Submitted:
02 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental Studies on Hydrocarbons and Pure Ethanol
2.1. Description of Experimental Work on Hydrocarbons
2.2. Description of Experimental Work on Ethanol
3. Assessment of Previous Correlations
4. Results and Discussion
4.1. Assessment of Existing Correlations
4.2. Comparison to Hydrocarbons Dataset
4.3. Comparison to Ethanol Dataset
4.4. Comparison to Propane (R290) Dataset
5. Conclusions
- A database was created based on 11 published papers from 10 independent laboratories for hydrocarbons (R290, R600, and R600a). This evaluation comprises 900 flow boiling heat transfer coefficient data points for hydrocarbons. Moreover, a dataset of 720 experimental data points was collected for the flow boiling heat transfer coefficients of ethanol.
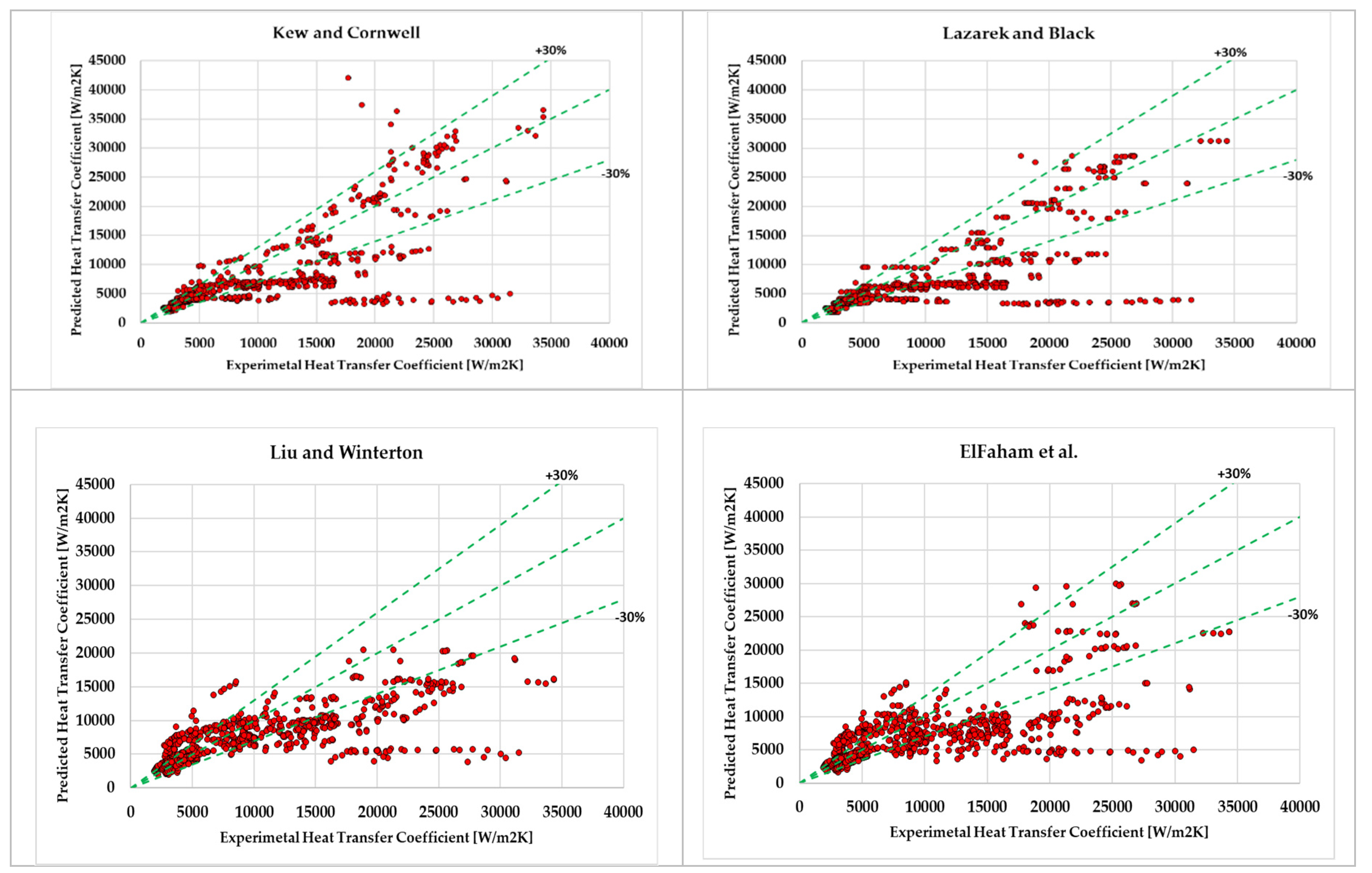
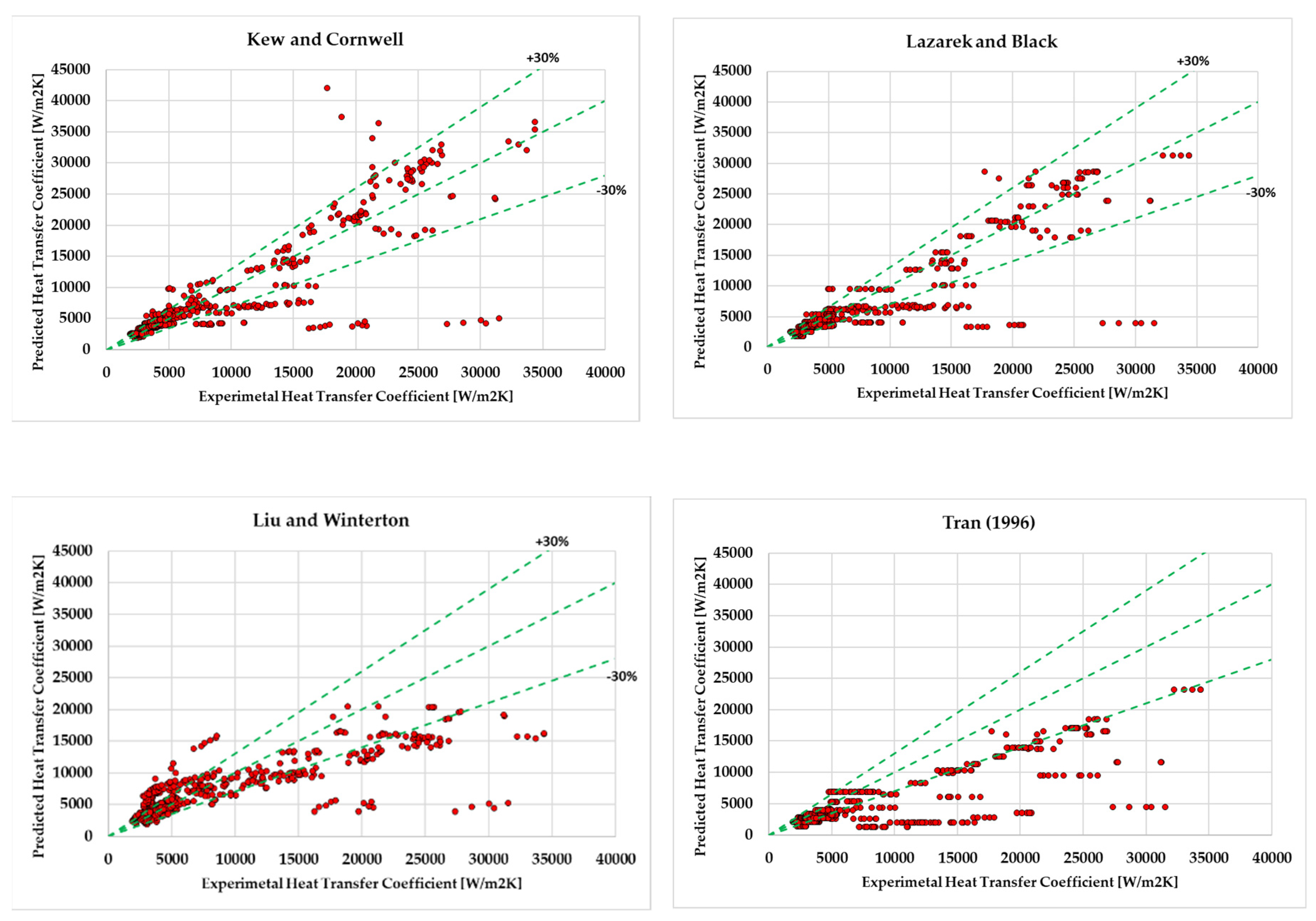
- It was found that for hydrocarbons Kew and Cornwell [39] (24.6%), Lazarek and Black [38] (25.7%) correlation has achieved the least mean absolute deviation which is less than 30%. However, Liu and Winterton [22] (33.1%), ElFaham and Tang [35] (36.7%), Tran (38.2%) had the tendency to show relatively low Mean Absolute deviation. On the other hand, Agostini et al. [46], Sun and Mishima [41] , Chaddock and Brunemann [58] , Bennet and Chen [33] were out of prediction and their results were unsatisfactory.
