Submitted:
05 June 2023
Posted:
05 June 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
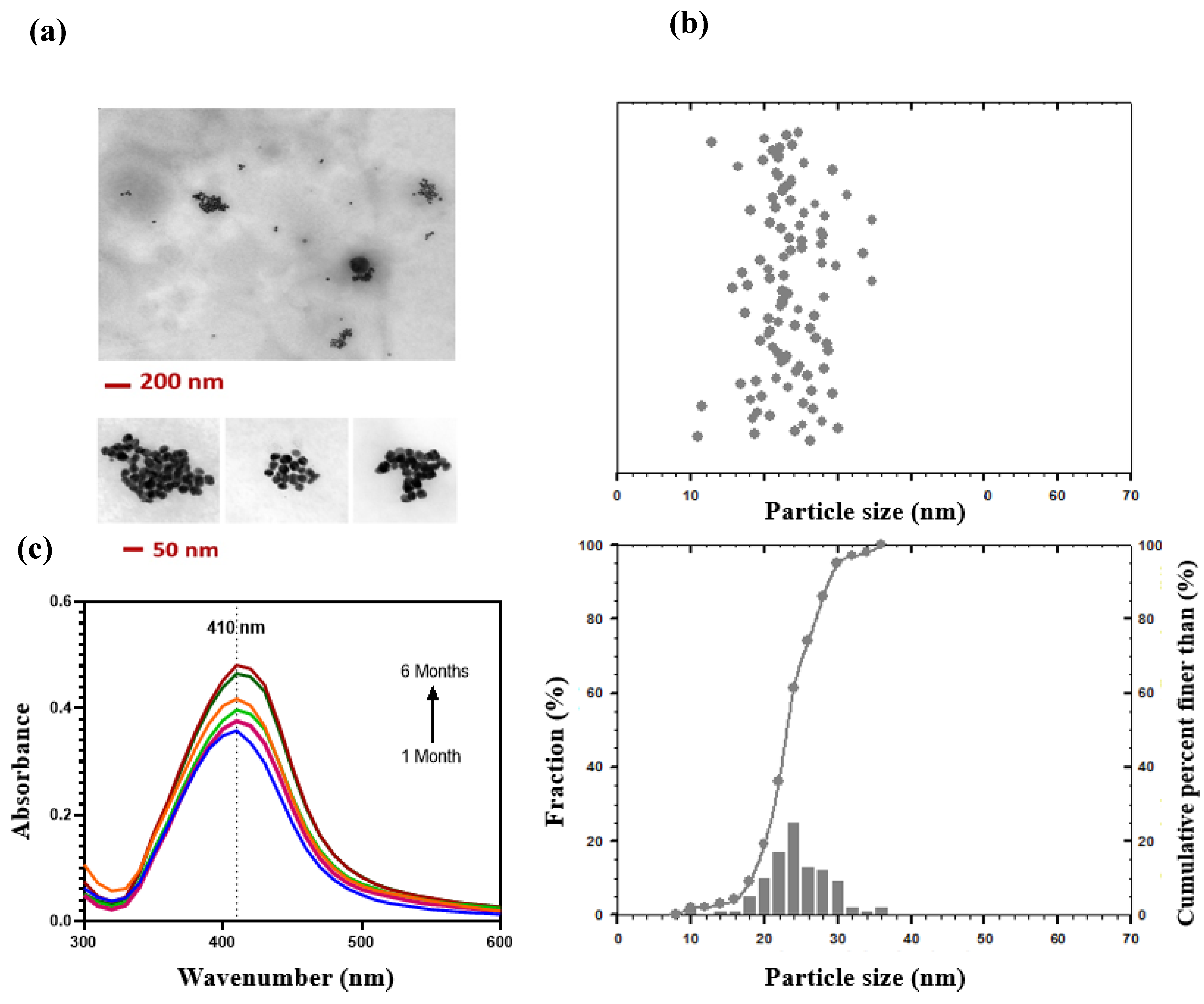
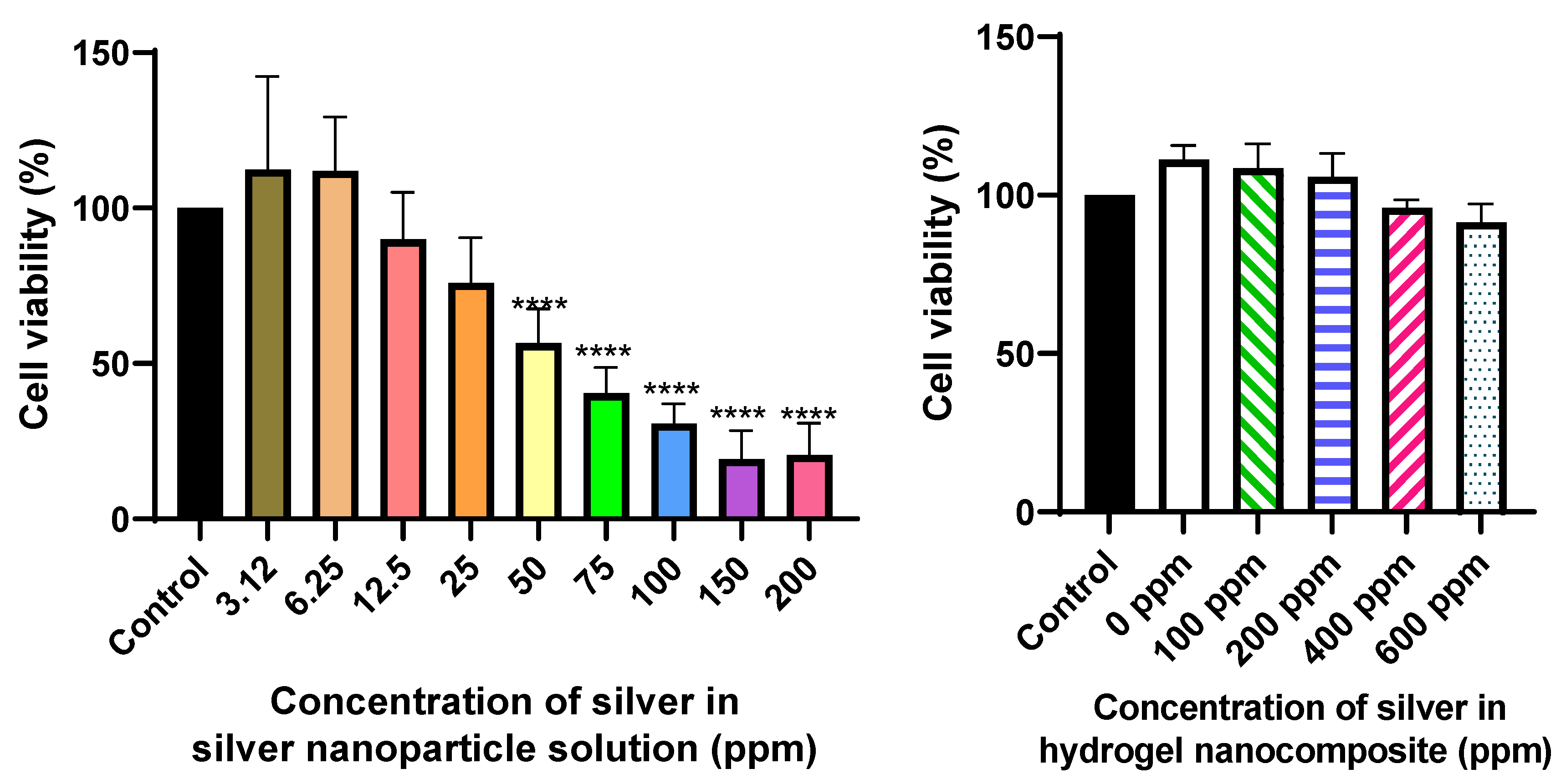
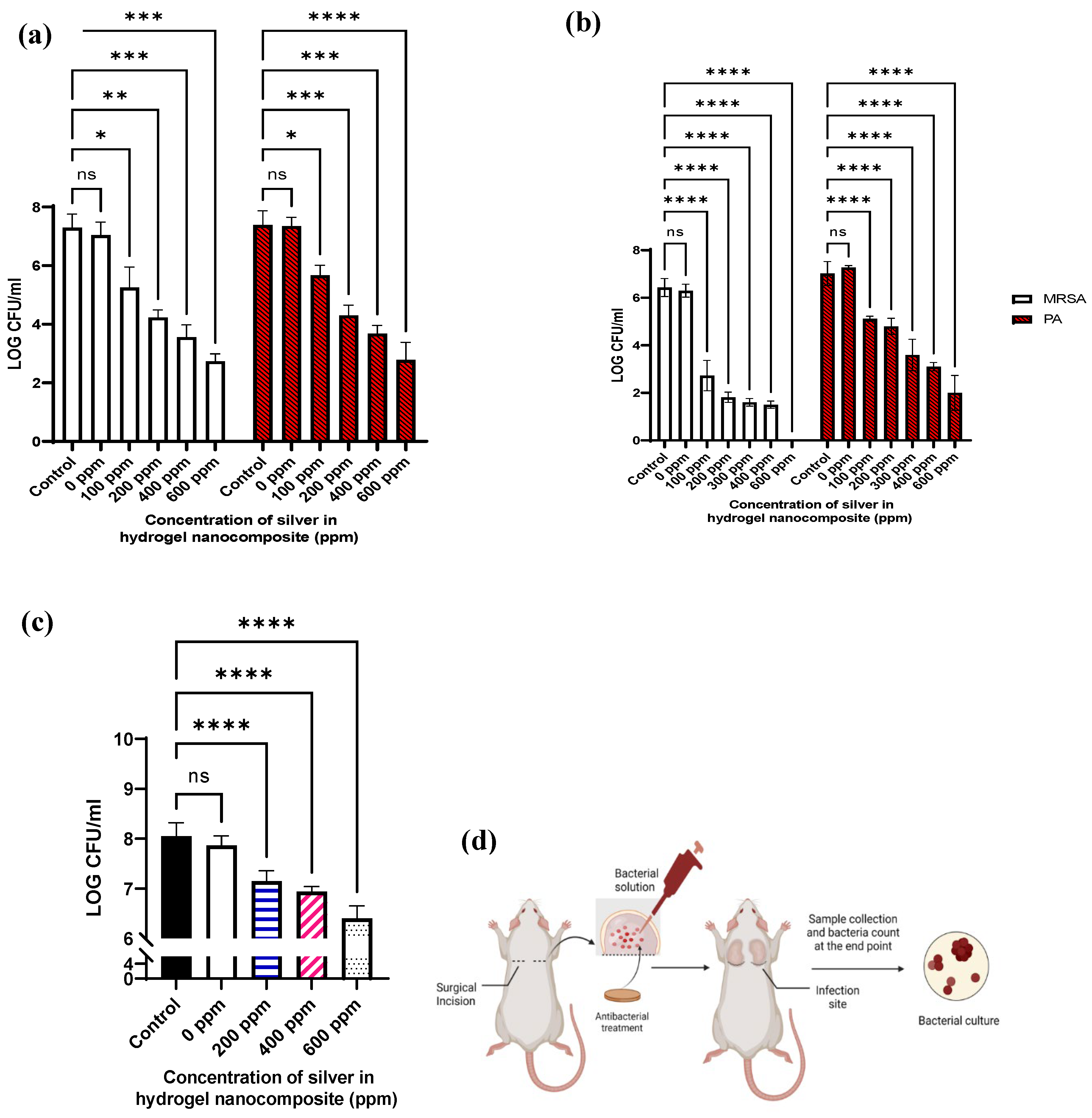
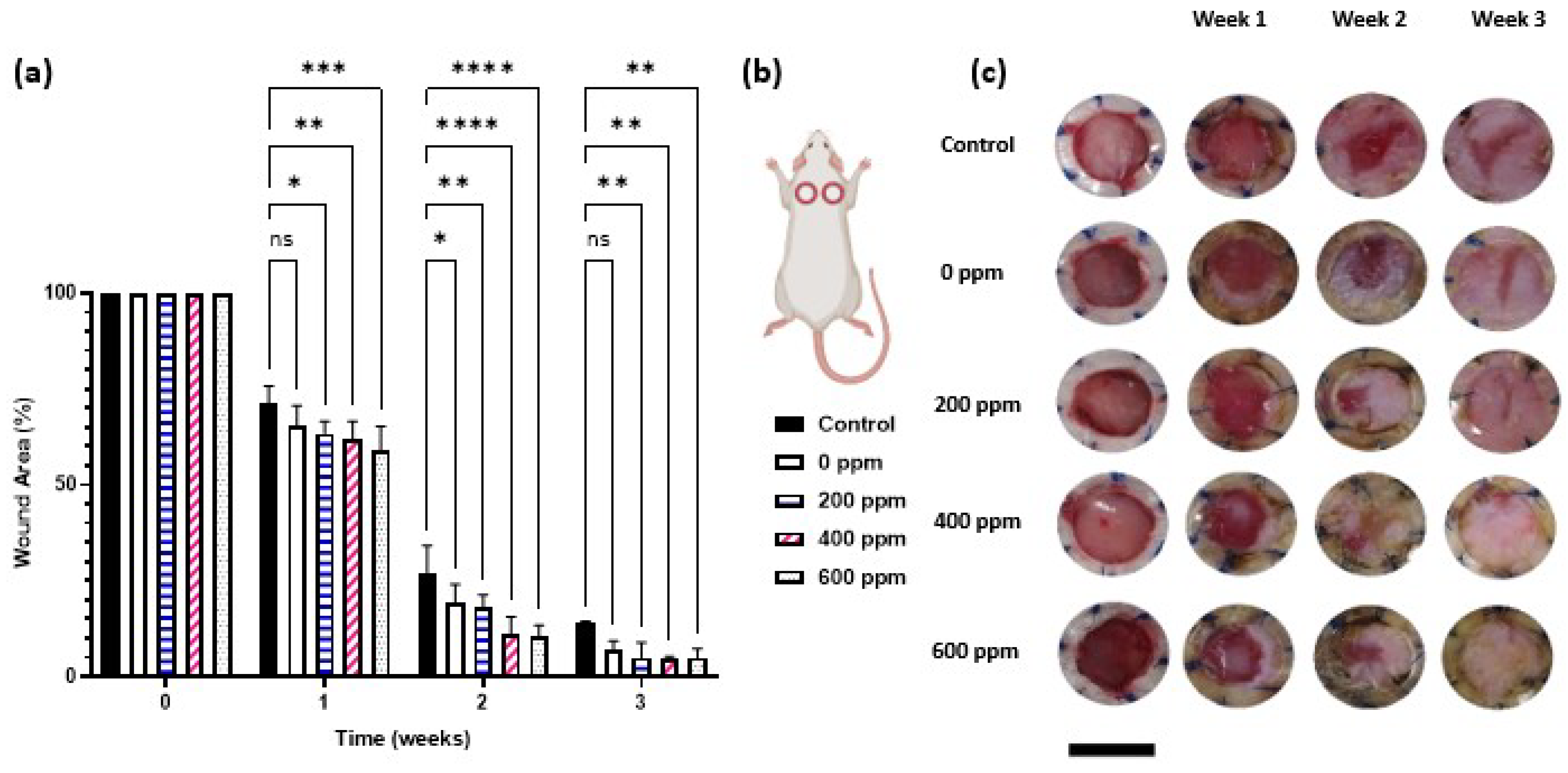
2. Results and discussion
3. Conclusions
4. Methods
4.1. Silver nanoparticle synthesis and Ag hydrogel nanocomposite preparation
4.2. In vitro cytocompatibility
4.2.1. Silver nanoparticle cell viability assay
4.2.2. Silver hydrogel nanocomposite cytocompatibility
4.3. Antibacterial activity
4.3.1. Minimum inhibitory concentration (MIC) of AgNPs solution
4.3.2. Antibacterial activity of Ag hydrogel nanocomposite formulations
4.4. In vivo study
4.4.1. Experimental Animals
4.4.2. In vivo antibacterial study
4.4.3. In vivo wound healing study
- Wound surface area calculation
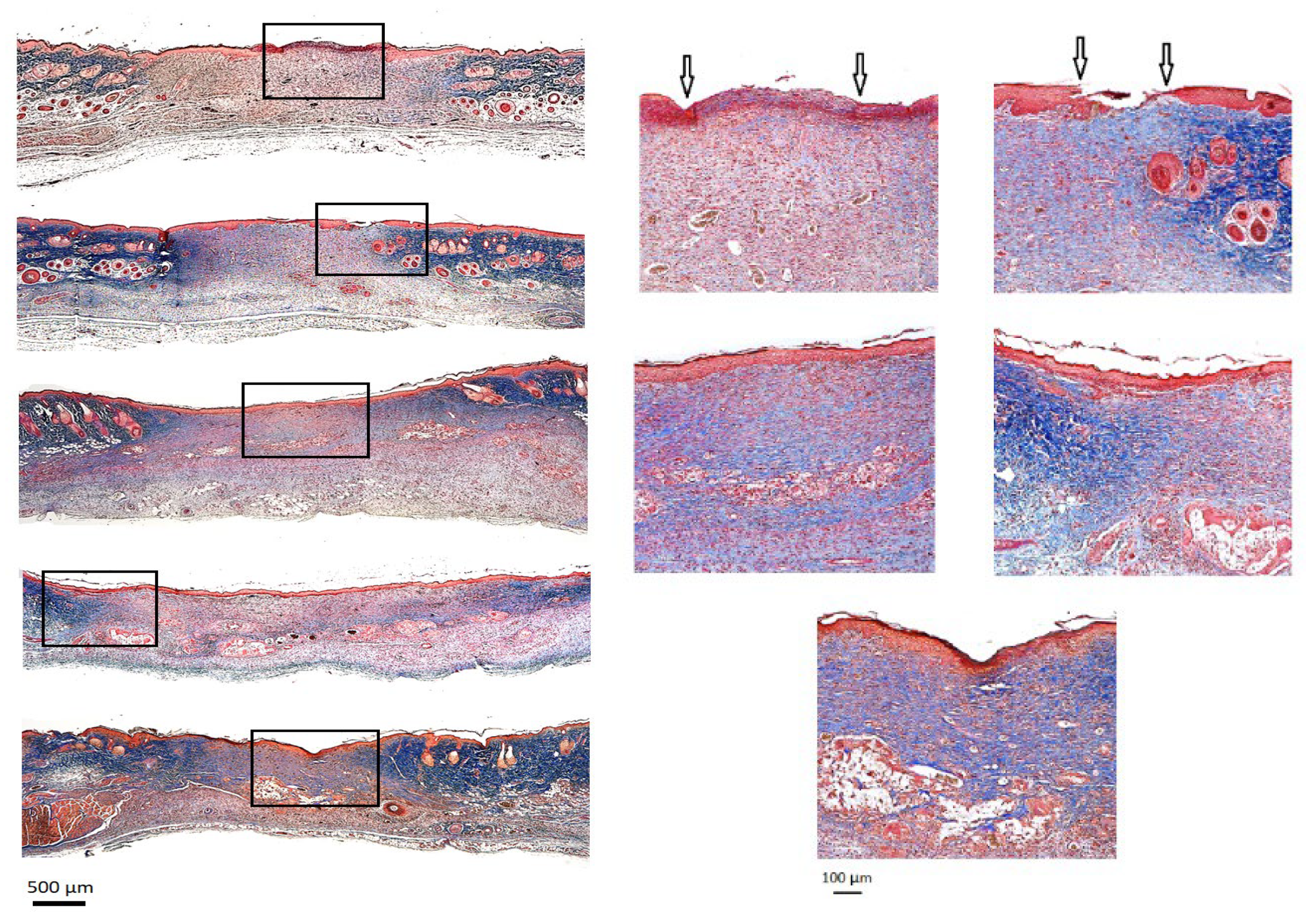
- Histological Analysis
- Hematology analysis and Biochemical assay
4.5. Statistical Analysis
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- V. Falanga, R.R. Isseroff, A.M. Soulika, M. Romanelli, D. Margolis, S. Kapp, M. Granick, K. Harding, Chronic wounds, Nat Rev Dis Primers. 8 (2022) 50. [CrossRef]
