1. Introduction
Considerable interest has been focused on the coordination chemistry of metal-based complexes and metallodrugs by chemistry, biology, and pharmacology researchers. Metallodrugs are compounds that contain a drug molecule and a metal ion, and numerous metallodrugs possess potential pharmaceutical properties, such as antibacterial, anticancer, and antiviral properties. Metallodrugs are useful as chemotherapeutics and therapeutic agents for treating various human diseases, including cancer, diabetes, neurological disorders, and infections [1-8]. Fluoroquinolones (FQs) are a class of compounds with strong and broad-spectrum antibacterial properties. Due to their low cost, high potency, and lack of cross-resistance, FQs have been widely used as antibiotics for over 30 years [9-12]. The complexation of FQs with different non-transition and transition metal ions has been extensively investigated to synthesize new metal-based complexes containing FQs, aiming to improve the pharmaceutical and biological characteristics of FQ antibiotics.
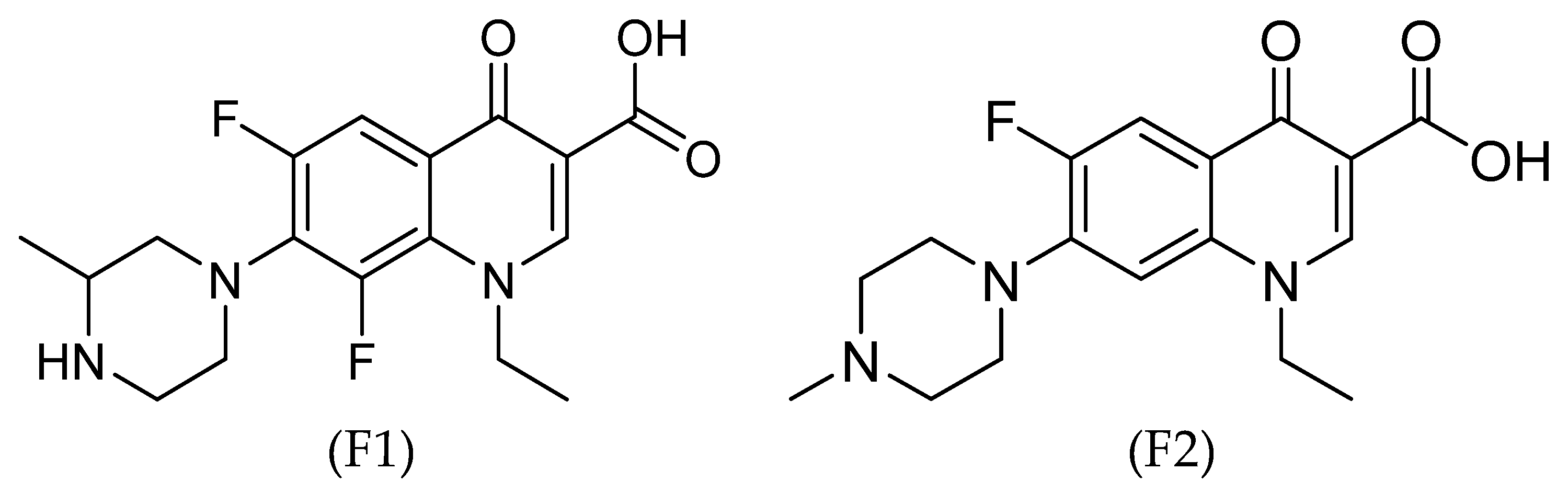
Two examples of the second-generation FQ antibiotic are lomefloxacin (F1) and pefloxacin (F2), which possess strong antibacterial, anti-inflammatory, and antifungal activities. Their structures are shown in
Figure 1. The antibacterial activity spectrum of F1 and F2 includes Gram-positive and -negative bacteria. F1 is commonly used to treat and prevent bacterial infections, urinary tract infections, and bronchitis, whereas F2 is used to treat Gram-negative-bacterial infections in the genitourinary tract and gastrointestinal system and treat gonococcal urethritis. The literature includes several examples of metal-based complexes of F1 and F2 containing several metal ions such as Zn(II), Cu(II), Ni(II), Co(II), Pt(II), Cr(III), Fe(III), Bi(IV), and UO
2(II) [13-16], some of these examples are listed in
Table 1. We believe metal-based complexes can be utilized to design and develop more biologically active drugs. Therefore, we aim to explore new insight into drugs F1 and F2 to form new metal-based complexes and examine the synthesized complexes’ pharmacological properties.
This study focused on several parameters:
(1) Synthesizing four metal-based complexes of F1 and F2 with Mg(II), Ca(II), Zn(II), and Fe(III) ions. The parameters of preparation were as follows: solvent - H2O:MeOH (1:1), reaction temperature - 60-70°C, media - neutral (pH 7-8), and molar ratio - 1:1 (ligand to ion).
(2) Employing several physicochemical approaches, including CHN elemental analyzer, ultraviolet/visible (UV-Visible), Fourier-transform infrared (FT-IR), and nuclear magnetic resonance (1H NMR) spectroscopies, to explore the complexation mode of F1 and F2 towards the metal ions under investigation.
(3) Observing the surface morphology, phase purity, shapes, and sizes of the synthesized complexes’ particles using scanning and transmission electron microscopy (SEM and TEM, respectively) and X-ray powder diffractometry (XRD).
(4) Screening the synthesized metal-based complexes in vitro for their antimicrobial properties using the Kirby-Bauer disc diffusion assay method. The investigated microbes were: Bacillus subtilis, Streptococcus pneumoniae, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, as well as three fungal microbes (Aspergillus niger, Penicillium sp., and Candida albicans).
2. Materials and Methods
2.1. Materials
The analytical grade metal ion slats were obtained from Sigma-Aldrich (St. Louis, MO, USA), whereas F1 and F2 were obtained from Merck KGaA (Darmstadt, Germany). The metal ions and the ligands under investigation were:
i) Mg(II): MgCl2 (95.21 g/mol; purity ≥ 98%)
ii) Ca(II): CaCl2 (110.98 g/mol; purity 99.99%)
iii) Zn(II): ZnCl2 (136.30 g/mol; purity ≥ 99.99 %)
iv) Fe(III): FeCl3·6H2O (270.30 g/mol; purity ≥ 98%)
v) F1: Lomefloxacin as HCl salt (C17H19F2N3O3⋅HCl; 387.81 g/mol; purity ≥ 98%)
vi) F2: Pefloxacin as mesylate salt (C17H20FN3O3⋅CH4O3S⋅2H2O; 465.49 g/mol; purity ≥ 98%)
2.2. Synthetic Methods
The Mg(II), Ca(II), Zn(II), and Fe(III) ions were complexed with F1 and F2 under the following conditions: Solvent: H2O:MeOH (1:1); Reaction temperature: 60–70°C; pH: neutral (7–8); Molar ratio: 1:1 (ligand to ion). The complexation process was as follows: First, four 100-mL beakers containing 20 mL of an aqueous solution of 2 mmol of MgCl2, CaCl2, ZnCl2, and FeCl3·6H2O were prepared. Next, 20 mL of F1 solution (2 mmol) dissolved in MeOH was gradually added to each beaker. The mixtures were stirred with a magnetic stirrer at 60-70°C. The pH of the mixtures was adjusted to ~7-8 using 5% ammonium solution, at which point colored precipitate began to form in each beaker. The four beakers were left on the hot plate for 10 minutes under continuous stirring and left overnight to ensure complete precipitation. The Mg(II), Zn(II), and Fe(III) ions formed yellow-white colored precipitates, whereas Ca(II) ions formed yellow-colored precipitates. The resulting colored precipitates were filtered, washed with MeOH and CH3CH2OCH2CH3, and subsequently dried in a vacuum desiccator over anhydrous CaCl2 for 48 hours. The Mg(II), Ca(II), Zn(II), and Fe(III) complexes of F2 were synthesized using the same procedure and were obtained as orange-yellow-colored powders for Mg(II) and Ca(II) ions, white-colored powder for Zn(II) ions, and brown-colored powder for Fe(III) ions. The eight synthesized metal-based complexes were subjected to elemental and spectral analyses, including UV-visible and FT-IR spectroscopy.
2.3. Characterization Methods
A Perkin-Elmer 2400 series CHN elemental analyzer, a Bruker DRX-250 Digital FT-NMR spectrometer, a Shimadzu FT-IR spectrophotometer, and a Perkin-Elmer Lambda 25 UV/Vis spectrophotometer were used to investigate the complexation mode of F1 and F2 with the metal ions. Elemental analysis data and spectra of 1H NMR (600 MHz; DMSO- d6), FT-IR (400–4000 cm-1), and UV/Vis (200–1000 nm) were analyzed. Surface morphology, phase purity, and the size and shape of the synthesized complexes’ particles were observed using a Quanta FEI 250 SEM, a JEOL JEM-1200 EX II TEM, and an X’Pert Philips X-ray diffractometer. SEM and TEM micrographs and the XRD patterns (2θ 5–90°) were used to analyze the synthesized complexes.
2.4. Antimicrobial Assays
The well-known Kirby-Bauer disc diffusion protocol [17-19] was used to evaluate the
in vitro antimicrobial activities (antibacterial and antifungal) of the synthesized metal-based complexes. The complexes were prepared at a 100 µg/mL concentration and screened against seven microbes isolated from clinical samples, including five bacterial species and three fungal species. The tested microbes were:
| Microbe |
Abbreviation |
| (A) Gram-positive bacterial strains |
| Bacillus subtilis |
B. subtilis |
| Streptococcus pneumoniae |
S. pneumoniae |
| Staphylococcus aureus |
S. aureus |
| (B) Gram-negative bacterial strains |
| Escherichia coli |
E. coli |
| Pseudomonas aeruginosa |
P. aeruginosa |
| (C) Fungal strains |
| Aspergillus niger |
A. niger |
| Penicillium sp. |
- |
| Candida albicans |
C. albicans |
The measured antibacterial susceptibility of the complexes was compared with that of standard antibiotic drugs (Streptomycin for antibacterial assays and ketoconazole for antifungal assays).
3. Results and Discussion
3.1. Compositions and UV-Visible Spectra
Four metal-based complexes of F1 and four metal-based complexes of F2, with the metal ions Mg(II), Ca(II), Zn(II), and Fe(III), were synthesized directly from the reaction of F1 and F2 with the metal ions. The chloride salts of the metal ions were dissolved in H
2O, and F1 and F2 were dissolved in MeOH solvent. The reactions were carried out under specific conditions: i) the solvent was H
2O:MeOH (1:1), ii) the reaction temperature was 60–70°C, iii) the media was neutral (pH 7–8), and iv) the molar reaction was 1:1 (ligand to ion). The complexes of F1 with Mg(II), Zn(II), and Fe(III) ions were yellow-white, and the complex with Ca(II) ions was completely yellow. The complexes of F2 with Mg(II) and Ca(II) ions were orange-yellow, the complex with Zn(II) ions was white, and the complex with Fe(III) ions was brown.
Table 1 provides the microanalytical results of the synthesized metal-based complexes, including the contents in (%) of C, H, N, and Cl, which were determined by a CHN elemental analyzer, as well as the contents in (%) of H
2O and metal, which were determined gravimetrically.