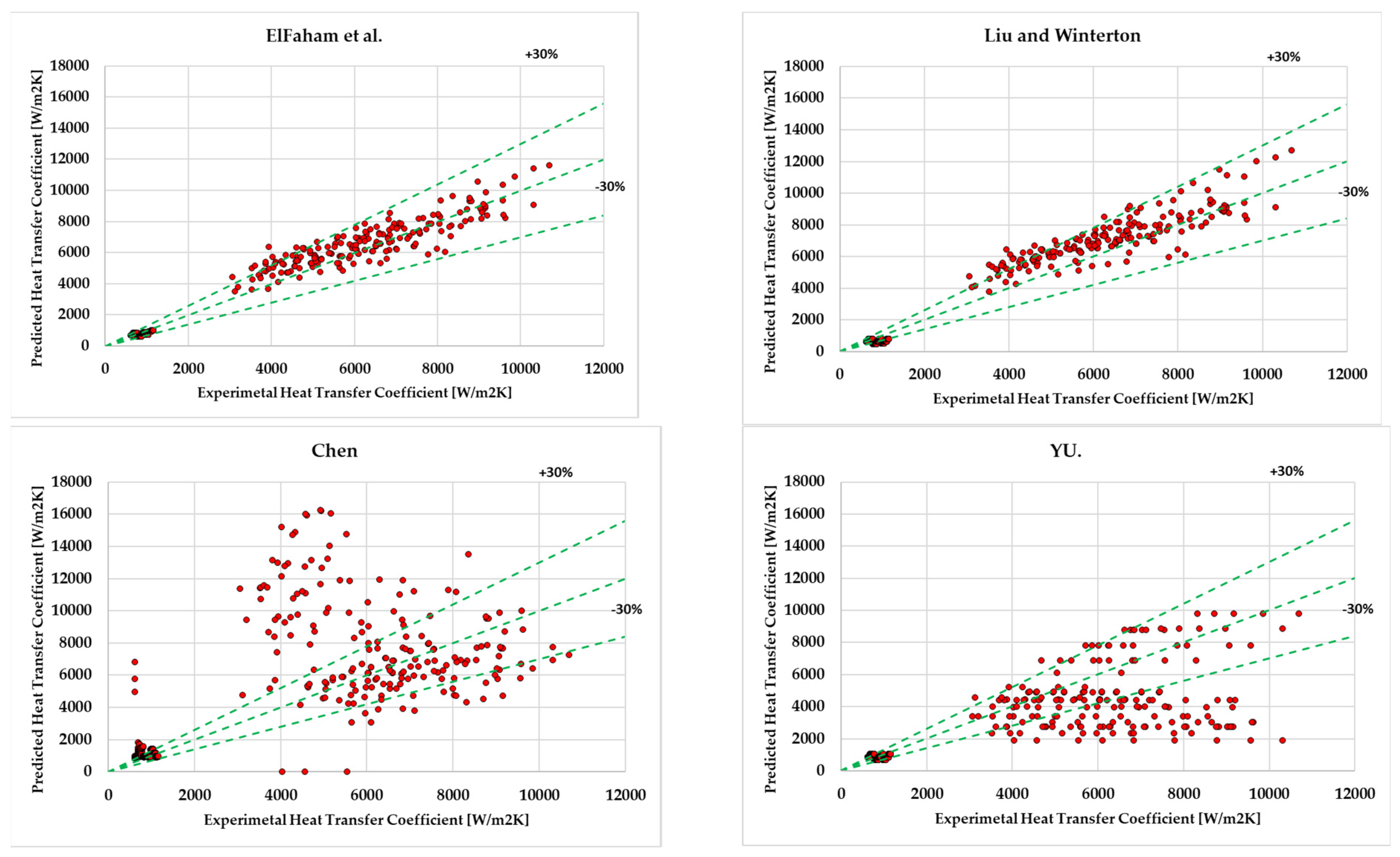
- It has been observed that among the assessed correlations for ethanol, ElFaham and Tang [35] achieved the lowest mean absolute deviation (15.3%). Nevertheless, Chen [30] (25%), Liu and Winterton [22] (25.1%), and YU [42] (25.7%) exhibited a range of mean absolute deviation less than 30%, which is considered to be in an outstanding position.
- There is an extreme shortage of experimental data on ethanol in the novel of literature. As a result, additional research should be conducted on ethanol, as it is a very promising fluid in many industrial applications. Furthermore, after analyzing the current data set of ethanol, it is recommended to utilize a heat flux range of 7 kW. m-2 to 20 kW. m-2 to fill the gap displayed in Figure 2.
- Due to the fact that each correlation developed using its own data, fluids, geometry, and operating conditions. As a result, no specific universal prediction method exists. This study used an assessment of the same correlations for different fluids to benchmark its findings, demonstrating that each fluid has a varied performance for prediction. Therefore, when comparing Table 6, Table 7 and Table 8, each correlation appears in a different place.
