Submitted:
02 June 2023
Posted:
02 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Section
2.1. Reagents and Instruments
2.2. Synthesis of Tb-MOFs and C460@Tb-MOFs
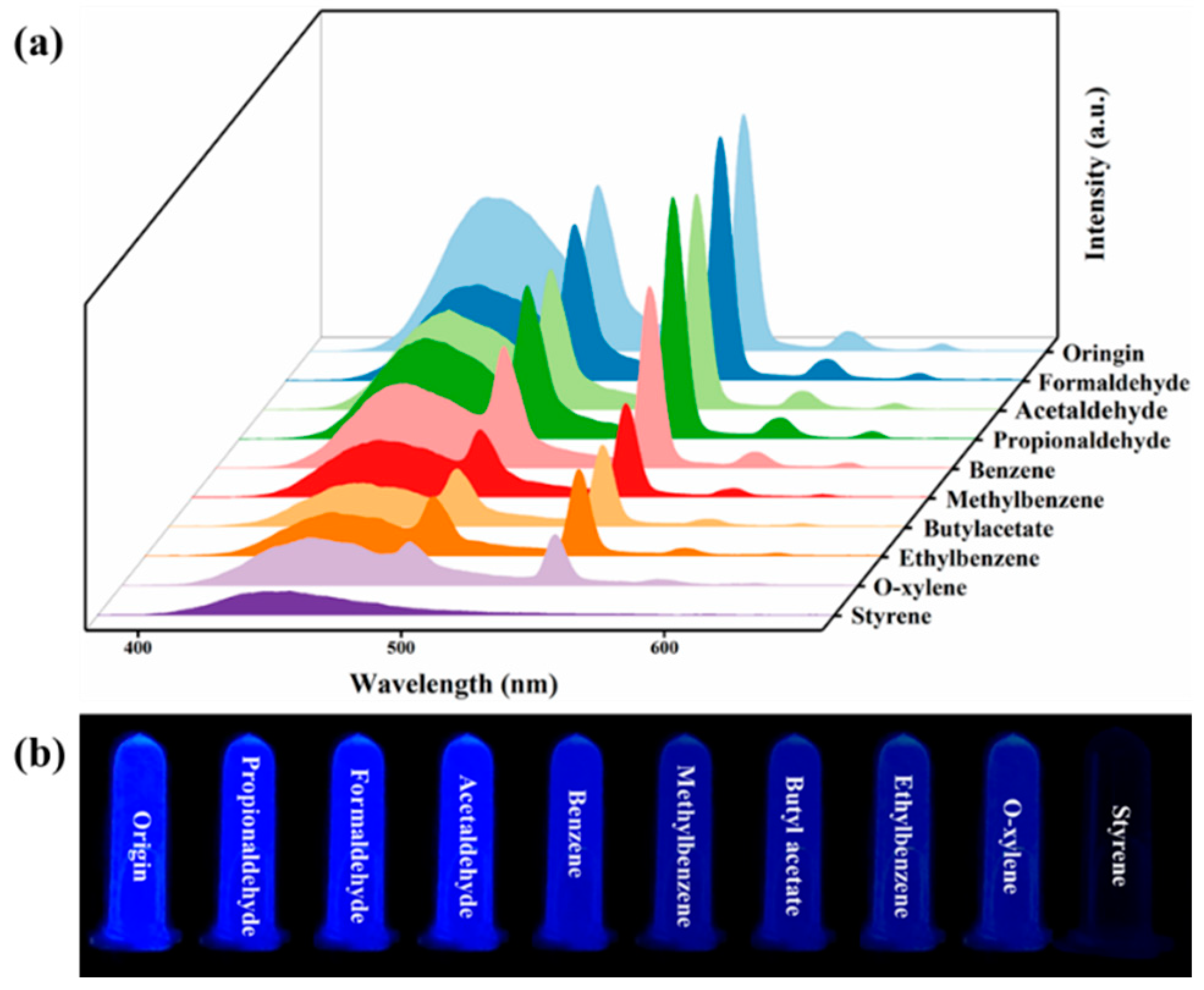
2.3. Fluorescence Sensing of Detection Styrene Liquid
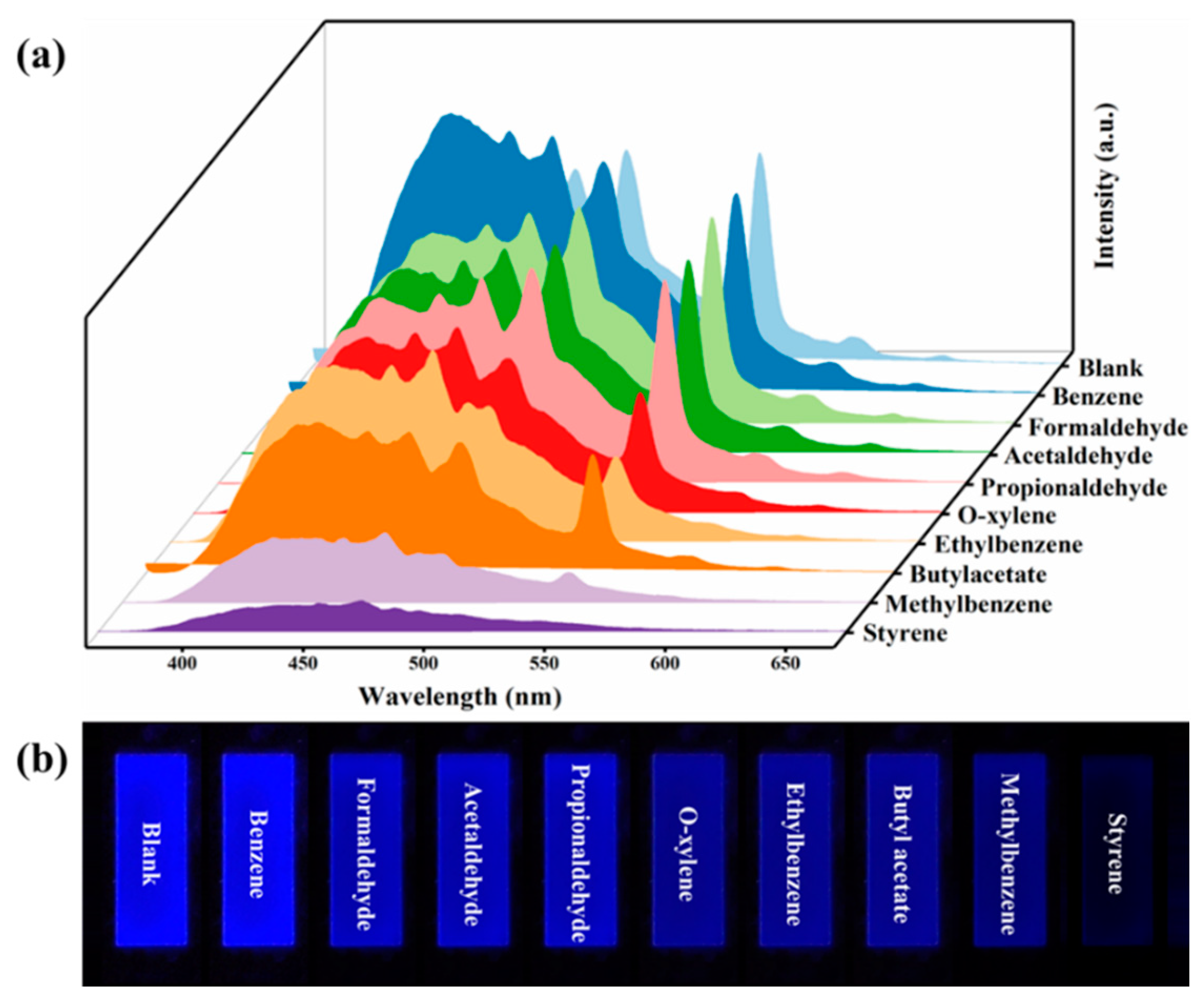
2.4. Fluorescence Sensing of Detection Styrene Gas
3. Results and Discussion
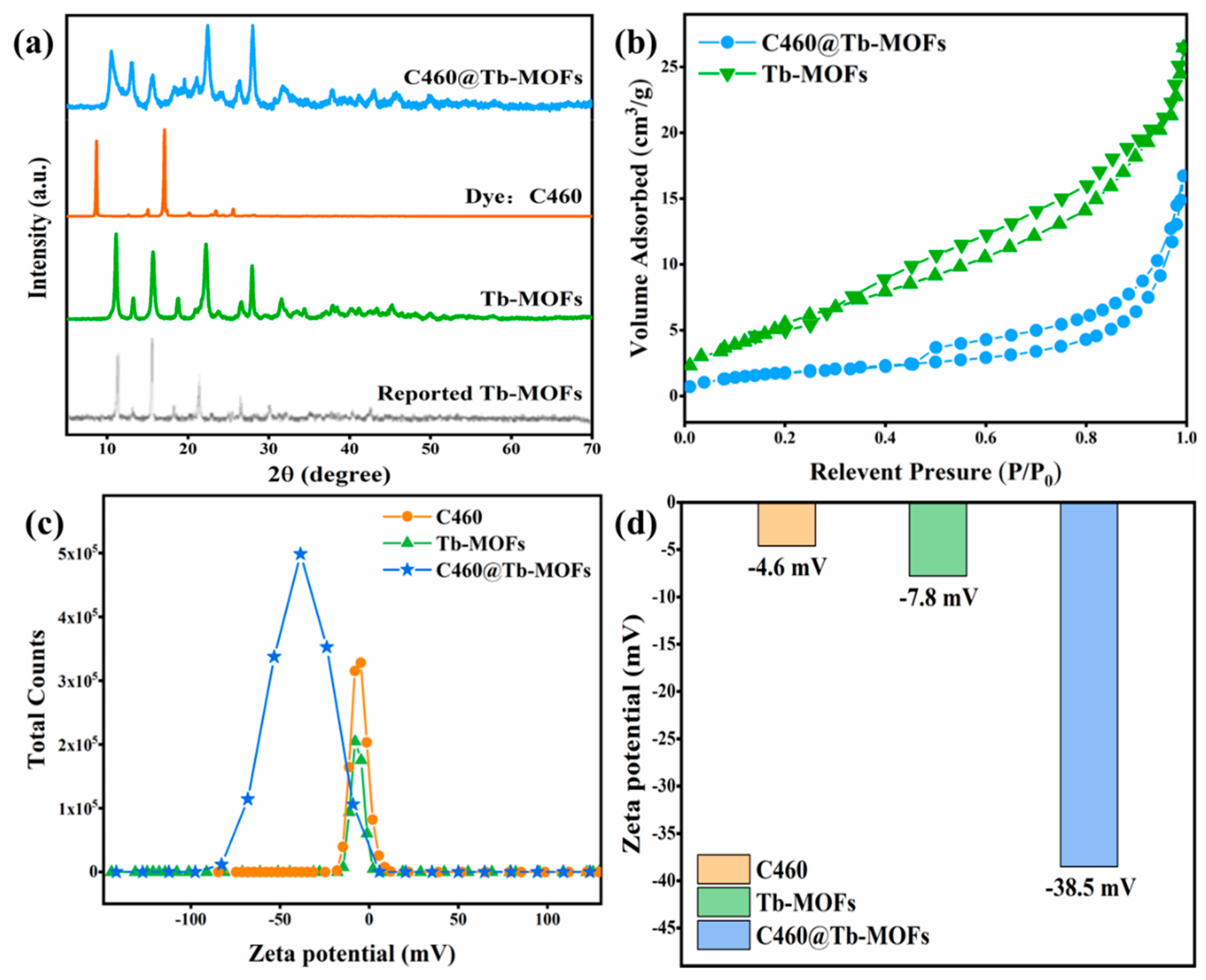
3.1. Characterization of C460@Tb-MOFs Fluorescent Probe
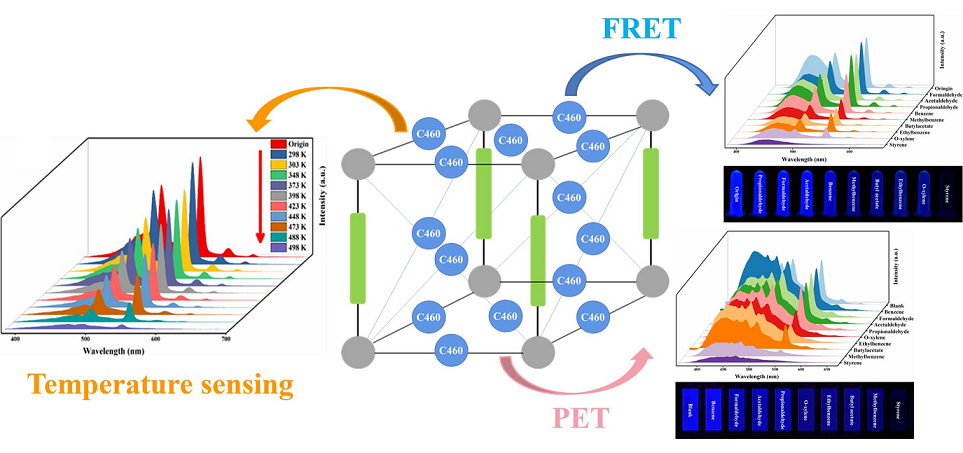
3.2. C460@Tb-MOFs for Real-Time Detection of Styrene Liquid and Styrene Gas
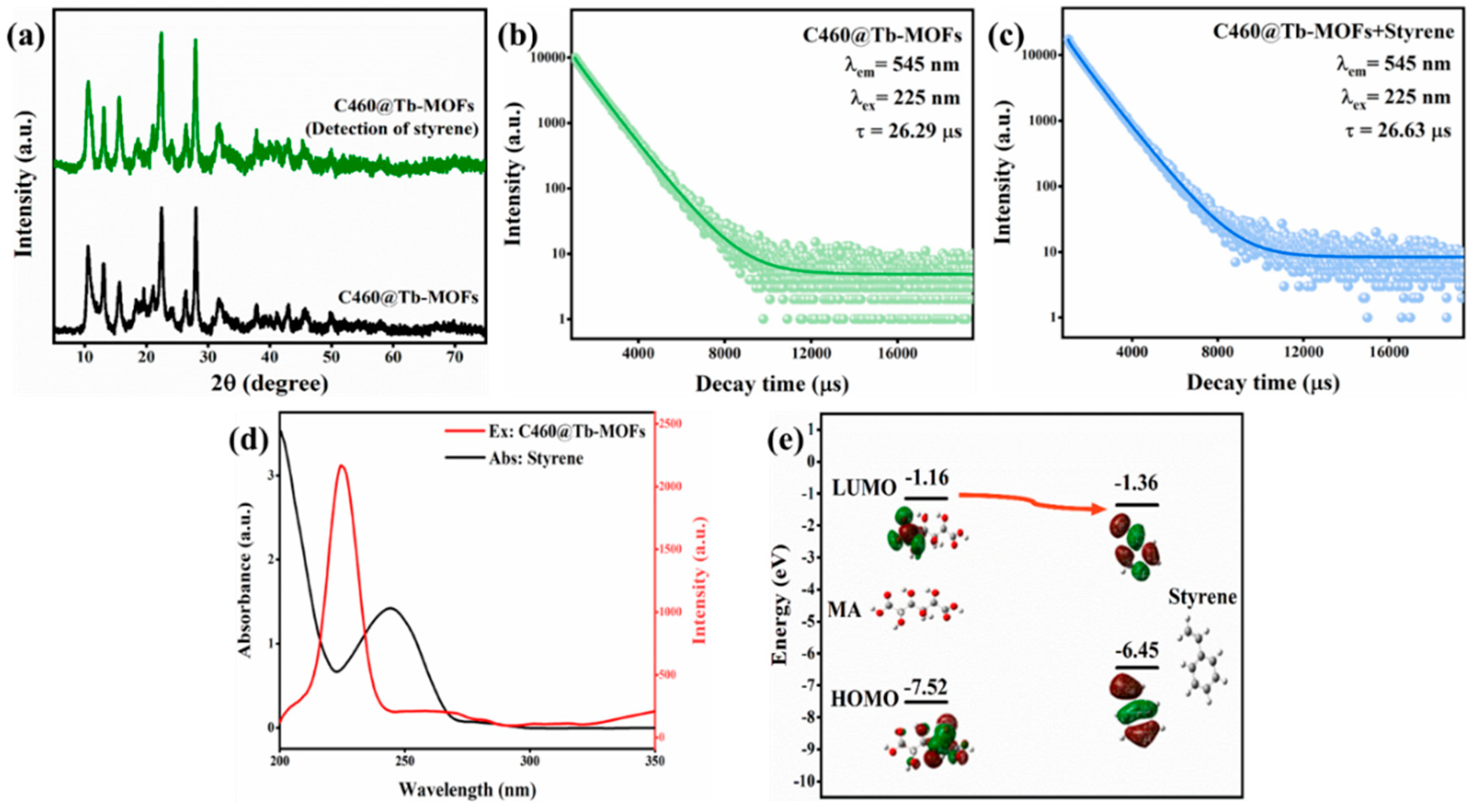
3.3. C460@Tb-MOFs Sensing Mechanism for Styrene
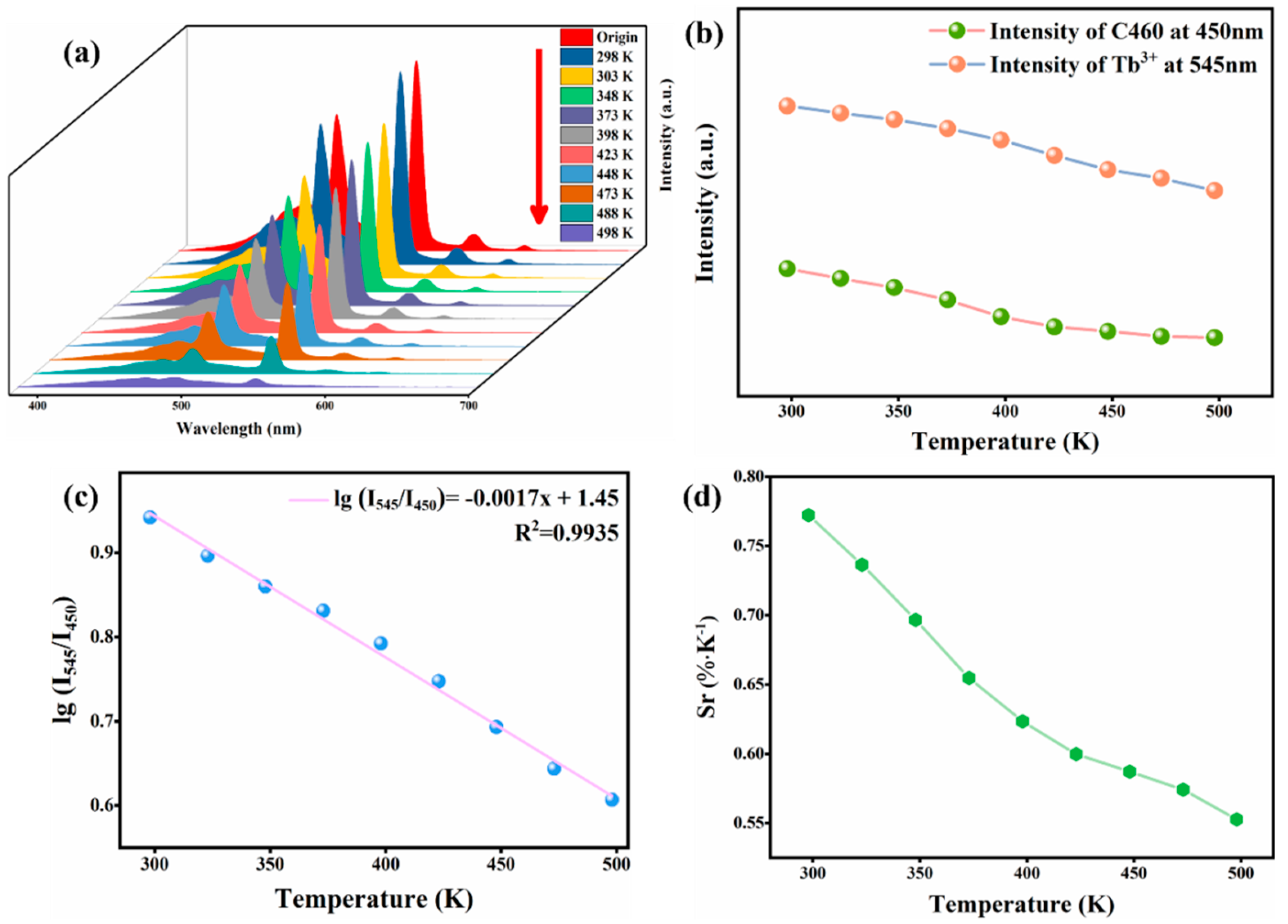
3.4. C460@Tb-MOFs for Temperature Sensing
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Li, Q.; Chen, H.; You, S.; Lin, Z.; Chen, Z.; Huang, F.; Qiu, B. Colorimetric and fluorescent dual-modality sensing platform based on UiO-66 for fluorion detection. Microchem. J. 2023, 186, 108318. [Google Scholar] [CrossRef]
- Liu, M.; Ren, X.; Meng, X.; Li, H. Metal-organic frameworks-based fluorescent nanocomposites for bioimaging in living cells and in vivo. Chin. J. Chem. 2021, 39, 473–487. [Google Scholar] [CrossRef]
- Cai, H.; Lu, W.; Yang, C.; Zhang, M.; Li, M.; Che, C.-M.; Li, D. Tandem Förster resonance energy transfer induced luminescent ratiometric thermometry in Dye-encapsulated biological metal–organic frameworks. Advanced Optical Materials. 2019, 7, 1801149. [Google Scholar] [CrossRef]