The data in
Table 1 suggest that the general compositions of the F1 metal-based complexes obtained with Mg(II), Ca(II), Zn(II), and Fe(III) ions were [MgF1(H
2O)Cl]⋅2H
2O, [CaF1(H
2O)Cl]⋅3H
2O, [ZnF1(H
2O)Cl], [FeF1(H
2O)
2Cl
2]⋅Cl⋅2H
2O, respectively. The corresponding gross formulas were C
17H
24F2N
3O
6MgCl (464.12), C
17H
26F2N
3O
7CaCl (497.89), C
17H
20F2N
3O
4ZnCl (469.19), and C
17H
27F2N
3O
7FeCl
3 (585.56 g/mol), respectively. The microanalytical results also suggest that the obtained complexes of F2 are formulated as [MgF2(H
2O)Cl]⋅2H
2O, [CaF2(H
2O)Cl]⋅3H
2O, [ZnF2(H
2O)Cl], [FeF2(H
2O)
2Cl
2]⋅Cl⋅2H
2O, corresponding to gross formulas of C
17H
25FN
3O
6MgCl (446.12), C
17H
28FN
3O
7CaCl (479.89), C
17H
22FN
3O
4ZnCl (451.19), and C
17H
29FN
3O
7FeCl
3 (567.56 g/mol), respectively.
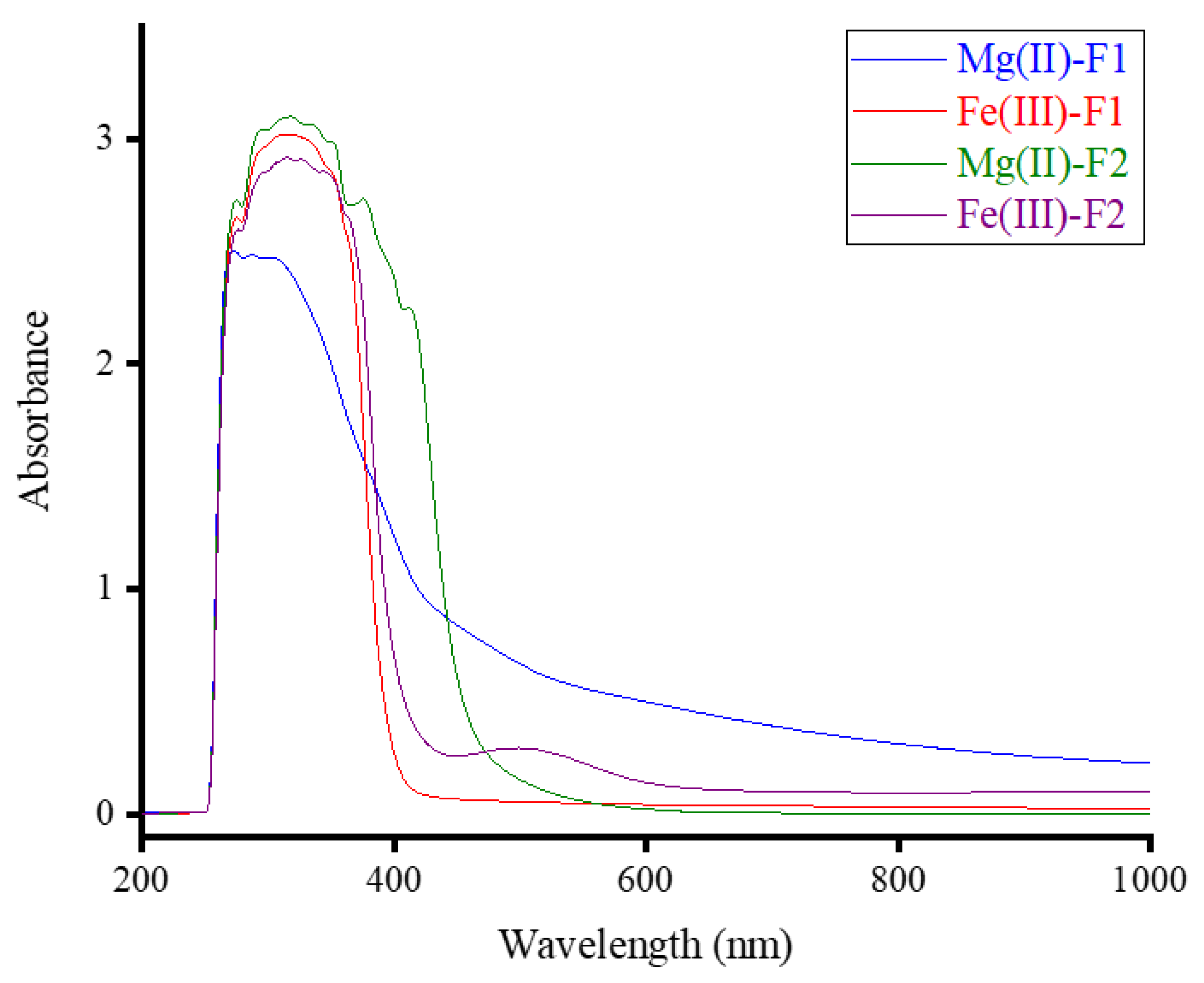
The complexes of F1 and F2 with Mg(II) and Fe(III) ions were dissolved in a dimethylsulfoxide (DMSO) solution, and their UV-visible spectra were recorded in the 200–1000 nm wavelength range, as shown in
Figure 2. The spectra of the four complexes displayed a strong, broad absorption band in the 260-500 nm range for Mg(II)-F1, 260-400 nm for Fe(III)-F1, 260–450 nm for Mg(II)-F2, and 260-600 nm for Fe(III)-F2 complex. These absorption bands, with a maximum wavelength (λ
max) at approximately ~320 nm, may be due to intra-ligand transitions (n→π*; π→π*). The UV-visible spectrum of the Fe(III)-F2 complex also exhibited a weak absorption band at 500 nm, which may be due to the Metal-to-ligand charge transfer band (MLCT) [20, 21]. The widening of the absorption bands decreased in a specific order: Mg(II)-F2 > Mg(II)-F1 > Fe(III)-F2 > Fe(III)-F1.
Table 1.
Microanalytical data of the F1 and F2 metal-based complexes with Mg(II), Ca(II), Zn(II), and Fe(III) ions.
Table 1.
Microanalytical data of the F1 and F2 metal-based complexes with Mg(II), Ca(II), Zn(II), and Fe(III) ions.
| Complex |
Elemental results (%)
Found (Calculated) |
| C |
H |
N |
Cl |
H2O |
Metal |
| Mg(II)-F1 |
44.06
(43.95) |
5.35
(5.17) |
8.93
(9.05) |
7.50
(7.64) |
11.45
(11.63) |
5.46
(5.24) |
| Ca(II)-F1 |
40.73
(40.97) |
5.10
(5.22) |
8.59
(8.44) |
7.30
(7.12) |
14.62
(14.46) |
7.94
(8.05) |
| Zn(II)-F1 |
43.60
(43.48) |
4.18
(4.26) |
9.10
(8.95) |
7.43
(7.56) |
4.00
(3.84) |
13.70
(13.93) |
| Fe(III)-F1 |
34.96
(34.84) |
4.31
(4.61) |
7.30
(7.17) |
17.94
(18.16) |
12.09
(12.30) |
9.43
(9.54) |
| Mg(II)-F2 |
45.60
(45.73) |
5.76
(5.60) |
9.57
(9.41) |
7.80
(7.95) |
12.32
(12.10) |
5.70
(5.45) |
| Ca(II)-F2 |
42.39
(42.51) |
5.99
(5.83) |
8.86
(8.75) |
7.56
(7.39) |
14.77
(15.00) |
8.26
(8.35) |
| Zn(II)-F2 |
45.35
(45.21) |
4.79
(4.88) |
9.45
(9.31) |
7.70
(7.86) |
3.92
(3.99) |
14.62
(14.49) |
| Fe(III)-F2 |
35.70
(35.94) |
5.00
(5.11) |
7.22
(7.40) |
18.95
(18.74) |
12.85
(12.69) |
9.97
(9.84) |
3.2. FT-IR Spectra
3.2.1. Complexes of F1
Figure 3 contains the FT-IR spectra for free F1 and its metal-based complexes. The IR spectrum of free F1 molecule was characterized by:
i) A strong, broad absorption band with a single head appeared at 3438 cm−1. This broadband could be assigned to the ν(O−H) vibrations of −the COOH group in the molecule and to moisture water.
ii) A medium-intensity absorption band resonated at 2938 cm
−1, and this band could be referred to the ν(N−H)
piperazine vibrations [
22].
iii) A strong board absorption band in the region from 2900 to 2600 cm−1 with multi-heads. These heads had different intensities and were located at 2892, 2841, 2760, 2703, and 2660 cm−1. All these heads originated from νasym(C−H) and νsym(C−H) vibrations of CH, CH3, and CH2 moieties.
iv) A group of three very strong and sharp absorption bands resonated at 1724, 1620, and 1492 cm
−1. The band of 1492 cm
−1 had a shoulder band with medium intensity located at 1529 cm
−1. The bands at 1719 and 1625 cm
−1 were generated from the ν(C=O)
COOH and ν(C=O)
pyridone vibrations, respectively. The band at 1492 cm
−1 was due to the δ
def(CH
2) vibrations, whereas its shoulder at 1529 cm
−1 could be referred to as the ν(C=C) vibrations [
23].
v) A group of medium-intensity bands resonated at 1333, 1257, 1212, 1096, 1042, and 807 cm
−1 were generated from the vibrations of δ
sciss(CH
2), ν
asym(C−N), ν(C−O), ν(C−F), ν
sym(C‒N), and δ
wag(CH
3), respectively [
24].
In the FT-IR spectra of F1 complexes with Mg(II), Ca(II), and Zn(II) ions, the absorption band due to the ν(C=O)
COOH, which appeared at 1724 cm
−1 in free F1 molecule, was absent, which suggests the formation of ionic bond between Mg(II), Ca(II), and Zn(II) metal ions with the oxygen atom of the carboxylate group of F1 molecule. The band originated from the ν(C=O)
pyridone vibrations, which appeared at 1625 cm
−1 in the FT-IR spectrum of free F1 molecule, was shifted to lower frequencies in the FT-IR spectra of Mg(II) (1610 cm
−1), Ca(II) (1609 cm
−1), and Zn(II) (1610 cm
−1) complex. This proposed that the C=O group of the pyridone ring in the F1 molecule participates in the chelation process with these metal ions [25, 26]. In the FT-IR spectrum of the F1 complex with Fe(III) ions, the bands due to the ν(C=O)
COOH and ν(C=O)
pyridone were slightly shifted to 1720 and 1623 cm
−1, respectively, suggest that carboxylic group (−COOH) and C=O group of pyridone ring were not participating in the coordination process, and these groups were far away from the place of coordination in F1 molecule with Fe(III) ions. The bands resulted from the ν(N−H)
piperazine, ν
asym(C−N), and ν
sym(C‒N) vibrations were shifted from 2938, 1257, 1096 cm
−1 in free F1 molecule to 2900, 1240, and 1052 cm
−1 in the Fe(III) complex. New medium-intensity absorption bands appeared in the FT-IR spectra of Mg(II), Ca(II), Zn(II), and Fe(III) complexes at (498 and 458 cm
−1), (491 and 450 cm
−1), (500 and 460 cm
−1), and (503 and 485 cm
−1), respectively. These two bands could be assigned to the ν(M−N) and ν(M−O) vibrations, respectively [
24]. All these IR spectral observations suggested that the F1 molecule was coordinated to the Mg(II), Ca(II), and Zn(II) ions via the oxygen atoms of the carboxylate group and the pyridone C=O group, whereas the F1 molecule was coordinated to the Fe(III) ions via the two nitrogen atoms of the piperazine ring.