References
- Lorentzen, G. The use of natural refrigerants: a complete solution to the CFC/HCFC predicament. Int. J. Refrig. 1995, 18, 190–197. [Google Scholar] [CrossRef]
- Cavallini, A. Working fluids for mechanical refrigeration—Invited paper presented at the 19th International Congress of Refrig-eration, The Hague, August 1995. International Journal of Refrigeration 1996, 19, 485–496. [Google Scholar] [CrossRef]
- Dzigbor, A.; Chimphango, A. Evaluating the potential of using ethanol/water mixture as a refrigerant in adsorption cooling system by using activated carbon - sodium chloride composite adsorbent. Int. J. Refrig. 2019, 97, 132–142. [Google Scholar] [CrossRef]
- Thome, J.R.; Cheng, L.; Ribatski, G.; Vales, L.F. Flow boiling of ammonia and hydrocarbons: A state-of-the-art review. Int. J. Refrig. 2008, 31, 603–620. [Google Scholar] [CrossRef]
- Granryd, E. , Hydrocarbons as refrigerants—an overview. International journal of refrigeration 2001, 24, 15–24. [Google Scholar] [CrossRef]
- Thome, J.R. Boiling of new refrigerants: a state-of-the-art review. Int. J. Refrig. 1996, 19, 435–457. [Google Scholar] [CrossRef]
- Liu, C.; et al. , Literature review of condensation and evaporation of R290. 2016.
- Del Col, D.; Bortolato, M.; Bortolin, S. Comprehensive experimental investigation of two-phase heat transfer and pressure drop with propane in a minichannel. Int. J. Refrig. 2014, 47, 66–84. [Google Scholar] [CrossRef]
- Tibiriçá, C.B.; Ribatski, G. Flow patterns and bubble departure fundamental characteristics during flow boiling in microscale channels. Exp. Therm. Fluid Sci. 2014, 59, 152–165. [Google Scholar] [CrossRef]
- Maqbool, M.H. , et al. Saturated flow boiling heat transfer characteristics of propane in a smooth vertical minichannel up to dryout incipience. in 23rd IIR Congress of Refrigeration, Prague, Czech Republic, 21–26 August 2011. [Google Scholar]
- Wang, S.; Gong, M.; Chen, G.; Sun, Z.; Wu, J. Two-phase heat transfer and pressure drop of propane during saturated flow boiling inside a horizontal tube. Int. J. Refrig. 2013, 41, 200–209. [Google Scholar] [CrossRef]
- Kandlikar, S.G.; Steinke, M.E. Flow Boiling Heat Transfer Coefficient in Minichannels− Correlation and Trends. In Interna-tional Heat Transfer Conference Digital Library.; Begel House Inc., 2022. [Google Scholar]
- Jung, D.; Lee, H.; Bae, D.; Oho, S. Nucleate boiling heat transfer coefficients of flammable refrigerants. Int. J. Refrig. 2004, 27, 409–414. [Google Scholar] [CrossRef]
- Choi, K.-I.; et al. Pressure drop and heat transfer during two-phase flow vaporization of propane in horizontal smooth minichan-nels. International Journal of Refrigeration 2009, 32, 837–845. [Google Scholar] [CrossRef]
- Maqbool, M.H., B. Palm, and R. Khodabandeh, Investigation of two phase heat transfer and pressure drop of propane in a ver-tical circular minichannel. Experimental Thermal and Fluid Science 2013, 46, 120–130. [Google Scholar] [CrossRef]
- Lee, H.-S.; Yoon, J.-I.; Kim, J.-D.; Bansal, P. Characteristics of condensing and evaporating heat transfer using hydrocarbon refrigerants. Appl. Therm. Eng. 2006, 26, 1054–1062. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, M.S.; Ro, S.T. Experimental study on forced convective boiling heat transfer of pure refrigerants and refriger-ant mixtures in a horizontal tube. International Journal of Refrigeration 1997, 20, 267–275. [Google Scholar] [CrossRef]
- Thome, J.; Shakir, S. A new correlation for nucleate pool boiling of aqueous mixtures, in Heat Transfer: Pittsburgh 1987, 1987.
- Gungor, K.E.; Winterton, R.H.S. A general correlation for flow boiling in tubes and annuli. Int. J. Heat Mass Transf. 1986, 29, 351–358. [Google Scholar] [CrossRef]
- Wen, M.-Y.; Ho, C.-Y. Evaporation heat transfer and pressure drop characteristics of R-290 (propane), R-600 (butane), and a mixture of R-290/R-600 in the three-lines serpentine small-tube bank. Appl. Therm. Eng. 2005, 25, 2921–2936. [Google Scholar] [CrossRef]
- Chien, N.-B.; et al. , An experimental investigation of convective boiling heat transfer using alternative and natural refrigerants inside horizontal microchannels. 2016.
