1. Introduction
Lung cancer is the second most common cancer in the world and the leading cause of cancer death worldwide [
1]. NSCLC is the most common type of lung cancer, accounting for 80% of all diagnosed lung cancer cases [
2,
3]. The treatment of NSCLC mainly includes radiotherapy (RT) and chemotherapy, surgical resection and immunotherapy. Nevertheless, for most patients, NSCLC remains an incurable disease [
4]. More than 50% of NSCLC patients receive RT during treatment [
5]. However, due to the radiation resistance of tumor tissue and radiation pneumonitis (RP) caused by RT on normal lung tissue adjacent to cancer, RT failure affects the quality of life of patients [
6,
7]. Therefore, the development of radiosensitizers that can effectively increase the toxicity of radiation to cancer tissues while reducing the damage to normal lung tissues will help to improve the clinical treatment effect of NSCLC.
The anti-cancer effect of RT is due by increasing DNA double-strand breaks (DSBs) of cancer cells [
8]. HR and NHEJ are main responsible pathways for DNA repair. The key repair proteins of HR and NHEJ include ATR, CHK1, DNA-PKcs, Ku70/Ku80, etc. [
9]. The dysregulation of DSB repair leads to RT resistance [
10]. Therefore, targeted inhibition of these RT related DNA repair molecules can help to increase the radiosensitivity. In addition, the characteristics of high-energy radiation lead to the ionization of water molecules (H
2O) in the lung tissue to produce a large amount of reactive oxygen species (ROS), and triggering oxidative stress and the aggregation of inflammatory cells [
11]. NF-κB is a mediator that promotes the induction of inflammatory genes, and its expression is increased after irradiation. Therefore, targeted inhibition of NF-κB can also be used as a radioprotective agent to reduce the inflammatory response in normal lung tissues induced by RT [
12,
13].
JWA gene, also known as arl6ip5, is originally cloned from retinoic acid (RA) -induced differentiation model in human bronchial epithelial (HBE) cells. JWA is an environmental response and tumor suppressor gene [
14,
15]. The low expression of JWA gene is associated with the poor prognosis of gastric cancer patients; and high expression of JWA inhibits the proliferation, invasion and migration of breast cancer, pancreatic cancer, esophageal squamous cell carcinoma, NSCLC and other cancers [
16,
17,
18,
19,
20]. Moreover, activation of JWA reverses cisplatin resistance in gastric cancer and trastuzumab-resistant breast cancer [
21,
22]. In addition, activation of JWA can inhibit oxidative stress and improve intestinal epithelial homeostasis in radiation enteritis and antagonize paraquat-induced neurotoxicity [
23,
24]. JWA plays a bidirectional regulatory role in DNA damage of cancer cells and normal cells caused by chemotherapy [
25].
Recent studies have shown that the mimic functional peptide of JWA protein can increase the toxicity of cisplatin on drug-resistant gastric cancer cells while reduce its toxicity on normal cells [
25]. JAC4, a specific agonist of JWA gene, can protect the intestine epithelial cells from radiation-induced enteritis through antioxidant/inflammatory signaling [
26]. However, it is unknown if JAC4 could exert synergistic and attenuated effects of RT on NSCLC. Herein, we investigated the role of JAC4 and the potential mechanism in NSCLC RT. We found that JAC4 in combination with RT increased DSBs, cell apoptosis and inhibited xenograft tumor growth by blocking DNA repair in NSCLC cells; at the same time, JAC4 protected normal bronchial epithelial cells from RT-induced ROS, DSBs, and inhibited NF-κB-mediated inflammatory response. The bidirectional role of JAC4 was further verified in JWA knockdown cells and validated in NSCLC xenograft RT model mice.
2. Materials and Methods
2.1. Compounds and Reagents
JAC4 (C16H13N3O3S, MW: 327.4) with purity over 98% was synthesized in our laboratory. In this study, polyethylene glycol (40%), ethanol (7.5%), 0.9% normal saline (52.5%) were used to prepare the solution for JAC4. Nuclear and cytoplasmic protein separation kit (P0027); total SOD activity assay kit (S0101S), malondialdehyde (MDA) assay kit (S0131S), catalase assay kit (S0051), and PMSF (100 mM) (ST506) were purchased from Beyotime Biotechnology Co., LTD. (Shanghai, China). Cell/Tissue Total RNA Rapid Extraction Kit (RC112-01), HiScript III RT SuperMix for qPCR (+gDNA wiper) (R323-01), AceQ qRT-PCR SYBR Green Master Mix (Without ROX) (Q121-02) were purchased from Vazyme Biotechnology Co., LTD. (Nanjing, China).
2.2. Cell Culture, X-ray Irradiation and Transfection
Human lung adenocarcinoma cells (SPCA-1) and human bronchial epithelial cells (BEAS-2B) were purchased from Shanghai Institute of Cell Biology, Chinese Academy of Sciences, and cultured in DMEM medium containing 10% fetal bovine serum and 100 g/mL ciprofloxacin in an incubator at 37°C and 5% CO2. The cells were fully attached to the wall and returned to normal morphology, pretreated with JAC4 (10 µM) for 24 h, and exposed to X-ray irradiation (4 Gy) at a dose rate of 1.25 Gy/min. Cell biology experiments were performed 2 h or 24 h later. For cell transfection, small interfering RNA (siRNA) for JWA (siJWA: 5’- UAAUAGAGCAGGUUGCUCACUACGC -3’, Generay, Shanghai, China) and its nonspecific control were synthesized. Both SPCA-1 and BEAS-2B cells were seeded into six-well plates. According to the Lipo 8000TM transfection reagent (Beyotime, Nanjing, China), when the cell density reached 70%, Opti-MEM® Medium 125 μL/well and siJWA (or sicontrol): Lipo 8000TM =100 pmol: 4 μL =25: 1 was mixed then cocultured with cells.
2.3. Cytotoxicity Assay
In total of 5,000 SPCA-1 and BEAS-2B cells were inoculated on 96-well plates for 24 h, and irradiated with 0, 4, 8, 12, 16 and 20 Gy, or treated with JAC4 of 0, 1, 5, 10, 20 and 50 μM for 24 h, respectively. CCK-8 reagent was added to each well and incubated in cell incubator for 1 h. Absorbance (OD) was measured at 450 nm. Cell viability calculation formula: cell viability (%) = (OD control group-OD treatment group) ×100% / (OD control group-OD blank hole).
2.4. Western Blotting Assay
The cell samples were prepared from lysates containing protease and phosphatase inhibitors; animal tumor tissues and lung tissues were fully ground in a mortar with liq-uid nitrogen, and appropriate amount of lysate containing PMSF and protease phosphatase inhibitor was added, which was fully cracked in a refrigerator at 4°C and centrifuged for use. The protein immunoblotting experiments were carried out according to the protocol [
27]. The antibodies used in this study are as follows: anti-JWA (1:100, AbMax, Beijing, China), anti-GAPDH (1:1000, Beyotime, Shanghai, China), anti-Tubulin (1:1000, Beyotime, Shanghai, China), anti-p-p65 (1:1000, Cell Signaling Technology, USA), anti-P65 (1:1000, Santa Cruz, CA, USA), anti-γ-H2AX (1:1000, Servicebio, Wuhan, China), anti-DNA-PKcs (1:1000, Abcam), anti-Ku70 (1:3000, Affinity), anti-p-ATR (1:1000, Cell Signaling Technology, USA), anti-p-CHK1 (1:1000, Cell Signaling Technology, USA).
2.5. Quantitative Reverse Transcription and Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted with the Cell/Tissue Total RNA Rapid Extraction Kit, the RNA concentration was determined, and 1 μg total RNA was synthesized into cDNA. AceQ qPCR SYBR Green Master Mix (Vazyme, Nanjing, China) was used in ABI 7900HT real-time PCR system (Applied Biosystems, Carlsbad, CA, USA) for PCR reaction procedure. The primer sequences were indicated as follows:
GAPDH: Forward: 5’- CATCACTGCCACCCAGAAGACTG -3’; Reverse: 5’- ATGCCAGTGAGCTTCCCGTTCAG -3’; IL-10: Forward: 5’- CGGGAAGACAATAACTGCACCC -3’; Reverse: 5’- CGGTTAGCAGTATGTTGTCCAGC -3’; TNF-α: Forward: 5’- GGTGCCTATGTCTCAGCCTCTT -3’; Reverse: 5’- GCCATAGAACTGATGAGAGGGAG -3’; IL-1β: Forward: 5’- TGGACCTTCCAGGATGAGGACA -3’; Reverse: 5’- GTTCATCTCGGAGCCTGTAGTG -3’; TGF-β1: Forward: 5’- TGATACGCCTGAGTGGCTGTCT -3’; Reverse: 5’- CACAAGAGCAGTGAGCGCTGAA -3’. With GAPDH as the internal parameter, normalization processing.