- Ruan, B.; Yang, J.; Zhang, Y.-J.; Ma, N.; Shi, D.; Jiang, T.; Tsai, F.-C. UiO-66 derivate as a fluorescent probe for Fe3+ detection. Talanta. 2020, 218, 121207. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yan, J.; Cui, Q.; Shang, R.; Zuo, Q.; Gong, L.; Zhang, W. Synthesis and Peroxide Activation Mechanism of Bimetallic MOF for Water Contaminant Degradation: A Review. Molecules. 2023, 28, 3622. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jiang, X.; Che, J.; Li, M.; Zhang, X.; Gao, D.; Bi, J.; Ning, Z. A Ratiometric Fluorescent Sensor Based on Dye/Tb (III) Functionalized UiO-66 for Highly Sensitive Detection of TDGA. Molecules. 2022, 27, 6543. [Google Scholar] [CrossRef]
- Liu, J.; Yue, X.; Wang, Z.; Zhang, X.; Xu, Y. Coumarin 7 functionalized europium-based metal–organic-framework luminescent composites for dual-mode optical thermometry. J. Mater. Chem. C. 2020, 8, 13328–13335. [Google Scholar] [CrossRef]
- Liu, L.; Lu, X.-Y.; Zhang, M.-L.; Ren, Y.-X.; Wang, J.-J.; Yang, X.-G. 2D MOF nanosheets as an artificial light-harvesting system with enhanced photoelectric switching performance. Inorg Chem Front. 2022, 9, 2676–2682. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Han, Y.; Li, H.; Kang, Q.; Wang, P.; Zhou, F. Immunoassay for cardiac troponin I with fluorescent signal amplification by hydrolyzed coumarin released from a metal–organic framework. ACS Applied Energy Materials. 