- J. Escandon, A.C. Vivas, J. Tang, K.J. Rowland, R.S. Kirsner, High mortality in patients with chronic wounds, Wound Repair and Regeneration. 19 (2011) 526–528. [CrossRef]
- R.G. Frykberg, J. Banks, Challenges in the Treatment of Chronic Wounds, Adv Wound Care (New Rochelle). 4 (2015) 560–582. [CrossRef]
- S.J.Mohd. Yussof, E. Omar, D.R. Pai, S. Sood, Cellular events and biomarkers of wound healing, Indian Journal of Plastic Surgery. 45 (2012) 220–228. [CrossRef]
- K. Raziyeva, Y. Kim, Z. Zharkinbekov, K. Kassymbek, S. Jimi, A. Saparov, Immunology of Acute and Chronic Wound Healing, Biomolecules. 11 (2021) 700. [CrossRef]
- G. Zhao, M.L. Usui, S.I. Lippman, G.A. James, P.S. Stewart, P. Fleckman, J.E. Olerud, Biofilms and Inflammation in Chronic Wounds, Adv Wound Care (New Rochelle). 2 (2013) 389–399. [CrossRef]
- Clinton, T. Carter, Chronic Wound Biofilms: Pathogenesis and Potential Therapies, Lab Med. 46 (2015) 277–284. [CrossRef]
- X. Zhang, M. Qin, M. Xu, F. Miao, C. Merzougui, X. Zhang, Y. Wei, W. Chen, D. Huang, The fabrication of antibacterial hydrogels for wound healing, Eur Polym J. 146 (2021) 110268. [CrossRef]