2.6. Apoptosis Assay
The cells were spread in 6-well plates for 24 h, pretreated with 10 μM JAC4 for 24 h, then treated with 4 Gy X-ray irradiation. After 24 h, the culture medium was abandoned and cells were washed twice with PBS, fixed with cell fixative for 10 min, removed cell fixative, cleaned twice with PBS, 3 min each time. 1 mL Hoechst 33342 cell staining solution (Beyotime, Shanghai, China) was added to each well and placed in a shaker for staining for 5 min. Discard the dyeing solution and washed with PBS twice, 3 min each time; a drop of anti-fluorescence quencher was then placed in the center of the petri dish and photographed under an inverted fluorescence microscope; and the proportion of apoptotic cells was analyzed. Apoptotic cells were defined as those whose nuclei were fragmented or densely stained. The intact nucleus is a healthy, normal cell. The experiment was carried out at least three times.
2.7. MDA, SOD and CAT Assays
The cells were planted on the 6-well plate, treated accordingly, and tested according to the operating instructions of the kits (Beyotime, Shanghai, China).
2.8. Immunofluorescence Assay
For immunofluorescence detection, the tissue sections and cells were incubated with primary anti body and then labeled with an anti-rabbit (or mouse) secondary antibody conjugated with Cy3 or FITC (1:1000) in the dark room for 1 h at room temperature. Cell nuclei were counterstained with 40, 60-diamidino-2-phenylindole (DAPI). Images were acquired under a laser scanning confocal microscope (Carl Zeiss Jena) or fluorescence inverted microscope (Nikon). The following antibodies were used: anti-γ-H2AX (1:200, Servicebio, Wuhan, China), anti-P65 (1:200, Santa Cruz, CA, USA), anti-JWA (1:50, AbMax, Beijing, China), anti-TNF-α (1:25, Cell Signaling Technology, USA), anti-TGF-β1 (1:200, Affinity), anti-DNA-PKcs (1:200, Abcam), anti-Ku70 (1:200, Affinity).
2.9. JC-1 Staining and Reactive Oxygen Species Assay
Mitochondrial membrane potential decline is a landmark event in the early stage of apoptosis. According to the fluorescence probe DCFH-DA entered the cell, the hydrolyzed DCFH was oxidized to produce fluorescent DCF to detect the intracellular ROS level. The cells were planted on six-well plates, treated accordingly, and tested according to the operating instructions of the mitochondrial membrane potential kit (Beyotime, Shanghai, China) and ROS detection kit (Beyotime, Shanghai, China). The intensity of fluorescent staining was quantified with Image J software.
2.10. Colony Formation Assay
SPCA-1 and BEAS-2B cells were seeded in six-well plates at a density of 500, 1,000 and 1, 500 cells/per well. The cells were treated with 10 μM JAC4 for 24 h and divided into different groups and treated with different doses of radiation (0, 2, 4 or 6 Gy). Fresh medium containing 10 μM JAC4 was replaced every 72 h and incubated in a cell incubator for 8-12 days until colony formation. The plates were then stained with crystal violet (Beyotime, Shanghai, China) and the number of colonies (≥ 50 cells) was counted. Cell colony formation rate, survival fraction and radiosensitization rate were calculated with reference to [
28].
2.11. SPCA-1 Cell Xenograft Tumor and Radiotherapy Mice Model
Male nude mice, aged 4-5 weeks, weighing 18-22 g, were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China) and housed in the Laboratory Animal Center of Nanjing Medical University. The mice were fed with food and water AD libitum for 12 h:12 h of night and day, in a SPF clean environment of 25 ± 2 °C and 50 % ± 10 % humidity. The animal study protocol was approved by the Animal Care Committee of Nanjing Medical University (Nanjing, China). After 7 days of acclimatization, SPCA-1 cells (5 × 106) were subcutaneously injected into the right armpit of the mice, level with the upper lung. When the tumor mass reached 60-100 mm3 (tumor volume = length×width2/2), the mice were randomly divided into control group, 100 mg/kg JAC4 group (daily administration), 3 Gy×5 fractions X-ray irradiation group (continuous 5 days of X-ray irradiation), and 100 mg/kg JAC4 + 3 Gy×5 fractions X-ray irradiation group; six mice in each group. RADSOURCE (USA) provided focused beams for localized irradiation of the tumor while ensuring that the whole lung of the mice could be irradiated. Body weight and tumor volume were measured daily. Each tumor tissue was divided into two parts, one half of which was fixed in 4 % paraformaldehyde, and the other half was frozen in a refrigerator at -80 °C for further use. The right lower lobe of each mouse was fixed with 4 % paraformaldehyde, and the remaining lung tissues were frozen in the refrigerator at -80 °C for later use. Routine H&E staining was used for pathological examination of major organs.
2.12. Statistical Analysis
GraphPad Prism 8.0 software was used to analyze the experimental data and draw statistical maps. All data are presented as mean ± S.D. One-way-ANOVA and Student’s t-test was separately used for comparison between multiple groups and two groups. p< 0.05 was considered statistically significant.
4. Discussion
RT is one of the main treatments for most cancers including NSCLC. Due to the rapid repair of DSBs in tumor tissue, cancer tissue can survive for a long time. There are few radiosensitizers that can simultaneously enhance efficacy and attenuate toxicity in RT. In this study, we reported for the first time that JAC4 has a synergistic and attenuate effect in RT for NSCLC. On the one hand, JAC4 enhanced the killing effect of RT on NSCLC both in vitro and
in vivo. On the other hand, JAC4 reduced the toxic effect of RT on normal lung tissue. The mechanistic evidence showed that JAC4 promoted apoptosis in NSCLC by blocking ATR-CHK1 and DNA-PKcs and further inhibited DNA repair therefore increased DNA double-strand damage (
Figure 8A). In addition, JAC4 enhanced antioxidant, anti-inflammatory abilities and prevented NF-κB nuclear translocation therefore attenuated radiation induced oxidative stress and inflammation in normal lung tissue (
Figure 8B). Our study may provide a new valuable target and strategy for lung cancer RT.
RT resistance of NSCLC is closely related to PI3K-AKT, HGF-cMet, cGAS-STING, HR and NHEJ signaling pathways [
32,
33,
34,
35]. A number of studies have shown that radiosensitizers that inhibit DNA double-strand break repair molecules can increase efficacy of RT on lung cancer [
36,
37,
38]. ATR is activated by TopBP1 or ETAA1 is recruited to RPA-ssDNA, phosphorylates and activates CHK1 to cause cell cycle arrest, leaving sufficient time for DNA damage to be checked and repaired [
39]. Ku binds to DNA-PKcs and triggered by DNA double-strand damage caused by radiation. Self-phosphorylation of DNA-PKcs changes its conformation and releases it from the DNA double-strand, resulting in NEHJ [
40]. In this study, JAC4 inhibited the expression of ATR/CHK1 and DNA-PKcs/Ku70 proteins by activating JWA gene expression, which increased DSBs and promoted apoptosis in lung cancer cells. In NSCLC, JWA and topoisomerase IIα have a negative feedback regulation on each other, and inhibition of topoisomerase IIα will lead to increase DNA damage [
20,
41]. In this study, JAC4 in combination with RT increased expression of JWA gene and inhibited the expression of ATR/CHK1 in NSCLC, which may be related to topoisomerase IIα. How JWA inhibits the expression of DNA-PKcs in NSCLC needs to be further elucidation.
