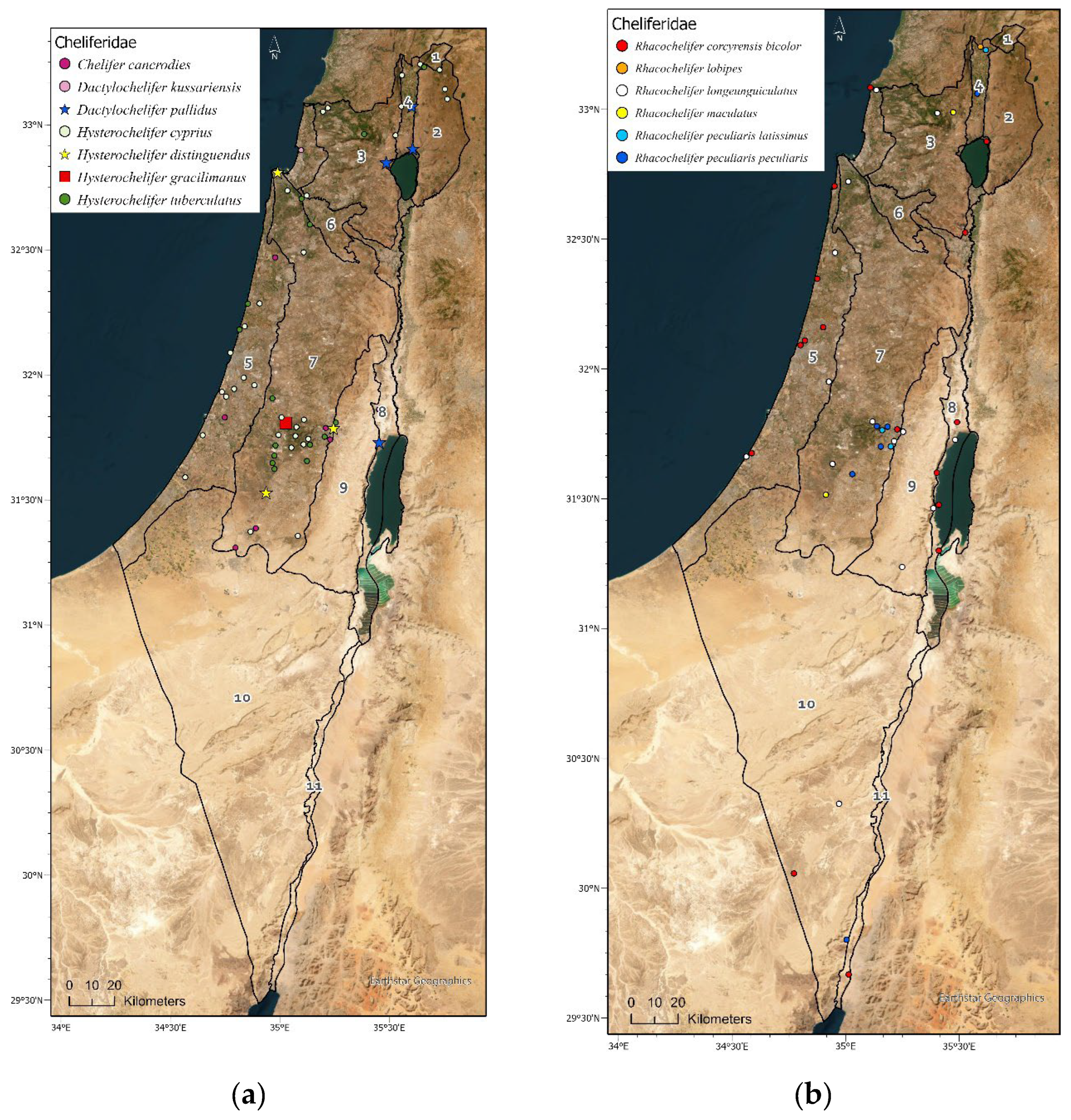
Cheliferidae is the largest and most common family deposited in the NNHC HUJ collection. Four genera and eighteen species and subspecies are documented from Israel (
Table 3,
Figure 4a,b). Only one species is Afrotropic, and the rest are Palearctic, with eleven Mediterranean, two Asian, two cosmopolitan and two endemic species (
Table 3). Cheliferids were collected all over the country, in all regions except the Hermon (
Figure 3,
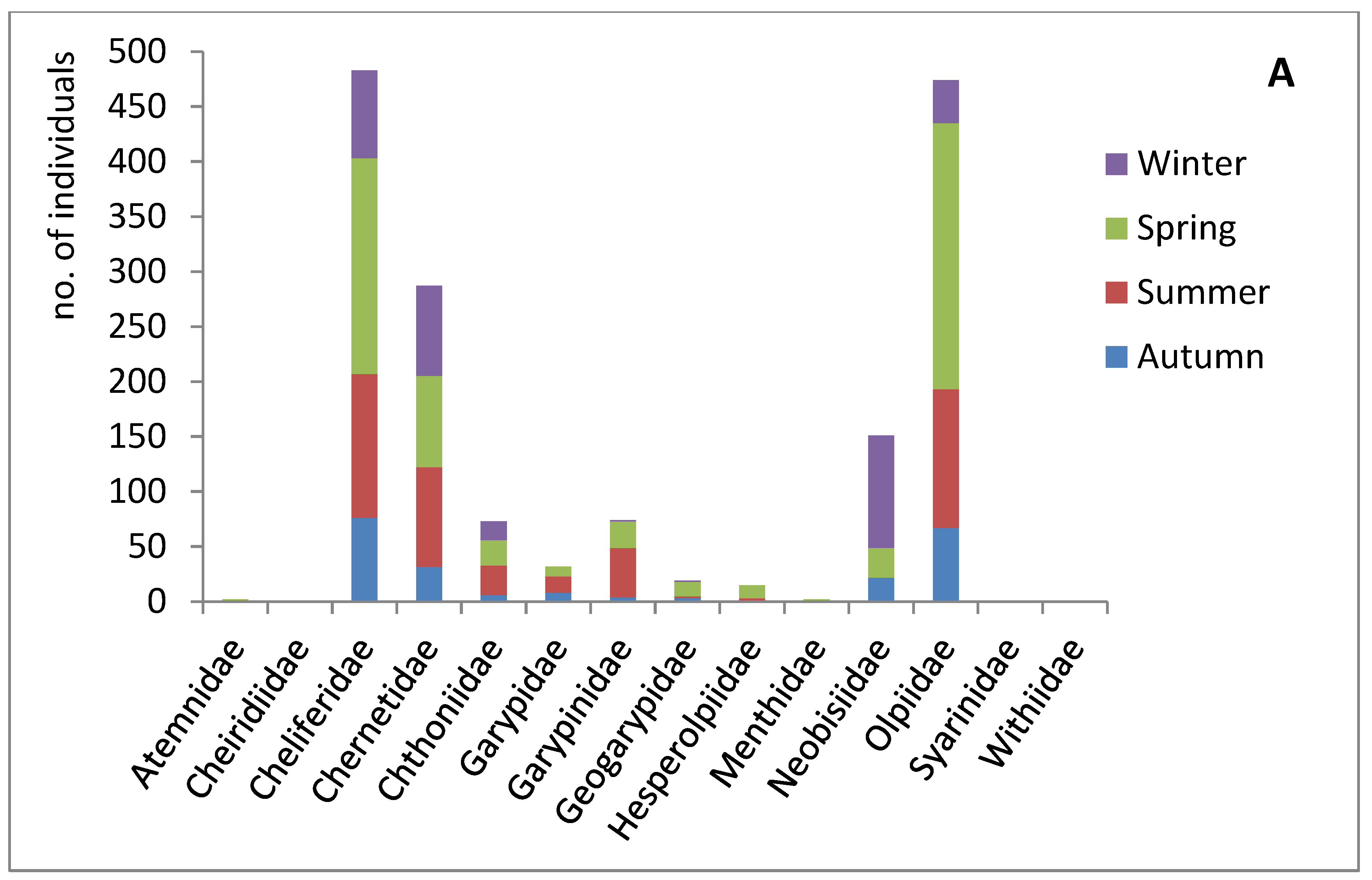
Figure 4a,b) and all year round. Most cheliferids were collected in spring and summer, and fewer individuals were collected in autumn and winter (
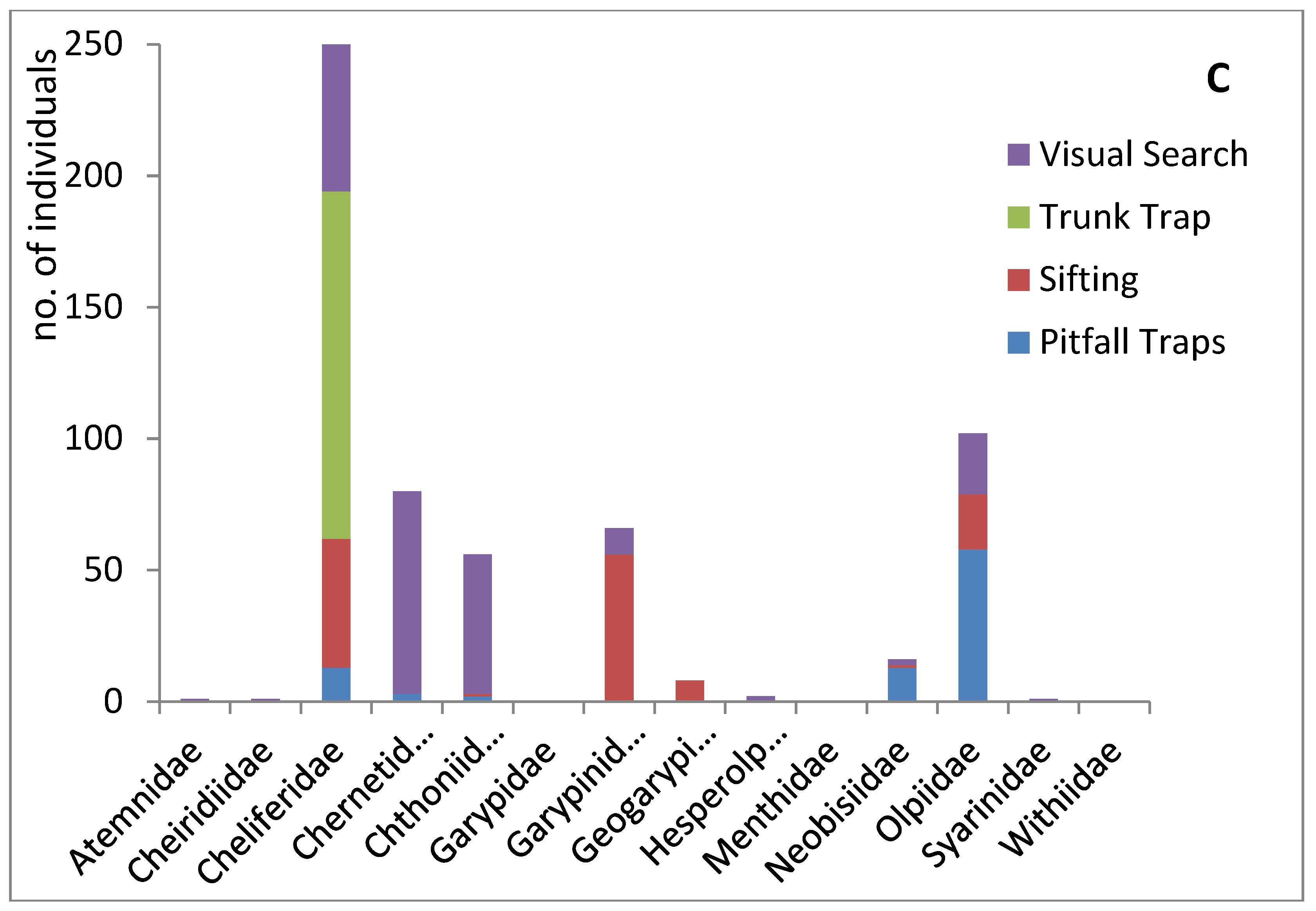
Figure 5A). Cheliferids were collected using all methods. They were often found under bark and in leaf litter (
Figure 5B) usually in habitats with trees. The subspecies
Rhacochelifer corcyrensis bicolor Beier, 1963 was found almost exclusively in the desert, mainly on
Acacia trees (
Vachellia Wight & Arn) using trunk traps (
Figure 4a,b,
Figure 5A,C). The cosmopolitan species
Chelifer cancroides (Linnaeus 1758) is phoretic and often found in beehives. The genus
Dactylochelifer Beier, 1932 (the species
D. kussariensis (Daday1889) and the endemic species
D. pallidus Beier, 1963) was typically found near wetlands (swamps and riverbanks) (
Figure 4a,
Figure 5A). The other endemic cheliferid
Hysterochelifer distinguendus (Beier, 1929) was found only in the Judea foothills (
Figure 4a). Several specimens of
Rhacochelifer longeunguiculatus Beier 1963 as well as unidentified species were collected from cave entrances but no troglobiont species were recorded from Israel thus far (
Figure 6A).