2019, 2, 7170–7177. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, T.; Zhong, T.; Zhang, C.; Tian, Y.; Wang, G. Coumarin-embedded MOF UiO-66 as a selective and sensitive fluorescent sensor for the recognition and detection of Fe3+ ions. J. Mater. Chem. C. 2021, 9, 16978–16984. [Google Scholar] [CrossRef]
- Shen, Y.; Tissot, A.; Serre, C. Recent progress on MOF-based optical sensors for VOC sensing. Chemical Science. 2022, 13, 13978–14007. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; Wang, P.; Yan, Y.; Ran, J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: a review. Sep. Purif. Technol. 2020, 235, 116213. [Google Scholar] [CrossRef]
- Tung, T.T.; Tran, M.T.; Feller, J.-F.; Castro, M.; Van Ngo, T.; Hassan, K.; Nine, M.J.; Losic, D. Graphene and metal organic frameworks (MOFs) hybridization for tunable chemoresistive sensors for detection of volatile organic compounds (VOCs) biomarkers. Carbon. 2020, 159, 333–344. [Google Scholar] [CrossRef]
- Joshi, M.; Nair, S. Hplc analysis of trigonella foenum-graecum seeds to assess phytoestrogens. Indian Journal of Occupational and Environmental Medicine. 2008, 12, 61–64. [Google Scholar] [CrossRef]
- Kim, S.-I.; Kim, A.-R.; Bae, H.J.; Kim, S.-Y.; Ravi, S.; Kim, K.C.; Bae, Y.-S. Cu(I)-incorporation strategy for developing styrene selective adsorbents. Chem. Eng. J. 2021, 425, 130601. [Google Scholar] [CrossRef]