JWA maintains redox homeostasis and suppresses inflammation process by inhibition of NF-κB to reduce ROS production [
30]. SOD is known enzyme that directly remove free radicals [
42]. SOD converts oxygen radicals into hydrogen peroxide. Catalase (CAT) decomposes hydrogen peroxide into water and oxygen molecules and is an important antioxidant [
43]. MDA is the product of peroxidation of polyunsaturated fatty acids, and the production of ROS can cause the production of MDA [
44]. Ionizing radiation produces a large number of various ROS in the body, resulting in oxidative stress and inflammatory reactions [
45]. Continuous ROS and oxidative stress cause DNA double-strand damage. Therefore, antioxidant system plays important role in the prevention and treatment of RP. In this study, after JAC4 treatment, JWA was activated to prevent the translocation of NF-κB into nucleus in normal cells, and the levels of ROS, MDA, pro-inflammatory factor TGF-β1, TNF-α and IL-1β were significantly decreased and the levels of SOD, CAT and anti-inflammatory factor IL-10 were increased in normal BEAS-2B cells, therefore obviously reduced irradiation induced DNA damages in normal cells. Studies have shown that inhibiting the activation of NF-κB can also enhance the sensitivity of RT to cancer cells [
46]. NF-κB may be the key point of bidirectional effect of JAC4 in protection of normal lung tissue from RT induced damage.
In lung tissue, ROS production is derived from multiple cells including endothelial cells, neutrophils, eosinophils, alveolar macrophages, and alveolar epithelial cells. The results of this study indicated that JAC4 inhibited ROS production in lung tissue, but the specific cell type targeted by JAC4 need to further identified. At present, the diagnosis of radiation-induced lung injury is still limited to early treatment and typical clinical manifestations [
47,
48], and lacks early predictive biomarkers. Our results showed that JAC4 through activation of JWA to alleviate radiation-induced oxidative stress, inflammation and DNA damages in lung tissues; at the same time, JAC4 enhances RT induced DNA damage and apoptosis in lung cancer cells. Therefore, whether JAC4 can be used as a protective agent for early intervention of radioactive lung injury; more importantly, further studies are needed to confirm whether the combination of JAC4 and radiotherapy can properly increase the radiation dose to further improve the therapeutic effect on lung cancer.
Single cell transcriptomics analysis indicate that many genes involved in mitochondrial depolarization are upregulated by RT, which may activate the cGAS-STING signaling pathway [
49]. In our study, JAC4 showed significantly different effects on RT-triggered mitochondrial membrane potential depolarization between SPCA-1 and BEAS-2B cells. This suggests that depolarization of mitochondrial membrane potential may be another key point of JAC4 bidirectional effect. It is well documented that cGAS-STING signaling pathway presents different expression states in cancer cells and normal lung tissues. Activation of cGAS-STING pathway enhances the radiosensitivity of NSCLC cells [
34]. Inhibition of cGAS-STING pathway can also be used to reduce radiation-induced lung injury [
50]. There is much evidence that some signal regulatory pathways are shared between cancer and normal cells, but the effects on phenotypes may be completely opposite, which is mostly due to the differential microenvironment between cancer and normal tissues. JWA may achieve the biological function of maintaining homeostasis by differentially regulating lung cancer cells and normal lung tissue cells damaged by radiation. Whether the bidirectional effects of JAC4 is related to the cGAS-STING pathway needs to be further clarified.
In conclusion, the synergistic and attenuated effects of JAC4 in NSCLC radiotherapy have been confirmed both in vitro and in vivo models. JWA differential regulation of oxidative stress and DNA repair in cancer and normal cells induced by radiotherapy is the key event for the bidirectional effect of JAC4 in combination with radiotherapy. The evidence from this study may provide translational new strategies for optimizing radiation therapy for lung cancer.
Figure 1.
JAC4 plays a bidirectional role in radiation-treated SPCA-1 and BEAS-2B cells. The viabilities of SPCA-1 (A) and BEAS-2B (B) cells exposed to different doses of X-ray irradiation (0, 4, 8, 12, 16, 20 Gy) after 24 h were measured by CCK-8 assay. The viabilities of SPCA-1 (C) and BEAS-2B (D) cells exposed to different doses of JAC4 (1, 5, 10, 20, 50 μM) or DMSO after 24 h were measured by CCK-8 assay. SPCA-1 (E) and BEAS-2B (F) cells were pretreated with JAC4 (10 µM) or DMSO for 24 h and then exposed to X-ray irradiation (4 Gy). After 24 h, the viabilities of cells were measured by CCK-8 assay. The radiosensitivities of JAC4 (10 μM) on SPCA-1(G) and BEAS-2B (I) cells treated with the indicated X-ray irradiation doses (0, 2, 4, 6 Gy) were evaluated by colony formation assay. The surviving fractions of SPCA-1 (H) and BEAS-2B (J) cells were calculated as described. Data are presented as the mean ± SD (n=3); (* p< 0.05, ** p< 0.01, *** p< 0.001, **** p< 0.0001, vs. control group and # p< 0.05, ## p< 0.01, vs. the X-ray irradiation group).
Figure 1.
JAC4 plays a bidirectional role in radiation-treated SPCA-1 and BEAS-2B cells. The viabilities of SPCA-1 (A) and BEAS-2B (B) cells exposed to different doses of X-ray irradiation (0, 4, 8, 12, 16, 20 Gy) after 24 h were measured by CCK-8 assay. The viabilities of SPCA-1 (C) and BEAS-2B (D) cells exposed to different doses of JAC4 (1, 5, 10, 20, 50 μM) or DMSO after 24 h were measured by CCK-8 assay. SPCA-1 (E) and BEAS-2B (F) cells were pretreated with JAC4 (10 µM) or DMSO for 24 h and then exposed to X-ray irradiation (4 Gy). After 24 h, the viabilities of cells were measured by CCK-8 assay. The radiosensitivities of JAC4 (10 μM) on SPCA-1(G) and BEAS-2B (I) cells treated with the indicated X-ray irradiation doses (0, 2, 4, 6 Gy) were evaluated by colony formation assay. The surviving fractions of SPCA-1 (H) and BEAS-2B (J) cells were calculated as described. Data are presented as the mean ± SD (n=3); (* p< 0.05, ** p< 0.01, *** p< 0.001, **** p< 0.0001, vs. control group and # p< 0.05, ## p< 0.01, vs. the X-ray irradiation group).
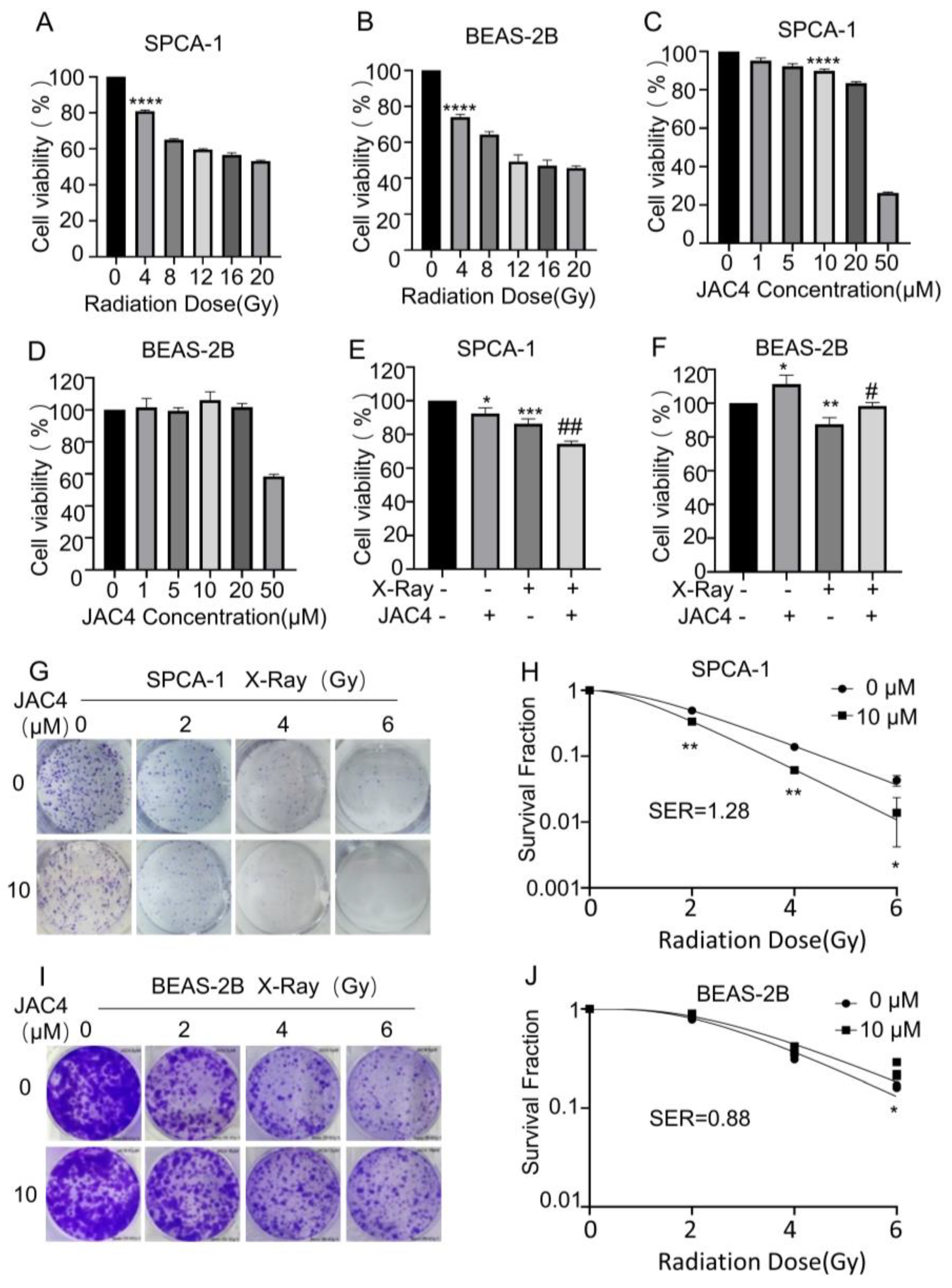
Figure 2.