- Liu, Z.; Winterton, R.H.S. A general correlation for saturated and subcooled flow boiling in tubes and annuli, based on a nucleate pool boiling equation. Int. J. Heat Mass Transf. 1991, 34, 2759–2766. [Google Scholar] [CrossRef]
- Yunos, Y.M.; Ghazali, N.M.; Pamitran, A.; Novianto, S. Analysis of the Two-Phase Heat Transfer Coefficient of Propane in a Small Channel. Energy Procedia 2017, 105, 4635–4640. [Google Scholar] [CrossRef]
- Kanizawa, F.T.; Tibiriçá, C.B.; Ribatski, G. Heat transfer during convective boiling inside microchannels. Int. J. Heat Mass Transf. 2016, 93, 566–583. [Google Scholar] [CrossRef]
- Wen, M.-Y.; Jang, K.-J.; Ho, C.-Y. The characteristics of boiling heat transfer and pressure drop of R-600a in a circular tube with porous inserts. Appl. Therm. Eng. 2014, 64, 348–357. [Google Scholar] [CrossRef]
- Copetti, J.; Macagnan, M.; Zinani, F. Experimental study on R-600a boiling in 2.6 mm tube. Int. J. Refrig. 2013, 36, 325–334. [Google Scholar] [CrossRef]
- Mastrullo, R.; Mauro, A.; Revellin, R.; Viscito, L. Flow boiling heat transfer and pressure drop of pure ethanol (99.8%) in a horizontal stainless steel tube at low reduced pressures. Appl. Therm. Eng. 2018, 145, 251–263. [Google Scholar] [CrossRef]
- Steiner, D.; Taborek, J. Flow boiling heat transfer in vertical tubes correlated by an asymptotic model. Heat Transf. Eng. 1992, 13, 43–69. [Google Scholar] [CrossRef]
- Vasileiadou, P.; Sefiane, K.; Karayiannis, T.G.; Christy, J.R. Flow boiling of ethanol/water binary mixture in a square mini-channel. Appl. Therm. Eng. 2017, 127, 1617–1626. [Google Scholar] [CrossRef]
- Chen, J.C. Correlation for Boiling Heat Transfer to Saturated Fluids in Convective Flow. Ind. Eng. Chem. Process. Des. Dev. 1966, 5, 322–329. [Google Scholar] [CrossRef]
- Saitoh, S.; Daiguji, H.; Hihara, E. Correlation for boiling heat transfer of R-134a in horizontal tubes including effect of tube diameter. Int. J. Heat Mass Transf. 2007, 50, 5215–5225. [Google Scholar] [CrossRef]
- Jung, D.; McLinden, M.; Radermacher, R.; Didion, D. A study of flow boiling heat transfer with refrigerant mixtures. Int. J. Heat Mass Transf. 1989, 32, 1751–1764. [Google Scholar] [CrossRef]
- Bennett, D.L.; Chen, J.C. Forced convective boiling in vertical tubes for saturated pure components and binary mixtures. AIChE J. 1980, 26, 454–461. [Google Scholar] [CrossRef]
- Choi, K.-I.; Pamitran, A.; Oh, J.-T. Two-phase flow heat transfer of CO2 vaporization in smooth horizontal minichannels. Int. J. Refrig. 2007, 30, 767–777. [Google Scholar] [CrossRef]
- ElFaham, M. , A development of a general heat transfer correlation for boiling two-phase flow of pure ethanol based on experimental datasets and correlations. 2022.
- Yoon, S.H.; Cho, E.S.; Hwang, Y.W.; Kim, M.S.; Min, K.; Kim, Y. Characteristics of evaporative heat transfer and pressure drop of carbon dioxide and correlation development. Int. J. Refrig. 2003, 27, 111–119. [Google Scholar] [CrossRef]
- Wattelet, J.; et al. , Evaporative characteristics of R-134a, MP-39, and R-12 at low mass fluxes. ASHRAE transactions, 1993.