- Yang, F.; Ma, J.; Zhu, Q.; Ma, Z.; Wang, J. Aggregation-induced luminescence based UiO-66: highly selective fast-response styrene detection. ACS Appl. Mater. Interfaces. 2022, 14, 22510–22520. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-I.; Kim, A.-R.; Kim, S.-Y.; Lee, J.-Y.; Bae, Y.-S. High styrene/ethylbenzene selectivity in a metal-organic framework with coordinatively unsaturated cobalt(II) sites. Sep. Purif. Technol. 2020, 242, 116758. [Google Scholar] [CrossRef]
- Sarigiannis, D.A.; Karakitsios, S.P.; Gotti, A.; Liakos, I.L.; Katsoyiannis, A. Exposure to major volatile organic compounds and carbonyls in european indoor environments and associated health risk. Environ. Int. 2011, 37, 743–765. [Google Scholar] [CrossRef]
- Ras, M.R.; Borrull, F.; Marcé, R.M. Sampling and preconcentration techniques for determination of volatile organic compounds in air samples. TrAC, Trends Anal. Chem. 2009, 28, 347–361. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Du, T.; Zhu, Z.; Zhang, J.; Liu, Q. A gas sensor array for the simultaneous detection of multiple VOCs. Sci. Rep. 2017, 7, 1960. [Google Scholar] [CrossRef]