3.2.2. Complexes of F2
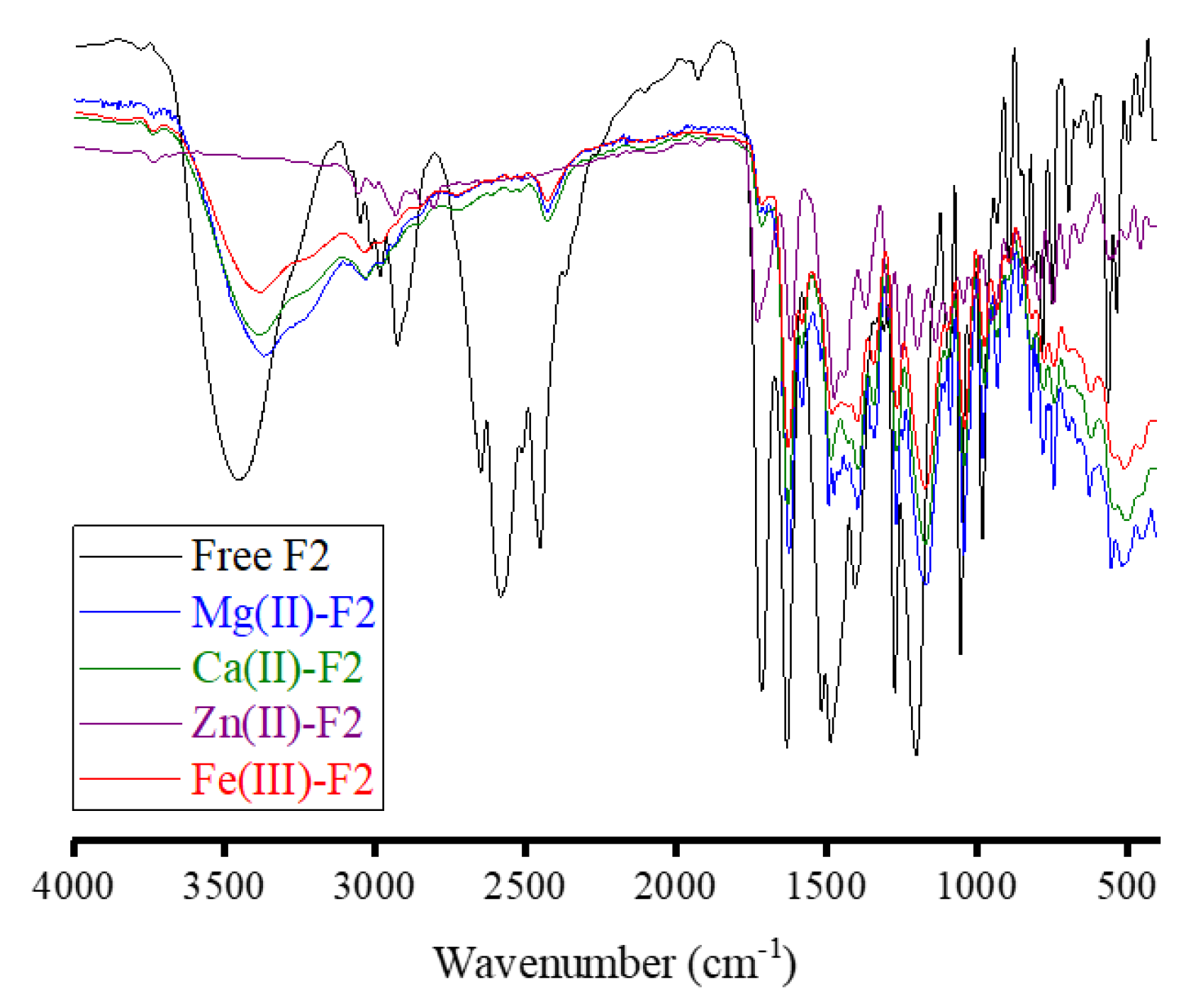
Figure 4 presents the FT-IR spectra for free F2 and its metal-based complexes. The IR spectrum of free F2 molecule was characterized by:
i) A strong, broad absorption band with a single head appeared at 3450 cm−1, originating from the vibrations of the O−H bond of −the COOH group in the molecule and moisture water.
ii) A medium-intensity board absorption band with three heads appeared at 3055, 2985, and 2930 cm−1. These heads were due to the νasym(C−H) and νsym(C−H) vibrations of CH, CH3, and CH2 moieties.
iii) A strong, board absorption band in the region from 2700 to 2400 cm−1 with three heads. These heads had different intensities and resonated at 2647, 2578, and 2454 cm−1. This broad band could be assigned to the ν(NH+)−CH3 vibrations.
iv) A group of three strong and sharp absorption bands located at 1715, 1635, and 1486 cm
−1. The band of 1486 cm
−1 had a shoulder band with medium intensity located at 1518 cm
−1. The absorptions at 1715, 1635, 1518, and 1486 cm
−1 were due to the ν(C=O)
COOH, ν(C=O)
pyridone, ν(C=C), and δ
def(CH
2) vibrations, respectively [
23].
v) Absorptions at 1405, 1273, 1202, 1093, and 1053 cm
−1, with different intensities and could be referred to as the vibrations of δ
sciss(CH
2), ν
asym(C−N), ν(C−O), ν(C−F), and ν
sym(C‒N), respectively [
24].
The absorption band originated from the ν(C=O)COOH vibration, which appeared at 1715 cm−1 in the FT-IR spectrum of free F2 molecule, was no longer observed in the FT-IR spectra of Mg(II), Ca(II), and Zn(II) complexes. The band due to the ν(C=O)pyridone was shifted from 1635 cm−1 in free F2 to around 1620 cm−1 in its complexes with Mg(II), Ca(II), and Zn(II) ions. This proposed that the F2 molecule captured these metal ions through the oxygen atom of the carboxylate group (ionic bond) and the oxygen atom of the pyridone C=O group (coordination bond) [25, 26]. As observed in the Fe(III)-F1 complex, in the Fe(III)-F2 complex, the absorption bands originated from the ν(C=O)COOH and ν(C=O)pyridone vibrations were slightly shifted. The bands resulted from the νasym(C−N), and νsym(C‒N) vibrations were shifted from 1273 and 1053 cm−1 in free F2 molecule to 1252 and 1030 cm−1 in the Fe(III) complex, respectively. The ν(M−N) and ν(M−O) vibrations were responsible for the new absorption bands that appeared at (508, 455 cm−1), (500, 470 cm−1), (495, 447 cm−1), and (505, 455 cm−1) in the spectra of Mg(II), Ca(II), Zn(II), and Fe(III) complexes, respectively. All these IR spectral observations suggested that the F2 molecule captured the Mg(II), Ca(II), and Zn(II) ions via the oxygen atoms of the carboxylate group and the pyridone C=O group, whereas the F2 molecule captured the Fe(III) ions via the two nitrogen atoms of the piperazine ring.
3.3. H NMR Spectral Analysis
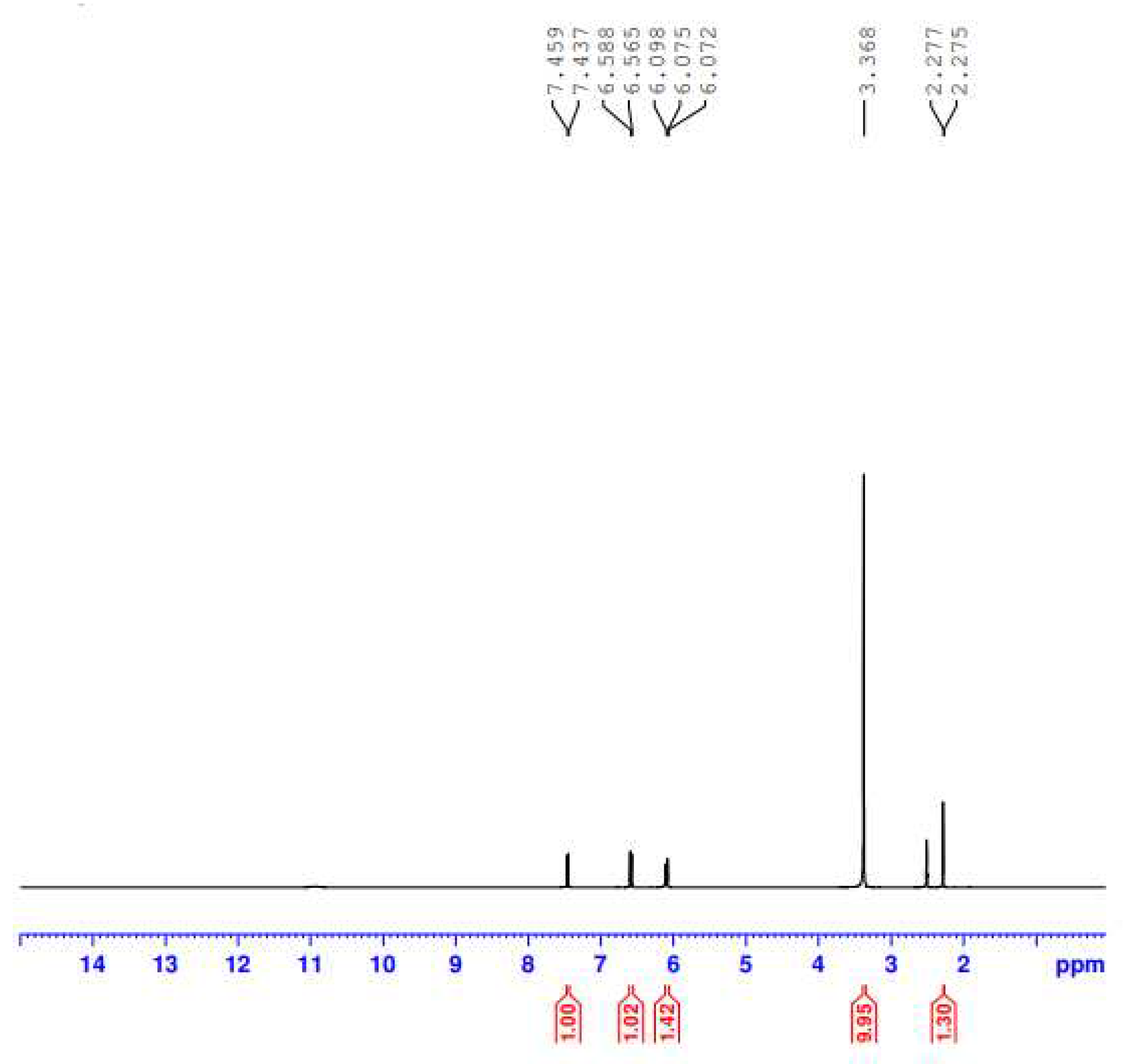
Figure 5 contains the
1H NMR spectrum of the F1-Fe(III) complex collected in DMSO-
d6 solvent at room temperature. The
1H NMR chemical shifts obtained from the complex’s spectrum are listed in
Table 2 and compared with those of the free F1 molecule [
27]. In the complex spectrum, the signals located at 2.275 and 2.277 ppm could be attributed to protons of −CH
3 moieties, and the protons of −CH
2 moieties could be responsible for the signals appearing at 6.565 and 6.588 ppm. The aromatic protons and the pyridine ring’s protons resonated in the 7.437–7.459 ppm range. The characteristic signal due to the (–COOH) proton, which is observed at δ 11.783 ppm in the spectrum of the free F1, is no longer observed in the spectrum of the complex. This suggested the deprotonation of the −COOH group and the participation in complexation with Fe(III) ion. The signal appeared at 3.368 ppm may be due to the protons of the coordinated water molecules.