- Lazarek, G.M.; Black, S.H. Evaporative heat transfer, pressure drop and critical heat flux in a small vertical tube with R-113. Int. J. Heat Mass Transf. 1982, 25, 945–960. [Google Scholar] [CrossRef]
- Kew, P.A.; Cornwell, K. Correlations for the prediction of boiling heat transfer in small-diameter channels. Appl. Therm. Eng. 1997, 17, 705–715. [Google Scholar] [CrossRef]
- Hamdar, M.; Zoughaib, A.; Clodic, D. Flow boiling heat transfer and pressure drop of pure HFC-152a in a horizontal mini-channel. Int. J. Refrig. 2009, 33, 566–577. [Google Scholar] [CrossRef]
- Sun, L.; Mishima, K. An evaluation of prediction methods for saturated flow boiling heat transfer in mini-channels. Int. J. Heat Mass Transf. 2009, 52, 5323–5329. [Google Scholar] [CrossRef]
- Yu, W.; France, D.; Wambsganss, M.; Hull, J. Two-phase pressure drop, boiling heat transfer, and critical heat flux to water in a small-diameter horizontal tube. Int. J. Multiph. Flow 2002, 28, 927–941. [Google Scholar] [CrossRef]
- Warrier, G.R.; Dhir, V.K.; Momoda, L.A. Heat transfer and pressure drop in narrow rectangular channels. Exp. Therm. Fluid Sci. 2002, 26, 53–64. [Google Scholar] [CrossRef]
- Kenning, D.; Cooper, M. Saturated flow boiling of water in vertical tubes. Int. J. Heat Mass Transf. 1989, 32, 445–458. [Google Scholar] [CrossRef]
- Tran, T.N.; Wambsganss, M.W.; France, D.M. Small circular- and rectangular-channel boiling with two refrigerants. Int. J. Multiph. Flow 1996, 22, 485–498. [Google Scholar] [CrossRef]
- Agostini, B.; Bontemps, A. Vertical flow boiling of refrigerant R134a in small channels. Int. J. Heat Fluid Flow 2005, 26, 296–306. [Google Scholar] [CrossRef]
- Tran, T. , et al. A correlation for nucleate flow boiling in small channels. In Compact Heat Exchangers for the Process Industries.; Begel House Inc., 1997. [Google Scholar]
- Forster, H.K.; Zuber, N. Dynamics of vapor bubbles and boiling heat transfer. AIChE J. 1955, 1, 531–535. [Google Scholar] [CrossRef]
- Stephan, K.; Abdelsalam, M. Heat-transfer correlations for natural convection boiling. Int. J. Heat Mass Transf. 1980, 23, 73–87. [Google Scholar] [CrossRef]
- Chisholm, D. A theoretical basis for the Lockhart-Martinelli correlation for two-phase flow. Int. J. Heat Mass Transf. 1967, 10, 1767–1778. [Google Scholar] [CrossRef]
- ElFaham, M.; Tang, C.C. Review of Datasets and Correlations for Two-Phase Flow Boiling Heat Transfer of Pure Ethanol and Ethanol/Water Binary mixtures. in Heat Transfer Summer Conference. American Society of Mechanical Engineers., 2022. [Google Scholar]
- Wojtan, L., T. Ursenbacher, and J.R. Thome, Investigation of flow boiling in horizontal tubes: Part II—Development of a new heat transfer model for stratified-wavy, dryout and mist flow regimes. International journal of heat and mass transfer 2005, 48, 2970–2985. [Google Scholar] [CrossRef]
- Pujol, L. and A.H. Stenning, coefficients have been measured in a heat transfer loop consisting of two upward and two downward sections formed by bending a twenty. Cocurrent Gas-Liquid Flow, 1969, 401.
- Li, W.; Wu, Z. A general criterion for evaporative heat transfer in micro/mini-channels. Int. J. Heat Mass Transf. 2010, 53, 1967–1976. [Google Scholar] [CrossRef]
- Oh, H.-K.; Son, C.-H. Evaporation flow pattern and heat transfer of R-22 and R-134a in small diameter tubes. Heat Mass Transf. 2011, 47, 703–717. [Google Scholar] [CrossRef]
- Hu, H.; Ding, G.; Huang, X.-C.; Deng, B.; Gao, Y.-F. Experimental investigation and correlation of two-phase heat transfer of R410a/oil mixture flow boiling in a 5-mm microfin tube. J. Enhanc. Heat Transf. 2011, 18, 209–220. [Google Scholar] [CrossRef]
- Lavin, J.G.; Young, E.H. Heat transfer to evaporating refrigerants in two-phase flow. AIChE J. 1965, 11, 1124–1132. [Google Scholar] [CrossRef]
- Chaddock, J.B.; Brunemann, H. Forced convection boiling of refrigerants in horizontal tubes: phase 3. 1967: laboratories.
