1. Introduction
Groundwater is the main freshwater reservoir on Earth, representing 98 % of freshwater outside of glaciers [
1]. Agriculture is the largest consumer of freshwater being responsible for 69 % of all withdrawals in the world, mainly for irrigation of food crops [
2] (p. 27). Use of groundwater for irrigation is increasing with agriculture intensification [
3] and climate change, in particular in southern Europe [
4] (p. 334). The quality of water in aquifer often referred to organic, chemical or microbial pollutions linked to human activity and to the risk associated with several water-borne human diseases [
5,
6]. There is a significant lack of information on the presence of plant pathogens in aquifer, the current knowledge being to consider the risk negligible [
7]. In fact, groundwater has received little attention from this perspective and we decided to evaluate the possibility that these waters harbor plant pathogens.
The region of Avignon is located southern France in a Mediterranean climate where vegetable and fruit crops are highly dependent on irrigation. The Avignon alluvial groundwater situated near the surface is directly linked to Durance and Rhône rivers and is used for irrigation. The PsyC is an emblematic bacterial model of environmental plant pathogens, isolated from many substrates and more particularly from components of the freshwater cycle [
8,
9]. Using genomic and phylogenetic approaches [
10] demonstrated that crop pathogens from the PsyC emerged from freshwater populations.
In this paper, we report evidence for the presence of plant pathogenic bacteria from the PsyC in groundwater of Avignon, at various places and dates. Even if their abundance was lower than in the surface water, most strains from groundwater belong to the most aggressively group of the PsyC (the phylogroup PG02). Therefore, aquifers must be considered as potential plant pathogenic reservoirs, especially when used for crop irrigation.
2. Materials and Methods
2.1. Groundwater Situation and Characteristics
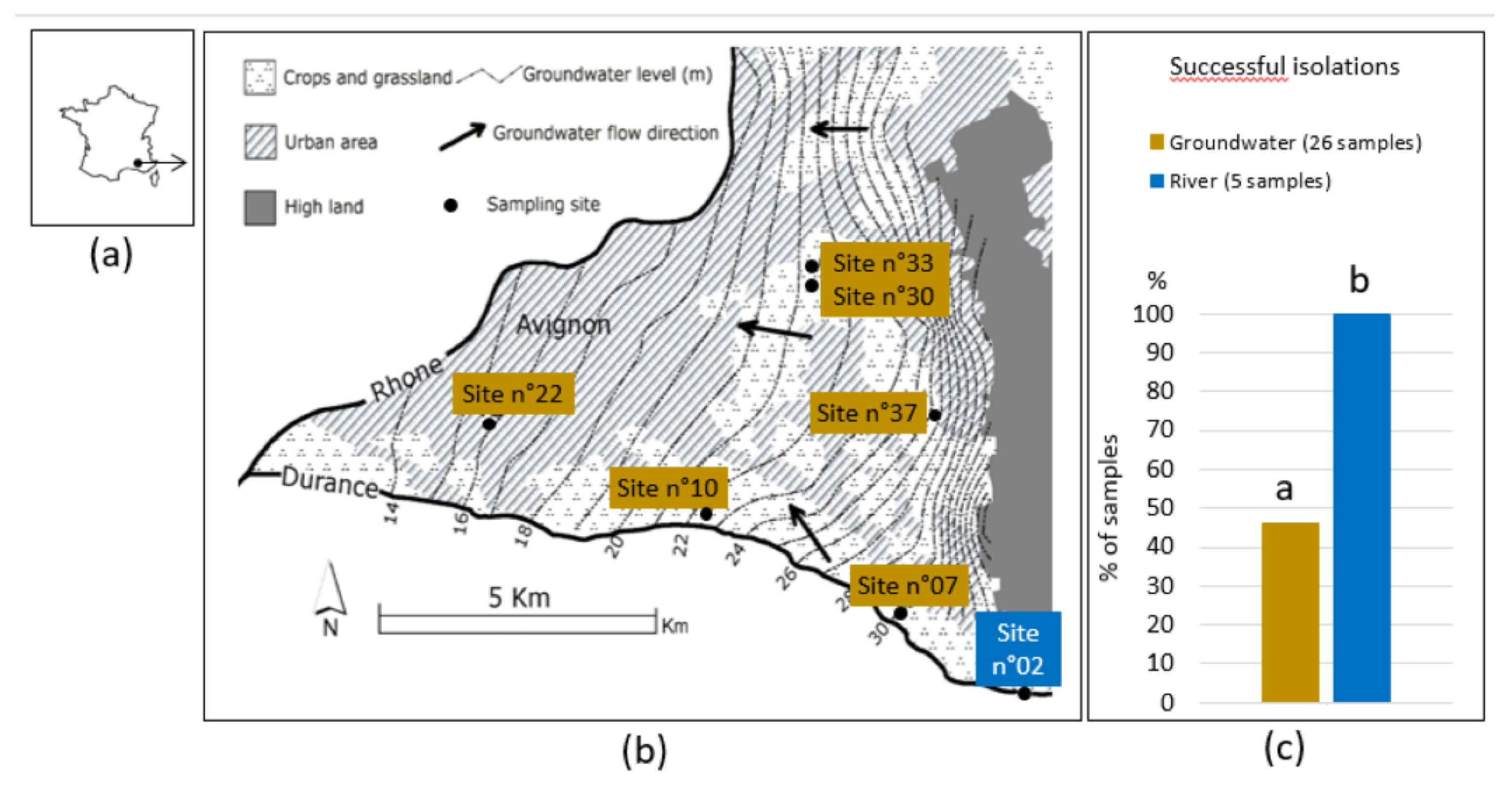
The groundwater studied is situated at Avignon city, in the south-eastern part of France (
Figure 1 (a)). The area over the groundwater is a cultivated alluvial plain extending between the Rhône and Durance rivers. The agricultural lands occupy 30 % of the total surface while the urban area of the city, which has grown significantly in recent decades, is the dominant land use today. This phreatic aquifer is characterized by rapid flow of several meters per day, directed roughly from east to west (
Figure 1 (b)). A silty layer, 1 - 4 m thick, covers the aquifer with a variable thickness that increases towards the confluence of the Durance and the Rhône rivers. The underlying geology contains sedimentary materials (silt, sand and gravel) of quaternary age. The bedrock of these alluvium formations is mainly composed of Miocene marns. From the seven sites of sampling, six has been described by Nofal
et al. [
11] and were chosen to explore groundwater, the last being situated in river Durance upstream the groundwater (
Figure 1 (b)). (see details of site characteristics in
sup Table S1).
2.2. Sampling and Analysis of Water