- Park, J.; Tabata, H. Gas sensor array using a hybrid structure based on zeolite and oxide semiconductors for multiple bio-gas detection. ACS Omega. 2021, 6, 21284–21293. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Okur, S.; Li, C.; Chandresh, A.; Mutruc, D.; Hecht, S.; Heinke, L. A photoprogrammable electronic nose with switchable selectivity for VOCs using MOF films. Chem. Sci. 2021, 12, 15700–15709. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, H.; Wang, Y.; Qiu, F.; Feng, Y.; Song, X.; Tang, X.; Zhang, G.; Liu, W. A family of mixed-lanthanide metal–organic framework thermometers in a wide temperature range. Dalton Trans. 2018, 47, 13384–13390. [Google Scholar] [CrossRef]
- Ding, Y.; Lu, Y.; Yu, K.; Wang, S.; Zhao, D.; Chen, B. MOF-nanocomposite mixed-matrix membrane for dual-luminescence ratiometric temperature sensing. Advanced Optical Materials. 2021, 9, 2100945. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, B. Ratiometric detection of temperature using responsive dual-emissive MOF hybrids. J. Mater. Chem. C. 2015, 3, 9353–9358. [Google Scholar] [CrossRef]
- Xia, T.; Cui, Y.; Yang, Y.; Qian, G. A luminescent ratiometric thermometer based on thermally coupled levels of a Dy-MOF. J. Mater. Chem. C. 2017, 5, 5044–5047. [Google Scholar] [CrossRef]
- Wong, K.-L.; Law, G.-L.; Yang, Y.-Y.; Wong, W.-T. A highly porous luminescent terbium–organic framework for reversible anion sensing. Advanced Materials. 2006, 18, 1051–1054. [Google Scholar] [CrossRef]