| Author/year | Fluids | Tube material/inside diameter (mm) | Saturation temperature/Vapor quality | Heat flux (kW/m2) |
Mass flux (kg/m2s) |
|---|---|---|---|---|---|
| Shin et al. (1997) [17] |
R22, R32, R134a, R290, R600a refrigerant mixtures |
Horizontal/Stainless steel tube din= 7.7 |
Tsat = 12 x = 0.05-0.7 |
q = 10-30 | G = 424-583 |
| Wang et al. (2014) [11] | R290 | Horizontal, copper tube din = 6 |
Tsat = -35 – -1.9 x = 0.14 - 0.75 |
q = 11.7-87.1 | G = 62 -104 |
| Wen et al. (2005) [20] | R290, R600, R290/R600 |
Horizontal/Copper tube din=2.46 |
Tsat = 6 x = 0-0.86 |
q = 5-21 | G = 250-500 |
| Chien et al. (2016) |
R290, R32, R410a | Horizontal/ Stainless steel tube(microchannel) din= 0.3mm, 1.5 |
Tsat = 10 x = 0.1 – dry out |
q = 10-20 | G = 200-500 |
| Yunos et al. (2017) [23] |
R290 | Horizontal/ single circular stainless-steel tube din= 7.6 |
Tsat = 6-20 x = 0.01-0.15 |
q = 5-22 | G = 200-650 |
| Kanizwa et al. (2016) [24] | R600a, R134a, R245fa | stainless steel tube din = 0.38-2.6 |
Tsat= 22 x = 0.01 - 0.69 |
q = 46-100 | G = 240-400 |
| Del Col et al. (2014) [8] | R290 | Horizontal, Copper mini-channel din =0.96 |
Tsat = 31 x =0.05-0.6 |
q = 10-315 | G = 100-600 |
| Wen et al. (2014) [25] | R600a | Horizontal, Circular pipe within dispersed-copper porous inserts. din =0.168-0.506 |
Tsat = 10 x = 0.076-0.87 |
q= 12-65 | G = 120-1100 |
| Maqbool et al. (2011) [10] | R290 | Vertical, stainless steel mini-channel din =1.7 |
Tsat = 23, 33, 43 x = 0-1 |
q = 5-280 | G = 100-500 |
| Copetti et al. (2013) [26] | R600a, R134a | Horizontal mini channel/ smooth stainless-steel tube din = 2.6 |
Tsat = 22 x = 0.076 - 0.87 |
q = 44-95 | G = 240-440 |
| Choi et al. (2009) [14] | R290 | Horizontal, smooth stainless steel mini channels din = 1.5, 3 |
Tsat =0,5,10 x = 0-1 |
q = 5-20 | G = 50-400 |
| Author/year | Fluids | Tube material/inside diameter (mm) | Saturation temperature/Vapor quality | Heat flux (kW/m2) |
Mass flux (kg/m2s) |
|---|---|---|---|---|---|
| Mastrullo et al. (2018) [27] | Ethanol | Horizontal stainless-steel tube din = 6.0 |
Tsat= 64.5-85.8 x = 0.11-0.91 |
q = 10-40.3 | G = 85-127 |
| Robertson et al. (1988) [28] | Ethanol | Vertical copper tube din = 10 |
Tsat = 88.6 x = 0.03-0.6 |
q = 25.5-1.4.6 | G = 145-290 |
| Vasileiadou et al. (2017) [29] | Ethanol, Deionized water, 5% v/v Ethanol/water | borosilicate glass square channel din = 5 |
Tsat= 40 | q = 2.8-6.1 | G = 0.3-1 |
| Author (Year) | Correlations |
|---|---|
| Chen (1966) [30] |
|
| Bennett and Chen (1980) [33] |
is calculated using Equation (1) is calculated using Equation (5) |
| ElFaham and Tang (2022) [35] |
is calculated using Equation (5) |
| Jung et al. (1989) [32] |
is calculated using Equation (5) |
| Saitoh et al. (2007) [31] |
is calculated using Equation (4) is calculated using Equation (5) |
| Choi et al. (2007) [34] |
is calculated using Equation (4) is calculated using Equation (5) |
| Liu-Winterton(1991) [21] |
is calculated using Equation (4) is calculated using Equation (5) |
| Yoon et al. (2004) [49] |
where is calculated using Equation (4) is calculated using Equation (5) |
| Wattelet et al. (1994) [46] |
is calculated using Equation (4) is calculated using Equation (5) |
| Author (Year) | Correlations |
|---|---|
| Lazarek and Black (1982) [36] | |
| Kew and Cornwell (1997) [37] | |
| Hamdar et al. (2010) [42] | |
| Sun and Mishima (2009) [38] | |
| Yu et al. (2002) [40] | |
| Warrier et al. (2002) [50] | |
| Kenning and Cooper (1989) [35] |
is calculated using Equation (5) |
| Tran et al. (1996) [39] | |
| Agostini et al. (2005) [41] | |
| Tran et al. (1997) [51] |
| Dimensionless Number | Equation |
|---|---|
| Reynolds number for liquid phase | |
| Boiling number | Bo = |
| Bond number | Bd = |
| Weber number for Liquid phase | |