Table 2.
1H NMR data (ppm) of free F1 molecule and its complex with Fe(III) ion.
Table 2.
1H NMR data (ppm) of free F1 molecule and its complex with Fe(III) ion.
| Free F1 |
F1-Fe(III) |
Assignments |
| 1.323-1.762 |
2.275, 2.277 |
δ 3H, -CH3; -CH2CH3
δ 3H, -CH3; attached to piperazine ring |
| 3.546-4.206 |
6.072, 6.075, 6.98 |
δ 7H, piperazine ring protons |
| 4.632-4.685 |
6.565, 6.588 |
δ 2H, -CH2; -CH2CH3
|
| 7.796, 8.902 |
7.437, 7.459 |
δ H, -CH; benzene ring and pyridine ring |
| 10.198-10.315 |
- |
δ H, -NH2+; piperazine ring |
| 11.783 |
- |
δ H, -COOH |
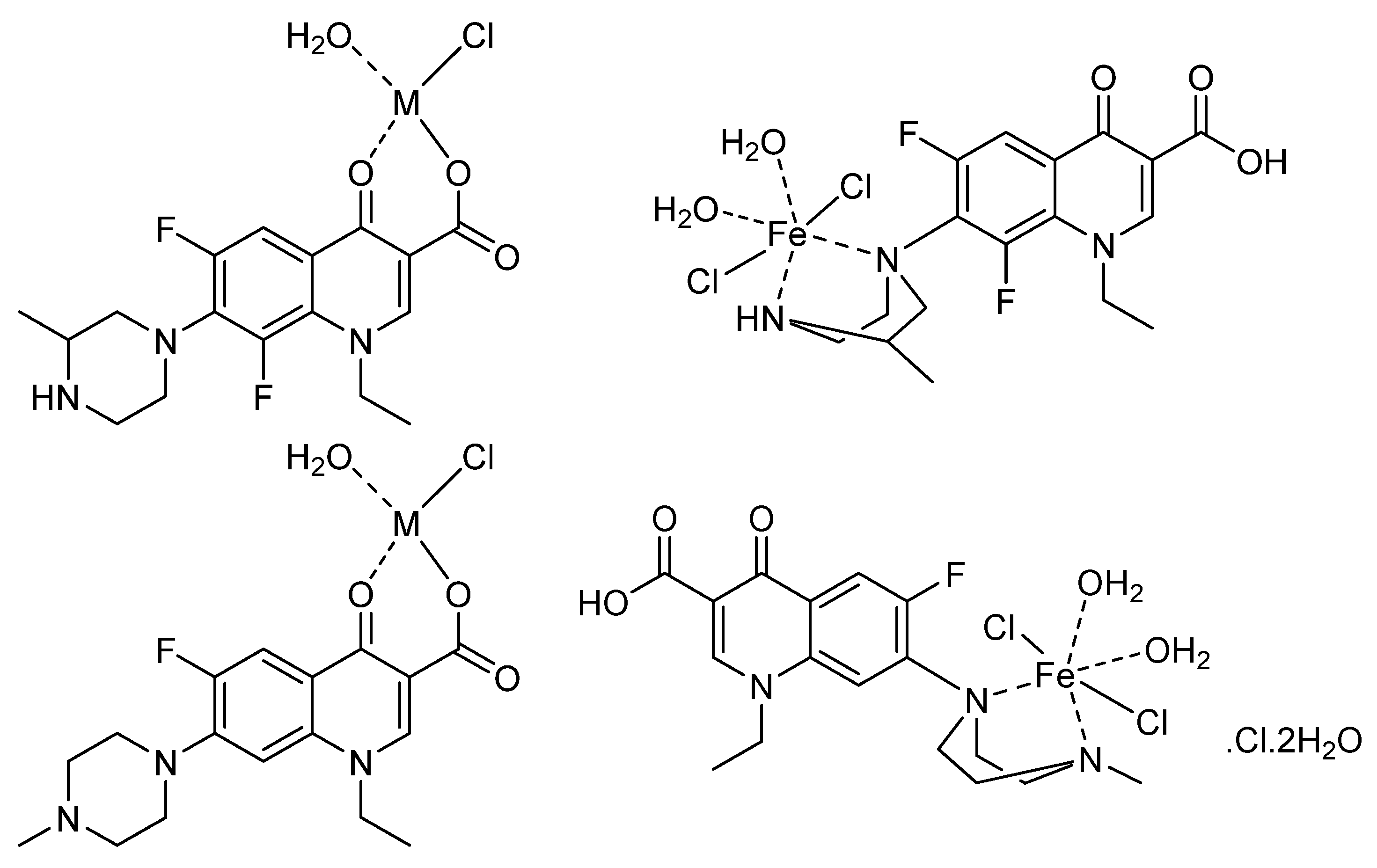
3.4. Proposed Structures
Structures of F1 and F2 metal-based complexes with Mg(II), Ca(II), Zn(II), and Fe(III) ions were proposed based on the analytical (C, H, N, Cl, Metal analysis) and spectral (UV−Visible, IR,
1H NMR) results and described in