JAC4 enhances radiation toxicity to SPCA-1 but reduces radiation toxicity to BEAS-2B cells. (A) The formation of γ-H2AX foci was determined by immunofluorescence assay at 2 h after 4 Gy of X-ray irradiation. The images of SPCA-1 cells are on the left panel, and the BEAS-2B cells are on the right panel. γH2AX (red) and DAPI (blue). The number of γ-H2AX foci of SPCA-1 (B) and BEAS-2B (C) cells were counted as described. (Scale bar, 20 μm) (D) The Western blotting images of γ-H2AX and JWA in SPCA-1 and BEAS-2B cells at 2 h after 4 Gy of X-ray irradiation. (E) SPCA-1 and BEAS-2B cells were pretreated with JAC4 (10 µM) or DMSO for 24 h and then exposed to X-ray irradiation (4 Gy). the Hoechst staining images in SPCA-1 and BEAS-2B cells. (Scale bars, 100 μm). (F-G) The quantitative data of apoptosis ratios. (H) JC-1 staining in SPCA-1 and BEAS-2B cells (Scale bars, 100 μm). (I-J) The ratio of red to green fluorescence intensity. Data are presented as the mean ± SD (n=3); (** p< 0.01, *** p< 0.001, **** p< 0.0001, vs. control group and # p< 0.05, ### p< 0.001, #### p< 0.0001, vs. the X-ray irradiation group).
Figure 2.
JAC4 enhances radiation toxicity to SPCA-1 but reduces radiation toxicity to BEAS-2B cells. (A) The formation of γ-H2AX foci was determined by immunofluorescence assay at 2 h after 4 Gy of X-ray irradiation. The images of SPCA-1 cells are on the left panel, and the BEAS-2B cells are on the right panel. γH2AX (red) and DAPI (blue). The number of γ-H2AX foci of SPCA-1 (B) and BEAS-2B (C) cells were counted as described. (Scale bar, 20 μm) (D) The Western