| Froude number for liquid phase | |
| Lockharte-Martinelli parameter | |
| Convection number | |
| Confinement number |
| Correlations (Year) | MAE (%) | MRE (%) |
|---|---|---|
| Kew and Cornwel [39] (1997) | 24.6 | -12.89 |
| Lazarek and Black [38] (1982) | 25.73 | -18.72 |
| Liu and Winterton [22] (1991) | 33.02 | -3.33 |
| ElFaham and Tang [35] (2022) | 36.69 | -6.16 |
| Tran [45] (1996) | 38.16 | -36.22 |
| Yoon [36] (2004) | 40.64 | 14.02 |
| Wojtan et al. [52] (2005) | 42.54 | -41.39 |
| Hamdar [40] (2010) | 47.01 | -5.01 |
| Warrier [43] (2002) | 51.25 | -40.72 |
| Pujol and Stenning [53] (1969) | 56.12 | 10.5 |
| Li and Wu [54] (2010) | 57.64 | 13.22 |
| Saitoh [31] (2007) | 60.57 | 27.7 |
| YU [42] (2002) | 65.03 | -9.56 |
| Wattelet [37] (1994) | 67.98 | 54.04 |
| Kenning Copper [44] (1989) | 68.59 | 38.71 |
| Oh and Son [55] (2011) | 73.27 | 19.26 |
| Hu et al. [56] (2011) | 80.39 | 15 |
| Gungor and Winterton [19] (1986) | 83.51 | 75.43 |
| Chen [30] (1966) | 83.75 | 75.31 |
| Lavin and Young [57] (1965) | 83.8 | 49.16 |
| Jung [32] (1989) | 94.97 | 61.01 |
| Choi [34] (2007) | 104.56 | 73.16 |
| Bennett and Chen [33] (1980) | 104.6 | 74.28 |
| Chaddock and Brunemann [58] (1967) | 104.8 | 83.69 |
| Sun and Mishima [41] (2009) | 116.8 | 96.85 |
| Kew and Cornwell [39] (1997) | 186.97 | 177.68 |
| Correlations (Year) | MAE (%) | MRE (%) |
|---|---|---|
| ElFaham and Tang [35] (2022) | 15.29 | -5.83 |
| Chen [30] (1966) | 25.02 | 20 |
| Liu and Winterton [22] (1991) | 25.12 | -14.81 |
| YU [42] (2002) | 25.7 | -8.17 |
| Saitoh [31] (2007) | 26.78 | -10.38 |
| Yoon [36] (2004) | 27.37 | -20.03 |
| Wattelet [37] (1994) | 28.39 | -8.35 |
| Sun and Mishima [41] (2009) | 29.69 | 18.58 |
| Wojtan et al. [52] (2005) | 40.57 | -40.57 |
| Jung [32] (1989) | 47.25 | -14.42 |
| Gungor and Winterton [19] (1986) | 52.28 | 51.61 |
| Hu et al. [56] (2011) | 56.76 | 56.01 |
| Hamdar [40] (2010) | 58.56 | -58.57 |
| Bennett and Chen [] (1980) | 59.6 | 59.75 |
| Kenning Copper [44] (1989) | 64.66 | -51.01 |
| Oh and Son [55] (2011) | 66.01 | -52.44 |
| Pujol and Stenning [53] (1969) | 73.54 | -73.34 |
| Lavin and Young [57] (1965) | 76.43 | -74.91 |
| Chaddock and Brunemann [58] (1967) | 77.27 | -29.97 |
| Warrier [43] (2002) | 79.67 | -79.65 |
| Tran [45] (1996) | 83.36 | -83.35 |
| Kew and Cornwell [39] (1997) | 83.91 | -83.91 |
| Tran et al. [47] (1997) | 84.02 | -84.02 |
| Lazarek and black [38] (1982) | 85.17 | -85.17 |
| Choi [34] (2007) | 119.07 | 118.89 |
| Li and Wu [54] (2010) | 574.76 | 569.49 |
| Correlations (Year) | MAE (%) | MRE (%) |
|---|---|---|
| Kew and Cornwell [39] (1997) | 17.66 | -1.85 |
| Lazarek and black [38] (1982) | 18.28 | -9.13 |
| Liu and Winterton [22] (1991) | 31.17 | 5.8 |
| Tran [45] (1996) | 32.4 | -30.4 |
| ElFaham and Tang [35] (2022) | 34.96 | 5.48 |
| Wojtan et al. [52] (2005) | 37.78 | -35.99 |
| Yoon [36] (2004) | 40.94 | 28.97 |
| Hamdar [40] (2010) | 43.11 | 13.32 |
| Warrier [43] (2002) | 48.09 | -35.09 |
| Pujol and Stenning [53] (1969) | 58.25 | 26.35 |
| Li and Wu [54] (2010) | 63.01 | 35.31 |
| Saitoh [31] (2007) | 67.88 | 4.8 |
| Wattelet [37] (1994) | 75.55 | 63.57 |
| Kenning Copper [44] (1989) | 79.25 | 39.85 |
| YU [42] (2002) | 80.66 | 66.6 |
| Oh and Son [55] (2011) | 87.04 | 36.72 |
| Chen [30] (1966) | 96.18 | 76.92 |
| Gungor and Winterton [19] (1986) | 102.31 | 99.98 |
| Hu et al. [56] (2011) | 102.34 | 99.97 |
| Lavin and Young [57] (1965) | 113.88 | 94.54 |
| Jung [32] (1989) | 123.46 | 99.67 |
| Tran et al. [47] (1997) | 128.08 | 111.93 |
| Choi [34] (2007) | 130.29 | 123.6 |
| Bennett and Chen [33] (1980) | 138.48 | 127.86 |
| Chaddock and Brunemann (1967) | 220.54 | 218.07 |
| Sun and Mishima [41] (2009) | 252.15 | 250.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