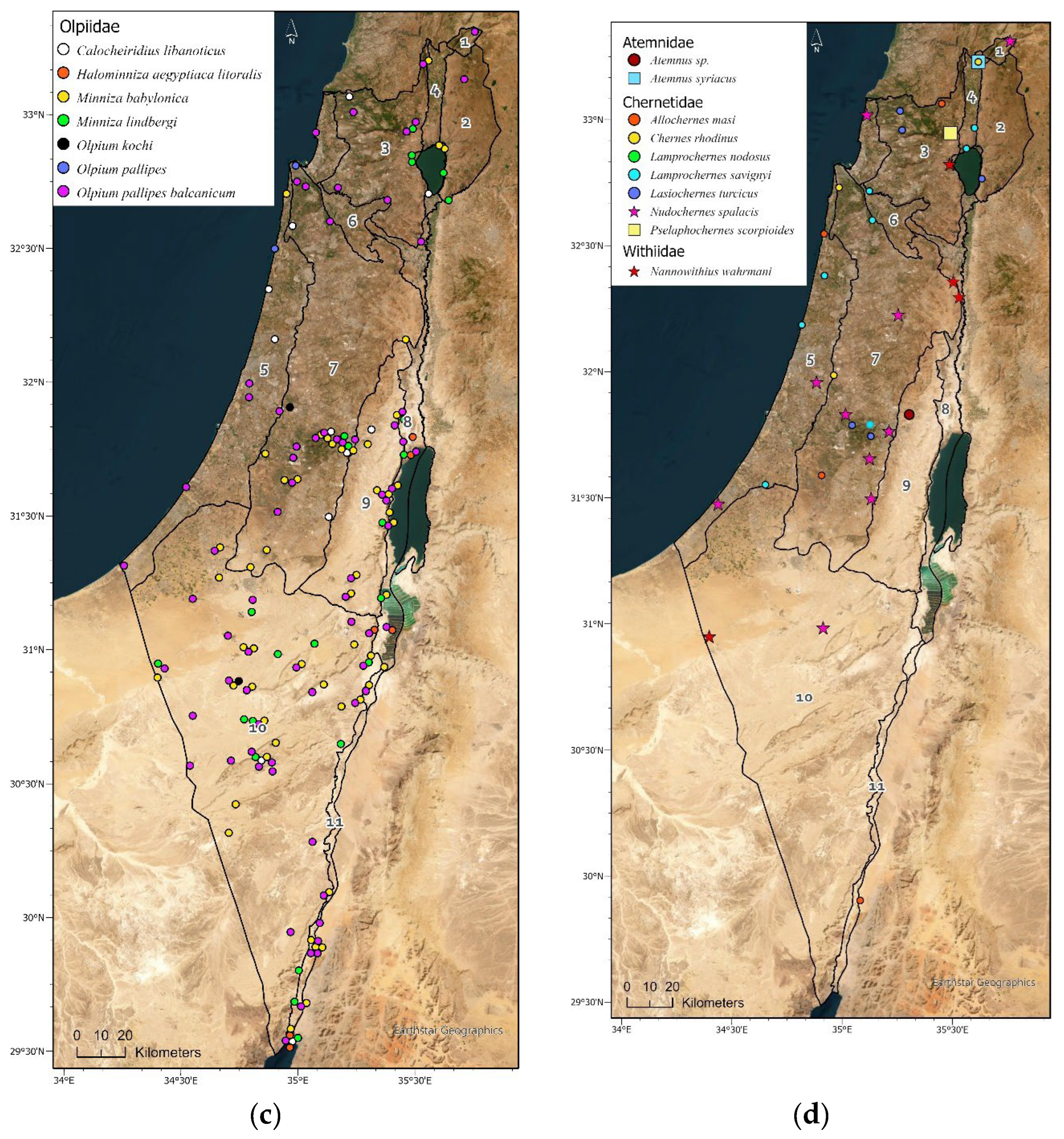
Olpiidae is the second largest and most common family deposited in the NNHC HUJ collection, both in number of specimens and number of species and subspecies. Four genera and ten species are documented from Israel (
Table 3,
Figure 4c). Although globally Olpiidae species are usually found in xeric habitats (desert and semi-desert), the species in Israel are mostly Palearctic, with eight species from the Mediterranean and two species from Central and Western Asia and the Arabian Peninsula, thus from both Palearctic and Afrotropic regions (
Table 3). Olpiids were collected all over the country, in all regions except the Hula Valley, but were significantly more common in the desert, where more than 75% of the specimens were found, while less than 10% were found in all the northern regions of Israel and the coastal plain (
Supplementary Materials Tables S2-S3). They were collected all year, but the highest number was collected in spring while very little were collected in winter (
Figure 5A). Specimens were collected by visual search under stones, sifting and in pitfall traps (
Figure 5C). None of the olpiid species are endemic to Israel, yet
Halominniza aegyptiaca litoralis (Beier, 1963) is endemic to the southern Levant (Israel, Jordan and Sinai), and was found in Israel only in the desert near the Dead Sea and the Red Sea, in association with sea (salty) water (
Figure 4c). The Asian species
Minniza babylonica Beier, 1931, which is found in both Palearctic and Afrotropic regions, is typically found in Israel in the desert (
Figure 4c). This family has not been recorded from caves or moist epigean habitats (
Figure 6B).
Chernetidae is the third largest and most common family deposited in the NNHC HUJ collection in both number of specimens and number of species, with six genera and eight species, in addition to undescribed species from caves. Five of these species are widely distributed and three are Mediterranean whereas one is endemic from an Afrotropic origin (
Table 3,
Figure 4d). Most of the specimens (80%), were collected in the mountains of central Israel and Jezreel valley, while hardly any were collected in the desert (
Figure 4d,
Supplementary Materials Tables S2-S3). Chernetids were found evenly throughout the year (
Figure 5A) and usually by visual search (
Figure 5C). Species of Chernetidae are often phoretic on rodents or insects (
Figure 5B) and tend to aggregate in their nests as evident by 80% of the family’s specimens in Israel:
Lamprochernes savignyi (Simon, 1881) is phoretic on flies,
Lasiochernes turcicus Beier, 1949 (
Figure 6C) is found in nests of
Apodemus Kaup, 1829, and
Nudochernes spalacis Beier, 1955 in nests of
Nannospalax species. Nearly half of the specimens collected from caves in Israel (44%) belong to the Chernetidae family, being a quarter of the family’s specimens collected in Israel, from the species
Allochernes masi,
Chernes rhodinus,
Lasiochernes turcicus and other unidentified species (
Figure 5B). They were found mainly in caves with bats, both frugivorous and insectivorous, and
L. turcicus also in an
Apodemus nest.