blotting images of γ-H2AX and JWA in SPCA-1 and BEAS-2B cells at 2 h after 4 Gy of X-ray irradiation. (E) SPCA-1 and BEAS-2B cells were pretreated with JAC4 (10 µM) or DMSO for 24 h and then exposed to X-ray irradiation (4 Gy). the Hoechst staining images in SPCA-1 and BEAS-2B cells. (Scale bars, 100 μm). (F-G) The quantitative data of apoptosis ratios. (H) JC-1 staining in SPCA-1 and BEAS-2B cells (Scale bars, 100 μm). (I-J) The ratio of red to green fluorescence intensity. Data are presented as the mean ± SD (n=3); (** p< 0.01, *** p< 0.001, **** p< 0.0001, vs. control group and # p< 0.05, ### p< 0.001, #### p< 0.0001, vs. the X-ray irradiation group).
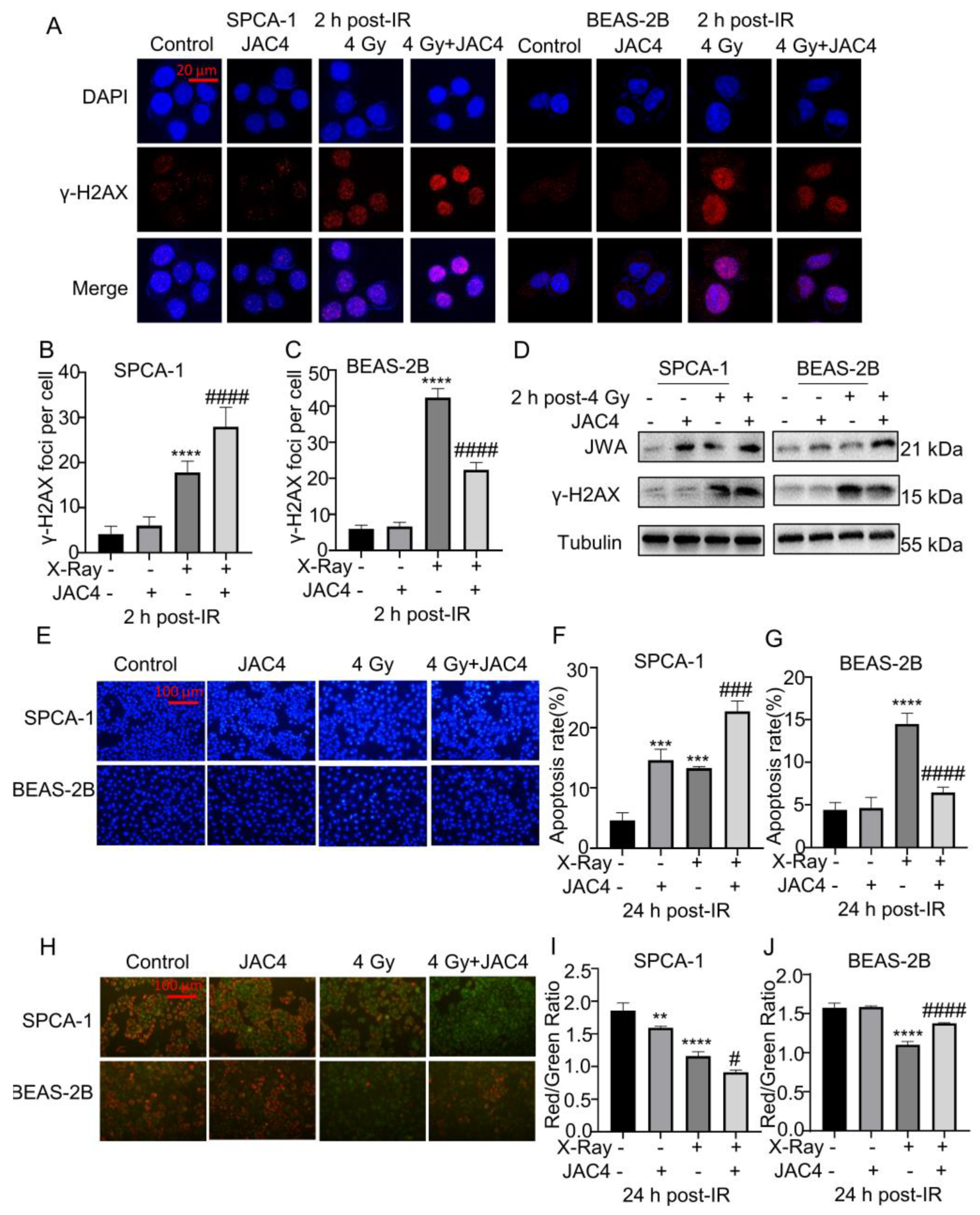
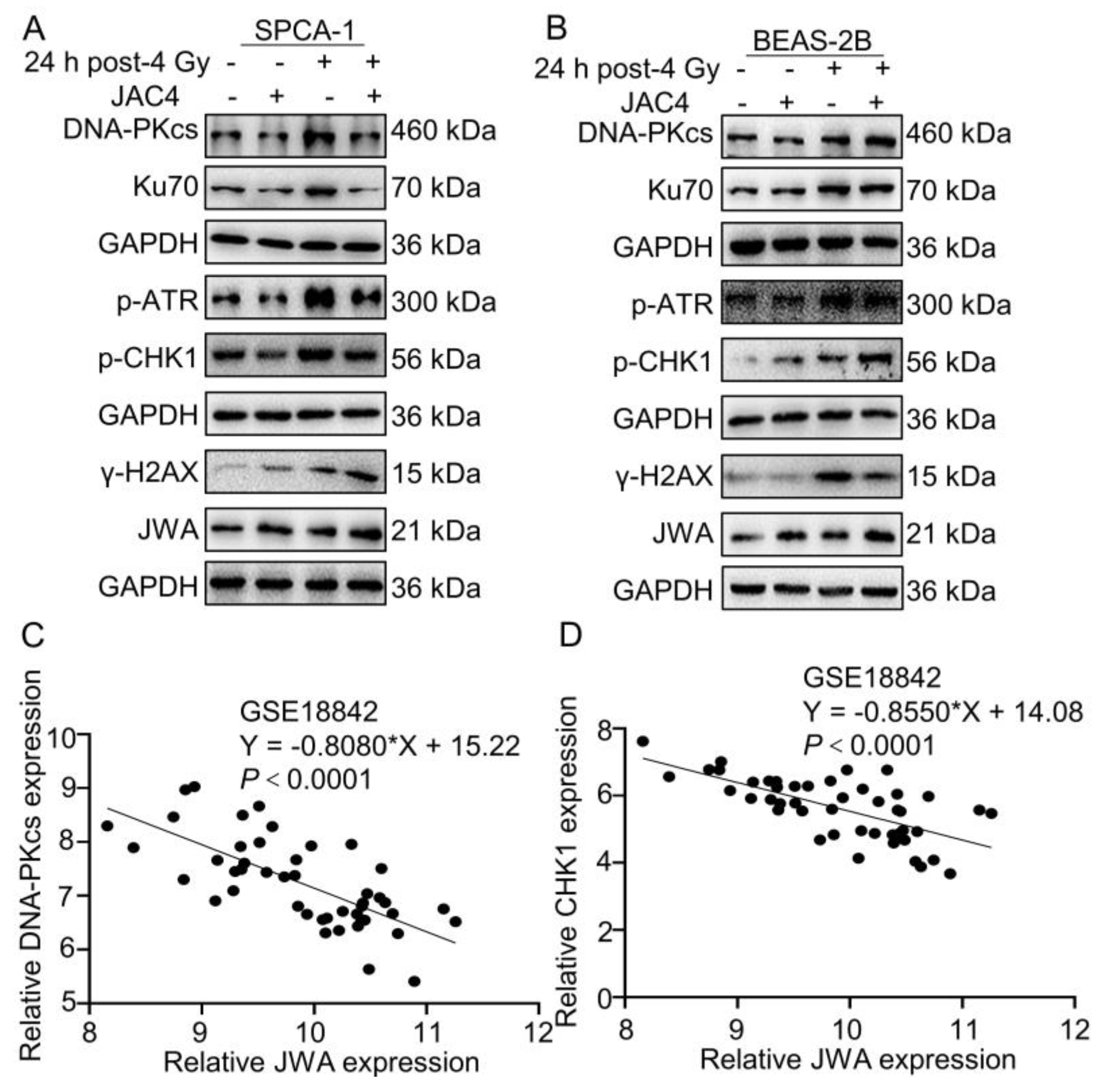
Figure 3.
JAC4 differentially regulates NHEJ and HR pathways in SPCA-1 and BEAS-2B cells. (A-B) The Western blotting images of DNA-PKcs, Ku70, p-ATR, p-CHK1, γ-H2AX, JWA and GAPDH in SPCA-1 and BEAS-2B cells at 24 h after 4 Gy of X-ray irradiation. (C-D) GSE18842 (Number of patients with NSCLC=46) database was analyzed for the correlation between JWA and DNA-PKcs and CHK1 mRNA expression.
Figure 3.
JAC4 differentially regulates NHEJ and HR pathways in SPCA-1 and BEAS-2B cells. (A-B) The Western blotting images of DNA-PKcs, Ku70, p-ATR, p-CHK1, γ-H2AX, JWA and GAPDH in SPCA-1 and BEAS-2B cells at 24 h after 4 Gy of X-ray irradiation. (C-D) GSE18842 (Number of patients with NSCLC=46) database was analyzed for the correlation between JWA and DNA-PKcs and CHK1 mRNA expression.
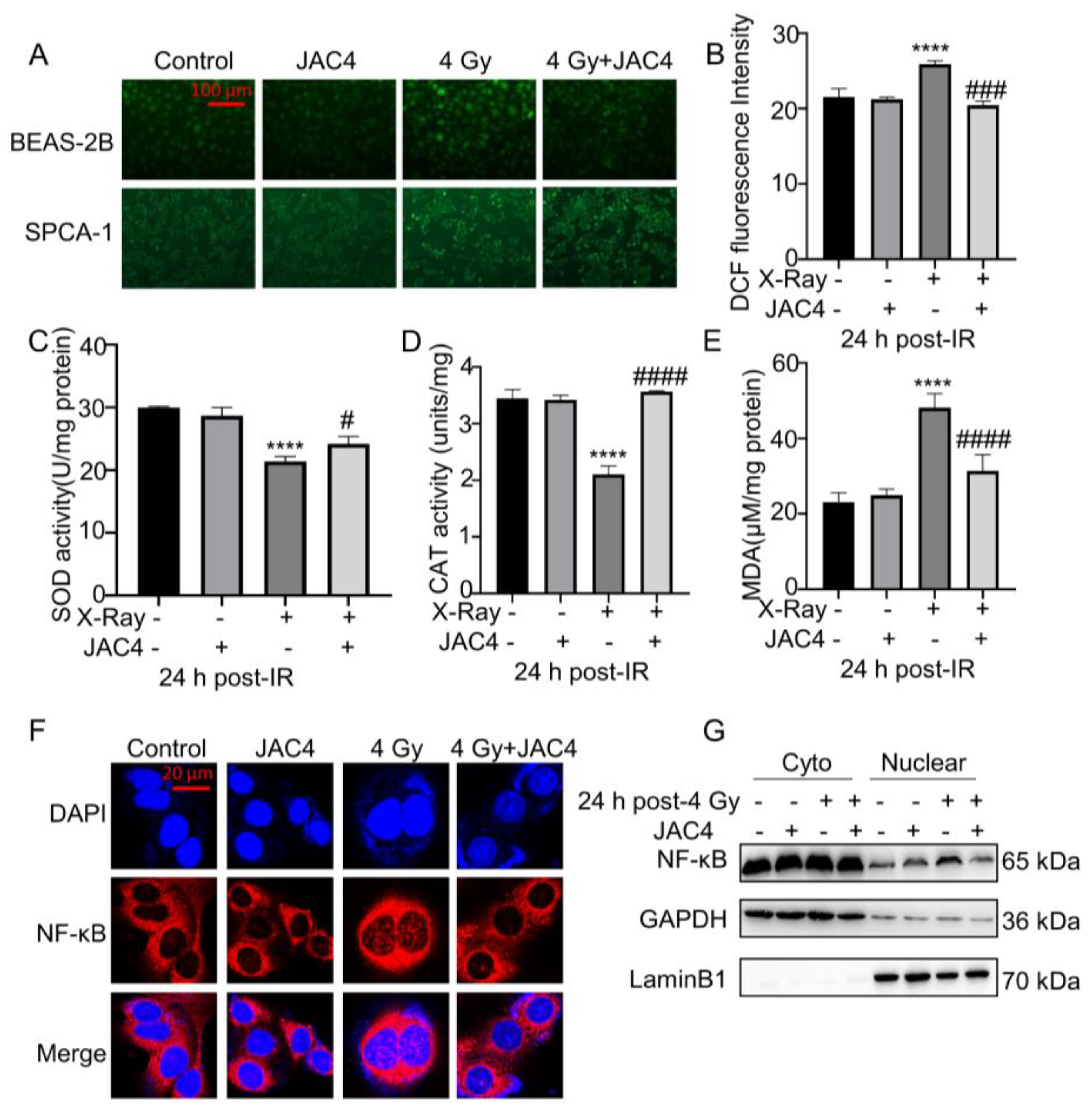
Figure 4.