Sampling campaign was carried out during the 2011-2012 years. Twenty-six samples were collected in Avignon groundwater using disinfected immersed pump and pipes and five samples were obtain by throwing a bucket in the river Durance. From 2 to 6 L were collected in clean zipped plastic bags, transported back to the laboratory at 4°C for further analyses. At each sampling, the level of groundwater was measured, and some physico-chemical parameters such as temperature, pH, electrical conductivity and some ions concentrations, were determined according to the methods of Rousset
et al. [
13] (see details on samples in
Sup Table S2).
2.3. Isolation and Characterization of Bacterial Population
Water subsamples (1.00 to 5.68 L) were filter-concentrated under vacuum from 800 to 2000 times and bacteria desorbed by washing filters with 2.5 or 5 ml of sterile water (see details in
sup-Table S2). Concentrate suspensions were diluted and plated on the semi-selective medium KBC, to enumerate and isolate putative strains of the
P. syringae Complex (PsyC). KBC is modified from King B medium [
14] and contains cycloheximide, cephalexin and boric acid [
15]. We determined the number of culturable bacteria by plating dilutions on 10 % Tryptic Soy Agar (TSA), a non-selective medium. The putative colonies of PsyC obtained on KBC plates were purified and stored in 20 % glycerol at - 80 °C. Classification of strains in the PsyC was confirmed by phylogenetic analysis of the
cts housekeeping gene following [
16]. Test of triggering an hypersensitive response (HR) on tobacco was performed for all strains, following Morris
et al. [
17]. It indicates the potentially pathogenicity of strains on plants. Richness and Shannon-Weaver diversity indexes of PsyC populations in ground and river Durance water were calculated.
3. Results
3.1. The P. syringae Complex Was Detected in 46% of Groundwater Samples
We sampled Avignon groundwater at five dates during one year. To be representative of the different situations of groundwater according to Nofal
et al. [
11], six sites were chosen among 43 possible sites where there is access to groundwater (
Figure 1 (b)). Sites n°30, 33 & 37, surrounded by planted grassland, are located in the upstream part of the groundwater table, rather far from both rivers Durance and Rhône. The fluctuation of their levels depends mainly on irrigation. Sites n°07 & 10 under wild grassland and vegetable crops respectively, are very close to the Durance river and under its influence and site n°22 is located downstream of the groundwater, near the river confluence in a urban zone. Water from the Durance river was sampled at site n°02 located upstream of the groundwater to represent the surface water. We were able to isolate PsyC strains from groundwater at each sampling date (not systematically in all sites), except in September 2011 (
sup Table S2). The detection thresholds for PsyC were respectively 2 CFU.L
-1 of groundwater and 5 CFU.L
-1 of river water. Compared to the river water where we isolated PsyC strains at all dates, the frequency of successful isolations of PsyC from groundwater samples was significantly lower than in the surface river water (
Figure 1 (c)).
