1. Introduction
Due to its severe major impact on women’s health and the fact that its physiopathology is yet incompletely known, endometriosis continues to be an important topic of study in the literature. The disease affects 10 to 15% of women of reproductive age, reaching higher incidence in cases with infertility or chronic pelvic pain [
1,
2]. The clinical manifestations of this condition can range from asymptomatic to its primary clinical manifestation, which remain infertility and severe pelvic pain. This disease continues to have a significant negative impact on patient’s wellbeing, limiting daily activity in affected women. Understanding its underlying causes and potential treatments are the main goals of research on this topic in literature. The condition implies the existence of tissue, glands, and stroma that resemble the endometrium located outside of the uterus.
Endometriosis is an estrogen-dependent chronic inflammatory illness that may affect the pericardium, lungs, and even the nervous system in addition to the pelvic region. There are some theories that try to grasp its mode of appearance and spread. Sampson’s theory makes an attempt to explain it by focusing on the retrograde menstrual cycle. Another explanation is the one Meyers emphasizes, which attributes the cause to the metaplasia of celomic epithelium [
1,
2,
3,
4].
Endometriosis continues to pose a challenge for clinicians whether they have access to advanced diagnostic tools or not, with a 4 to 11-year delay between the onset of symptoms and a proper diagnosis due to the condition’s nonspecific wide range of clinical manifestations as well as the absence of specific tests or biomarkers. The American Society for Reproductive Medicine (ASRM) classification system, which has four stages (stages I - minimum; stage II - mild; stage III - moderate; and stage IV - severe), is the most extensively used classification system for endometriosis [
5].
The first line of treatment for this medical condition, which is hormonally dependent, is hormonal therapy. All of the published guidelines for treating this disease’s pain recommend this course of action, yet there are few therapeutic alternatives and no universally accepted medical or surgical approach when dealing with infertility [
1,
6].
Few researches in the literature have examined vitamin D’s potential therapeutic benefits for endometriosis. In addition to being a well-known secosteroid that controls calcium metabolism and bone mineralization, vitamin D and its receptor also has substantial effects on the immune system and reduces inflammation in the body. These two additional roles caught the attention of specialists that began studying its effects in endometriosis [
6,
7].
Some studies from the literature showed that vitamin D is an inhibitor of cellular proliferation in carcinomas (breast, colon, skin, brain and other) [
8].
Studies on vitamin D have examined the impact of medical interventions in order to elucidate the expected improvements in endometriosis-related inflammation. A small subset of current research focuses on vitamin D therapy for endometriosis. With its immunomodulatory, anti-inflammatory, anti-proliferative, and anti-invasive properties, vitamin D has been suggested to potentially play an important role in the multifactorial pathogenesis of endometriosis [
8,
9].
The vitamin D nuclear receptor (VDR), which is involved in the transcription process of more than 900 genes, mediates the biological effects of vitamin D. This receptor is engaged in a number of immunological processes along with the active form of vitamin D, 1,25(OH)
2 vitamin D [
10,
11].
PHH3 is a protein that is an essential part of the nucleosome, which is the fundamental building block of chromatin and plays a role in the structure and operation of DNA. PHH3 can be altered chemically through processes including acetylation, methylation, and phosphorylation, which can change how DNA is organized and regulated. The family of proteins known as histones is essential in regulating gene expression and chromatin structure [
12,
13].
PHH3 alterations may play a role in the onset and progression of endometriosis, according to recent investigations. For instance, one study discovered that endometrial tissue from women with endometriosis had unusual high levels of PHH3 acetyl group, which may have an impact in the development and survival of abnormal endometrial cells outside of the uterus. Additionally, PHH3 acetylation levels in endometriotic lesions tissue were significantly higher than in healthy endometrial tissue, and this increase has a genetic basis because it is linked to the activation of genes essential for cell survival and proliferation [
13,
14]. According to another study, PHH3 was discovered to be hypermethylated in endometriotic tissue lesions. This may be a factor in the development of well-known endometriosis characteristics such as altered gene expression and cellular function [
15]. Endometriotic tissue’s altered histone 3 methylation patterns resulting in lower levels of the suppressive H3K27me3 mark and higher levels of the activating H3K4me3 mark, possibly causing abnormal endometrial tissue growth and survival outside of the uterus [
14,
16,
17].
Specific histone-modifying enzymes have also been linked to the onset and development of endometriotic lesions, in addition to these chemical global changes in histone structure [
14,
18]. Gujral et al.’s study, for instance, discovered that deacetylating the histone through HDAC3 enhanced the progression of endometriosis by controlling the expression of genes linked to inflammation and angiogenesis [
17]. Another study that addressed this issue found that JMJD1A, a histone demethylase, was overexpressed in endometriotic tissue and contributed to the onset of endometriosis by controlling the transcription of genes involved in the cell cycle [
19].
Overall, these studies have thoroughly examined the consequences of this protein alteration that may play a part in the pathogenesis of endometriosis, even though the precise role of PHH3 in this disease is still not yet entirely fully understood [
14,
15,
16,
17,
18,
19]. To better comprehend all of the mechanisms and to pinpoint further treatment strategies, additional research is required.
The main motivation behind our research is to identify PHH3 in endometriotic lesions in an effort to shed some light on the physio-pathogenesis of this complex and enigmatic disease, as well as tissue levels of vitamin D in an effort to validate its autocrine and/or paracrine actions. Furthermore, we believe that the markers PHH3 and vitamin D represent a promising new research direction in the pathogenesis of endometriosis.
2. Materials and Methods
2.1. Patients and Tissue Samples
All patients were given written informed consent before being enrolled in the study and the Hospital Ethics Committee approved it (No 11062/2020 and No 6/2021). When 60 female endometriosis patients underwent surgery at the Obstetrics and Gynecology Hospital “Cuza-Voda” in Iasi and the Obstetrics and Gynecology Hospital “Panait Sirbu” in Bucharest, formalin-fixed, paraffin-embedded tissue samples were collected from each patient. All the women included had stage III or IV endometriotic lesions. The samples were collected between January 2021 and January 2022.
24 of the 60 patients (18 to 42 years old) agreed to get progestin treatment for three months prior to surgery.
Inclusion criteria:
We collected tissue samples from women who had endometriosis that had been laparoscopically detected and later histologically confirmed. The tissue samples that we used in the analysis came from endometriotic ovarian cysts. We collected samples from both women who had undergone surgery and underwent hormone therapy (with dienogest 2mg daily) for three months and from women without any treatment.