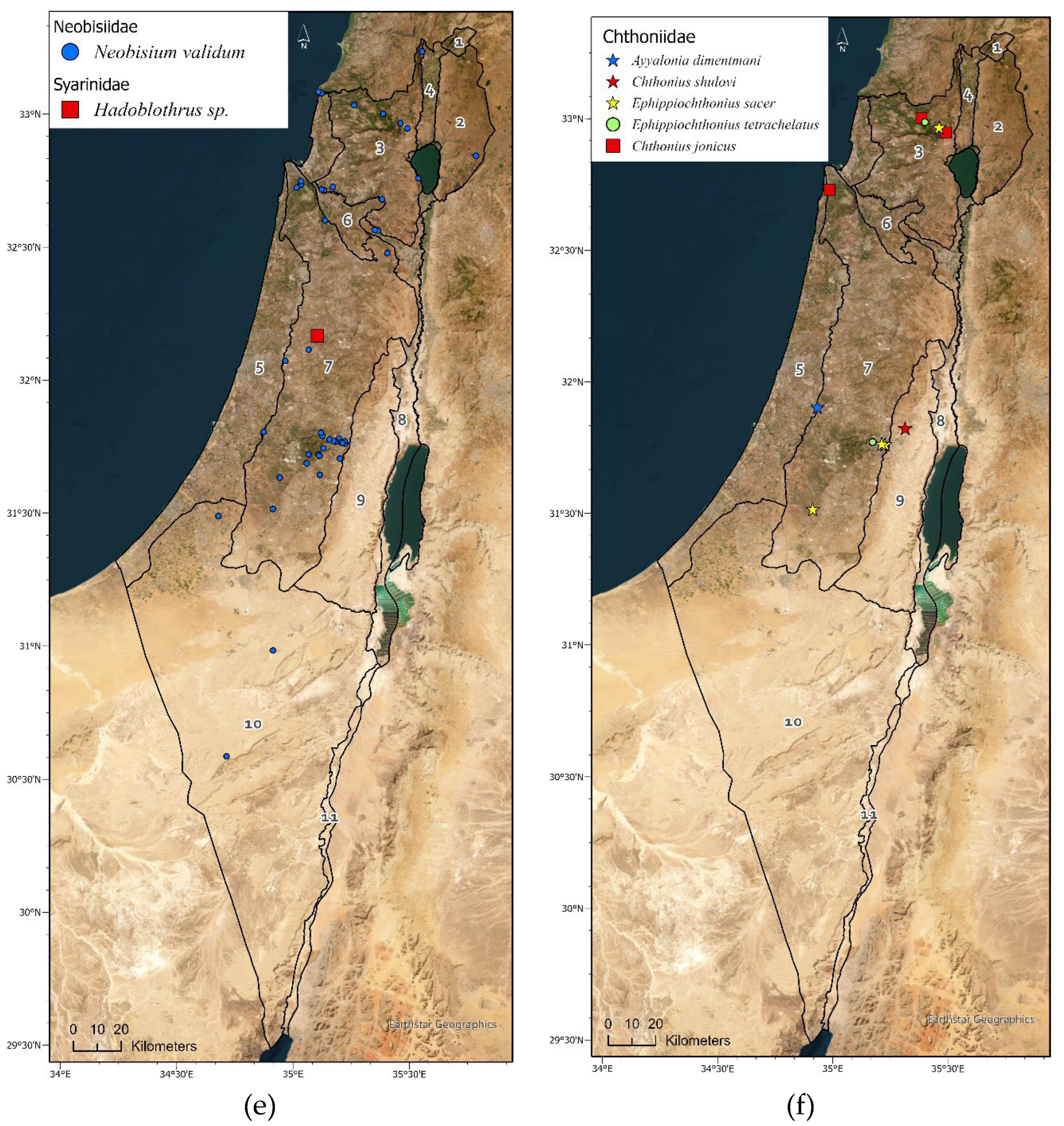
Neobisiidae is the fourth most common family deposited in the NNHC HUJ collection, in terms of number of specimens; however, it is represented in Israel with only one genus and one species throughout the country, the Palearctic
Neobisium validum (L. Koch, 1873) (
Figure 6D), which is found in the East-Mediterranean as well as Central and Western Asia (
Table 3,
Figure 4e), with the type locality in Syria. Only 4% of the specimens collected from the desert, while 93% were collected in the mountains of north and central Israel and Jezreel valley (
Figure 4e,
Supplementary Materials Tables S2-S3). The remaining 3% were collected in the coastal region. Preliminary DNA sequencing (not presented here) indicates a species complex, which needs to be examined further. As a hygrophilous family, Neobisiidae specimens were collected four times more in winter than in spring and autumn, while none were collected during the summer (
Figure 5A). They were usually found by sifting soil and leaf litter, visual search under stones or using pitfall traps (
Figure 5B,C). One specimen of a presently unidentified troglobitic species was found in a cave.
Chthoniidae is represented in Israel by five species from three genera in addition to several undescribed species from caves. Three species are endemic, one Mediterranean and one cosmopolitan (
Table 3,
Figure 4f). Chthoniids were found exclusively in the mountains of north and central Israel (
Figure 3,
Figure 4f,
Supplementary Materials Tables S2-S3) or in caves (
Figure 5B). Half of the pseudoscorpion specimens we found in Israel’s caves belong to the Chthoniidae, representing 75% of the family’s specimens collected in Israel. Being a family with high short-range endemism [
6], we expect several new species to science among the unidentified cave specimens (
Figure 6E). As a hygrophilous family, only a few Chthoniidae specimens were collected in the driest season in Israel, autumn (
Figure 5A). Chthoniids were collected mostly by visual search under stones, and on bats guano in caves (
Figure 5B,C), but they also occur in leaf litter, and some were collected by sifting and pitfall traps.
Ayyalonia dimentmani Ćurčić, 2008 is an example of an endemic relict species found only in a single unique chemoautotrophic cave. Another endemic species,
Chthonius shulovi Beier, 1963 was found only once in one locality in Wadi Qelt [
15]. Both species were never found again. However, the endemic species
Ephippiochthonius sacer (Beier, 1963) seems to be more widespread, and was found several times in the Mediterranean region of north and central Israel.
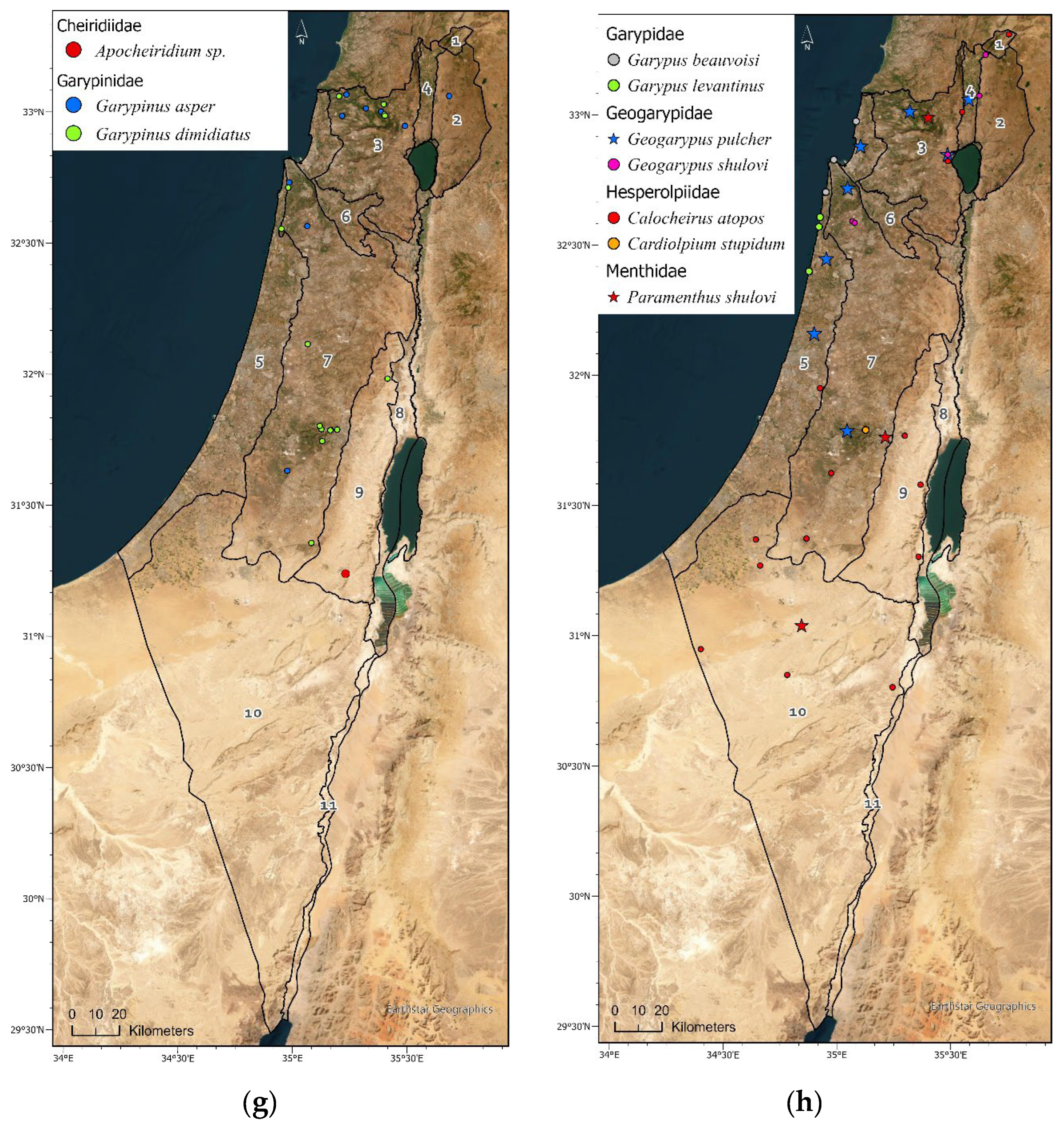
Garypidae is represented in Israel by two species of
Garypus L. Koch, 1873 that are halophilous and widespread in seashore habitats of the Mediterranean (
Table 3,
Figure 4h). Both were found in all seasons but winter (
Figure 5A), exclusively in the salty habitat of the Mediterranean coast (
Figure 3,
Figure 4h,
Figure 5B). Some specimens were found in algae debris at the coastline.