- H. Nosrati, R. Aramideh Khouy, A. Nosrati, M. Khodaei, M. Banitalebi-Dehkordi, K. Ashrafi-Dehkordi, S. Sanami, Z. Alizadeh, Nanocomposite scaffolds for accelerating chronic wound healing by enhancing angiogenesis, J Nanobiotechnology. 19 (2021) 1–21. [CrossRef]
- M. (Sam) Pakyari, R.B. Jalili, R.T. Kilani, N. Amiri, E. Brown, A. Ghahary, Studying the in vivo application of a liquid dermal scaffold in promoting wound healing in a mouse model, Exp Dermatol. 31 (2022) 715–724. [CrossRef]
- R. Hartwell, B. Chan, K. Elliott, H. Alnojeidi, A. Ghahary, Polyvinyl alcohol-graft-polyethylene glycol hydrogels improve utility and biofunctionality of injectable collagen biomaterials, Biomedical Materials. 11 (2016) 35013. [CrossRef]
- R. Hartwell, V. Leung, C. Chavez-Munoz, L. Nabai, H. Yang, F. Ko, A. Ghahary, A novel hydrogel-collagen composite improves functionality of an injectable extracellular matrix, Acta Biomater. 7 (2011) 3060–3069. [CrossRef]
- R. Hartwell, M.S. Poormasjedi-Meibod, C. Chavez-Munoz, R.B. Jalili, A. Hossenini-Tabatabaei, A. Ghahary, An in-situ forming skin substitute improves healing outcome in a hypertrophic scar model, Tissue Eng Part A. 21 (2015) 1085–1094. [CrossRef]
- M. Verly, E. Mason, S. Sheikh-Oleslami, R. Jalili, B. Russ, R.T. Kilani, A. Ghahary, A Pilot Trial Assessing the Feasibility and Efficacy of a Novel Powder for Rapid Wound Healing, European Burn Journal. 2 (2021) 238–248. [CrossRef]
- Khansa, A.R. Schoenbrunner, C.T. Kraft, J.E. Janis, Silver in Wound Care-Friend or Foe?: A Comprehensive Review., Plast Reconstr Surg Glob Open. 7 (2019) e2390. [CrossRef]
- C.A. Lariviere, A.B. Goldin, J. Avansino, Silver toxicity with the use of silver-impregnated dressing and wound vacuum-assisted closure in an immunocompromised patient., J Am Col Certif Wound Spec. 3 (2011) 8–12. [CrossRef]
- S. Lee, B.-H. Jun, Silver Nanoparticles: Synthesis and Application for Nanomedicine, Int J Mol Sci. 20 (2019) 865. [CrossRef]
- Y. Qing, L. Cheng, R. Li, G. Liu, Y. Zhang, X. Tang, J. Wang, H. Liu, Y. Qin, Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies, Int J Nanomedicine. Volume 13 (2018) 3311–3327. [CrossRef]