Exclusion criteria:
Due to the possibility that these factors could affect our research, we eliminated patients with a body mass index (BMI) > 30, malignancy or other tumoral lesions, diabetes, depression, genetic syndromes, and pregnancy.
Histomorphological and immunohistochemistry (IHC) testing confirmed the pathological diagnosis in every case. Routinely prepared hematoxylin and eosin (H&E) sections have been examined, and IHC has been independently evaluated by two pathologists to confirm the diagnosis.
2.2. Immunohistochemistry
IHC staining was performed on tissues that has been formalin-fixed and paraffin-embedded.
Four-micrometer-thick serial sections on coated sections were prepared in citrate buffer (pH 6) following deparaffinization in xylene and rehydration in ethanol series. 0.3% H2O2 was used to inhibit endogenous peroxidase activity for 20 minutes at room temperature. Monoclonal anti-PHH3 antibodies (BioSB, Santa Barbara, USA) were diluted to 1:250 for working purposes, and vitamin D anti-receptor dilution 1:3000 (Abcam, Cambridge, UK) was incubated for an overnight period at 4°C. The sections were washed, exposed to the secondary antibody for 45 minutes at 37 degrees, and then thoroughly cleaned with
phosphate-buffered saline (PBS) (
Table 1). Hematoxylin was used as a counterstain in the standard avidin-biotin-peroxidase technique, which used a liquid DAB (diaminobenzidine) substrate and chromogen system for viewing. As a negative control, primary antibodies were left out. Human lymph node tissue section was utilized as a positive reference for PHH3 and human jejunum was utilized for VDR.
Sixty samples with endometriosis with their tissues’ stromal and epithelial components were examined for PHH3 and VDR. Patients who received treatment were compared to those without treatment. PHH3 and VDR have been identified to be present in the normal fallopian tube and follicular ovarian cysts.