- Yang, J.; Che, J.; Jiang, X.; Fan, Y.; Gao, D.; Bi, J.; Ning, Z. A novel turn-on fluorescence probe based on Cu(II) functionalized metal– organic frameworks for visual detection of uric acid. Molecules. 2022, 27, 4803. [Google Scholar]
- Che, J.; Jiang, X.; Fan, Y.; Li, M.; Zhang, X.; Gao, D.; Ning, Z.; Li, H. A Novel Dual-Emission Fluorescence Probe Based on CDs and Eu3+ Functionalized UiO-66-(COOH)2 Hybrid for Visual Monitoring of Cu2+. Materials. 2022, 15, 7933. [Google Scholar] [CrossRef]
- Wang, X.R.; Wang, X.Z.; Du, J.; Huang, Z.; Liu, Y.Y.; Huo, J.Z.; Liu, K.; Ding, B. Post-synthetic dual-emission rhodamine B@ZIF-365 hybrid material and enzymatic biosensor enzyme@ZIF-365: ratiometric temperature sensing, biomolecule nicotinamide detection and sensing platform for lactose and Al3+. J. Solid State Chem. 2019, 279, 120949. [Google Scholar] [CrossRef]
- Li, M.; Dong, C.; Yang, J.; Yang, T.; Bai, F.; Ning, Z.; Gao, D.; Bi, J. Solvothermal synthesis of La-based metal-organic frameworks and their color-tunable photoluminescence properties. Journal of Materials Science: Materials in Electronics. 2021, 32, 9903–9911. [Google Scholar] [CrossRef]
- Dong, C.-L.; Li, M.-F.; Yang, T.; Feng, L.; Ai, Y.-W.; Ning, Z.-L.; Liu, M.-J.; Lai, X.; Gao, D.-J. Controllable synthesis of Tb-based metal–organic frameworks as an efficient fluorescent sensor for Cu2+ detection. Rare Metals. 2021, 40, 505–512. [Google Scholar] [CrossRef]