Geogarypidae is represented in Israel by two species from one genus:
Geogarypus shulovi Beier, 1963 is Palearctic from Central and Western Asia, and
Geogarypus pulcher Beier, 1963 is endemic to Israel (
Table 3,
Figure 4h). Most of the specimens were found in the mountains of north and central Israel, but some also in the coastal plain and Hula valley (
Figure 3,
Figure 4h,
Figure 5B,
Supplementary Materials Tables S2-S3). They were usually found by sifting leaf litter, mostly in spring (
Figure 5B,C).
Hesperolpiidae is represented in Israel by two species from two genera,
Calocheirus atopos Chamberlin, 1930 is Afrotropic from the Arabian Peninsula and Sudan, and
Cardiolpium stupidum (Beier, 1963) is Palearctic from East-Mediterranean and Central-West Asia (
Table 3,
Figure 4h). They are found mostly in spring (
Figure 5A), in small numbers but in a wide distribution along Israel (
Figure 3,
Figure 4h,
Figure 5B).
Menthidae is an infrequently collected family and represented in our collection by only three specimens; all belong to the endemic species
Paramenthus shulovi Beier, 1963; an Afrotropic genus. Each of the specimens was collected from a different sub-region of the country; the Negev desert, the Central Mountains, and the Galilee (
Table 3,
Figure 4 h).
Atemnidae is represented in our collection by only two specimens, each from a different species and a different region:
Atemnus syriacus (Beier, 1955) in the north of Israel, and an undescribed species from the Judean desert (
Table 3,
Figure 4d) .
Withiidae is represented in our collection by the endemic species
Nannowithius wahrmani (Beier, 1963), which belongs to the Afrotropic genus
Nannowithius Beier. It is a small myrmecophilous species that was found in nests of the ant
Messor semirufus (André, 1883) in the Negev desert, under a stone near the sea of Galilee, and phoretic on the myrmecophilous scorpion
Birulatus israelensis which lives only in
Messor ebeninus Santschi, 1927 nests (
Figure 4d,
Figure 6f) [
27].
Cheiridiidae is represented in our collection by a single specimen that was collected in a cave in the Judean desert (
Figure 4g).
Syarinidae is represented in our collection by a single specimen that was collected in a cave in the Central Mountains (
Figure 4e).
Figure 6.
Habitus of live pseudoscorpion representatives from Israel. A. Hysterochelifer cyprius (Cheliferidae). B. Olpium pallipes balcanicum (Olpidae). C. Lasiochernes turcicus (Chernetidae). D. Neobisium validum (Neobisiidae). E. Ephippiochthonius sp. (Chthoniidae). F. Nannowithius wahrmani (Withiidae) phoretic on Birulatus israelensis. Photos A, B, D, E, F by S. Aharon, C by J. A. Ballesteros.
Figure 6.
Habitus of live pseudoscorpion representatives from Israel. A. Hysterochelifer cyprius (Cheliferidae). B. Olpium pallipes balcanicum (Olpidae). C. Lasiochernes turcicus (Chernetidae). D. Neobisium validum (Neobisiidae). E. Ephippiochthonius sp. (Chthoniidae). F. Nannowithius wahrmani (Withiidae) phoretic on Birulatus israelensis. Photos A, B, D, E, F by S. Aharon, C by J. A. Ballesteros.
3.1.3. A dichotomous key to the pseudoscorpion families and genera of Israel (applying to adults) (in parentheses no. of genera and no. of species in the family).
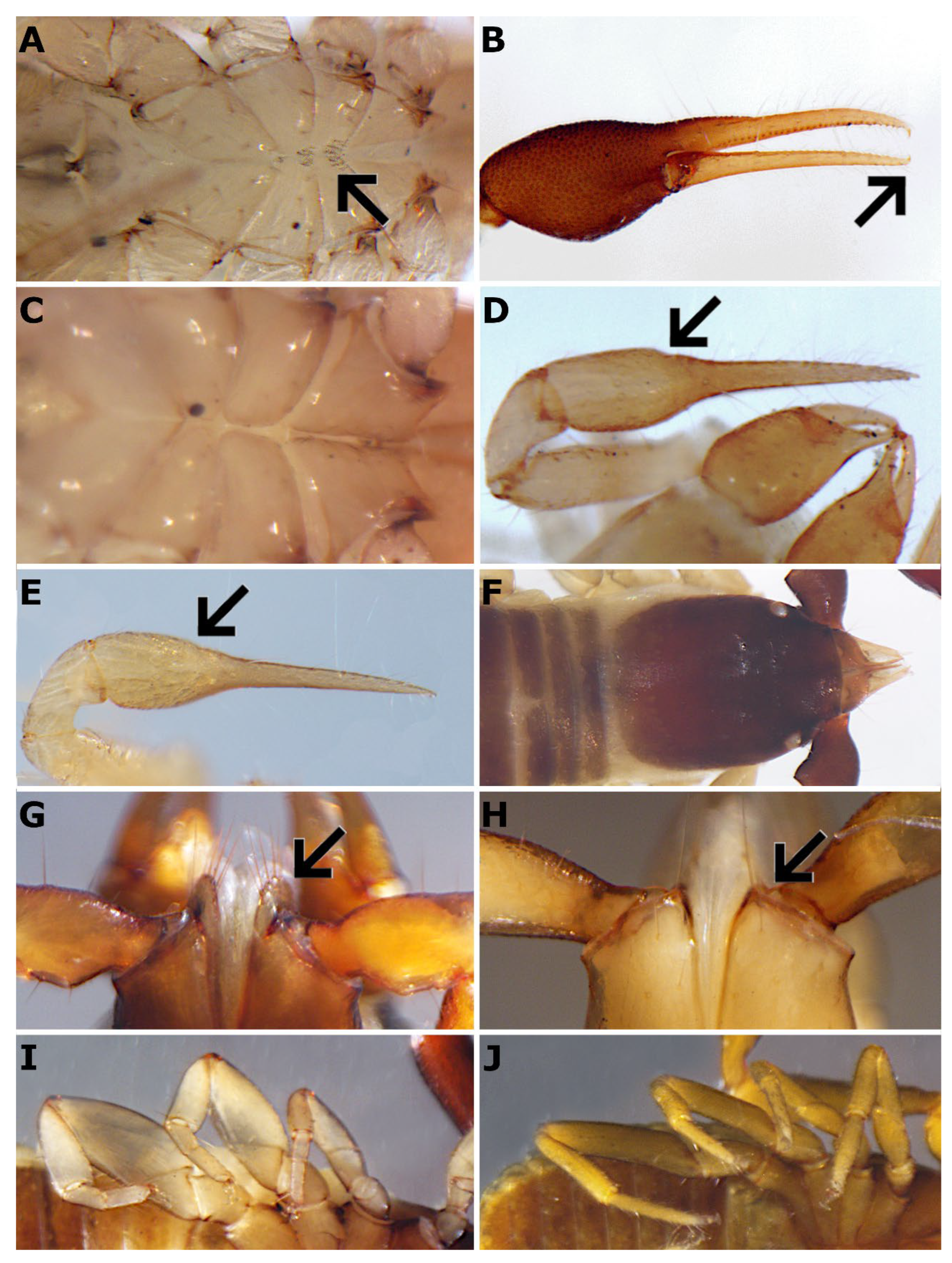
1. a. Legs with different number of tarsal segments: legs I and II with one tarsal segment and legs III and IV with two tarsal segments; pedipalpal chelal fingers slender and without venom apparatus; two trichobothria (
ib and
isb) medially on dorsal face of pedipalpal chela hand; coxal spines present (
Figure 7A) . . . . . . . . . . . . . . . . . . . . . . . . .