3.2. Water Physico-Chemical Characteristics Partially Determine Density of P. syringae Complex Population in Groundwater
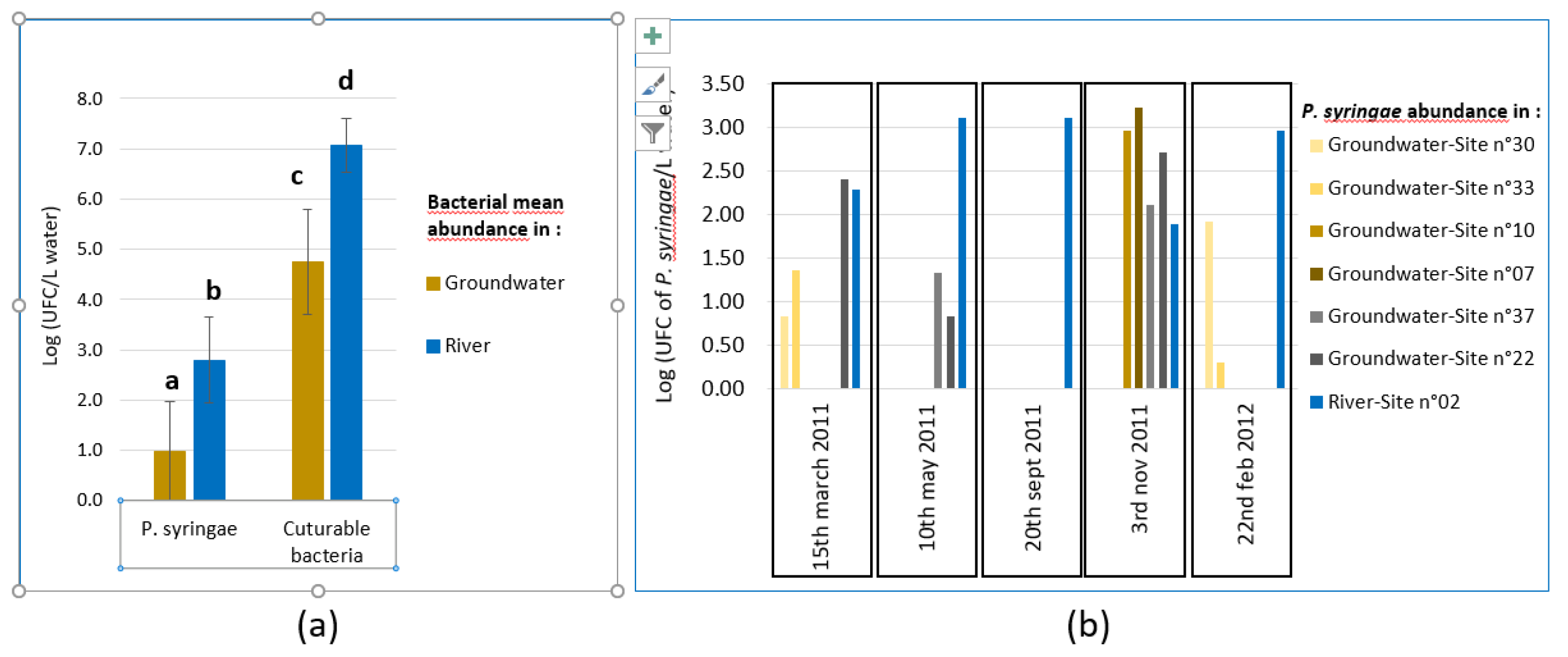
Densities of PsyC populations were significantly less in groundwater than in river water (
Figure 2 (a)) even if highly variable (
Figure 2 (b)). In fact, PsyC abundance was often the lowest in groundwater as expected. However, the opposite situation could also occur occasionally, as exemplified in November 2011 (
Figure 2 (b) and
sup Table S2).