- Ashwin, B.C.M.A.; Sivaraman, G.; Stalin, T.; Yuvakkumar, R.; Muthu Mareeswaran, P. Selective and sensitive fluorescent sensor for Pd2+ using coumarin 460 for real-time and biological applications. J. Photochem. Photobiol. B. 2018, 183, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Dong, C.; Li, M.; Li, L.; Jiang, X.; Gao, R.; Wang, R.; Zhang, L.; Ning, Z.; Gao, D.; Bi, J. Terbium-based metal-organic frameworks: highly selective and fast respond sensor for styrene detection and construction of molecular logic gate. J. Hazard. Mater. 2020, 388, 121816. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, D.; Zeng, J.; Gan, N.; Cuan, J. A luminescent lanthanide-free MOF nanohybrid for highly sensitive ratiometric temperature sensing in physiological range. Talanta. 2018, 181, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Cadiau, A.; Brites, C.D.S.; Costa, P.M.F.J.; Ferreira, R.A.S.; Rocha, J.; Carlos, L.D. Ratiometric nanothermometer based on an emissive Ln3+-organic framework. ACS Nano. 2013, 7, 7213–7218. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Freslon, S.; Daiguebonne, C.; Pollès, L.L.; Calvez, G.; Bernot, K.; Yi, X.; Huang, G.; Guillou, O. A family of lanthanide-based coordination polymers with boronic acid as ligand. Inorg. Chem. 2015, 54, 5534–5546. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Konno, Y.; Nakanishi, T.; Kobayashi, A.; Kato, M.; Fushimi, K.; Hasegawa, Y. Chameleon luminophore for sensing temperatures: control of metal-to-metal and energy back transfer in lanthanide coordination polymers. Angew. Chem. Int. Ed. 2013, 52, 6413–6416. [Google Scholar] [CrossRef]