Figure 6. In these structures, F1 and F2 coordinate the Mg(II), Ca(II), and Zn(II) ions in a bidentate manner using the oxygen atoms of the carboxylate group and the pyridone C=O group, whereas F1 and F2 bind to the Fe(III) ion using the two nitrogen atoms of the piperazine ring. The coordination spheres around the metal ions are complemented by water molecules. The capturing of the metal ions under investigation by F1 and F2 resulting in complexes with the proposed molecular formulas [MgF1(H
2O)Cl]⋅2H
2O, [CaF1(H
2O)Cl]⋅3H
2O, [ZnF1(H
2O)Cl], [FeF1(H
2O)
2Cl
2]⋅Cl⋅2H
2O, [MgF2(H
2O)Cl]⋅2H
2O, [CaF2(H
2O)Cl]⋅3H
2O, [ZnF2(H
2O)Cl], and [FeF2(H
2O)
2Cl
2]⋅Cl⋅2H
2O.
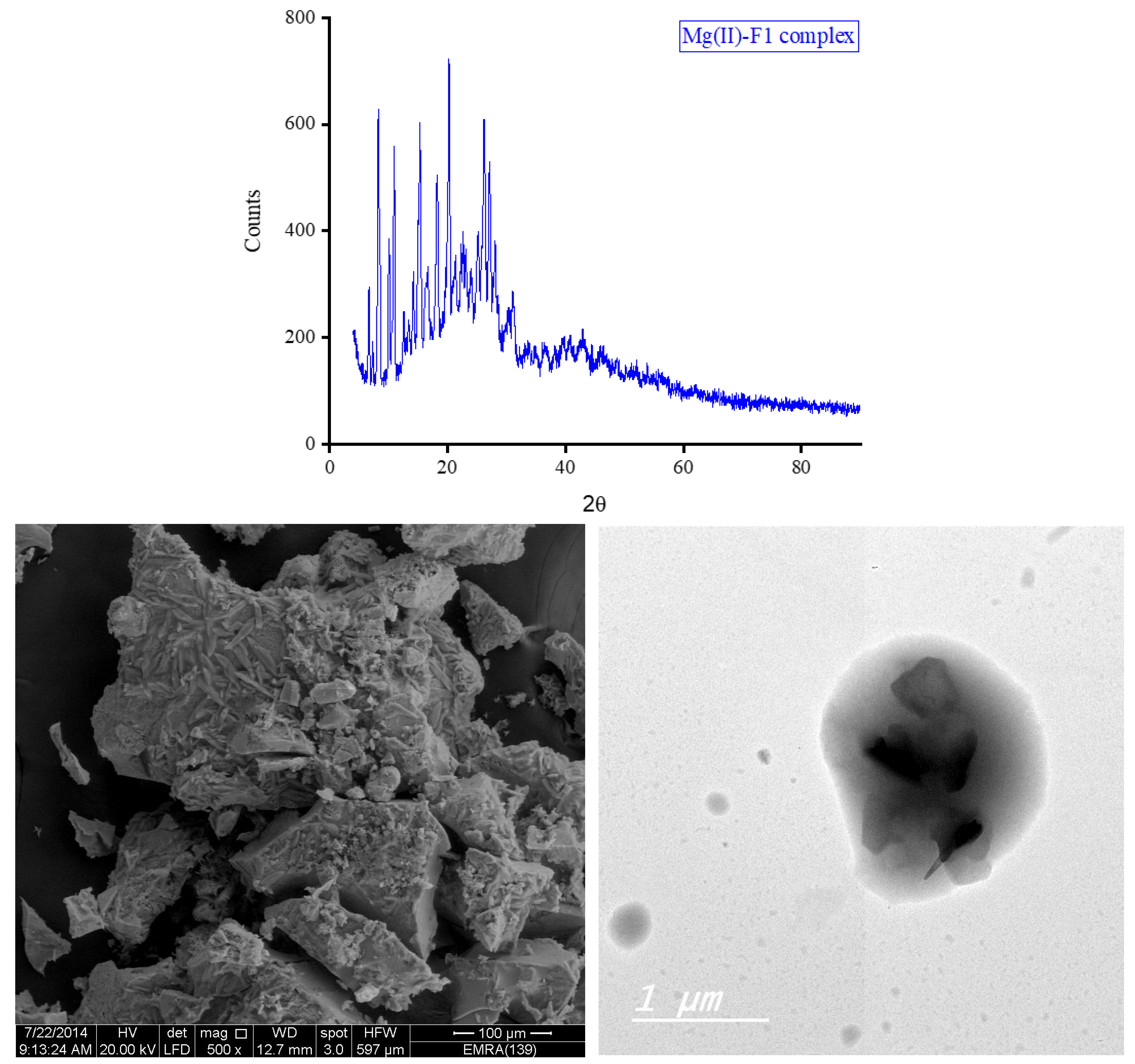
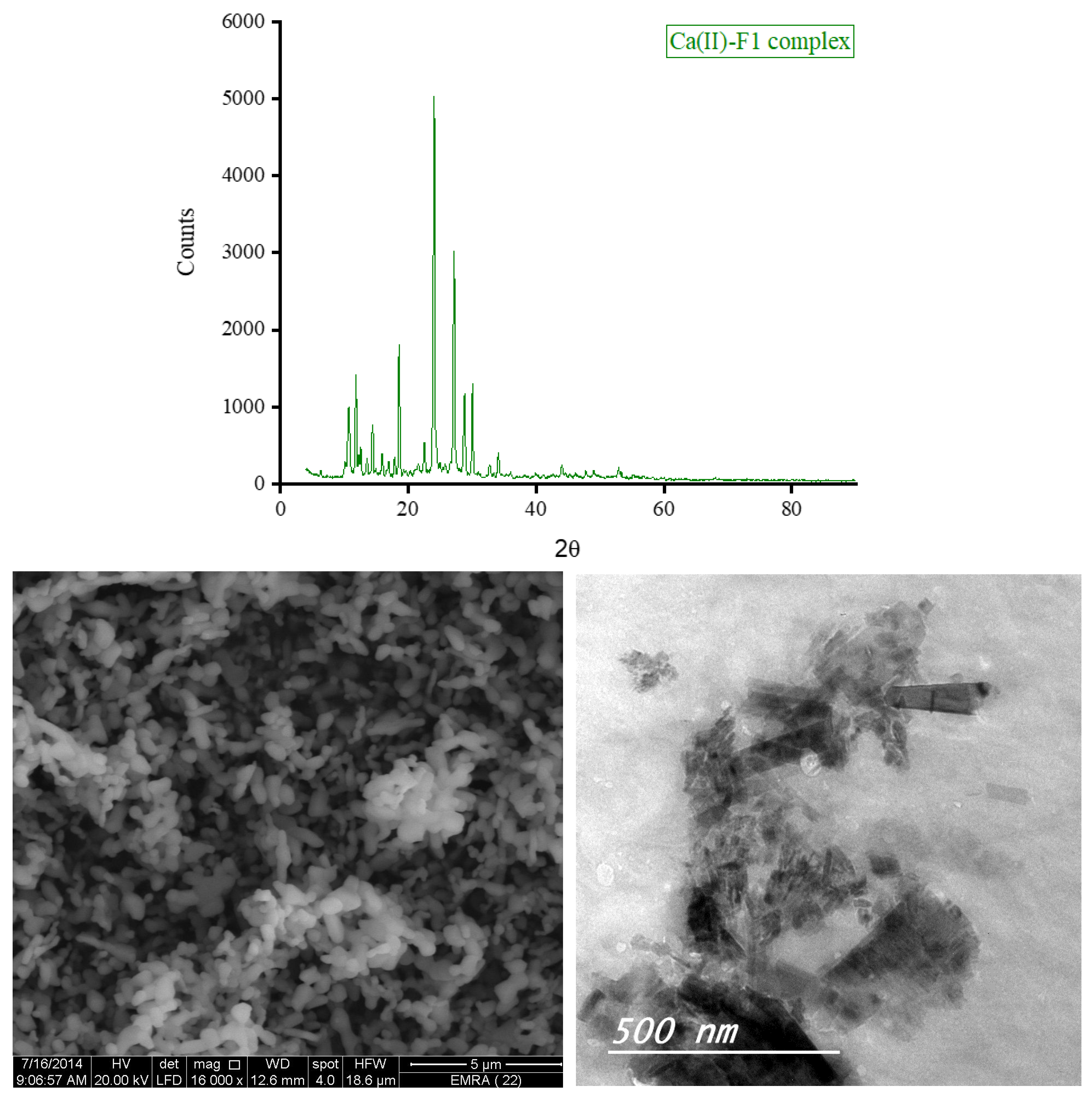
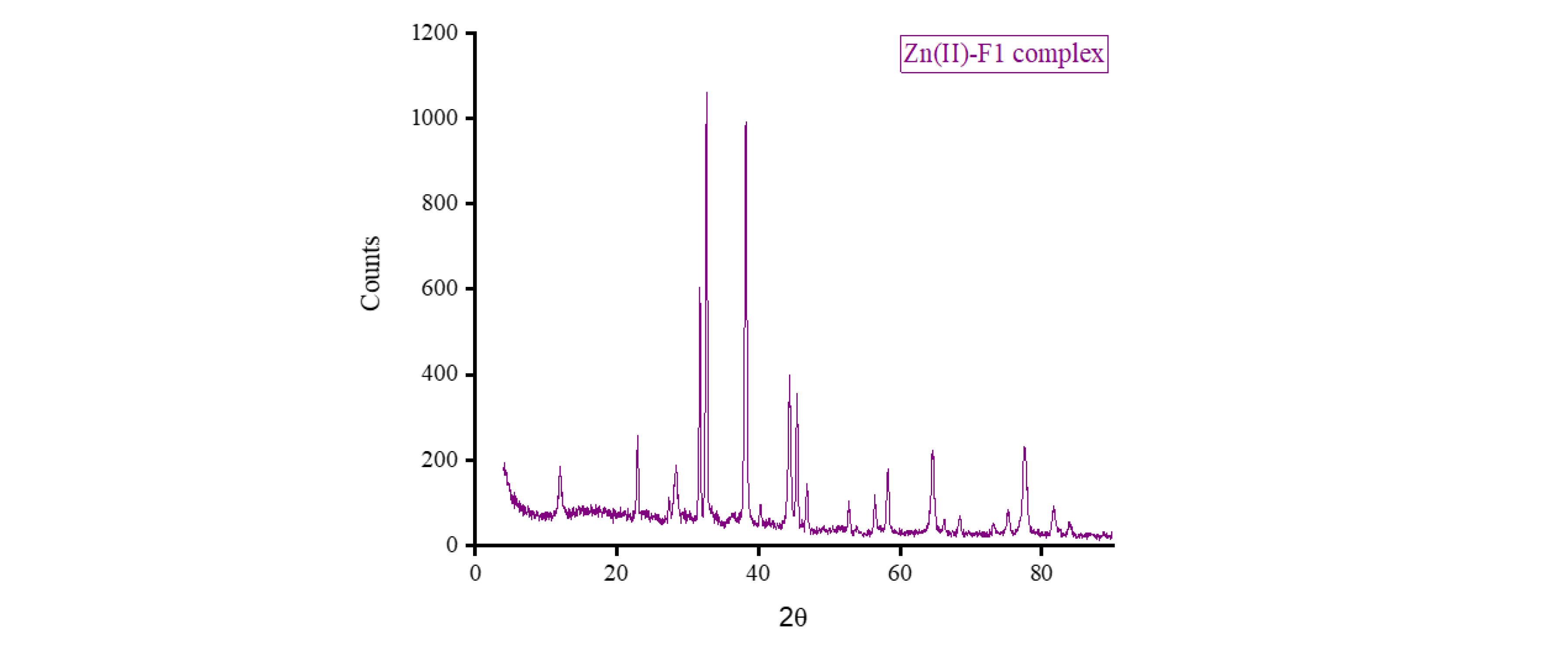
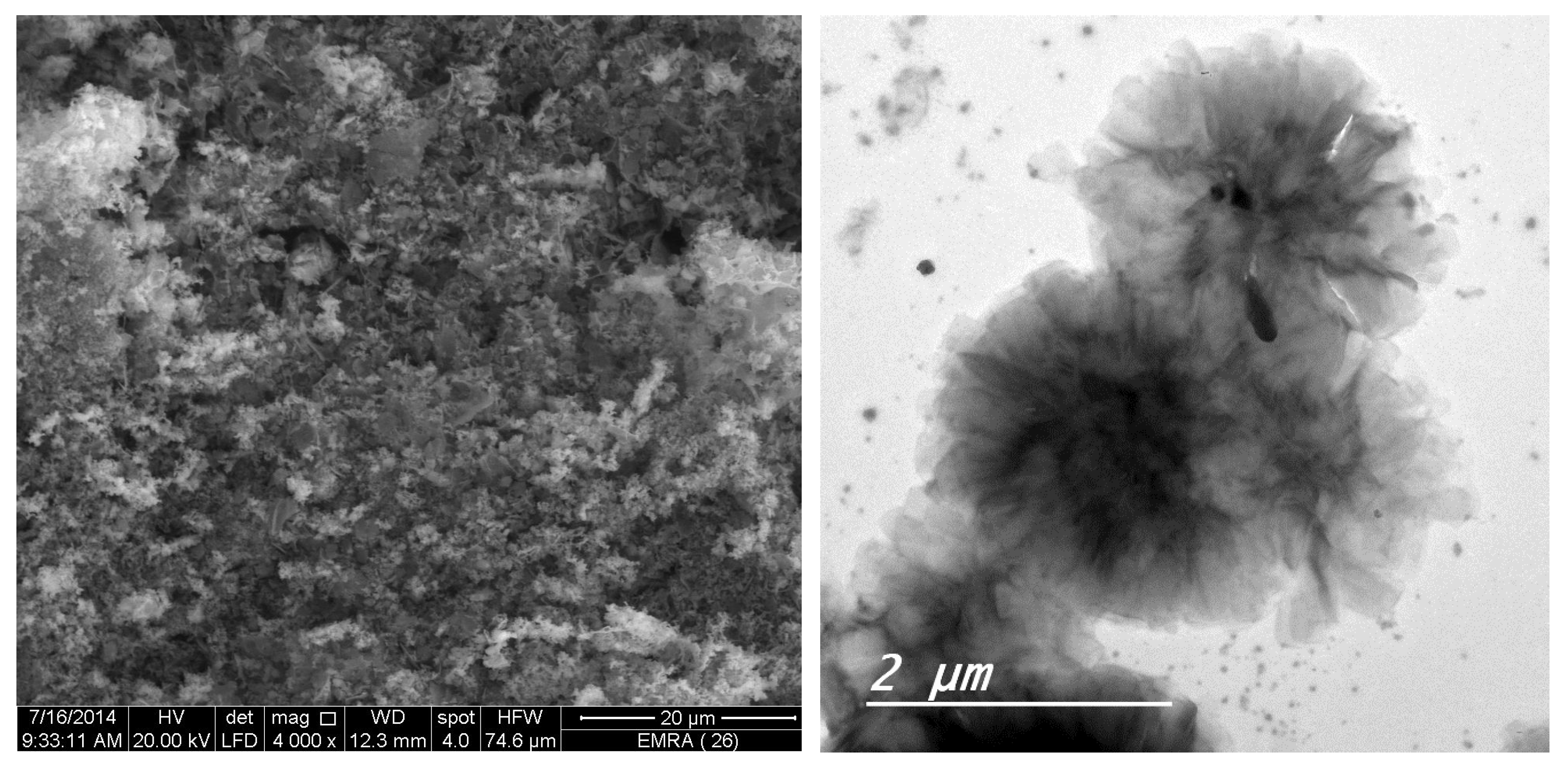
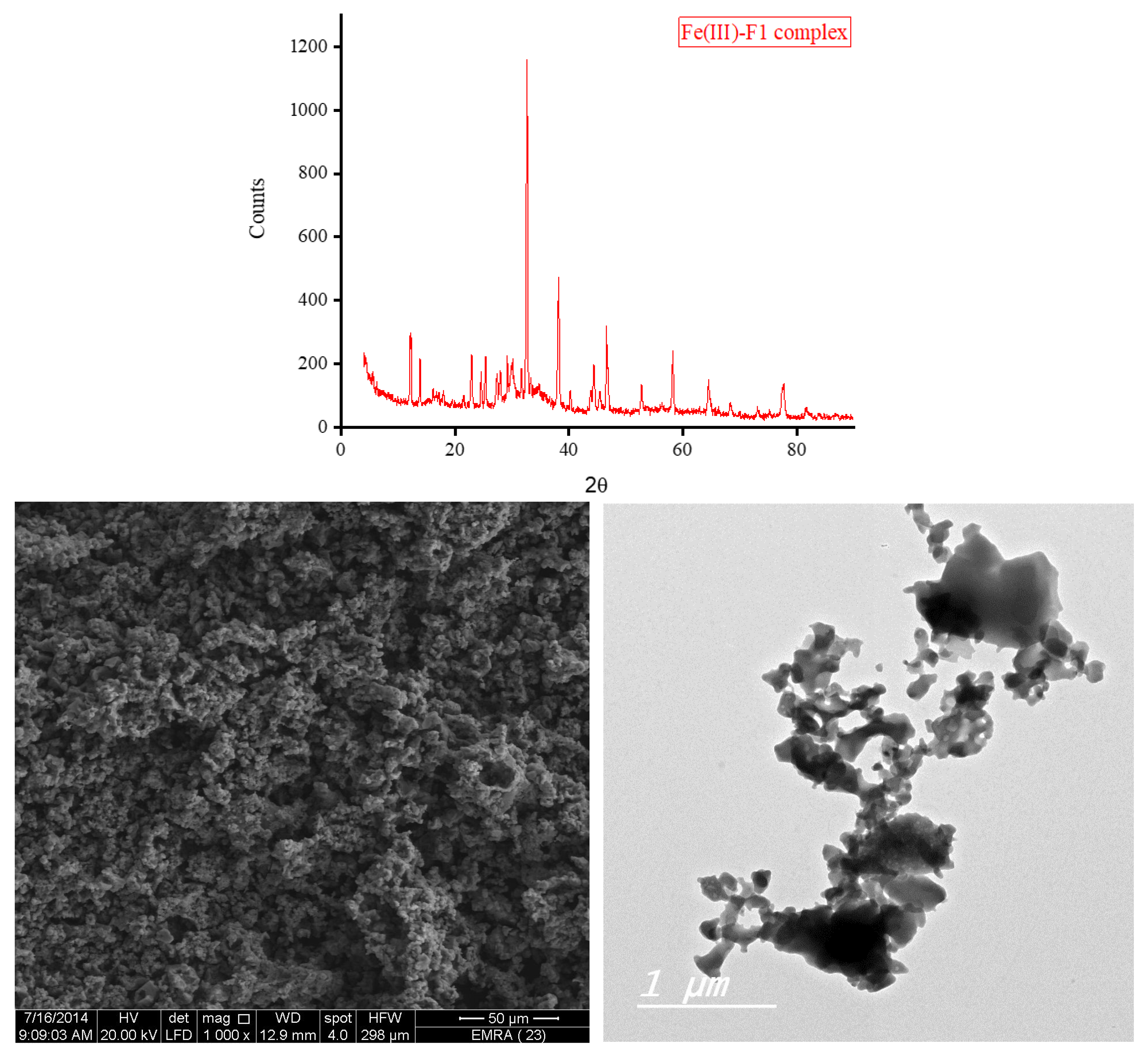
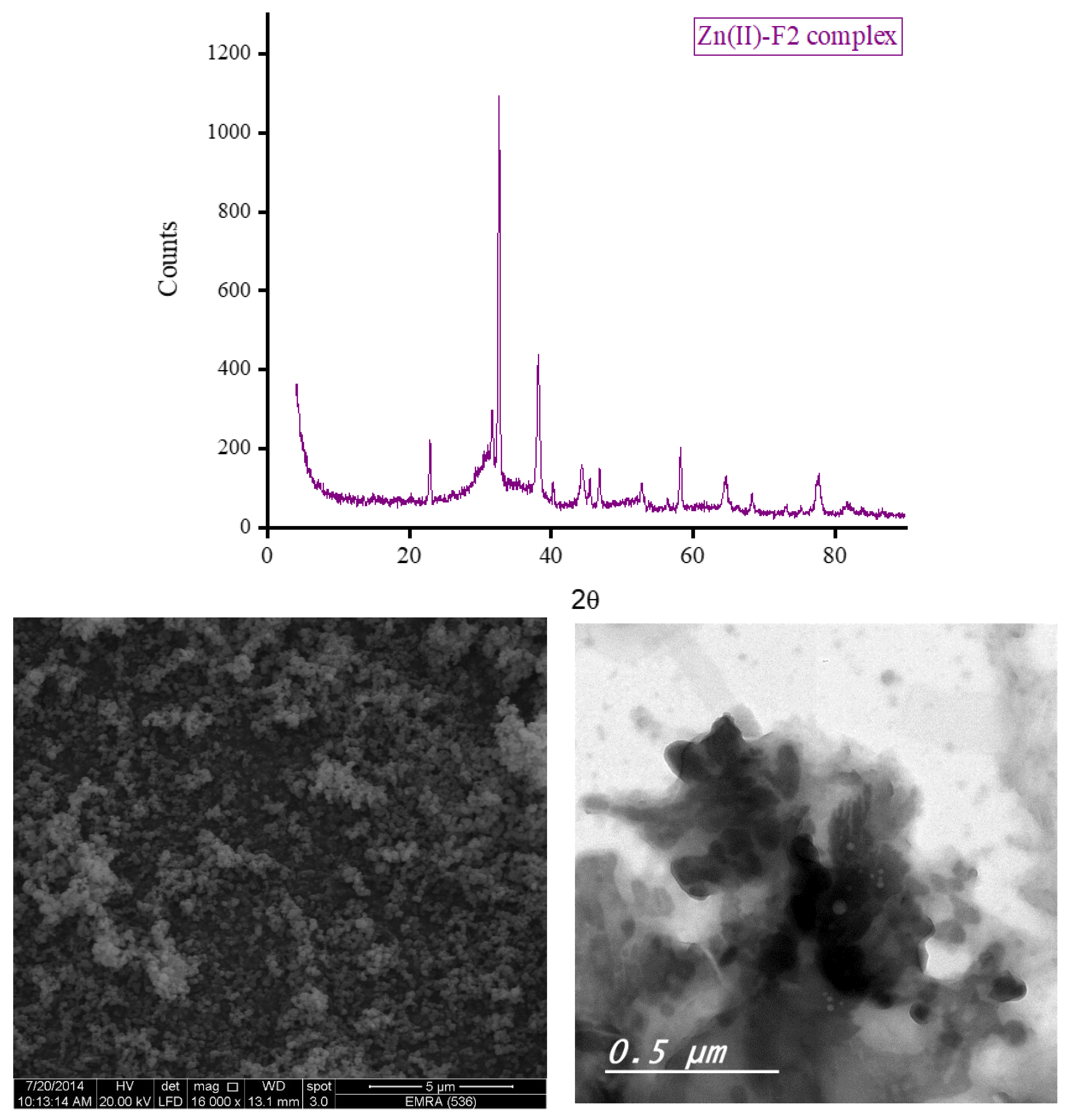
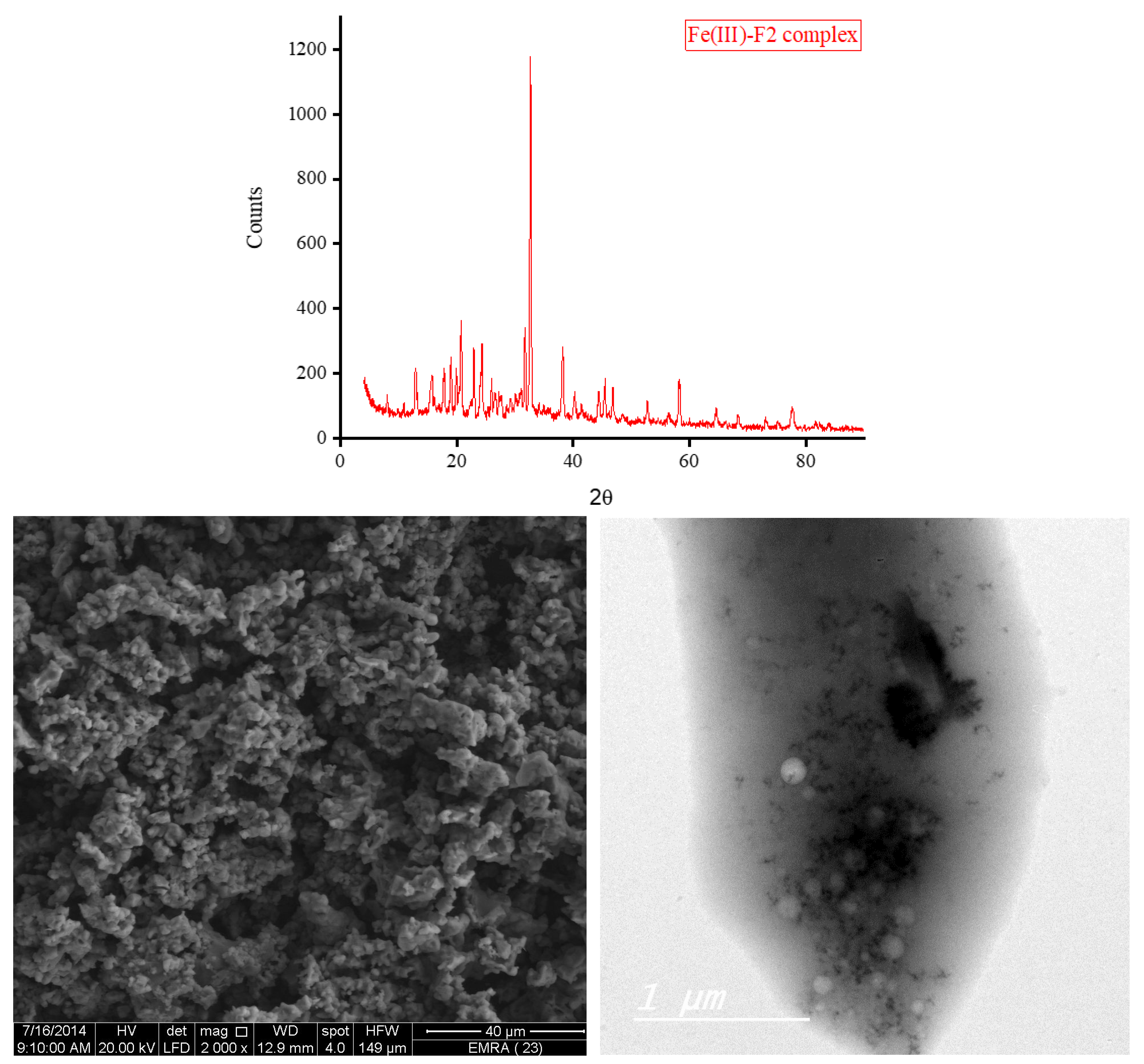
3.5. XRD, SEM, and TEM Results
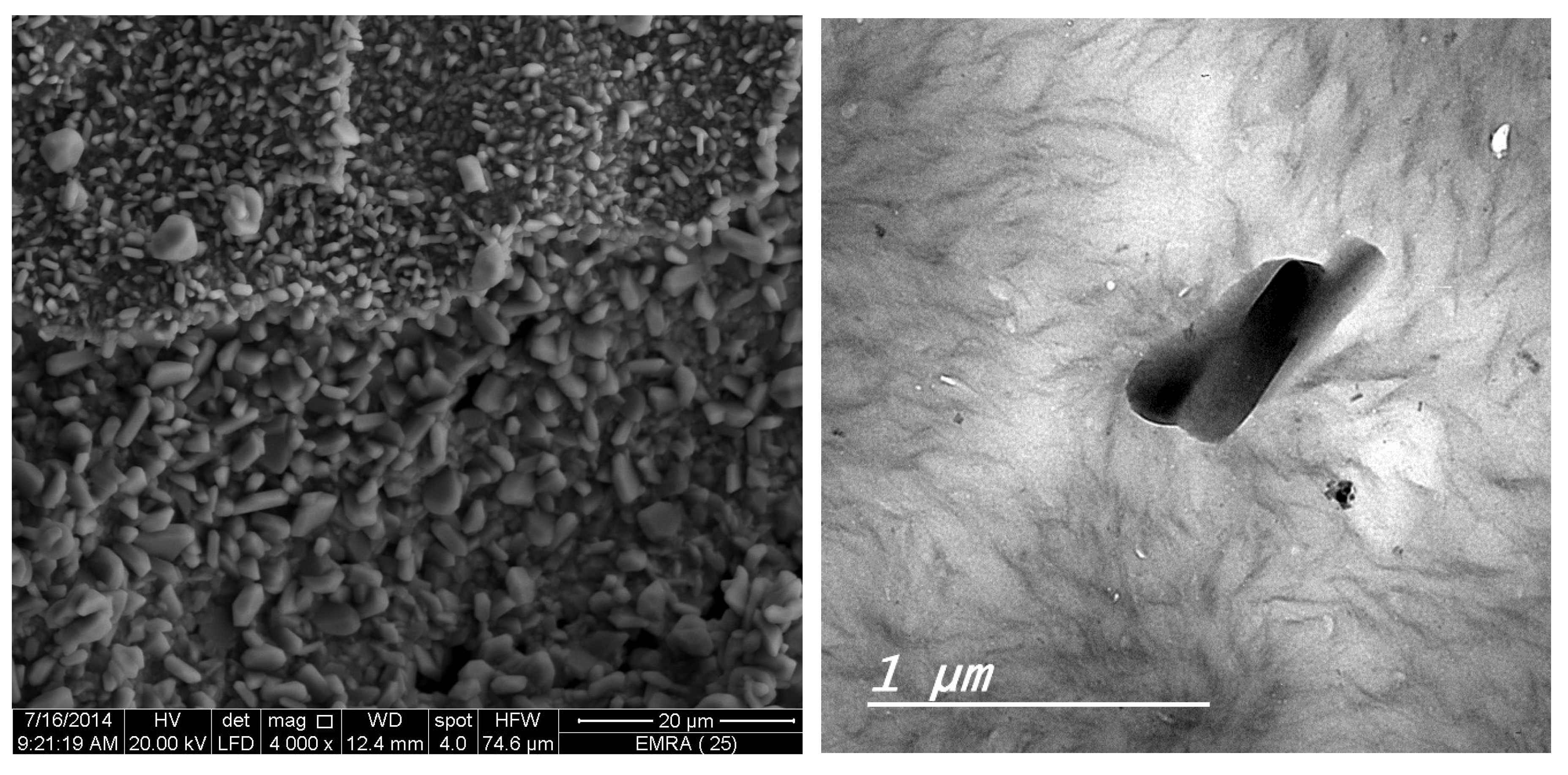