- R. Vazquez-Muñoz, N. Bogdanchikova, A. Huerta-Saquero, Beyond the Nanomaterials Approach: Influence of Culture Conditions on the Stability and Antimicrobial Activity of Silver Nanoparticles., ACS Omega. 5 (2020) 28441–28451. [CrossRef]
- J. Franková, V. Pivodová, H. Vágnerová, J. Juráňová, J. Ulrichová, Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model, J Appl Biomater Funct Mater. 14 (2016) e137–e142. [CrossRef]
- Avalos, A.I. Haza, D. Mateo, P. Morales, Interactions of manufactured silver nanoparticles of different sizes with normal human dermal fibroblasts, Int Wound J. 13 (2016) 101–109. [CrossRef]
- E. Szczepańska, A. Bielicka-Giełdoń, K. Niska, J. Strankowska, J. Żebrowska, I. Inkielewicz-Stępniak, B. Łubkowska, T. Swebocki, P. Skowron, B. Grobelna, Synthesis of silver nanoparticles in context of their cytotoxicity, antibacterial activities, skin penetration and application in skincare products, Supramol Chem. 32 (2020) 207–221. [CrossRef]
- S. Loganathan, K. Selvam, M.S. Shivakumar, S. Senthil-Nathan, P. Vasantha-Srinivasan, D. Gnana Prakash, S. Karthi, F. Al-Misned, S. Mahboob, A. Abdel-Megeed, A. Ghaith, P. Krutmuang, Phytosynthesis of Silver Nanoparticle (AgNPs) Using Aqueous Leaf Extract of Knoxia Sumatrensis (Retz.) DC. and Their Multi-Potent Biological Activity: An Eco-Friendly Approach, Molecules. 27 (2022) 7854. [CrossRef]
- P. Thangaraju, S.B. Varthya, ISO 10993: Biological Evaluation of Medical Devices, in: Medical Device Guidelines and Regulations Handbook, Springer International Publishing, Cham, 2022: pp. 163–187. [CrossRef]
- S. Tang, J. Zheng, Antibacterial Activity of Silver Nanoparticles: Structural Effects, Adv Healthc Mater. 7 (2018) 1–10. [CrossRef]
- S. Agnihotri, S. Mukherji, S. Mukherji, Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy, RSC Adv. 4 (2014) 3974–3983. [CrossRef]
- S. Shah, S. Gaikwad, S. Nagar, S. Kulshrestha, V. Vaidya, N. Nawani, S. Pawar, Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa, Biofouling. 35 (2019) 34–49. [CrossRef]
- P. Singh, S. Pandit, J. Garnæs, S. Tunjic, V. Mokkapati, A. Sultan, A. Thygesen, A. Mackevica, R.V. Mateiu, A.E. Daugaard, A. Baun, I. Mijakovic, Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition, Int J Nanomedicine. Volume 13 (2018) 3571–3591. [CrossRef]