Regarding hydrochemical properties, the concentrations of PsyC in water were inversely correlated (Pearson test, p < 0.01) with conductivity values as previously shown in stream water by Monteil et al. [
18] (
Sup Table S3). The pH and dominant anions and cations were correlated with conductivity. These variables reflects the differences in geological substratum of the groundwater. Sites n°30, 33 & 37 situated at the eastern part of the studied area had the highest conductivity and the lowest pH values, site n°22 from the western part had an intermediate value of conductivity and sites n°07 & 10 near the Durance, and river water itself (n°02) had the lowest conductivity values. These variables explained 27 % of PsyC densities variations. We observed a correlation between the percentage of dissolved oxygen and the bacterial concentrations. A lowest oxygen content in water could be an indication of captivity of the groundwater isolated from the surface, coherent with a lowest number of bacteria coming from the surface, but this correlation was non-significant. Most of the variability remains unexplained and is probably multifactorial. PsyC and culturable bacteria densities were also significantly correlated (R
2 = 42 %) (
Sup Table S3) and we can assume that generic mechanisms such as filtration, predation, or starvation influence abundances of populations, whatever the species. In a survey during the year 2016 and 2017, Morris et al. [
19] found that PsyC densities were correlated to culturable bacteria densities only in the upper and middle Durance basins of the Durance catchment and surprisingly not in the lower basin where our site is located. It suggests that the factors that influence the densities of PsyC and total culturable bacteria may vary in time. In contrast with the regularly present populations of PsyC in river water [
18], in groundwater the presence and variability of PsyC densities is difficult to predict. Our results could reflect the lack of method sensitive enough to measure the size of PsyC populations when they are very small. Using ultrafiltration device [
20] that allow filtering 30 liters of water in less than one hour, could help to study these populations accurately.
3.3. The Population of P. syringae Complex Was Less Diverse in Groundwater than in River Water
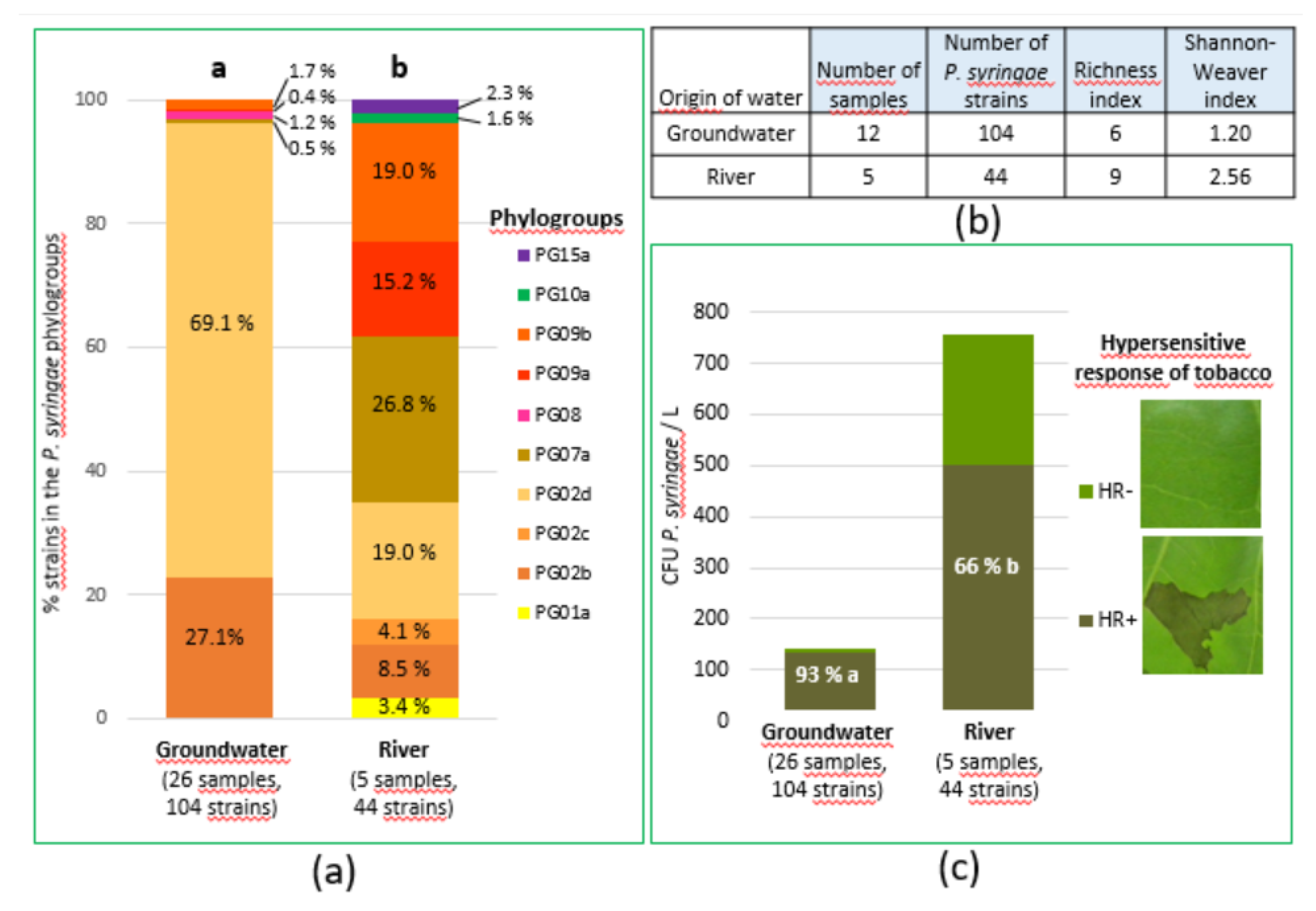
We isolated and purified 148 strains of PsyC, 104 from groundwater and 44 from water from Durance river for which phenotypic and genotypic information is reported in
sup Table S4. The PsyC is very diverse, including probably more than 20 bacterial species among which only 14 have been formerly described. In our study, we have used the framework proposed by Berge et al. [
16] for a clear, simple and reliable classification of PsyC strains based on the phylogeny of partial cts gene sequences. Following this framework, we were able to affiliate the 148 strains into 10 clades from 7 phylogroups (
sup Table S4). Given the stochasticity of the PsyC detection in groundwater, we combined data from groundwater on one side and river data on the other side, whatever site and date. Abundances (Colony Forming Unit per liter (CFU.L
-1)) of each clade were determined in each sample by combining clade % and PsyC abundance (
Sup Table S5). Abundances of clades in samples were cumulated giving the global clade abundances in both groundwater and river water (
Figure 3 (a)).