The presence of positive cells (brown or yellowish-brown color in the nucleus) in a continuous cell count in the area of the epithelial and stroma compartments under a light microscope were characterized as PHH3 and VDR positive. Regardless of staining intensity, and number of cells, nuclear pattern of stained cells was considered to be positive.
3. Results
Before surgery, 24 (40%) patients had hormonal treatment, while 36 (60%) patients received no treatment at all. To evaluate the effect of the PHH3 and VDR on the ovarian endometriosis cells, we used to establish their pattern of expression and distribution.
In this study, 24 (47%) of the 60 endometriosis areas showed PHH3 protein expression.
In contrast to the negative expression of PHH3 (32 cases – 16 without treatment and 16 with treatment), which was similar in both groups 44% and 66% respectively, the expression level of PHH3 in the group without treatment 20 (71%) was higher than in the group with treatment 8 (29%) (
Table 2).
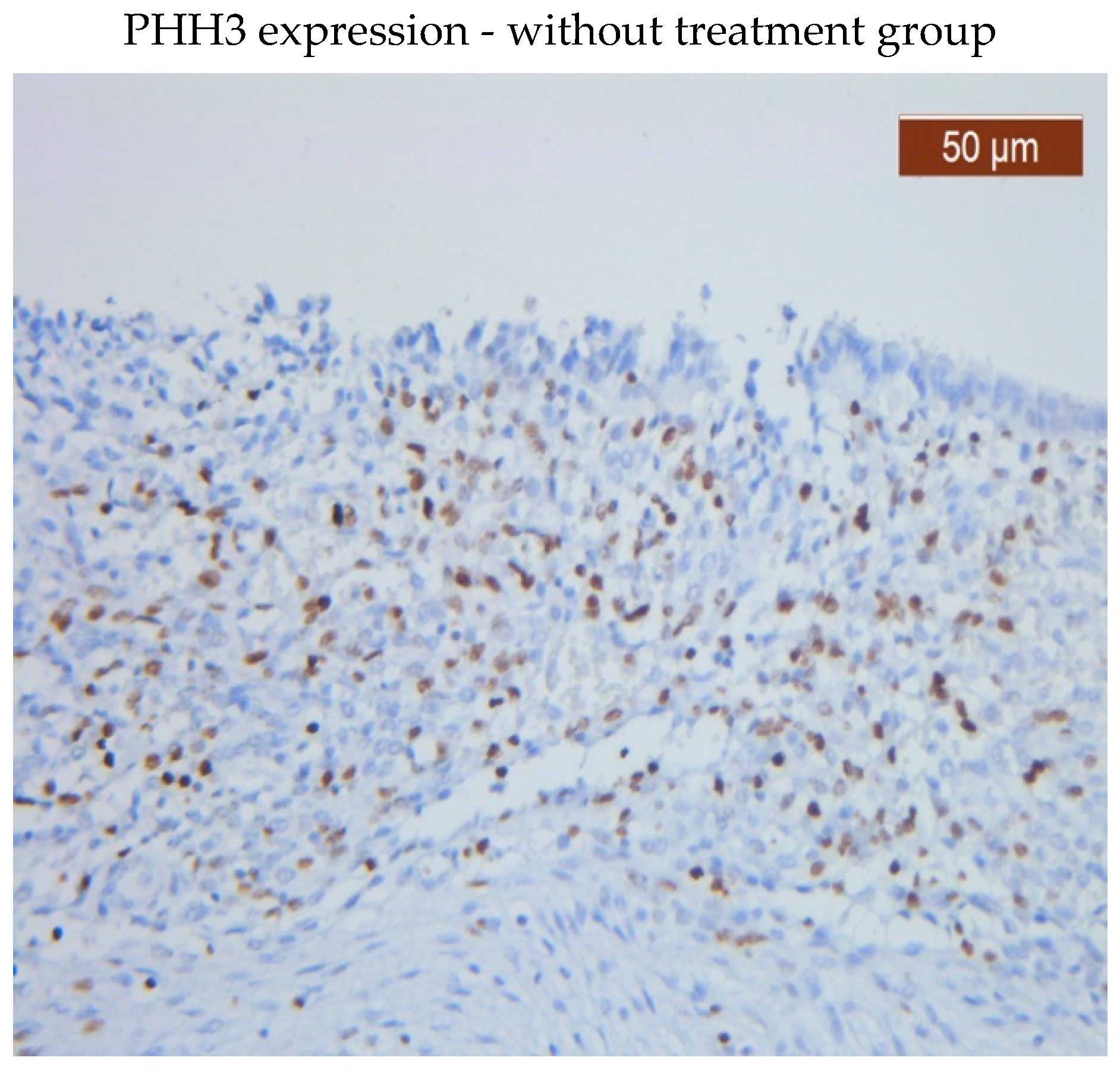
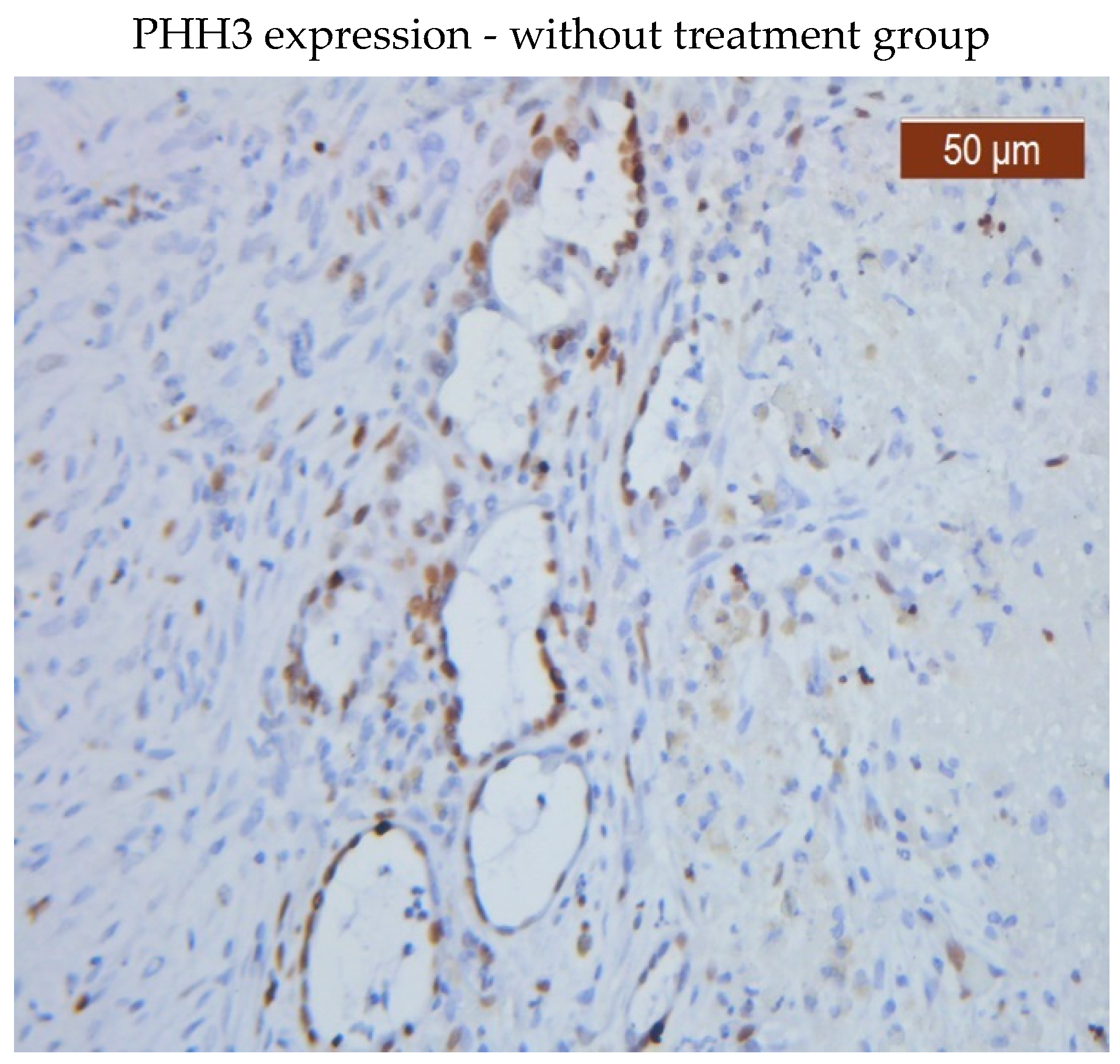
PHH3 was expressed in both the glandular epithelium and endometrial stroma in the group that received no therapy. While PHH3 was expressed in the glandular epithelium in both groups, patients receiving medication had a lower level of PHH3 expression in the stroma than patients not receiving treatment.
PHH3 showed diffuse positive nuclear expression in the stromal (
Figure 1) and epithelial compartments (
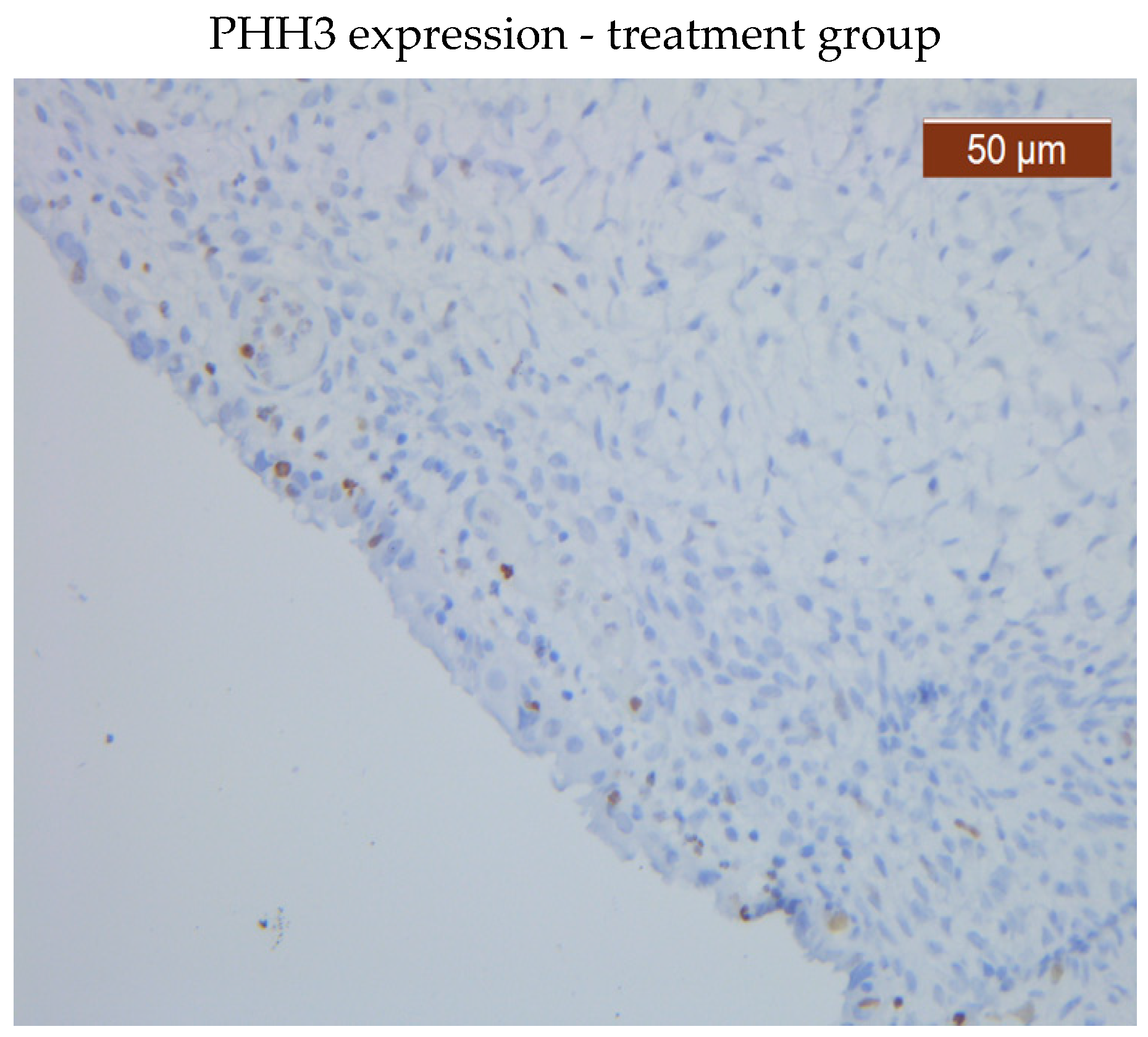
Figure 2), however, PHH3 expression levels in the treated group stroma were lower than those in the untreated one (
Figure 3 and 4).