- Zhao, S.-N.; Li, L.-J.; Song, X.-Z.; Zhu, M.; Hao, Z.-M.; Meng, X.; Wu, L.-L.; Feng, J.; Song, S.-Y.; Wang, C.; Zhang, H.-J. Lanthanide ion codoped emitters for tailoring emission trajectory and temperature sensing. Adv. Funct. Mater. 2015, 25, 1463–1469. [Google Scholar] [CrossRef]
- Meng, X.; Song, S.-Y.; Song, X.-Z.; Zhu, M.; Zhao, S.-N.; Wu, L.-L.; Zhang, H.-J. , A Eu/Tb-codoped coordination polymer luminescent thermometer. Inorg Chem Front. 2014, 1, 757–760. [Google Scholar] [CrossRef]

| MOFs | Temperature range (K) | Sr (% K-1) | Max temperature (K) | Ref |
|---|---|---|---|---|
| Rh101@UiO-67 | 293~333 | 1.19 | 333 | [35] |
| Tb0.99Eu0.01(BDC)1.5(H2O)2 | 300~320 | 0.37 | 320 | [36] |
| Cdots&RB@ZIF-8 | 293~353 | 0.74 | 353 | [24] |
| Eu@Uio-(bpydc) | 293~353 | 0.31 | 353 | [25] |
| Dycpia | 298~473 | 0.42 | 473 | [26] |
| [Eu0.7Tb0.3(cam)(Himdc)2(H2O)2]3 | 100~450 | 0.079 | 450 | [37] |
| [Tb0.99Eu0.01(hfa)3(dpbp)]n | 200~300 | 0.52 | 300 | [38] |
| Tb0.9Eu0.1L | 40~300 | 0.11 | 300 | [39] |
| [(Tb0.9382Eu0.0616)(bpcd)2(NO3)2]Cl | 25~200 | 0.34 | 200 | [40] |
| Tb0.92Eu0.08-HPIDC-OX | 303~473 | 0.36 | 473 | [23] |
| C460@Tb-MOFs | 298~498 | 0.55 | 498 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