The results show that groundwater harbored 5 clades from 4 phylogroups but the PsyC population was highly dominated by phylogroup PG02, representing 96.2 %, subdivided into clade PG02d (69.1 %) and clade PG02b (27.1 %) (
Figure 3 (a);
sup Table S5). In contrast, river water harbored 8 clades from 5 phylogroups, none of them dominating the populations. Phylogroups PG09, PG02 and PG07 represented 34, 32 and 27 % of PsyC population from the river, respectively (
Figure 3 (a)). Regardless the population size, based on isolated PsyC strains, Morris et al. [
19] found also various phylogroups in river Durance catchment, the most abundant being PG02 (45 %), PG07 (14 %), PG10 (12 %), PG13 (10 %) without one dominating as in groundwater. They described a widespread haplotype named DD.1 (a lineage into the PG02b clade) present in all sites and representing 10 % of all PsyC strains they isolated. In this study, haplotype DD.1 was isolated from groundwater (4.8 % of isolated strains) and river Durance (6.8 % of isolated strains) that confirms its ubiquity (
Sup Table S6). The diversity indexes were lower in groundwater than in river water and more particularly the evenness Shannon-weaver index (
Figure 3 (b)). In terms of population size, PG02b and PG02d were not markedly different in groundwater (respectively 36 and 100 CFU.L
-1) compared to river water (resp 64 and 144 CFU.L
-1) (
sup Table S5). Phylogroup PG02 is dominant in groundwater. It is known to be frequent in cultivated and non-cultivated areas [
17]. Clades PG02b is considered as the “true” P. syringae species and PG02d is a very close species not yet named [
16,
22]. Together these clades contain mostly plant pathogenic strains producing syringomicin-like toxins, and able to nucleate ice [
16]. Three other clade/phylogroups were also present in groundwater but at very low frequency (< 1 %) including PG07 which corresponds to the species P. viridiflava being an environmental plant pathogenic bacteria with pectinolytic acivity, PG08 close to PG07, PG09a and PG09b that are potentially pathogenic, but have never been isolated from diseased plants [
16]. In river water, in addition to PG02b, 2d, 07a, 09a and 09b, we found PG01a that corresponds to the pathogenic group of P. syringae pv tomato and PG02c a non-pathogenic group (named “SZ30” by Diallo et al. [
23]) close to P. congelans. Moreover, we isolated PG10a and PG15 (
sup Table S4), two groups that have never been isolated from diseased plants, the last being reported for the first time by Morris et al. [
19].
3.4. Phytopathogenic Strains of Phylogroup 2 Dominated the Population of the P. syringae Complex in Groundwater
Pathogenic potential of strains was assessed via induction of a Hypersensitive Reaction (HR) on tobacco. In both groundwater and river water, the majority of strains induced an HR on tobacco and so are considered potentially pathogenic on plants. Surprisingly, the percentage of the PsyC population producing an HR was significantly higher in groundwater (93 %) than in river water (66 %) (
Figure 3 (c)). However, taking into account the size of the PsyC population, abundance of potentially pathogenic PsyC was five time higher in river water than in groundwater (
Figure 3 (c)). Groundwater seems to be a reservoir of plant pathogenic PsyC, but it harbors a lower concentration of this bacterial group on average, than river water. However, it can occasionally contain a high concentration of phytopathogenic bacteria as in November 2011 (
Sup Table S2 & S4).