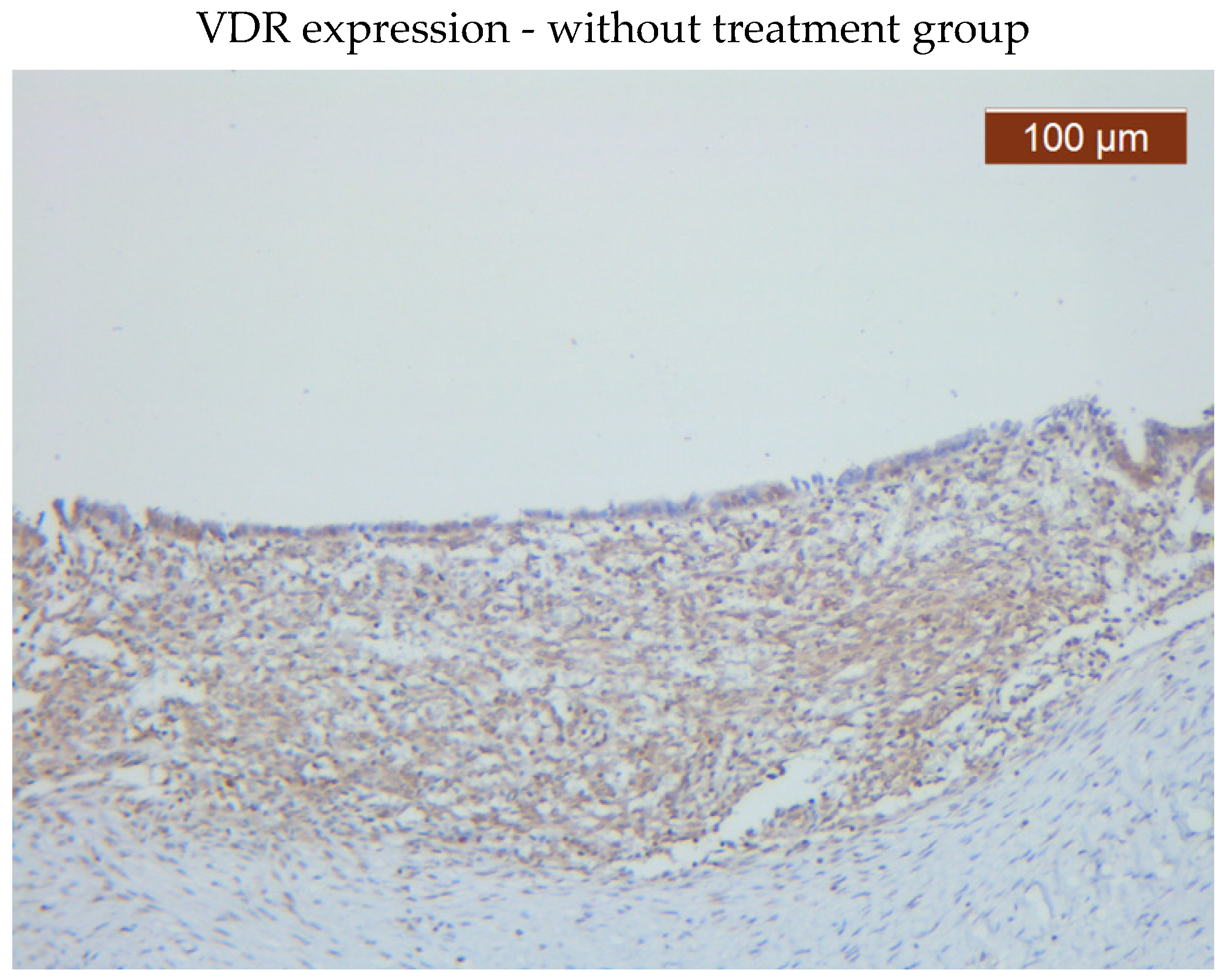
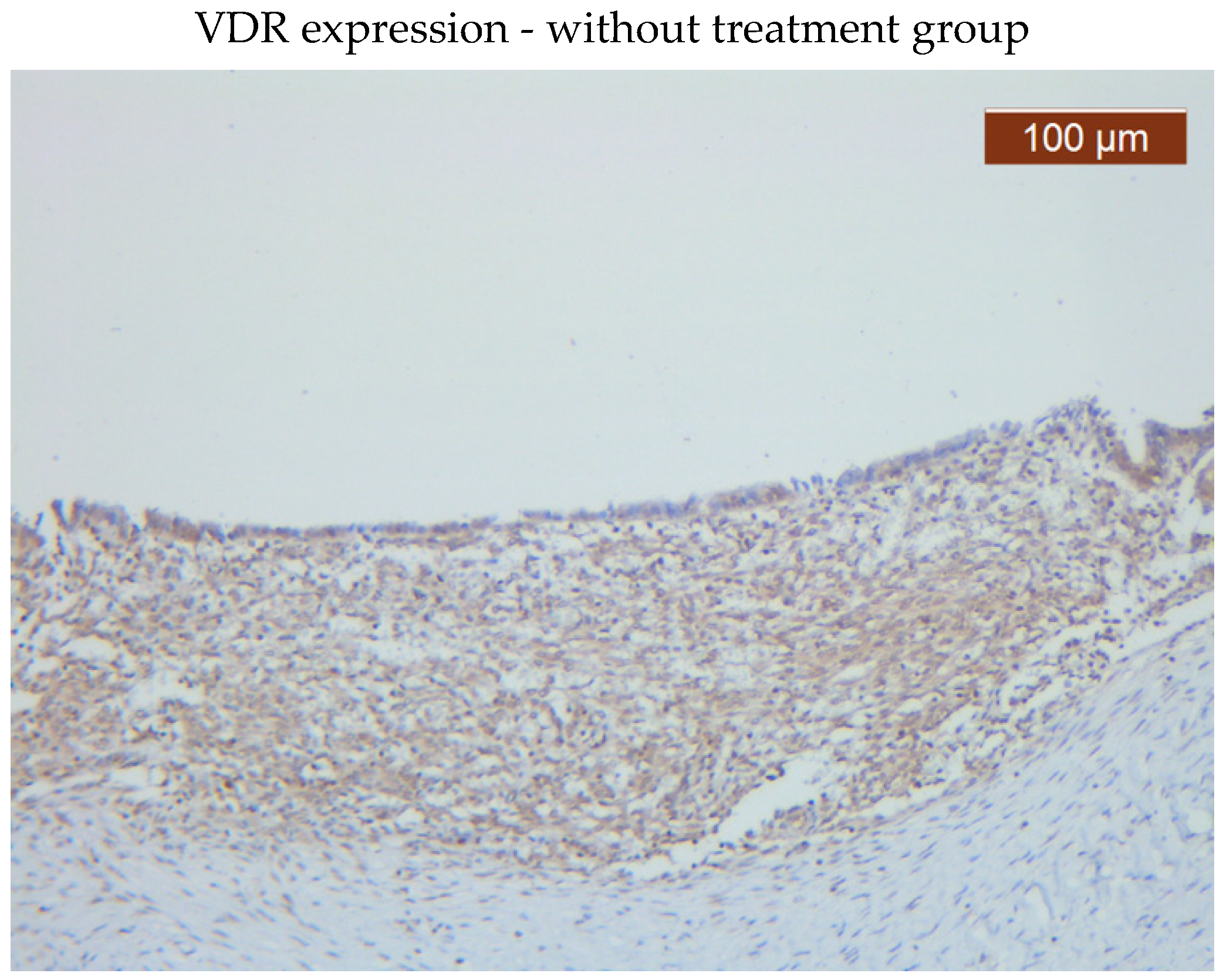
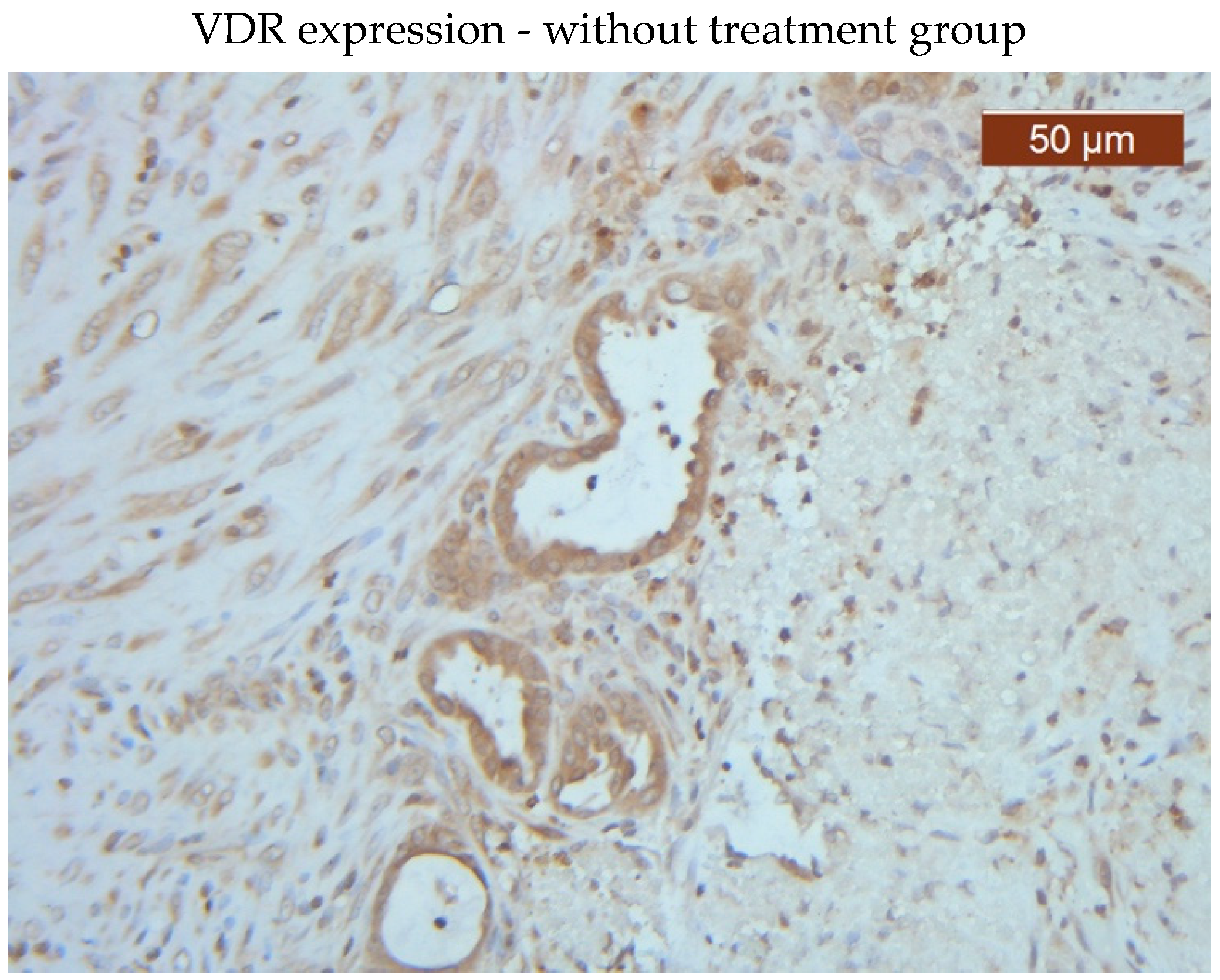
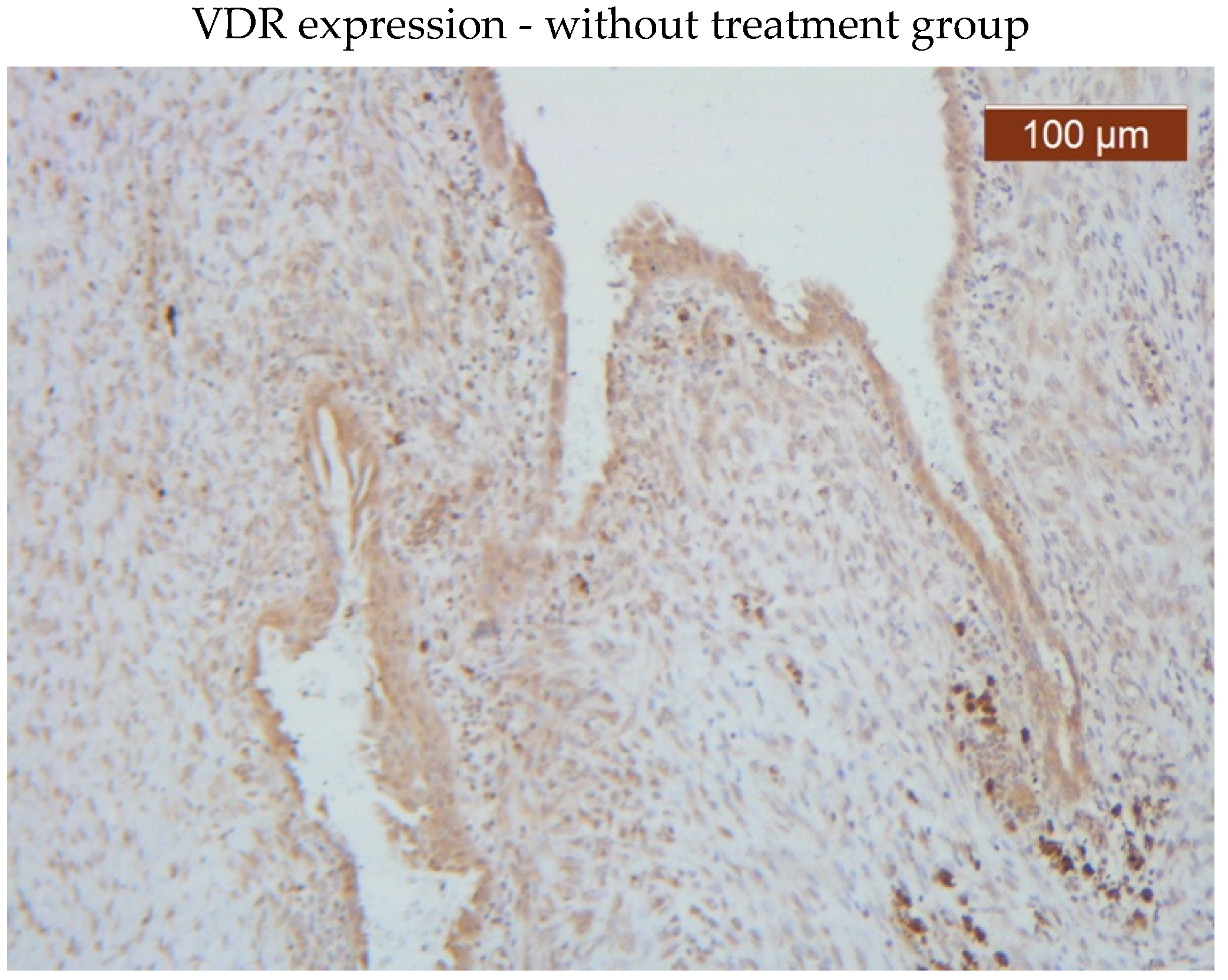
VDR showed diffuse positive nuclear expression both in the epithelial (
Figure 5) and stromal (
Figure 6) compartments, however, VDR expression levels in the treated group stroma tend to be lower than those in the untreated one (
Figure 7–8).
Comparative images of the immunohistochemical staining in the two groups (with or without treatment)
4. Discussion
There are limited data about PHH3, a protein thought to play a role in a number of biological processes related to endometriosis. The PHH3 protein, which is known to be involved in DNA-templated reactions to the nucleosome, is thought to undergo several genetic alterations over the course of this disease’s development and progression [
2,
18].
Information about PHH3 expression in endometriosis remains scarce.