- S. Liao, Y. Zhang, X. Pan, F. Zhu, C. Jiang, Q. Liu, Z. Cheng, G. Dai, G. Wu, L. Wang, L. Chen, Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa, Int J Nanomedicine. Volume 14 (2019) 1469–1487. 146. [CrossRef]
- S. Tang, J. Zheng, Antibacterial Activity of Silver Nanoparticles: Structural Effects, Adv Healthc Mater. 7 (2018) 1701503. [CrossRef]
- T.C. Dakal, A. Kumar, R.S. Majumdar, V. Yadav, Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles, Front Microbiol. 7 (2016). [CrossRef]
- Mandal, S. Sekar, N. Chandrasekaran, A. Mukherjee, T.P. Sastry, Synthesis, characterization and evaluation of collagen scaffolds crosslinked with aminosilane functionalized silver nanoparticles: in vitro and in vivo studies, J Mater Chem B. 3 (2015) 3032–3043. [CrossRef]
- M.J. Kühn, L. Talà, Y.F. Inclan, R. Patino, X. Pierrat, I. Vos, Z. Al-Mayyah, H. Macmillan, J. Negrete, J.N. Engel, A. Persat, Mechanotaxis directs Pseudomonas aeruginosa twitching motility, Proceedings of the National Academy of Sciences. 118 (2021). [CrossRef]