4. Discussion
Future investigations are needed to understand why populations of
P. syringae complex are different in groundwater and river. Groundwater recharge can involve various processes [
11]. Firstly, the Durance river feeds the aquifer by horizontal flow and we could hypothesize in that case, that populations of the PsyC are homogeneously transferred from river to groundwater. Sampling effort at sites close to the river such as n°10 & 07 (
Figure 1) could validate this hypothesis. A second way that groundwater is recharged occurs mainly in the summer via flood irrigation used by farmers on their hay meadows and orchards, mainly in agricultural area, such as at sites n°30, 33, 37. Water used for irrigating comes from the Durance river and is distributed by a complex network of irrigation canals. Populations of the PsyC from the river could be then modified by canalization transport, and more likely by the contact with cultivated plants, leaf litter and transport through the soil. Monteil
et al. [
18,
24] have shown that in subalpine grasslands, leaf litter was an important source of very diverse PsyC populations. These populations were effectively transported with water infiltrating through the soil. In addition, in lab experiments they showed that percolation could have different efficiencies for different strains of PsyC and that indigenous soil populations of PsyC possibly contribute to the populations of bacteria transferred through the column of soil. Consequently, populations of PsyC transferred in the Avignon aquifer through flood irrigation could be a mix between those from river, litter, plants and soil, with these mixed populations filtered by soil depending on its characteristics. That could explain the low diversity in groundwater. Sampling leaf litter, plants, soil and irrigating water in meadows over the groundwater and testing the transfer of the PsyC bacteria from these different sources through column of soil sampled at the same site could help to clarify this hypothesis. A third recharge source is the rain. Monteil
et al. [
25] estimated that 70 % of rain samples collected near Avignon contain populations of PsyC with an average size of 734 CFU.L
-1 that could contribute strains to the water table. However only 78 % of the PsyC strains collected from rain in that study induced an HR on tobacco. This is lower frequency than we observed in groundwater (93 %). Therefore the main effect of rain is likely to act as a carrier of populations, from litter, plants and soil to the water table in the same way as irrigation.
Another hypothesis to explain the differences in population densities in ground and river water is that differences in water parameters favor the domination of groundwater populations by phylogroup PG02. In a preliminary experiment, we found that pure strains of PG02 inoculated into sterilized water sampled from Avignon groundwater have a better fitness than pure strains of PG07 that is one of the most abundant phylogroup in river water. In sterilized river water their fitnesses were similar and lower than in groundwater for PG02 (data not shown). We could hypothesize that there is an interaction between physiology and/or genetic expression of PG02 strains and physical, chemical or organic water parameters. Temperature could be one of them, being less fluctuating in groundwater (14 to 19 °C) than in river (5 to 19 °c) (
sup Table S2). The quantity and nature of water organic substrates could also be involved. Groundwater is a very oligotrophic environment containing low levels of organic substrates [
13]. River water carries various substances in particular in areas subjected to anthropogenic influences such as agriculture and waste water [
26] that could limit specifically the survival of PG02. Biologic parameters are also involved in the regulation of aquatic bacterial populations. Predation and competition have a huge impact on populations [
27] and could have a role to play. It is necessary to pursue further to test the survival of strains isolated from groundwater both in ground and river water at various temperatures.
5. Conclusions
Our results highlight the presence of PsyC populations dominated by plant pathogenic strains of phylogroup PG02 in alluvial Avignon groundwater. The size of these populations was rather low compared to that of the river water. Most of time, using groundwater for crop irrigation remains probably safer than irrigating with river water. However, abundances of P. syringae were quite variable and unpredictable and occasionally abundances were comparable to that of river water. Overall, groundwater is clearly a reservoir of P. syringae complex.
The way that plant pathogenic bacteria enter groundwater is probably complex: it could originate from river through underground infiltrations or from surface water through irrigation or rain. Their presence could originate also from pathogens colonizing plants on the top of aquifers, washed away by rain or irrigation water and carried from surface to groundwater. The physiological state of strains and their characteristics such as biofilm production or surface properties, will determine their capacity to pass across soil or geological substrates and to survive in stressed conditions such as oligotrophy, toxic organic substrates, competitors or predators. The dominance of PsyC PG02d and PG02b clades in groundwater is probably linked to their adaptation to these kind of conditions. Future research is needed to explore their ecology in this compartment and will lead to understanding and quantifying fluxes, sources and fluctuations of these pathogens and their impacts on health of irrigated crops.