JAC4 enhances the antioxidant capacity of BEAS-2B cells induced by irradiation. BEAS-2B and SPCA-1 cells were pretreated with JAC4 (10 µM) or DMSO for 24 h and then exposed to X-ray irradiation (4 Gy). (A) The intracellular ROS test images of SPCA-1 and BEAS-2B cells (Scale bars, 100 μm). (B) The ROS fluorescence intensity analysis in BEAS-2B cells. (C-E) The activity of SOD, CAT and the level of MDA in BEAS-2B cell lysates. (F)The immunofluorescence staining of P65 in BEAS-2B cells (Scale bars, 20 μm). (G) Nuclear-cytoplasmic separation Western blotting assay was carried out to confirm the position change of P65 protein in BEAS-2B cells. Data are presented as the mean ± SD (n=3); (**** p< 0.0001, vs. control group and # p< 0.05, ### p< 0.001, #### p< 0.0001, vs. the X-ray irradiation group).
Figure 4.
JAC4 enhances the antioxidant capacity of BEAS-2B cells induced by irradiation. BEAS-2B and SPCA-1 cells were pretreated with JAC4 (10 µM) or DMSO for 24 h and then exposed to X-ray irradiation (4 Gy). (A) The intracellular ROS test images of SPCA-1 and BEAS-2B cells (Scale bars, 100 μm). (B) The ROS fluorescence intensity analysis in BEAS-2B cells. (C-E) The activity of SOD, CAT and the level of MDA in BEAS-2B cell lysates. (F)The immunofluorescence staining of P65 in BEAS-2B cells (Scale bars, 20 μm). (G) Nuclear-cytoplasmic separation Western blotting assay was carried out to confirm the position change of P65 protein in BEAS-2B cells. Data are presented as the mean ± SD (n=3); (**** p< 0.0001, vs. control group and # p< 0.05, ### p< 0.001, #### p< 0.0001, vs. the X-ray irradiation group).
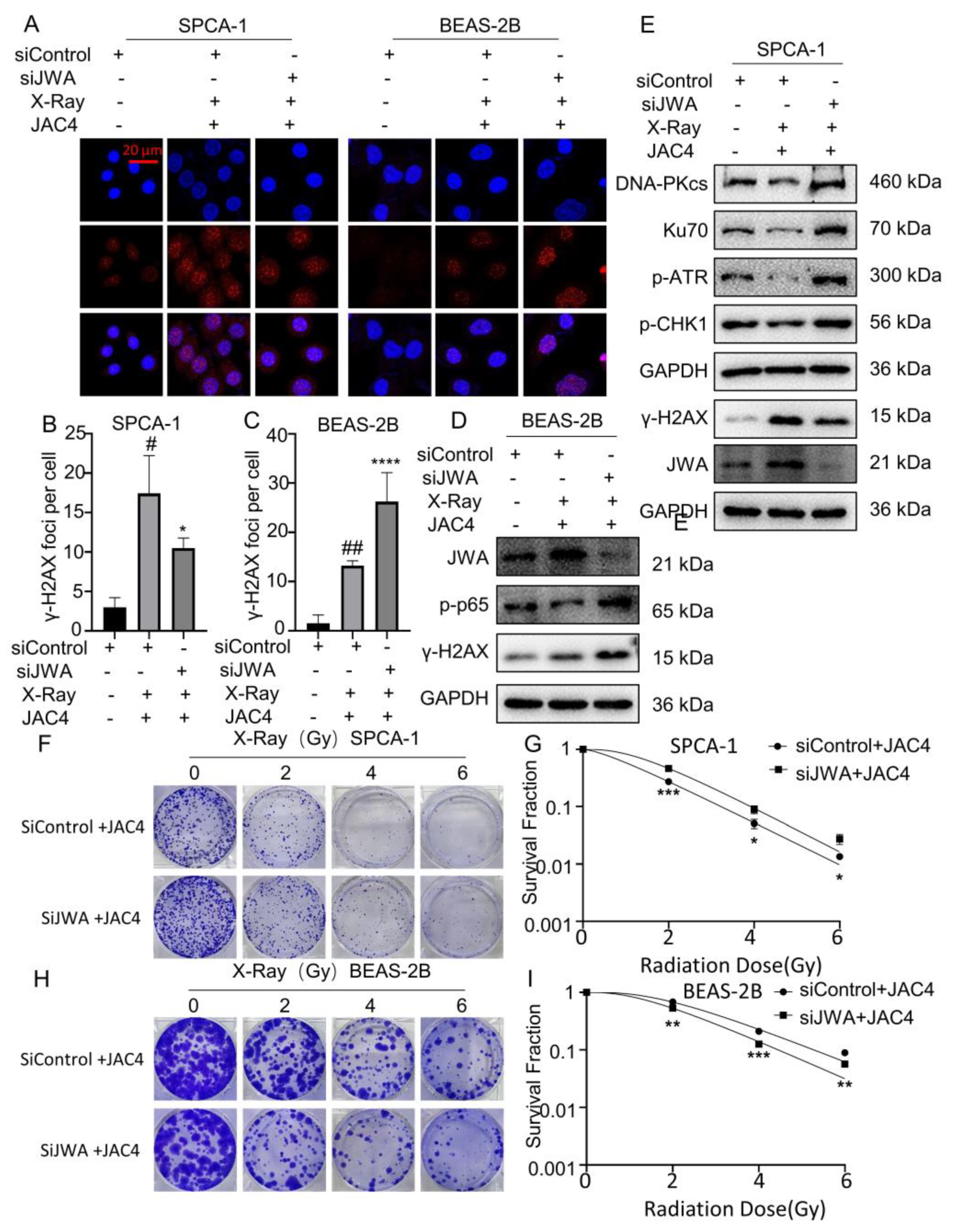
Figure 5.
Knockdown of JWA mitigates damages of X-ray on SPCA-1 but enhances the injuries on BEAS-2B cells. (A) γ-H2AX foci were tested by immunofluorescence assay in SPCA-1 and BEAS-2B cells transfected with sicontrol, sicontrol plus JAC4 and siJWA plus JAC4 groups 24 h after irradiation. The number of γ-H2AX foci of SPCA-1 (B) and BEAS-2B (C) cells were counted as described (Scale bar, 20 μm). (D) Protein levels of JWA, γ-H2AX and p-p65 in BEAS-2B cells of each group 24 h post-X-ray. (E) Protein levels of JWA, γ-H2AX, DNA-PKcs etc. in SPCA-1 cells of each group 24 h post-X-ray. (F-I) The radiosensitivity of JWA knockdown in SPCA-1 and BEAS-2B cells was verified by colony formation assay after the cells were irradiated with different doses (0, 2, 4 or 6 Gy). (n=3); (* p< 0.05, ** p< 0.01, *** p< 0.001, **** p< 0.0001 vs. sicontrol group and # p< 0.05, ## p< 0.01 vs. the siJWA plus JAC4 group).
Figure 5.