The XRD diffractograms and the SEM and TEM micrographs of F1 metal-based complexes are shown in
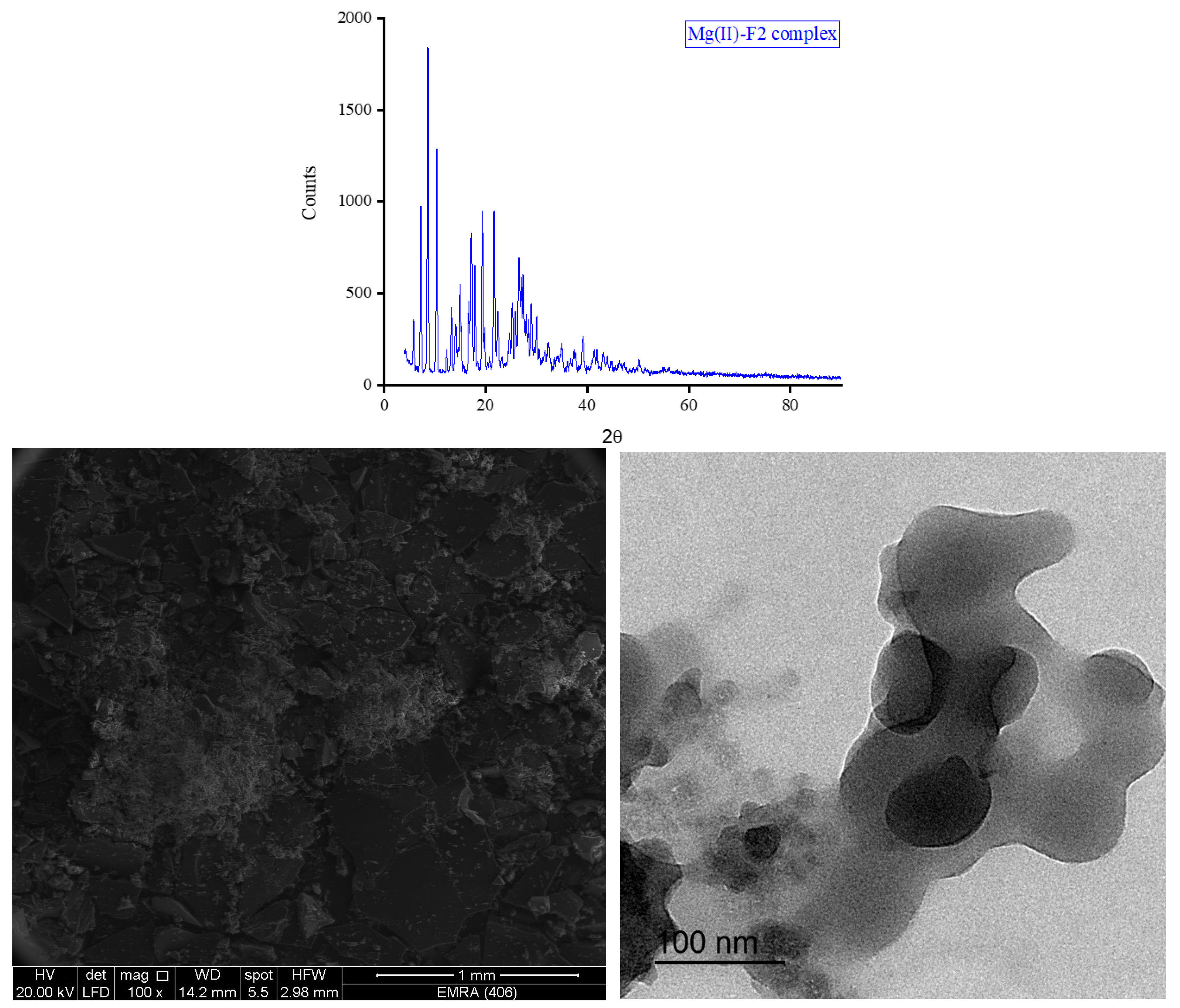
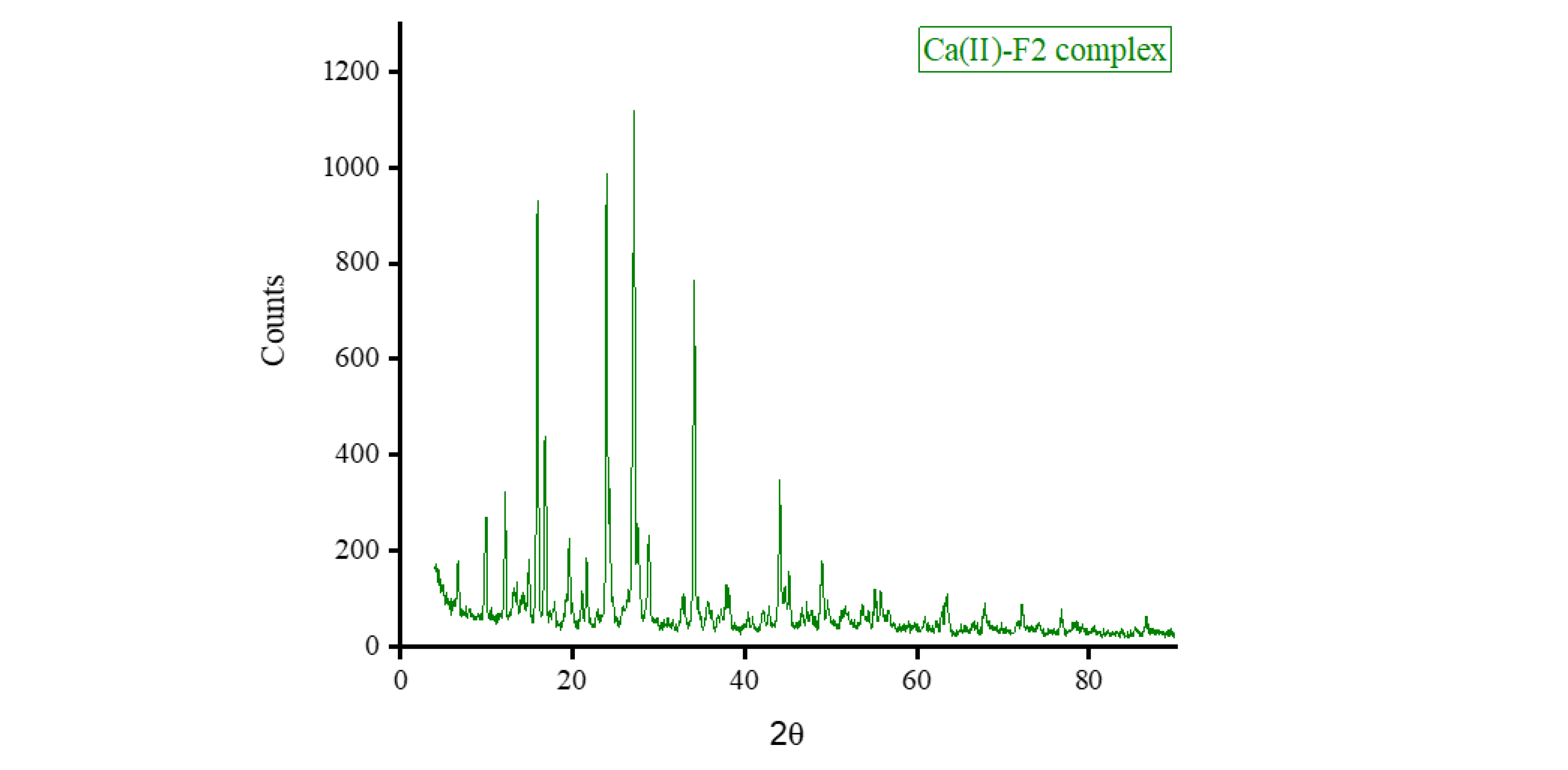
Figure 7. Those of the F2 metal-based complexes are given in
Figure 8. The XRD diffraction profiles suggested that the Ca(II), Zn(II), and Fe(III) ions tend to form good crystallinity complexes with F1 and F2 with well-defined morphology. The Mg(II) ion forms amorphous complexes with F1 and F2. The strongest diffraction line observed in the XRD patterns for F1 complexes with Mg(II), Ca(II), Zn(II), and Fe(III) located at Bragg’s angle 2
θ of 20.212°, 24.008°, 32.668°, and 32.627°, respectively. For F2 complexes, the strongest diffraction line was located at Bragg’s angle 2
θ of 8.434°, 27.107°, 32.585°, and 32.750° for Mg(II), Ca(II), Zn(II), and Fe(III) complex, respectively. Morphologically, the Ca(II)-F1 complex had the most interesting surface topography. Its particles had homogenate short rod-like shaped structures, and these short rods were clustered together to form a tree shape. The Ca(II)-F2 complex rods were longer than those of the Ca(II)-F1 complex and less homogenate. The Ca(II)-F2 rods do not tend to form a tree shape but accumulate together to form a rough mat. The particles of Fe(III) complexes with F1 and F2 had a spherical-like morphology. These spherical particles were fused together to form coral reef-like structures. The particles of Mg(II)-F1 and Mg(II)-F2 complexes tended to form big agglomerates. Complexes of Zn(II) ions with F1 and F2 had cotton-like structures. According to the TEM micrographs, most of the particles of the F1 and F2 complexes exhibit diameters in the range of 30 to 90 nm.
Figure 7.
a. The XRD spectrum, SEM and TEM micrographs of Mg(II)-F1 complex.
Figure 7.
a. The XRD spectrum, SEM and TEM micrographs of Mg(II)-F1 complex.