Understanding the sources and reservoirs of crop pathogens is crucial for developing methods to reduce their impact on crop production. These data are part of the knowledge about the ecology of plant pathogens that will be required for anticipation and response to plant disease emergence, in the context of global change. It could be included in prediction models and in new approaches to disease forecasting and surveillance and lead to adaptation of agricultural practices.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Odile Berge, Salah Nofal, Anne-Laure Cognard-Plancq and Cindy E. Morris; Data curation, Odile Berge, Salah Nofal, Frédérique Razan, Caroline Guilbaud and Anne-Laure Cognard-Plancq; Formal analysis, Odile Berge, Salah Nofal and Cindy E. Morris; Investigation, Odile Berge, Salah Nofal, Frédérique Razan, Charlotte Chandeysson, Caroline Guilbaud, Anne-Laure Cognard-Plancq and Cindy E. Morris; Supervision, Odile Berge and Anne-Laure Cognard-Plancq; Validation, Odile Berge, Anne-Laure Cognard-Plancq and Cindy E. Morris; Visualization, Odile Berge, Salah Nofal and Cindy E. Morris; Writing – original draft, Odile Berge; Writing – review & editing, Odile Berge, Salah Nofal, Frédérique Razan, Charlotte Chandeysson, Caroline Guilbaud, Anne-Laure Cognard-Plancq and Cindy E. Morris.
Funding
This research received no external funding and was funded by INRAE and Avignon University.
Data Availability Statement
Data are available in article supplementary material. Sequences are deposit in NCBI database (in progress).
Acknowledgments
The authors wish to thank Michel Daniel and Roland Simler for their assistance in the physico-chemical analyses and data acquisition, the staff in the Plant Growth facilities at the INRAE Plant Pathology Research Unit for their support in preparing plant materials, Caroline L. Monteil, Vincent Marc and Marina Gillon for fruitful discussions. B. O. would like to thank Véronique Lefebvre for her support, encouragement and advice during the writing process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shiklomanov, I.A. Appraisal and Assessment of World Water Resources. Water Int., 2000, 25(1), 11-32. [CrossRef]
- FAO, Water for Sustainable Food and Agriculture. A report produced for the G20 Presidency of Germany. 2017, Food and Agriculture Organization of the United Nations. https://www.fao:3/i7959e/i7959e.pdf.
- Foster S.S.D.; Chilton P.J. Groundwater: the processes and global significance of aquifer degradation. Philos. Trans. R. Soc. Lond., B, Biol. Sci. PHILOS T R SOC B, 2003, 358(1440), 1957-1972. [CrossRef]
- EEA, Climate change, impacts and vulnerability in Europe 2016. An indicator-based report, in EEA Report. 2017. Climate change impacts and vulnerabilities 2016 THAL17001ENN.pdf.
- Korbel K.L.; Hose G.C. A tiered framework for assessing groundwater ecosystem health. Hydrobiologia, 2011, 661(1), 329-349. [CrossRef]
- Martin, M.S.; Santos, I.C.; Carlton, D.D.; Stigler-Granados, P.; Hildenbrand, Z.L.; Schug, K.A. Characterization of bacterial diversity in contaminated groundwater using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Sci. Total Environ., 2018, 622, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Gu G.Y.; Yin H.B.; Ottesen A.; Bolten S.; Patel J.; Rideout S.; Nou X.W. Microbiomes in Ground Water and Alternative Irrigation Water, and Spinach Microbiomes Impacted by Irrigation with Different Types of Water. Phytobiomes J., 2019, 3(2), 137-147. [CrossRef]
- Morris C.E.; Sands D.C.; Vinatzer B.A.; Glaux C.; Guilbaud C.; Buffiere A.; Yan S.C.; Dominguez H.; Thompson B.M. The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle. Isme J., 2008, 2(3), 321-334. [CrossRef]
- Pietsch R.; Vinatzer B.A.; Schmale D.G. Diversity and abundance of ice nucleating strains of Pseudomonas syringae in a freshwater lake in Virginia, USA Front. Microbiol., 2017, 8, 318. [CrossRef]
- Monteil, C.L.; Yahara, K.; Studholme, D.J.; Mageiros, L.; Méric, G.; Swingle, B.; Morris, C.E.; Vinatzer, B.A.; Sheppard, S.K. Population-genomic insights into emergence, crop adaptation and dissemination of Pseudomonas syringae pathogens. Microb. Genom., 2016, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Nofal, S.; Travi Y.; Cognard-Plancq A.L.; Marc, V. Impact of infiltrating irrigation and surface water on a Mediterranean alluvial aquifer in France using stable isotopes and hydrochemistry, in the context of urbanization and climate change. Hydrogeology J., 2019. 27(6), 2211-2229. [CrossRef]
- SCP, Etude pédologique et Aptitudes des sols à la mise en valeur. In S.-O. Atlas n°1, carte pédologique 1/50 000. Editor, SCP Société du Canal de Provence, Le Tholonet, France 1974.