Knockdown of JWA mitigates damages of X-ray on SPCA-1 but enhances the injuries on BEAS-2B cells. (A) γ-H2AX foci were tested by immunofluorescence assay in SPCA-1 and BEAS-2B cells transfected with sicontrol, sicontrol plus JAC4 and siJWA plus JAC4 groups 24 h after irradiation. The number of γ-H2AX foci of SPCA-1 (B) and BEAS-2B (C) cells were counted as described (Scale bar, 20 μm). (D) Protein levels of JWA, γ-H2AX and p-p65 in BEAS-2B cells of each group 24 h post-X-ray. (E) Protein levels of JWA, γ-H2AX, DNA-PKcs etc. in SPCA-1 cells of each group 24 h post-X-ray. (F-I) The radiosensitivity of JWA knockdown in SPCA-1 and BEAS-2B cells was verified by colony formation assay after the cells were irradiated with different doses (0, 2, 4 or 6 Gy). (n=3); (* p< 0.05, ** p< 0.01, *** p< 0.001, **** p< 0.0001 vs. sicontrol group and # p< 0.05, ## p< 0.01 vs. the siJWA plus JAC4 group).
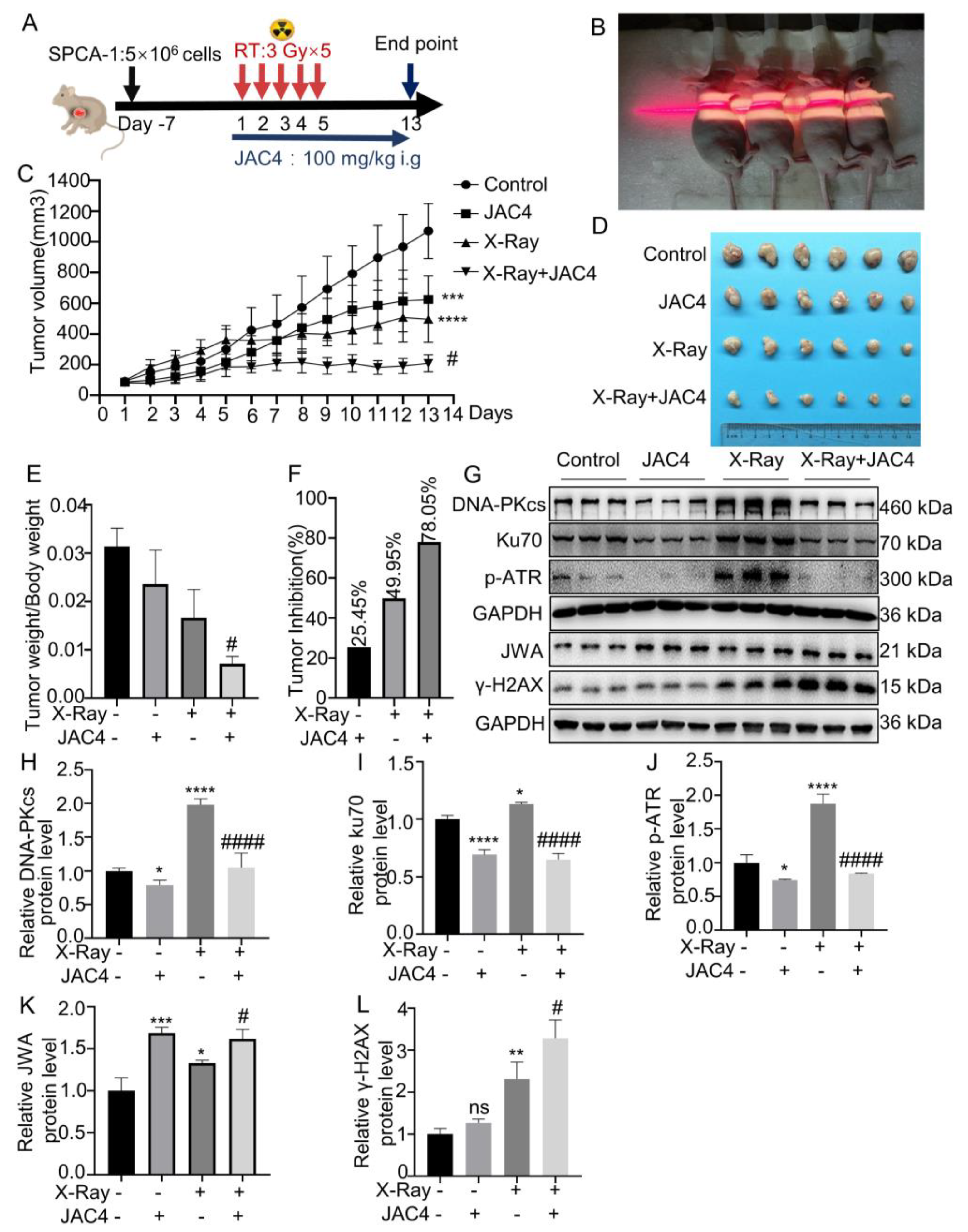
Figure 6.
JAC4 has a synergistic effect on X-ray-treated tumors in vivo. (A) Once the xenograft tumors reached specific volume, the mice were given oral JAC4 (100 mg/kg, once a day until the model end), and irradiated (3 Gy×5 fractions). (B) Irradiation therapy image (the irradiated areas included mice with subcutaneous tumors and the whole chest). (C) The tumor growth curves of xenograft model in control, JAC4, X-Ray and the combined groups (n=6). (D) Image of tumors in each group of mice. (E) The ratio of tumor weight to body weight on day 13. (F) The tumor inhibition rate in each group. (G) The Western blotting images of DNA-PKcs, Ku70, p-ATR, JWA and γ-H2AX in tumor tissues. (H-L) Quantification of DNA-PKcs, Ku70, p-ATR, JWA and γ-H2AX protein levels in tumor tissues. Data are presented as the mean ± SD (n=3); (* p< 0.05, ** p< 0.01, *** p< 0.001, **** p< 0.0001 vs. control group and # p< 0.05, ## p< 0.01, ### p < 0.001 vs. the X-ray irradiation group).
Figure 6.
JAC4 has a synergistic effect on X-ray-treated tumors in vivo. (A) Once the xenograft tumors reached specific volume, the mice were given oral JAC4 (100 mg/kg, once a day until the model end), and irradiated (3 Gy×5 fractions). (B) Irradiation therapy image (the irradiated areas included mice with subcutaneous tumors and the whole chest). (C) The tumor growth curves of xenograft model in control, JAC4, X-Ray and the combined groups (n=6). (D) Image of tumors in each group of mice. (E) The ratio of tumor weight to body weight on day 13. (F) The tumor inhibition rate in each group. (G) The Western blotting images of DNA-PKcs, Ku70, p-ATR, JWA and γ-H2AX in tumor tissues. (H-L) Quantification of DNA-PKcs, Ku70, p-ATR, JWA and γ-H2AX protein levels in tumor tissues. Data are presented as the mean ± SD (n=3); (* p< 0.05, ** p< 0.01, *** p< 0.001, **** p< 0.0001 vs. control group and # p< 0.05, ## p< 0.01, ### p < 0.001 vs. the X-ray irradiation group).