Figure 7.
b. The XRD spectrum, SEM and TEM micrographs of Ca(II)-F1 complex.
Figure 7.
b. The XRD spectrum, SEM and TEM micrographs of Ca(II)-F1 complex.
Figure 7.
c. The XRD spectrum, SEM and TEM micrographs of Zn(II)-F1 complex.
Figure 7.
c. The XRD spectrum, SEM and TEM micrographs of Zn(II)-F1 complex.
Figure 7.
d. The XRD spectrum, SEM and TEM micrographs of Fe(III)-F1 complex.
Figure 7.
d. The XRD spectrum, SEM and TEM micrographs of Fe(III)-F1 complex.
Figure 8.
a. The XRD spectrum, SEM and TEM micrographs of Mg(II)-F2 complex.
Figure 8.
a. The XRD spectrum, SEM and TEM micrographs of Mg(II)-F2 complex.
Figure 8.
b. The XRD spectrum, SEM and TEM micrographs of Ca(II)-F2 complex.
Figure 8.
b. The XRD spectrum, SEM and TEM micrographs of Ca(II)-F2 complex.
Figure 8.
c. The XRD spectrum, SEM and TEM micrographs of Zn(II)-F2 complex.
Figure 8.
c. The XRD spectrum, SEM and TEM micrographs of Zn(II)-F2 complex.
Figure 8.
d. The XRD spectrum, SEM and TEM micrographs of Fe(III)-F2 complex.
Figure 8.
d. The XRD spectrum, SEM and TEM micrographs of Fe(III)-F2 complex.
3.6. Biological Screening
3.6.1. Antibacterial Activity
The well-known Kirby-Bauer disc diffusion protocol assessed the antibacterial properties of the synthesized metal-based complexes
in vitro towards three Gram-positive bacterial species and two -negative bacterial species. All these bacterial microbes were isolated from clinical samples, and the streptomycin antibiotic drug was used as a positive (+) control for comparison.
Table 3 tabulated the zone of inhibition diameter (mm/mg sample) results of the antibacterial potential for the controls [(+); standard drug and (−); DMSO solvent] and the synthesized metal-based complexes.
a) Screening toward Gram-positive bacteria
The inhibition zones (in mm/mg) observed for the standard drug streptomycin were 18.0, 18.0 and 20.0 mm/mg towards B. subtilis, S. pneumoniae, and S. aureus, respectively. Complexes of F1 with Mg(II), Ca(II), and Zn(II) ions showed no or very low activity against the tested Gram-positive microbes. The complex of F1 with Fe(III) ion showed remarkable activity against S. pneumoniae and S. aureus strains with potency equal to that of the standard drug streptomycin. Interestingly, when Fe(III) ion complexed with the F2 molecule, the resulting complex (Fe(III)-F2) had low activity against the tested Gram-positive microbes. The screening results indicated that the complex of F2 with Mg(II) ion displayed remarkable activity against B. subtilis and S. pneumoniae species with potency equal to that of the standard drug streptomycin. Complexes of F2 with Mg(II), Ca(II), and Zn(II) ions were strongly active against the S. aureus strain.
b) Screening toward Gram-negative bacteria
The zones of inhibition (in mm/mg) of the standard drug streptomycin towards E. coli and P. aeruginosa were 22.0 and 27.0 mm/mg, respectively. After it showed remarkable activity towards two species of Gram-positive bacteria, the Fe(III)-F1 complex was also strongly active against the tested Gram-negative bacterial strains. The rest of the F1 complexes [Mg(II), Ca(II), Zn(II)] had low activity against the tested Gram-negative bacteria. This is not the same for F2 complexes, F2 complexes with Mg(II), Ca(II), and Zn(II) ions had strong potency towards the two tested Gram-negative bacteria.
3.6.2. Antifungal Activity
The well-known Kirby-Bauer disc diffusion protocol was also used to assess the antifungal properties of the synthesized metal-based complexes
in vitro towards three species;
A. niger,
Penicillium sp., and
C. albicans. Ketoconazole which was used as positive (+) control, had inhibition values of 18.0, 21.0, and 21.0 mm/mg against the tested fungal species, respectively. Data listed in
Table 4 demonstrated that the Fe(III)-F1 complex is the only complex that showed remarkable activity against all tested fungi with a potency equal to that of the standard drug ketoconazole. Complexes of F1 with Mg(II), Ca(II), and Zn(II) ions had very low-to-low inhibitory activity against the tested fungal species. Complexes of F2 with Mg(II) and Zn(II) ions were strongly active against
Penicillium sp. and
C. albicans strains.
4. Conclusions
The structural and pharmacological properties of Mg(II), Ca(II), Zn(II), and Fe(III) metal ions combined with two fluoroquinolone antibiotics (F1 and F2) were investigated. The metal-based complexes were prepared in neutral media (pH ~ 7–8) at approximately 60–70°C using a 1:1 stoichiometric molar ratio and were obtained as white, yellow, and brown powders. The Mg(II), Ca(II), and Zn(II) ions were captured by the oxygen atoms of the carboxylate group and the pyridone C=O group of the F1 and F2 molecules. The F1 and F2 molecules utilized the two nitrogen atoms of the piperazine ring to capture the Fe(III) ion. The Kirby-Bauer disc diffusion assay was employed to determine the in vitro antimicrobial activity of the synthesized metal-based complexes against a broad spectrum of microbes. Among all the metal-based complexes, the Fe(III)-F1 complex exhibited the most potent antimicrobial profile. This complex inhibited the growth of all tested bacterial and fungal species with potency equal to that of the standard drugs, streptomycin and ketoconazole. The next stage of this research will focus on screening the synthesized metal-based complexes (especially those containing Fe(III) ions) for their cytotoxic activity against various human cancer cell lines.
Funding
This research was funded by the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia through project no. (IFKSURC-1-0127).
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research. (IFKSURC-1-0127).
References
- A.C. Tella, J.A. A.C. Tella, J.A. Obaleye, M.D. Olawale, J.M.V. Ngororabanga, A.S. Ogunlaja, S.A. Bourned, C.R. Chimie 22 (1) (2019) 3-12.
- G.L. Eichhorn, L.G. G.L. Eichhorn, L.G. Marzilli, Advances in Inorganic Biochemistry Models in Inorganic Chemistry, PTR Prentice-Hall, Inc, New Jersey, 1994.
- M.N. Hughes, The Inorganic Chemistry of Biological Processes, 2nd ed., Wiley, Chichester [England], 1984.
- E. Alessio, Bioinorganic Medicinal Chemistry, Wiley-VCH Verlag GmbH and Co. KGaA, 2011.
- F. Trudu, F. F. Trudu, F. Amato, P. Vaňhara, T. Pivetta, E.M. Peña-Méndez, J. Havel, J. Appl. Biomed. 13 (2) (2015) 79.
- U. Singh, A.M. U. Singh, A.M. Malla, I.A. Bhat, A. Ahmad, M.N. Bukhari, S. Bhat, S. Anayu-tullah, A. A. Hashmi, Microb. Pathog. 93 (2016) 172.
- P.P. Netalkar, S.P. P.P. Netalkar, S.P. Netalkar, V.K. Revankar, Polyhedron 100 (2015) 215.
- M.A. Ragheb, M.A. M.A. Ragheb, M.A. Eldesouki, M.S. Mohamed, Spectrochim. Acta A 138 (2015) 585.
- S.P. Fricker, Dalton transactions, 43 (2007) 4903.
- A. Jurowska, K. A. Jurowska, K. Jurowski, J. Szklarzewicz, B. Buszewski, T. Kalenik, W. Piekoszewski, Cur. Med. Chem., 23(29) (2016) 3322.
- E.K. Efthimiadou, H. Thomadaki, Y. Sanakis, C.P. Raptopoulou, N. Katsaros, A. Scorilas, A. Karaliota, G. Psomas, J. Inorg. Biochem. 101 (2007) 64.
- L.M.M. Vieira, M.V. de Almeida, M.C.S. Lourenço, F.A.F.M. Bezerra, A.P.S. Fontes, Eur. J. Med. Chem., 44 (2009) 4107-4111.
- S.A. Sadeek, W.H. S.A. Sadeek, W.H. El-Shwiniy, J. Mol. Struct., 98 (2010) 130.
- H.F. Abd El-Halim, G.G. H.F. Abd El-Halim, G.G. Mohamed, M.M.I. El-Dessouky, W.H. Mahmoud, Spectrochim. Acta A, 82 (2011) 8.
- W. Qi, J. W. Qi, J. Huang, Z. An, Acta Crystallogr. 64 (2008) m302.
- P. Drevenšek, J. P. Drevenšek, J. Košmrlj, G. Giester, T. Skauge, E. Sletten, K. Sepčić, I. Turel, J. Inorg. Biochem. 100 (2006) 1755.
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- J.J. Biemer, Antimicrobial susceptibility testing by the Kirby-Bauer disc diffu-sion method, Ann. Clin. Lab. Sci. 3 (1973) 135-140.
- M.C. Serrano, M. Ramírez, D. Morilla, A. Valverde, M. Chávez, A. Espinel-Ingroff, R. Claro, A. Fernández, C. Almeida, E. Martín-Mazuelos, A comparative study of the disc diffusion method with the broth microdilution and Etest methods for voriconazole susceptibility testing of Aspergillus spp., J. An-timicrob. Chemo-ther. 53 (2004) 739-742.
- Allan, J.R.; Baird, N.D.; Kassyk, A.L. Some first row transition metal complexes of nicotinamide and nicotinic acid. J. Therm. Anal. Calorim. 1979, 16, 79–90. [Google Scholar] [CrossRef]
- Öztirk, .F.; Şekerci, M.; Özdemir, E. Preparation of complexes of 1-amino-6,7-O-cyclohexylidene-4-azaheptane with transition metal acetates. Russ. J. Gen. Chem. 2006, 76, 33–36. [Google Scholar] [CrossRef]
- Refat, M.S.; El-Sayed, M.Y.; Hassan, R.F. Study of the chemical structure and the microbial effect of iron(III) metal ions with four consecutive generations of quinolones in a nanometric form for the purpose of increasing the efficacy of antibacterial and antifungal drugs. Appl. Organomet. Chem. 2017, 32, e4195. [Google Scholar] [CrossRef]
- G.B. Deacon, R. Phillips, Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination, Coord. Chem. Rev. 33 (1980) 227-250.
- K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, Wiley Interscience, John Wiley & Sons, New York, NY, USA, 2nd edition, 1970.
- El-Megharbel, S.M.; Hegab, M.S.; Manaaa, E.-S.A.; Al-Humaidi, J.Y.; Refat, M.S. Synthesis and physicochemical characterizations of coordination between palladium(ii) metal ions with floroquinolone drugs as medicinal model against cancer cells: novel metallopharmaceuticals. New J. Chem. 2018, 42, 9709–9719. [Google Scholar] [CrossRef]
- S.M. El-Megharbel, M.A. Hussien, M.S. Refat, In-Situ Copper(II) Complexes of Some Quinolone Drug Ligands Were Discussed for Their Molecular Structures: Syn-thesis in Binary Solvent, Journal of Computational and Theoretical Nanoscience 14 (1) (2017) 561-576.
- M.W. Nassar, K.A.M. Attia, R.A. Said, A. El-Olemy, M.A. Hasan, Second De-rivative Synchronous Spectrofluorimetric Determination of Lomefloxacin Hydrochlo-ride in Presence of Its Decarboxylated Degradation Product INTRODUCTION, Ijppr. Human 11 (1) (2017) 397-418.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).