suborder Heterosphyronida . . . . . . . . . . . . . . . . . . . . .
superfamily Chthonioidea . . . . . . . . .
family Chthoniidae (3, 7)
2
1. b. All legs with same number of tarsal segments; at least one pedipalpal chelal finger with venom apparatus (
Figure 7B); trichobothria
ib and
isb usually located on the lateral face of the fixed chelal finger (occasionally
ib is on the dorsal surface); coxal spines absent (
Figure 7C) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
suborder Iocheirata 4
2. a. Coxae III without coxal spines; only coxae II each with 11 fine elongated coxal spines, middle spines longest; apex of pedipal pal coxa with three setae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Ayyalonia
2. b. Coxae II and III with coxal spines; apex of pedipal pal coxa with two setae . . . . . . . . . . Chthonius-related genera 3
3. a. Teeth of pedipalpal chelal fingers pointed, aligned, erect and spaced; pedipal pal hand with dorsal step-like outline (dorsodistal saddle-shaped constriction) (
Figure 7D) between the groups of trichobothria
ib-isb ang
eb-esb-ist . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Ephippiochthonius
3. b. Teeth of pedipalpal chelal fingers blunt, dense and distinctly reclined backwards; pedipal pal hand with evenly rounded weakly curved dorsal outline (
Figure 7E) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Chthonius
4. a. Rectangular carapace (anterior and posterior margins similar in length); robust chelicerae, their bases almost as wide as posterior side of carapace; all legs with two tarsal segments; two pairs of eyes (epigean species) or none (caves); setae present on the apical margin of pedipalp coxa (
Figure 7F,G) . . . . . . . . . . . . . . . .
infraorder Hemictenata . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
superfamily Neobisioidea 5
4. b. Carapace not rectangular (anterior margin shorter than posterior margin); width of chelicerae bases smaller than posterior margin of carapace (less than half its length) (
Figure 7H); the legs have either all one tarsal segment or all two tarsal segments; either no eyes or one or two pairs of eyes or eyespots; setae on the apical margin of pedipalp coxa may be present or absent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
infraorder Panctenata 6
5. a. Apex of pedipalpal coxa rounded and bears three or more setae (
Figure 7F) . . . . . . . . . . .
family Neobisiidae (1, 3)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Neobisium
5. b. Apex of pedipalpal coxa not rounded and bears two long subequal setae (
Figure 7G) . . . .
family Syarinidae (1, 1)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Hadoblothrus
6. a. All legs with two tarsal segments (
Figure 7I); two pairs of eyes with lenses; usually elongated carapace (longer than wide); pedipalpal femur with or without trichobothria . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
7
6. b. All legs with one tarsal segment (
Figure 7J); one pair of eyes or eye-spots, or eyes absent; carapace sub-triangular; pedipalpal femur without trichobothria . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
16
Figure 7.
A. Ephippiochthonius tetrachelatus with coxal spines present. B. Geogarypus pulcher with venom apparatus on both pedipalpal chelal fingers. C. Neobisium validum with no coxal spines. D. Ephippiochthonius sp. with dorsal step-like outline of pedipalpal hand. E. Cthonius sp. with evenly rounded weakly curved dorsal outline of pedipalpal hand. F. Neobisium validum with five setae on rounded pedipalp coxa apex. G. Hadoblothrus sp. pedipalp coxal apex not rounded and bears two long subequal setae. H. Garypinus sp. with the width of the chelicerae bases smaller than the width of the posterior margin of carapace. I. Garypinus sp. with two tarsal segments in all legs. J. Hysterochelifer tuberculatus with one tarsal segment in all legs.
Figure 7.
A. Ephippiochthonius tetrachelatus with coxal spines present. B. Geogarypus pulcher with venom apparatus on both pedipalpal chelal fingers. C. Neobisium validum with no coxal spines. D. Ephippiochthonius sp. with dorsal step-like outline of pedipalpal hand. E. Cthonius sp. with evenly rounded weakly curved dorsal outline of pedipalpal hand. F. Neobisium validum with five setae on rounded pedipalp coxa apex. G. Hadoblothrus sp. pedipalp coxal apex not rounded and bears two long subequal setae. H. Garypinus sp. with the width of the chelicerae bases smaller than the width of the posterior margin of carapace. I. Garypinus sp. with two tarsal segments in all legs. J. Hysterochelifer tuberculatus with one tarsal segment in all legs.