Our research showed that the PHH3 signature is present in both the stromal and epithelial components and is associated with an increased risk of developing severe endometriosis. PHH3 was expressed in both the treated and untreated groups. Positive correlations and substantial associations between patients without therapy and positive PHH3 expression were identified.
There are only a few studies in literature evaluating VDR expression in endometriosis. It’s also debatable whether vitamin D levels and endometriosis are linked. The published reports regarding vitamin D in the literature showed a great variability of the available expression as follows: in endometrial receptivity during the menstrual cycle before embryo transfer [
20], in endometriotic heterotopies and endometrium of patients with genital endometriosis [
21], and the study of vitamin D in ovarian endometriosis compared to the three phases of the normal endometrium [
8]. Contrarily, our approach has been to use vitamin D that promotes secretion and regulates normal cellular growth at a local level, focusing on the role of vitamin D in defining endometriosis. This has allowed us to determine the ability of both epithelial and stromal cells to synthesize vitamin D in accordance with available treatment options.
Contradictory results have been found in human investigations, despite the fact that in vitro and animal studies have supported the immunomodulatory effects of vitamin D in endometriosis, with the regression of implants. The extremely varied nature of endometriosis may be a factor in the inconsistent nature of study findings. Additionally, 1,25(OH)
2 vitamin D, the active form of vitamin D, has a short half-life, making it challenging to accurately measuring it [
7,
8,
20,
21]. Agic et al. have proposed that, in contrast to its well-known endocrine function, vitamin D may have more local effects, autocrine and/or paracrine that contribute to endometriosis. He detected higher levels of vitamin D in ovarian endometriosis cysts compared to normal ovarian tissue. We measured VDR expression in tissue samples (both in epithelium and stroma) from women with endometriosis in an effort to take a further step in the direction suggested by Agic et al., 2007 [
22].