- Rousset, L.; Gillon, M.; Duport, C.; Clavel, T.; Lagree, M.; Traikia, M.; Panagiotopoulos, C.; Berge, O. A first inventory of the labile biochemicals found in Avignon groundwater: can we identify potential bacterial substrates? E3S Web Conf. i-DUST 2018 – Inter-Disciplinary Underground Science & Technology, 2019, 88, 02001. [Google Scholar] [CrossRef]
- King E.O.; Ward M.K.; and Raney D.E. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med., 1954, 44(2), 301-307.
- Schaad, N. Serological identification of plant pathogenic bacteria. Annu. Rev. Phytopathol., 1979, 17, 23–47. [Google Scholar] [CrossRef]
- Berge O.; Monteil C.L.; Bartoli C.; Chandeysson C.; Guilbaud C.; Sands D.C.; Morris C.E. A User’s Guide to a Data Base of the Diversity of Pseudomonas syringae and Its Application to Classifying Strains in This Phylogenetic Complex. PLoS ONE, 2014, 9(9). [CrossRef]
- Morris C.E.; Sands D.C.; Vanneste J.L.; Montarry J.; Oakley B.; Guilbaud C.; Glaux C. Inferring the Evolutionary History of the Plant Pathogen Pseudomonas syringae from Its Biogeography in Headwaters of Rivers in North America, Europe, and New Zealand. Mbio, 2010, 1(3), 107–110. [CrossRef]
- Monteil C.L.; Lafolie F.; Laurent J.; Clement J.C.; Simler R.; Travi Y.; Morris C.E. Soil water flow is a source of the plant pathogen Pseudomonas syringae in subalpine headwaters. Environ. Microbiol., 2014. 16(7), 2038-2052. [CrossRef]
- Morris C.E.; Lacroix C.; Chandeysson C.; Guilbaud C.; Monteil C.; Piry S.; Rochelle Newall E.; Fiorini S.; Van Gijsegem F.; Barny M.A.; Berge O. Comparative seasonal abundance and diversity of populations of the Pseudomonas syringae and Soft Rot Pectobacteriaceae species complexes throughout the Durance River catchment from its French Alps sources to its delta. bioRxiv, 2022. 09.06.506731. [CrossRef]
- Knappett P.S.K.; Layton A.; McKay L.D.; Williams D.; Mailloux B.J.; Huq M.R.; Alam M.J.; Ahmed K.M.; Akita Y.; Serre M.L.; Sayler G.S.); van Geen A. Efficacy of Hollow-Fiber Ultrafiltration for Microbial Sampling in Groundwater. Ground Water, 2011, 49(1), 53-65. [CrossRef]
- Kumar S.; Stecher G.; Li M.; Knyaz C.; Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol., 201, 35(6), 1547-1549. [CrossRef]
- Gomila, M.; Busquets, A.; Mulet, M.; Garcia-Valdes, E.; Lalucat, J. Clarification of Taxonomic Status within the Pseudomonas syringae Species Group Based on a Phylogenomic Analysis. Front. Microbiol., 2017, 8, 2422. [Google Scholar] [CrossRef] [PubMed]
- Diallo M.D.; Monteil C.L.; Vinatzer B.A.; Clarke C.R.; Glaux C.; Guilbaud C.; Desbiez C.; Morris C.E. Pseudomonas syringae naturally lacking the canonical type III secretion system are ubiquitous in nonagricultural habitats, are phylogenetically diverse and can be pathogenic. Isme J., 2012, 6(7), 1325-1335. [CrossRef]
- Monteil C.L.; Guilbaud C.; Glaux C.; Lafolie F.; Soubeyrand S.; and Morris C.E. Emigration of the plant pathogen Pseudomonas syringae from leaf litter contributes to its population dynamics in alpine snowpack. Environ. Microbiol., 2012, 14(8), 2099-2112. [CrossRef]
- Monteil C.L.; Bardin M.; Morris C.E. Features of air masses associated with the deposition of Pseudomonas syringae and Botrytis cinerea by rain and snowfall. Isme J., 2014, 8(11), 2290-2304. [CrossRef]
- Kamjunke, N.; Hertkorn, N.; Harir, M.; Schmitt-Kopplin, P.; Griebler, C.; Brauns, M.; von Tuempling, W.; Weitere, M.; Herzsprung, P. Molecular change of dissolved organic matter and patterns of bacterial activity in a stream along a land-use gradient. Water Res., 2019, 164, 114919. [Google Scholar] [CrossRef] [PubMed]
- Wanjugi P.; Fox G.A.; Harwood V.J. The Interplay Between Predation, Competition, and Nutrient Levels Influences the Survival of Escherichia coli in Aquatic Environments. Microbial Ecology, 2016, 72(3), 526-537. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).