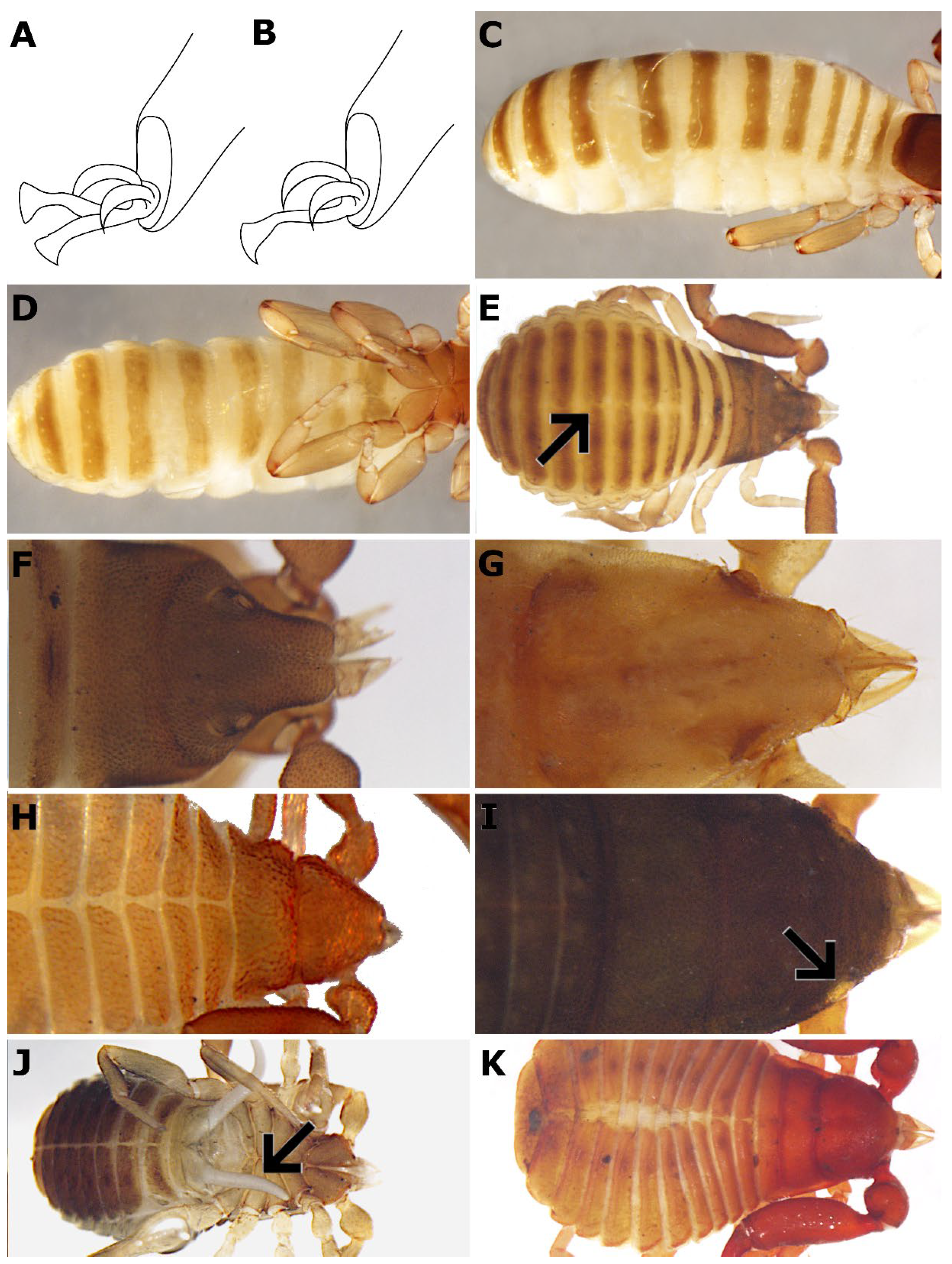
7. a. Arolia bifurcate (
Figure 8A); at least a few tergites and sternites are divided. . . . . . . . . .
superfamily Garypinoidea
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . family Garypinidae (1, 2)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Garypinus
7. b. Arolia simple (
Figure 8B) (not bifurcate); tergites and sternites can (in certain families) be all undivided . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . superfamily Garypoidea . . . . . . . . . . . . . . . . . .8
8. a. Venom apparatus present only in the fixed chelal finger; fixed chelal finger with 11 trichobothria, of which two are on the dorsal surface of the hand; specialised joint present between coxae II and III . . . . . . family Menthidae (1, 1)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Paramenthus shulovi
8. b. Venom apparatus present in both chelal fingers; fixed chelal finger with 8 or (rarely) 7 trichobothria, including 3 on internal face; dorsal surface of the hand without trichobothria; articulation furrow between coxae II and III absent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
9. a. All tergites and sternites undivided (
Figure 8C,D); abdomen oblong, not much broader than the carapace; the carapace sub-rectangular and slightly tapering orally; eyes positioned near anterior margin of carapace, not sitting on mounds; arolia longer than claws; vestitural bristles of pedipalps rather long, erect, and thinly pointed; pleural membrane of abdomen with smooth and even longitudinal striation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
10
9. b. At least a few tergites and sternites divided (
Figure 8E); abdomen broadly oval, distinctly broader than the carapace; carapace triangular and strongly tapering orally; eyes protruding, sitting on mounds, removed from anterior margin of the carapace; arolia can be longer or shorter than the claws; vestitural bristles of pedipalps very short, fine, bent, and abruptly pointed; pleural membrane of abdomen granulate or with short wavy undulating striation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
15
10. a. Palpal fingers distinctly bent; palpal femur with or without two fairly long dorsal tactile bristles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . family Olpiidae (4, 10) . . . . . . . .11
10. b. Palpal fingers straight; palpal femur with a single fairly long dorsosubbasal tactile bristle . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .family Hesperolpiidae (2, 2) . . . . . . . 14
11. a. Carapace without submedian transverse furrow, longer than broad maximum 1.5 times . . . . . . . . . . . . . . . . . . . .12
11. b. Carapace with a submedian transverse furrow; either just slightly or 1.7 to 1.8 times longer than broad. . . . . . . 13
12. a. Carapace and pedipalps dark brown; palpal femur 0.6 to 0.7 mm long; fingers about as long as the hand with pedicel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Olpium
12. b. Carapace and pedipalps light yellowish-brown; palpal femur 0.9 to 1 mm long; fingers much longer than hand with pedicel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Halominniza
13. a. Body length about 2 to 3 mm; carapace 1.7 to 1.8 times longer than broad; femur, tibia and chela of pedipalps dark brown, with pedicel and tips reddish, rarely entirely reddish; desert species with worm-shaped abdomen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Minniza
13. b. Body length 1.5 to 1.7 mm; carapace but slightly longer than broad; femur and tibia of pedipalps light brownish-yellow, chela blackish-olivebrown . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Calocheiridius
14. a. Body length 2.3 to 2.8 mm; eyes about half their diameter distant from one another; carapace with a weak transverse furrow; palpal hand very broad, abruptly constricted medially and laterally towards the base of the fingers; fingers at least as long as the femur; trichobothria est situated near the middle of fixed finger; three trichobothria ist, it and et form a group near tip of finger . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Calocheirus
14. b. Body length 1.2 to 1.9 mm; eyes touching, the anterior eyes slightly excavated behind, the posterior eyes conical and flattened; carapace without a transverse furrow; palpal hand not very broad, medially obliquely narrowed towards the base of fingers; fingers shorter than femur; trichobothria est situated rather basally, proximal of the middle, near trichobothria isb, close to the group formed of eb-esb-ib-isb . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Cardiolpium
15. a. Carapace triangular and medially concave (
Figure 8E); maxilla of palpal coxae with developed shoulder; coxal area not expanding posteriorly: coxa IV approximately same width as coxa I (broad and short); body 2 to 2.5 mm long; arolia longer than the claws; tergites usually with dark spots; occur in leaf litter and under stones or bark in the Mediterranean shrubland . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
family Geogarypidae (1, 2) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Geogarypus
15. b. Carapace triangular but not medially concave (
Figure 8F); maxillar shoulder of palpal coxae not developed; coxal area expanding posteriorly: i.e. coxa IV distinctly longer and narrower than coxa I; body 5 to 6 mm long; arolia shorter than claws; tergites usually with dark bands; occur only in littoral or supralittoral zones . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
family Garypidae (1, 2)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Garypus
16. a. Small body size (< 2 mm); carapace triangular and coarsely granulate with one medial deep transverse furrow (
Figure 8G); one pair of small eyes, if not absent, distinctly removed from anterior margin; femur and patella of all legs fused, with suture between them hardly visible; coxa IV much wider than coxa I; pedipalps with reduced number of trichobothria: at most seven on fixed chelal finger and one or two on moveable finger. . .