There is scarce research analyzing the tissue levels of VDR in endometriosis-affected women. Due to numerous discrepancies found in human and animal studies about the serum vitamin D levels and its effects in endometriosis patients, we made the decision to conduct this analysis at the tissue level. In another investigation on human endometriotic stromal cells, Miyashita et al. found that vitamin D treatment did not only reduce inflammation but also significantly decreased the viability of the aforementioned cell type, rather than having a proapoptotic effect [
23]. The next step in this direction is revealed by our study that proved high VDR expression in our endometriotic tissue samples in women without hormonal therapy. This aspect, combined with the low serum vitamin D levels found in endometriosis patients in most studies and the lower tissue levels observed in ours in women receiving hormonal therapy, suggests that vitamin D supplementation is necessary in patients with this condition in order to slow down the spread and severity of the disease [
7,
9,
23,
24,
25].
In their 2021 investigation, Yarmolinskaya et al. took into account the polymorphism of vitamin D nuclear receptor while evaluating the expression of VDR in the eutopic and ectopic endometrium in 32 women with endometriosis and in 20 healthy controls. In an effort to confirm the favorable effects of hormonal treatment in this still enigmatic disease, we only intended to examine the receptor presence in endometriosis-affected women who were receiving progestine treatment or not. The Russian researchers discovered no cyclic fluctuations with the menstrual cycle phases compared to controls and a lower VDR expression in the ectopic endometrium than in the eutopic one in women with endometriosis. We made an effort to go further into this area by examining VDR expression in the endometriotic cyst tissue of women who had already received endometriosis diagnoses and contrasting women with treatment with those who had not received treatment. High amounts of VDR were found in women who weren’t receiving treatment [
21].
We are aware of no other studies on VDR expression in women’s endometriotic ectopic tissue outside the aforementioned Russian study [
22,
26,
27].
The increased expression of VDR in the endometrium of endometriosis-suffering women without therapy shows that the molecular pathway is actively involved in the development and pathophysiology of endometriosis. Nevertheless, there is a strong correlation between the presence of hormonal action and the low expression of VDR in the group receiving treatment.
Vitamin D functions as a cellular signal transducer to suppress proliferation in the group receiving treatment due to its lower receptor expression in epithelial and stromal components. Based on their findings, the authors of an extensive review of the available vitamin D literature from 2018 came to the conclusion that vitamin D is a steroid hormone with progesterone-like activity. These findings, along with ours, suggest that VDR may be linked to some forms of endometrioses’ aggressiveness in individuals who are not receiving treatment [
28]. Additionally, by highlighting the anti-fibrotic properties of vitamin D, Monastra et al. suggest a new line of inquiry for us: whether vitamin D supplementation, in addition to the standard progestin therapy, might help endometriosis patients avoid the fibrosis and adhesions that are characteristic of their condition and alleviate pain-related symptoms [
29].
We discovered that patients receiving treatment had lower levels of PHH3 and VDR, indicating a protective effect of the hormone therapy. The role of PHH3 in risk stratification and prognostic of endometriosis is basically equivalent to the direct protein expression. The use of PHH3 IHC analysis makes it more convenient for clinicians to detect endometriosis.
It is hypothesized that PHH3, which functions as a nucleosome for DNA synthesis, plays a role in endometriosis patients’ increased proliferation and decreased apoptosis rates in the epithelial and stromal areas.
In endometriosis patients, PHH3 knockdown contributes to preventing cellular division and proliferation, which raises the apoptosis rate of the epithelial and stromal endometriosis areas.
PHH3 plays a role, equivalent to the direct protein expression, in endometriosis risk assessment and prognostication. Clinicians can more easily identify endometriosis when PHH3 IHC detection is used.
According to these findings, the current investigation revealed that an important percentage of the group not receiving therapy expressed PHH3.
Study Limitations
Our research has some limitations. The first is illustrated by the small number of patients whose tissue samples were examined and included in our analysis. We need extensive additional studies in this area so we can understand the variation in previous findings on vitamin D levels in endometriosis patients. The second aspect is represented by the fact that only endometriosis cysts were used for sample collection in all cases. This disease’s phenotype cannot be regarded as typical of an illness as complex as endometriosis. As a further direction, we will try to correlate these results with serum vitamin D and PHH3 levels in these patients.
5. Conclusions
PHH3 overexpression has been associated with the group that received no therapy, and additional research has not supported the specificity of anti-PHH3 antibody immunostaining. From what we know, this is the first study that evaluated tissue VDR expression in women with endometriosis with and without treatment. Vitamin D overexpression is linked to patients’ poor prognoses in the absence of treatment. Additionally, this analysis of the relationship between PHH3 and VDR tissue expression in endometriosis-affected women was analyzed for the first time, according to our knowledge.
Author Contributions
Conceptualization, D.R.M. and L.L.; methodology, C.E.M. and A.I.G.B.; software, I.E.B.; validation, I.E.B., C.E.M. and A.I.G.B.; formal analysis, L.L. and A.E.C.; investigation, E.B.; resources, E.B.; data curation, A.U.; writing—original draft preparation, D.R.M.; writing—review and editing, A.U.; D.R.M. and L.L.; visualization, C.E.M.; supervision, A.U.; project administration, D.R.M.; funding acquisition, D.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the UNIVERSITY OF MEDICINE AND PHARMACY “GR. T. POPA”, IAŞI, Romania, grant number 10310/29.06.2020.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the UNIVERSITY OF MEDICINE AND PHARMACY “GR. T. POPA”, IAŞI (10/28.09.2020) and of the UNIVERSITY OF MEDICINE AND PHARMACY “CAROL DAVILA:, BUCHAREST (06/21.12.2021).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data used to support the findings of this study are available upon request to the corresponding author.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsamantioti, E.S.; Mahdy, H. Endometriosis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023[Updated 2023 Jan 23].https://www.ncbi.nlm.nih.gov/books/NBK567777/.