- Travan, C. Pelillo, I. Donati, E. Marsich, M. Benincasa, T. Scarpa, S. Semeraro, G. Turco, R. Gennaro, S. Paoletti, Non-cytotoxic Silver Nanoparticle-Polysaccharide Nanocomposites with Antimicrobial Activity, Biomacromolecules. 10 (2009) 1429–1435. [CrossRef]
- N. Amiri, S. Ajami, A. Shahroodi, N. Jannatabadi, S. Amiri Darban, B.S. Fazly Bazzaz, E. Pishavar, F. Kalalinia, J. Movaffagh, Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing, Int J Biol Macromol. 162 (2020) 645–656. [CrossRef]
- C. Tyavambiza, M. Meyer, S. Meyer, Cellular and Molecular Events of Wound Healing and the Potential of Silver Based Nanoformulations as Wound Healing Agents, Bioengineering. 9 (2022) 712. [CrossRef]
- X. Liu, P. Lee, C. Ho, V.C.H. Lui, Y. Chen, C. Che, P.K.H. Tam, K.K.Y. Wong, Silver Nanoparticles Mediate Differential Responses in Keratinocytes and Fibroblasts during Skin Wound Healing, ChemMedChem. 5 (2010) 468–475. [CrossRef]
- K. Zhang, V.C.H. Lui, Y. Chen, C.N. Lok, K.K.Y. Wong, Delayed application of silver nanoparticles reveals the role of early inflammation in burn wound healing, Sci Rep. 10 (2020) 6338. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