superfamily Cheiridioidea . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
family Cheiridiidae (1, 1)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Apocheiridium
16. b. Body size larger than 1.5 mm; eyes (if not absent) positioned near the anterior margin of the carapace (
Figure 8H); femur and patella of legs not fused; coxa IV not wider than coxa I; pedipalps usually with eight trichobothria on fixed chelal finger and four on moveable finger . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
superfamily Cheliferoidea. . . .
17
17. a. Venom apparatus present in both chelal fingers; one pair of eyes or eye spots; males with or without coxal sacs or ram’s horn organs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .18
17. b. Venom apparatus absent from one chelal finger; no eyes or one pair of eyes or eye spots; males without coxal sacs or ram’s horn organs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .22
18. a. Articulation of femur and patella of leg I and II narrow and transverse, therefore these joints scarcely movable; male (and occasionally females) abdominal posterior sternites with discrete patches of glandular sensory setae; hind coxae of male without coxal sacks and ram’s horn organs absent; very small myrmecophilous specimens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .family Withiidae (1, 1)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Nannowithius wahrmani
18. b. Articulation of femur and patella of leg I and II well developed (
Figure 8I), great and oblique and therefore well movable; male abdominal sternites without patches of sensory setae; hind coxae of male excavate caudally, male genitalia with coxal sacks and ram’s horn shaped organs (
Figure 8I); not myrmecophilous . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . .
family Cheliferidae (4, 18) . . . . . . . . . . . . . . . .
19
19. a. Carapace with dense granulation and greater bristle-bearing granules; abdominal tergites of male with lateral carinae and elongate hind-angles. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
19. b. Carapace with dense granulation without greater granules; abdominal tergites of male without lateral carinae, their hind-angles not elongate. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21
20. a. Pedipalpal femur with coarse bristle-bearing medial granules; subbasal bristle of chelicera present . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Hysterochelifer
20. b. Pedipalpal femur without coarse medial granules; no subbasal bristle of chelicera . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Chelifer
21. a. At least submedian transverse furrow of the carapace deeply incised; pedipalps slender. . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Dactylochelifer
21. b. Both transverse furrows of carapace shallow; pedipalps clumsy and strong . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Rhacochelifer
22. a. No eyes or eye spots (
Figure 8J); venom apparatus developed only in movable chelal finger; carapace with grooves; robust (
Figure 8J); abdomen broadly oval, distinctly broader than the carapace . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . family Chernetidae (6, 9) . . . . . . . . . . .23
22. b. One pair of eyes or eye spots; venom apparatus developed only in fixed chelal finger; longitudinal grooves may be present; slender and elongate abdomen, not broader than the carapace . . . . . . . . . . . . . . . . . . family Atemnidae (1, 2)
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Atemnus
23. a. Body and pedipalp setae long, thin and pointed; carapace almost smooth; subbasal transverse furrow on carapace indistinct; tibia and hand of pedipalps with fairly long lateral pseudotactile setae; often phoretic on flies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Lamprochernes
23. b. Body and pedipalp setae short, stout, dentate or clavate; carapace granulate; tibia and hand of pedipalps without lateral pseudotactile setae; not phoretic on flies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 24
24. a. Tarsus of leg IV with long erect tactile seta near the middle, distinctly longer than width of tarsus . . . . . . . . . . . 25
24. b. Tarsus of leg IV without erect tactile seta near the middle . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
25. a. Carapace rather coarsely granulated, with two distinct transverse furrows, of which the posterior is flatter than the anterior; body and pedipalp setae always slightly but clearly clavate, relatively long; male pedipalp without dense long setation; palpal femur at most 0.5 mm long; small 1.5-2 mm; in ground litter and detritus, in moist places . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Pselaphochernes
25. b. Carapace with dense and fine granulation with posterior transverse furrow situated scarcely nearer to hind border than to the anterior furrow; body setae dentate, but not clavate; palpal femur longer than 0.7 mm; body length 2-4 mm; in nests of small mammals and in caves with bats . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . .26
26. a. Tactile bristle near the middle of the hind tarsus long and simple; body setae rather long serrated to slightly truncate not clavate, those of the pedipalps only serrated; setae on femur and tibia of the pedipalps of the male very long and dense like a mane; fairly large and robust: body length 4 mm, palpal femur at least 1 mm long; in nests of Apodemus. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Lasiochernes
26. b. Tactile bristle near the middle of the hind tarsus dentate and scarcely twice as long as the other bristles and about one third as long as width of tarsus; body setae very short; medial bristles of the palpal femur of the male but scarcely more dense than in the female; body length 2-3 mm, femur length 0.69-0.74 mm; in nests of the rodent Nannospalax . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Nudochernes
27. a. Carapace quite coarsely granulated; body and pedipalp setae serrated and strongly culled usually quite short; slightly clavate; pedialps are moderately slender, granulated . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Allochernes
27. b. Carapace moderately coarsely granulated, sometimes partially reticulated; body and pedipalp setae dentate and more or less truncate; pedialps robust, granulated; pedipalp coxae usually not granulated . . . . . . . . . . . . . . . . . . Chernes
Figure 8.
A. Bifurcate arolia. B. Simple arolia. C. Minniza babylonica with all tergites undivided. D. Minniza babylonica with all sternites undivided. E. Geogarypus sp. with five tergites divided. F. Geogarypus sp. with carapace triangular and medially concave. G. Garypus beauvoisi with carapace triangular but not medially concave. H. Cheiridiidae sp. with one medial deep transverse furrow in the coarsely granulate and triangular carapace. I. Hysterochelifer tuberculatus with eyes positioned near the anterior margin of the carapace. J. Hysterochelifer tuberculatus with articulation of femur and patella of leg I and II well developed and male genitalia with ram’s horn shaped organs. K. Chernetidae sp. robust with two grooves in the carapace and without eyes or eye spots.
Figure 8.
A. Bifurcate arolia. B. Simple arolia. C. Minniza babylonica with all tergites undivided. D. Minniza babylonica with all sternites undivided. E. Geogarypus sp. with five tergites divided. F. Geogarypus sp. with carapace triangular and medially concave. G. Garypus beauvoisi with carapace triangular but not medially concave. H. Cheiridiidae sp. with one medial deep transverse furrow in the coarsely granulate and triangular carapace. I. Hysterochelifer tuberculatus with eyes positioned near the anterior margin of the carapace. J. Hysterochelifer tuberculatus with articulation of femur and patella of leg I and II well developed and male genitalia with ram’s horn shaped organs. K. Chernetidae sp. robust with two grooves in the carapace and without eyes or eye spots.