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int J Mol Sci 2021, 22, 10554. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; Arnal, J.F.; Lenfant, F. Estrogen Receptors and Endometriosis. Int J Mol Sci 2020, 21, 2815. [Google Scholar] [CrossRef]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Sivajohan, B.; Elgendi, M.; Menon, C.; Allaire, C.; Yong, P.; Bedaiwy, M.A. Clinical use of artificial intelligence in endometriosis: a scoping review. NPJ Digit Med 2022, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzopoulos, D.R.; Samartzis, N.; Kolovos, G.N.; Mareti, E.; Samartzis, E.P.; Eberhard, M.; Dinas, K.; Daniilidis, A. Treatment of endometriosis: a review with comparison of 8 guidelines. 2021; 21. [Google Scholar] [CrossRef]
- Lopes, V.M.; Lopes, J.R.; Brasileiro, J.P.; Oliveira, I.; Lacerda, R.P.; Andrade, M.R.; Tierno, N.I.; Souza, R.C.; Motta, L.A. Highly prevalence of vitamin D deficiency among Brazilian women of reproductive age. Arch Endocrinol Metab 2017, 61, 21–27. [Google Scholar] [CrossRef] [PubMed]
- de Pascali, F.; Casarini, L.; Kuhn, C.; Simoni, M.; Mahner, S.; Jeschke, U.; von Schonfeldt, V. Nuclear expression of VDR and AHR is mutually exclusive in glandular cells in endometriosis. Histochem Cell Biol 2021, 156, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzopoulos, D.R.; Lempesis, I.G.; Athanasaki, F.; Schizas, D.; Samartzis, E.P.; Kolibianakis, E.M.; Goulis, D.G. Association between vitamin D and endometriosis: a systematic review. Hormones (Athens) 2020, 2020 19, 109–121. [Google Scholar] [CrossRef]
- Kongsbak, M.; Levring, T.B.; Geisler, C.; von Essen, M.R. The vitamin d receptor and T cell function. Front Immunol 2013, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Tan, X.; Peng, X.; Bai, R.; Xiao, Q.; Zou, T.; Tan, J.; Zhang, H.; Wang, C. The relationships of vitamin D, vitamin D receptor gene polymorphisms, and vitamin D supplementation with Parkinson’s disease. Transl Neurodegener 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Attar, E.; Bulun, S.E. Aromatase inhibitors: the next generation of therapeutics for endometriosis? FertilSteril 2006, 85, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.J.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev 2016, 5, CD012179. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, Z.; Qu, Q.; Li, X.; Lu, X.; Zhang, H. Exosomal lncRNA HOTAIR Promotes the Progression and Angiogenesis of Endometriosis via the miR-761/HDAC1 Axis and Activation of STAT3-Mediated Inflammation. Int J Nanomedicine 2022, 17, 1155–1170. [Google Scholar] [CrossRef]
- Nasu, K.; Kawano, Y.; Kai, K.; Aoyagi, Y.; Abe, W.; Okamoto, M.; Narahara, H. Aberrant histone modification in endometriosis. Front Biosci (Landmark Ed) 2014, 19, 1202–1214. [Google Scholar] [CrossRef]
- Rytkönen, K.T.; Faux, T.; Mahmoudian, M.; Heinosalo, T.; Nnamani, M.C.; Perheentupa, A.; Poutanen, M.; Elo, L.L.; Wagner, G.P. Histone H3K4me3 breadth in hypoxia reveals endometrial core functions and stress adaptation linked to endometriosis. iScience 2022, 25, 104235. [Google Scholar] [CrossRef]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A. P Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod Biol Endocrinol 2020, 18, 84. [Google Scholar] [CrossRef]
- Psilopatis, I.; Vrettou, K.; Fleckenstein, F.N.; Theocharis, S. The Impact of Histone Modifications in Endometriosis Highlights New Therapeutic Opportunities. Cells 2023, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.Y.; Ryu, Y.; Pornour, M.; Qi, J. Histone demethylase JMJD1A in cancer progression and therapeutic resistance. Mol Carcinog 2022, 61, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, S.; Whang, P.; Ren, H.; Li, Y. Characterization of VDR and CYP27B1 expression in the endometrium during the menstrual cycle before embryo transfer: implications for endometrial receptivity. Reprod Biol Endocrinol 2020, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinskaya, M.; Denisova, A.; Tkachenko, N.; Ivashenko, T.; Bespalova, O.; Tolibova, G.; Tral, T. Vitamin D significance in pathogenesis of endometriosis. Gynecol Endocrinol 2021, 37, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Agic. , A.; Xu, H.; Altgassen, C.; Noack, F.; Wolfler, M.M.; Diedrich, K.; Friedrich, M.; Taylor, R.N.; Hornung, D. Relative expression of 1,25-dihydroxyvitamin D3 receptor, vitamin D 1 alpha-hydroxylase, vitamin D 24-hydroxylase, and vitamin D 25-hydroxylase in endometriosis and gynecologic cancers. Reprod Sci 2007, 14, 486–497. [Google Scholar] [CrossRef]
- Miyashita, M.; Koga, K.; Izumi, G.; Sue, F.; Makabe, T.; Taguchi, A.; Nagai, M.; Urata, Y.; Takamura, M.; Harada, M.; Hirata, T.; Hirota, Y.; Wada-Hiraike, O.; Fujii, T.; Osuga, Y. Effects of 1,25-Dihydroxy Vitamin D3 on Endometriosis. J Clin Endocrinol Metab 2016, 101, 2371–2379. [Google Scholar] [CrossRef]
- Abbas, M.A.; Taha, M.O.; Disi, A.M.; Shomaf, M. Regression of endometrial implants treated with vitamin D3 in a rat model of endometriosis. Eur J Pharmacol 2013, 715, 72–75. [Google Scholar] [CrossRef]
- Akyol, A.; Simsek, M.; Ilhan, R.; Can, B.; Baspinar, M.; Akyol, H.; Gul, H.F.; Gursu, F.; Kavak, B.; Akin, M. Efficacies of vitamin D and omega-3 polyunsaturated fatty acids on experimental endometriosis. Taiwan J Obstet Gynecol 2016, 55, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Zelenko, Z.; Aghajanova, L.; Irwin, J.C.; Giudice, L.C. Nuclear receptor, coregulator signaling, and chromatin remodeling pathways suggest involvement of the epigenome in the steroid hormone response of endometrium and abnormalities in endometriosis. Reprod Sci 2012, 19, 152–162. [Google Scholar] [CrossRef]
- Bergada, L.; Pallares, J.; Arcidiaconu, M.V.; Cardus, A.; Santacana, M.; Valls, J.; Cao, G.; Fernandez, E.; Dolcet, X.; Dusso, A.S.; Matias-Guiu, X. Role of local bioactivation of vitamin D by CYP27A1 and CYP2R1 in the control of cell growth in normal endometrium and endometrial carcinoma. Lab Invest 2014, 94, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Monti, N.; Cucina, A. Fibrosis: A Role for Vitamin D. Organisms. Journal of Biological Sciences, 2020, 4, 26–41. [Google Scholar] [CrossRef]
- Monastra, G.; De Grazia, S.; De Luca, L.; Vittorio, S.; Unfer, V. Vitamin D: a steroid hormone with progesterone-like activity. Eur Rev Med Pharmacol Sci 2018, 22, 2502–2512. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).