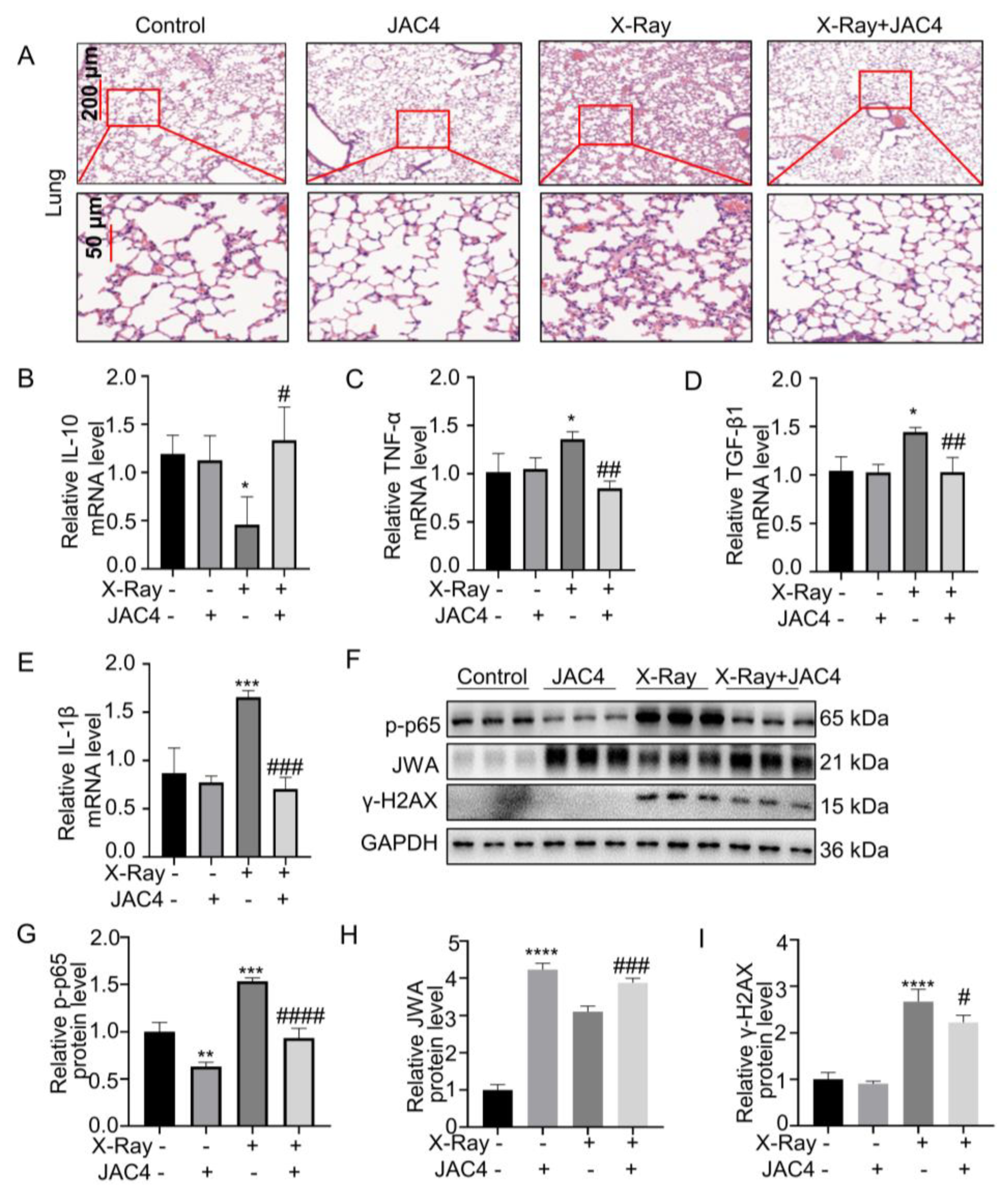
Figure 7.
JAC4 inhibits X-ray-induced inflammatory and injury of lung tissues in vivo. (A) H&E staining of lung tissues in each group. (B-E) The mRNA levels of IL-10, TNF-α, TGF-β1 and IL-1β in lung tissues were determined by qRT-PCR assay. (F) The Western blotting images of JWA, γ-H2AX and p-p65 protein in lung tissues. (G-I) Quantification of p-p65, JWA and γ-H2AX protein levels in lung tissue. Data are presented as the mean ± SD (n=3); (ns: p> 0.05; * p< 0.05, ** p< 0.01, *** p< 0.001, **** p< 0.0001 vs. control group; # p< 0.05, ### p < 0.001, #### p< 0.0001 vs. the X-ray irradiation group).
Figure 7.
JAC4 inhibits X-ray-induced inflammatory and injury of lung tissues in vivo. (A) H&E staining of lung tissues in each group. (B-E) The mRNA levels of IL-10, TNF-α, TGF-β1 and IL-1β in lung tissues were determined by qRT-PCR assay. (F) The Western blotting images of JWA, γ-H2AX and p-p65 protein in lung tissues. (G-I) Quantification of p-p65, JWA and γ-H2AX protein levels in lung tissue. Data are presented as the mean ± SD (n=3); (ns: p> 0.05; * p< 0.05, ** p< 0.01, *** p< 0.001, **** p< 0.0001 vs. control group; # p< 0.05, ### p < 0.001, #### p< 0.0001 vs. the X-ray irradiation group).
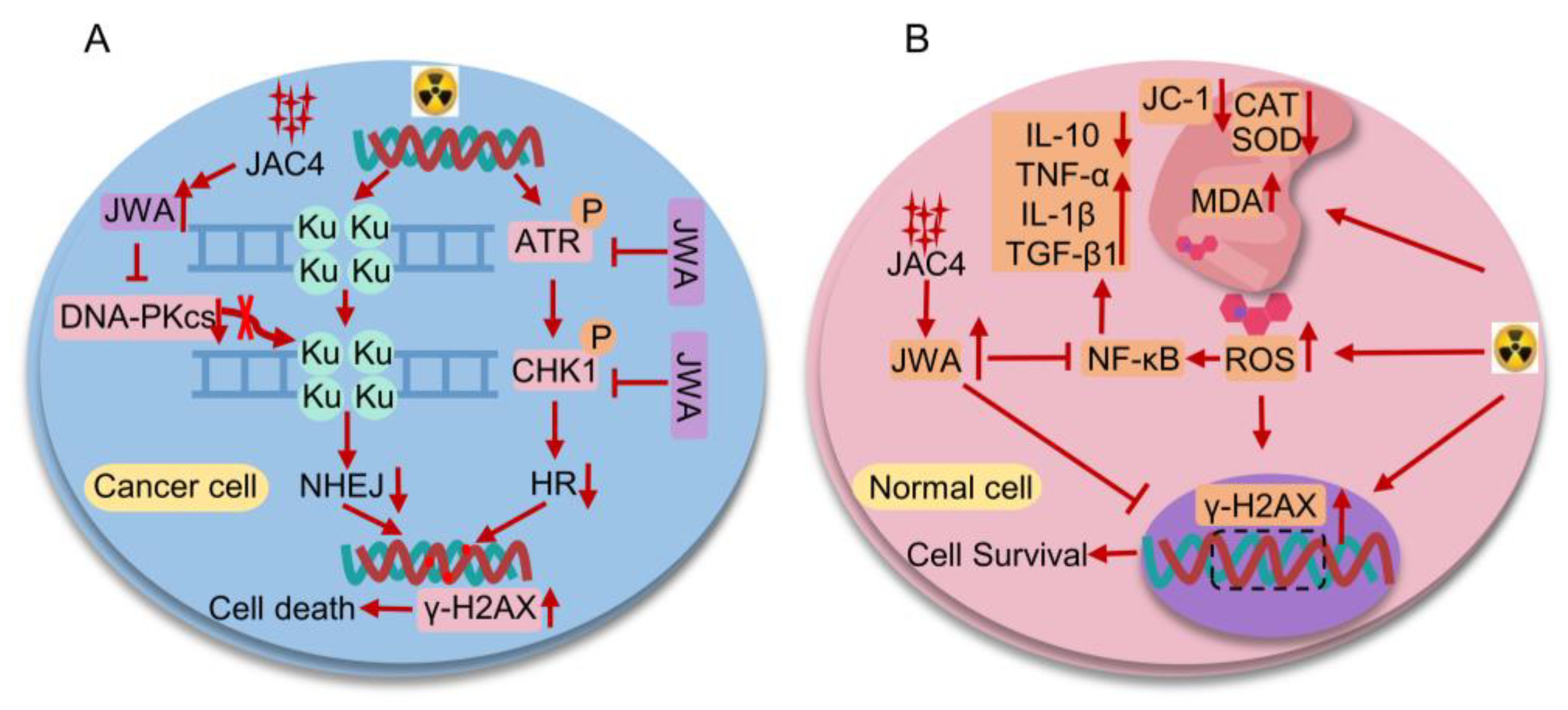
Figure 8.
The bidirectional mechanisms diagram of JAC4 in combination with radiotherapy for lung cancer. (A) Mechanism of action how JAC4 increases damages of X-ray on lung cancer cells. (B) Mechanism of action how JAC4 reduces damages of X-ray on normal lung epithelial cells.
Figure 8.
The bidirectional mechanisms diagram of JAC4 in combination with radiotherapy for lung cancer. (A) Mechanism of action how JAC4 increases damages of X-ray on lung cancer cells. (B) Mechanism of action how JAC4 reduces damages of X-ray on normal lung epithelial cells.